Published online Jul 9, 2022. doi: 10.5492/wjccm.v11.i4.201
Peer-review started: January 20, 2022
First decision: February 8, 2022
Revised: February 24, 2022
Accepted: May 17, 2022
Article in press: May 17, 2022
Published online: July 9, 2022
Processing time: 167 Days and 19.1 Hours
Recent research has demonstrated that critically ill patients with coronavirus disease 2019 (COVID-19) show significant immune system dysregulation. Due to that, some nutrients that influence immunomodulation have been suggested as a form of treatment against the infection. This review collected the information on the impact of vitamins on the prognosis of COVID-19, with the intention of facilitating treatment and prevention of the disease risk status in patients. The collected information was obtained using the PubMed electronic database by searching for articles that relate COVID-19 and the mechanisms/effects of the nutrients: Proteins, glucose, lipids, vitamin B12, vitamin D, calcium, iron, copper, zinc, and magnesium, including prospective, retrospective, and support articles. The findings reveal an optimal response related mainly to omega-3, eicosap
Core Tip: Immunomodulation has a considerable influence on the response to severe acute respiratory syndrome coronavirus 2 infection. Therefore, the medical team must acknowledge different resources to improve the immune system. In the current situation of prevalence coronavirus disease 2019, knowing the potential risks and benefits of nutritional supplementation can improve patients' response and avoid severe conditions, facilitating the process of healing. For that purpose, this article brings nutrients which might help and those which worsen the immunological regulation and other body functions, pursuing to mitigate the response against the virus.
- Citation: Costa BTD, Araújo GRL, da Silva Júnior RT, Santos LKS, Lima de Souza Gonçalves V, Lima DBA, Cuzzuol BR, Santos Apolonio J, de Carvalho LS, Marques HS, Silva CS, Barcelos IS, Oliveira MV, Freire de Melo F. Effects of nutrients on immunomodulation in patients with severe COVID-19: Current knowledge. World J Crit Care Med 2022; 11(4): 201-218
- URL: https://www.wjgnet.com/2220-3141/full/v11/i4/201.htm
- DOI: https://dx.doi.org/10.5492/wjccm.v11.i4.201
Among nutrition studies, some emphasize the importance of vitamins, trace elements, and long-chain fatty acids in supporting the immune system, keeping it able to protect against infections such as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)[1-3]. Therefore, the analysis of micronutrient supplementation is necessary to consider the effective optimization of the immune function and its use as adjuvant treatment in some cases[1].
Inadequate and insufficient intake of iron, zinc, vitamins B, C, and E can affect the immunological function of the organism and allows the presence of high levels of free radicals favoring oxidative stress[4]. Importantly, according to ESPEN expert statements and practice guidelines for the nutritional management of individuals with SARS-CoV-2 infection[5], oral nutritional supplements (ONS) should be preferred over enteral (EN) and parenteral nutrition, whenever possible to meet the patient’s needs. EN should be considered in polymorbid medical inpatients and elderly patients with a reasonable prognosis when ONS are not possible. ONS must provide at least 400 kcal/d, including 30 g or more of protein per day, for at least 1 mo[5].
On the other hand, a diet rich in vitamin C and zinc improves neutrophil phagocytosis, monocytic activity, and immune cell locomotion, and vitamin D is related to the mediation of interleukins (ILs) essential for immune defense, acting in the induction of antimicrobial peptides in macrophages[5]. The strong qualitative T-cell response is crucial against SARS-CoV-2, and lymphopenia is associated with elevated mortality[6,7]. Both CD4+ and CD8+ T-cell responses are present in infection, although the latter is inefficient[6]. Elevated IL-2 associated with decreased IFNγ levels have been observed in these cells, increasing the severity and chronic course of the disease[8].
Successful immune regulation of innate and adaptive immunity is a predictor for avoiding severe responses to SARS-CoV-2 infection[6]. Critically ill infected patients showed increased neutrophil counts, tissue damage, activation of the coagulation cascade, and decreased hemoglobin and lymp
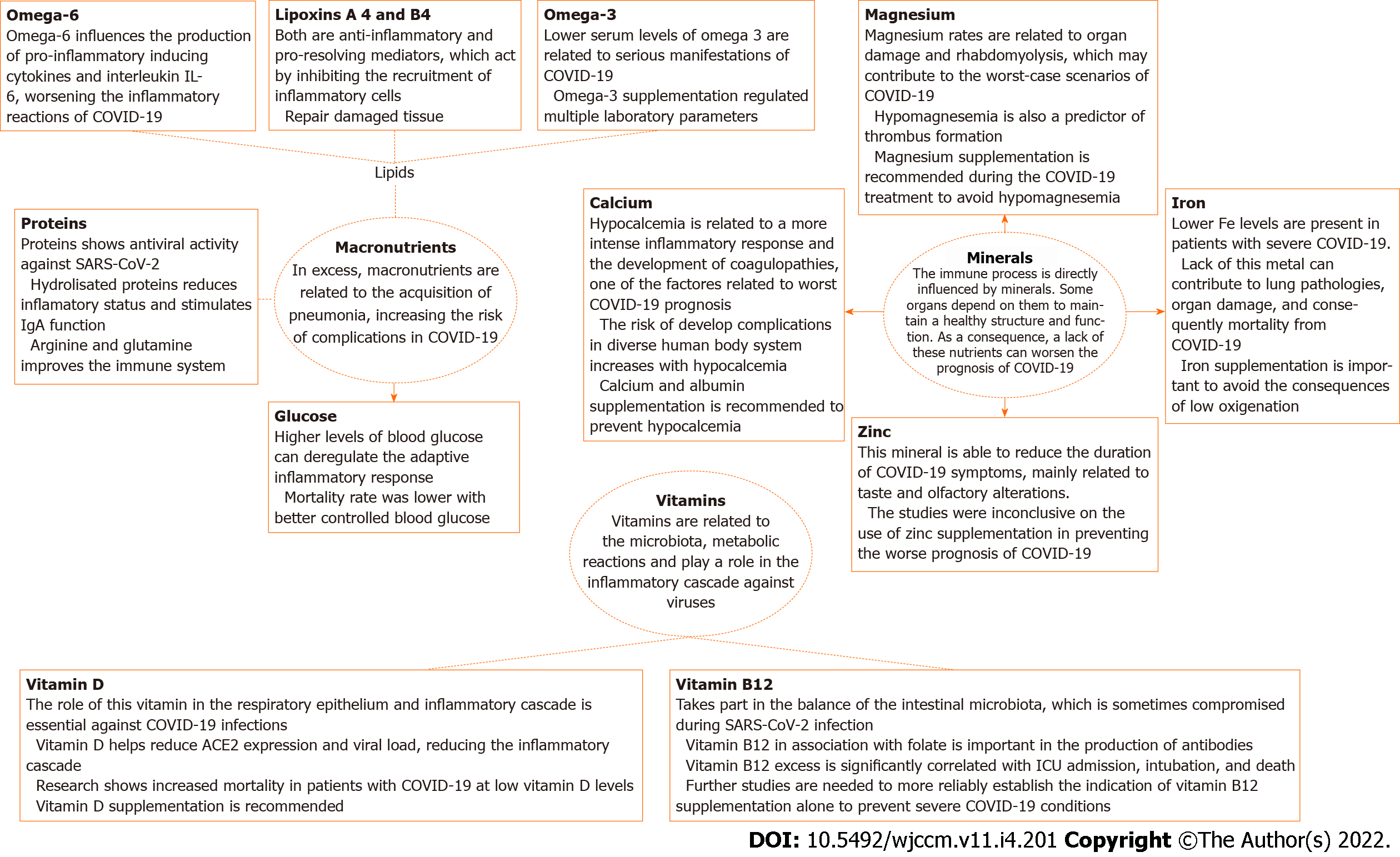
Some nutrients such as carbohydrates, proteins, omega 3, vitamin B12, vitamin D, iron, copper, calcium, zinc, and magnesium are the focus of this article for being directly linked to the host immune response in coronavirus disease 2019 (COVID-19) cases. Apart from these, conjugated linoleic acid and vitamins A and E regulate cytokine production as well as the proliferation and differentiation of specific leukocyte populations, in addition to acting on immunoglobulin production and lymphocyte differentiation[10-12]. In this review, we summarize the mechanisms of immunomodulation promoted by micro- and macro-nutrients in COVID-19.
This methodological review was conducted by two investigators, working independently with the guidance and support of a research advisor. Both prospective or retrospective trials and support articles were identified using The United States National Library of Medicine (PubMed). Between October 4, 2021 and February 15, 2022, we searched the relevant articles published in English using the following specific descriptors: COVID-19; SARS-CoV-2; immune system; immune response; vitamin B12; cobalamin; macronutrients; micronutrients; carbohydrate; protein; lipid; intensive care; vitamin D; iron; copper; zinc; magnesium and calcium; severe; nutrition; therapy; critically ill patients; coronavirus; immunomodulation; pro-resolving mediators; and inflammation. The descriptors were used alone and/or in combination in the PubMed database. No restriction was made as to the date of publication of the articles, nor was a target age range defined. Articles not written in English and not addressing these topics in the title and/or abstract were excluded. Original articles describing prospective, retrospective, and cross-sectional studies were included, as well as secondary research, such as systematic and narrative reviews. Guidelines were also included. Commentaries, editor letters, book chapters, and manuals were not included. Finally, 3316 articles were identified, of which 122 were included in this minireview.
The dietary factor that leads to the weakening of immune functions is the failure of macro- and micro-nutrient intake. In addition, clinical studies have shown that malnutrition, weight imbalance, and fragility and dysbiosis of the gut microbiota are the main factors involved in the deterioration of immune functions in infected patients[13].
The use of immunonutrients aims to increase the production of less potent inflammatory mediators and reduce those highly inflammatory, besides minimizing the production of free radicals and modulating the generalized inflammatory response[14]. For diabetic patients, this formulation is suggested, as it is a supplement already used. Once a product is removed from the formula, fruit is added to reach the caloric goal and improve palatability. Protein is the most important macronutrient for maintaining immune function and preserving muscle mass[1].
Proteins are types of macromolecules made of amino acids (AA) that perform various important functions for the body, for example, acting as antibodies, enzymes, messengers, transporters, and structural components in the body[15,16]. Some studies indicate that protein supplementation stimulates the immune system, which specifically improves infectious disease surveillance[17].
Studies with hydrolyzed proteins have shown that they are able to reduce the inflammatory state and stimulate IgA function and production. Also, arginine and glutamine are both non-essential amino acids that enhance the action of the immune system. The former is associated with macrophages in the generation of nitric oxide, and the latter provides energy for immune cell utilization[18].
Proteins show antiviral activities against enveloped and non-enveloped viruses. They inhibit virus entry into the cell by adhering to cell receptors[19]. Viruses need some enzymes, including DNA or RNA polymerases, reverse transcriptase, and integrase for replication, and some evidence suggests that proteins can inhibit the activity of these enzymes and eventually prevent virus replication[20,21].
On the other hand, increased consumption of saturated fats, refined carbohydrates, and alcohol, and low levels of fiber, unsaturated fats, micronutrients, and antioxidants significantly impair adaptive immunity while increasing innate immunity, which leads to chronic inflammation and severe damage to the host defense against viral pathogens[1]. These dietary patterns might have a detrimental effect on immune responses and are involved in the development of several inflammatory diseases[22]. Excessive macronutrient intake contributes to the propensity to acquire pneumonia, which is the most common high-risk complication of COVID-19[23].
The high mortality from COVID-19 in obese people points to an important role in nutrition[24]. Food can influence cytokine gene expression levels and thus modulate inflammation and oxidative stress[25]. Cytokines such as tumor necrosis factor (TNF)-alpha and IL-6 when produced excessively have been related to dysregulation of the inflammatory response and stimulation of cytokine storms[26]. Furthermore, increased adipose tissue contributes to greater leptin production, which is related to macrophage activation and proliferation, while reduced adiponectin levels decrease the synthesis of anti-inflammatory compounds. In addition, there is an increase in the release of non-esterified fatty acids into the bloodstream, which also leads to the perpetuation of the chronic inflammatory process[27]. Health-related consequences in populations affected by economic outages, quarantines, and curfews due to SARS-CoV-2 infection include psychological distress[28-30], which is associated with an increase in carbohydrate and lipid intake[31] and a decrease in physical exercise[32], resulting in weight gain and increased rates of overweight and obesity. Adipose tissue, besides storing energy, is responsible for producing certain substrates that, in excess, can stimulate a state of constant oxidative stress and contribute to the severity of clinical manifestations during SARS-CoV-2 infection.
Some comorbidities have emerged as risk factors for the severe development of COVID-19, including type 2 diabetes, increased body weight, hypertension, and dyslipidemia. In this sense, increased glucose concentrations may be responsible for the reported poor outcome. A recent study reported that type 2 diabetes was associated with a higher mortality rate due to COVID-19, although the mortality rate was lower with better controlled blood glucose[33]. Furthermore, diabetes mellitus (DM) can impair the adaptive inflammatory response by delaying T-cell activation, as well as negatively impact neutrophil chemotaxis and contribute to cytokine storm, leading to dysregulation of the immune response, susceptibility to infection, and an increased chance of severe clinical manifestation during SARS-CoV-2 infection[34]. On the other hand, DM was related to the overexpression of angiotensin-converting enzymes in some organs such as the heart, lungs, liver, and pancreas, increasing the severity of the cases and leading to organ failure during infection[35]. Consequently, diabetes was significantly associated with the development of acute respiratory distress syndrome, with a hazard ratio of 2.3[36].
The few articles available that mention supportive care in COVID-19 recommend that nutritional status should be assessed in all infected patients on hospital admission[1,5,37] and that patients at nutritional risk should receive nutritional support as early as possible, especially through increasing the protein intake by ONS[5,37].
Moreover, studies, including SPEN statements[5], highlight that even patients with COVID-19 who are not at risk of malnutrition should maintain an adequate intake, especially regarding adequate amounts of protein (1.5g/d) and calories (25-30 kcal/d), as well as oral supplementation with whey protein (20g/d) and intravenous solutions of multivitamins, multiminerals, and trace elements (goal: satisfaction of recommended dietary intake on admission). The choice of whey proteins is based on their anabolic and antioxidant properties combined with high digestibility[38,39]. Its potential clinical benefits have been highlighted in cancer cachexia[40] and were recently demonstrated in a randomized controlled trial of malnourished patients with advanced cancer[41]. Whey proteins also have immunomodulatory properties[42] and potential antiviral activity[43]. Furthermore, whey protein supplementation has been associated with improved immune recovery in HIV patients during the first 3 mo of antiretroviral treatment[44].
Figure 1 provides a summary of mechanisms of action of proteins and other nutrients in targeting SARS-CoV-2 infection.
Decreased or absent lipids in nutritional support can cause essential fatty acid deficiency, especially in preterm infants, and result in insufficient synthesis of omega-3 fatty acids, docosahexaenoic acid (DHA), and omega-6 fatty acid arachidonic acid (ARA)[45]. Lipids also play an important role in the delivery of fat-soluble vitamins such as vitamins A, D, E, and K[46]. Physiological processes such as metabolism, immune response, oxidative stress, blood clotting, organ function, and wound healing have a direct association with fatty acid availability[46,47]. However, this process needs to be well balanced, given that excess lipids can cause undesirable consequences. The excess of linoleic acid (LA) may be associated with exacerbation of inflammation, manifested mainly by increased levels of CRP, although other biomarkers such as IL-6, adiponectin, and adhesion molecules have not shown significant changes related to higher levels of LA consumption[48]. For this, studies have evaluated the impact of the use of substances able to reduce the expression of cytokines that contribute to the gravity of the infection and the enhancement of the inflammatory state in SARS-CoV-2 infection[49].
Omega-6 polyunsaturated fatty acids (PUFAs) can metabolize LA and further desaturate and form ARA, the main PUFA in cell membranes involved in inflammation in humans[50]. Omega-6 PUFAs may influence inflammation due to the fatty acid composition of the cell membrane phospholipids, which modulates cellular responses and cellular function[50,51]. Membrane phospholipids produce second messengers, such as diacylglycerols, endocannabinoids, and platelet activating factor, that act on biological activity[52]. These second messengers also modulate gene expression and physiological and metabolic responses, affecting the immune and inflammatory response, disease severity, and clinical outcome[53]. Moreover, ARA composes peripheral blood mononuclear cells, such as lymphocytes, neutrophils, and monocytes[52]. ARA also acts as a substrate for the enzymes cyclooxygenase, lipoxygenase, and cytochrome P450, constituting eicosanoid mediators such as leukotriene B4 (LTB4) and prostaglandin E2, which induces pro-inflammatory cytokines and IL-6[53,54]. LTB4 promotes leukocyte chemotaxis, adhesion, and degranulation, increases vascular permeability, and produces inflammatory mediators, leading to a pro-inflammatory effect[51,54]. ARA metabolism also results in the production of lipoxin A4 (LXA4) and lipoxin B4 (LXB4)[55,56]. LXA4 is an anti-inflammatory and pro-resolution mediator that acts by inhibiting inflammatory cell recruitment, cytokine production, and NADPH oxidase function, and restoring normal physiological function in damaged tissue, which leads to decreased inflammation[57,58]. Studies suggest that LXA4 can suppress leukocyte-mediated injury and promote chemotaxis of monocytes, and phagocytosis of apoptotic neutrophils[59]. LXB4 is generated by mucosal tissues in the upper respiratory tract and lower airways, and acts by regulating neutrophil activation[60].
In contrast, PUFAs, such as omega-3, are lipid compounds with potent anti-inflammatory activity, responsible for the homeostasis of the organism and regulation of various biological functions. It can be produced in small quantities by the human organism; however, it is possible to obtain this nutrient through foods such as fish, nuts, and soy oil, and the intake of 250 to 2000 mg/d is recommended for adults and 200 to 250 mg/d for children[61]. Lipid and carbohydrate requirements are adapted using the energy ratio of fat and carbohydrates between 30:70 in patients without respiratory impairment and 50:50 in patients on mechanical ventilation[5]. Linolenic acid, eicosapentaenoic acid (EPA), and DHA correspond to the representatives of this group of essential fatty acids, and their metabolism results in substances such as protectins and resolvins that regulate platelet coagulation and the inflammatory process[62].
EPA and DHA sources have shown potential anti-inflammatory activity, in addition to promoting immune function and improving liver metabolism[63]. Studies have shown that resolvins are able to reduce the inflammatory response by decreasing neutrophil invasion and reducing the synthesis of pro-inflammatory cytokines via inhibition of nuclear factor kappa B (NF-κB), in addition to promoting monocyte recruitment and increasing phagocytosis of apoptotic neutrophils and macrophage clearance[46,64]. Resolvins are specialized pro-resolving lipid mediators (SPMs), endogenous lipid mediators that include protectin, maresins, and lipoxins (LXs). SPMs are involved in the pathophysiology of respiratory diseases, such as COVID-19, and play a role in signaling events during the inflammatory process[65-67]. Studies have also shown their potential in tissue repair, regression of inflammation by increasing anti-inflammatory mediators such as IL-10, and regulating the adaptive immune response[67,68]. Several studies using animal disease models have shown the potential of SPMs to decrease lung inflammation and tissue damage, and to be able to disrupt the cytokine storm. Furthermore, SPMs do not act as an immunosuppressive agent[65,66,69,70]. Thus, regarding COVID-19, SPMs may in the near future be used to treat inflammation with the active precursors 18-HEPE, 17-HDHA, and 14-HDHA[66,69,71]. Furthermore, omega-3 PUFAs have been linked to reduced expression of cyclooxygenase 2 and decreased levels of pro-inflammatory cytokines such as IL-6, IL-8, and IL-1 beta and free radicals[72].
PUFAs are responsible for altering the composition of cell membranes, modulating cell signaling, and influencing immune responses[73,74]. They are present in the cell membrane, taking part in the formation of the phospholipids and assisting in the maintenance of both cell structure and functionality. Thereby, alterations in the composition and homeostasis of these compounds are able to influence cellular responses[75]. Thus, studies have shown that, due to their lipophilic capacity, PUFAs can bind to the cell membrane, altering the permeability of this structure, interfering with the virus' binding to the angiotensin-converting enzyme 2 (ACE2) receptor, and also interrupting its action as a receptor[76]. In addition, omega-3 PUFAs could contribute to alterations in the structure of the lipid rafts that carry the ACE2, being able to modify the ability of the virus to bind to its receptor and reduce replication rates[77]. This lipid could directly regulate and alter the amount, size, and the proteins expressed in the rafts by modulating the binding between the virus and its receptors[78]. Finally, the viral spike protein, which is responsible for interacting with ACE 2 and allowing entry into the cell, could be inactivated by PUFAs when they bind, thus blocking infection[79].
During cases of infection, adequate management of the patient's nutritional status must be performed, since systemic inflammation is capable of increasing the demand for nutrients and propitiating a picture of malnutrition that may worsen the clinical picture generated by COVID-19[80]. As a result of the aforementioned, lipid nutritional support emerges as a possible element in medical nutrition therapy for critically ill patients with COVID-19[73,74]. Studies have shown that there is a possible association between omega-3 levels in the body and reduced fatal outcomes caused by COVID-19[79]. A cross-sectional study observed a possible relationship between low omega-3 PUFA levels in the body and clinical manifestations of COVID-19. However, there is a need for further research that evaluates a larger population and standardizes the levels of this lipid as a possible predictor of risk in the bloodstream during infection[80]. Of note, patients admitted to intensive care units (ICUs) with respiratory distress syndrome had improved oxygenation and reduced length of stay after administration of this lipid[81,82]. Similarly, a randomized clinical trial with 101 patients reported that during omega-3 PUFA supplementation, there was regulation of some laboratory parameters such as normalization of arterial pH, bicarbonate level, and base excesses, as well as improvement in renal function[83].
Vitamin B12, also known as cobalamin, is a micronutrient obtained mainly through the consumption of animal source foods and absorbed in the gastrointestinal tract through metabolic pathways involving substances such as hydrochloric acid, pepsin, and intrinsic factor. It is a micronutrient with well-established functions in red blood cell synthesis, cell growth, the nervous system, and DNA synthesis. The active forms of cobalamin are hydroxocobalamin and methylcobalamin, which are closely linked to folic acid and adenosylcobalamin[84,85]. In addition, studies suggest that vitamin B12 plays an important role in the immune system by assisting in balancing the gut microbiota, which is sometimes compromised during SARS-CoV-2 infection[4]. Yet, cobalamin modulates the immune system by exerting influence on T lymphocytes, participating in their differentiation and proliferation and, thus, being important in maintaining the ratio between cytotoxic and helper T cells, in addition to influencing the activity of natural killer cells. By playing a role in cell division, vitamin B12 can have a direct influence on the rapid proliferation of B lymphocytes. Furthermore, vitamin B12 in association with folate is important in the production of antibodies[86]. Considering that vitamin B12 participates in metabolic reactions involving carbon-1, with interactions occurring with folate metabolism, in individuals with low levels of vitamin B12, 5-methyl-tetrahydrofolate (THF), produced by an irreversible reaction, results in an inactive form of folate. 5-methyl-THF can result in secondary folate deficiency, impairing purine and thymidine synthesis. This results in changes in DNA and RNA synthesis and, consequently, in the secretion of immunoglobulins[87].
Thus, insufficiency or deficiency of micronutrients such as vitamin B12 may affect the host immune response against viral infections and inflammatory activity, as well as influence the clinical outcomes of patients with COVID-19 in both immunological, microbiological, and hematological forms[88,89]. A single-center study[90] noted that patients who died from SARS-CoV-2 infection had less vitamin B12 when compared to those hospitalized in ICUs, but no significant differences were observed between them. Another study that evaluated serum micronutrient levels and disease severity in COVID-19 patients reported that some of these substances, such as cobalamin, were reduced in these individuals[91]. On the other hand, some patients may also have increased B12 levels, especially those who were intubated and deceased, with excess vitamin B12 being significantly correlated with a worse prognosis, such as ICU admission, intubation, and death[92]. Similarly, this increase was also observed in patients with poor clinical outcomes in another study[92,93]. The liver is responsible for cobalamin storage and damage to this organ in hospitalized patients may be the cause for the high levels of this vitamin found in certain individuals. However, despite the high plasma concentration of cobalamin, these patients may have neurological and hematological conditions, which are common in patients with low concentrations of the micronutrient. There are two possible pathways for the occurrence of this paradoxical effect: Tissue lysis reduces the intracellular concentration of cobalamin and increases the plasma concentration; thus, the high concentration ends up interfering in the transport of the substance and, consequently, in the intracellular uptake[94].
Given this scenario, studies linked to vitamin B12 supplementation are scarce and show inconclusive results. In this sense, some authors advocate supplementation associated with other micronutrients, making it difficult to analyze their results in isolation. In non-COVID-19 situations, vitamin B12 deficiency is classically treated with parenteral injection therapy of 1000 μg for 1 to 2 wk, followed by monthly administration. Intramuscular injections are uncomfortable and painful in children, as well as expensive. Thus, oral preparations are being investigated[95]. In a study, children over 6 years were treated with a daily pill containing thiamine 250 mg, pyridoxine 250 mg, and cyanocobalamin 1000 μg for 3 mo, and those under 6 years old with an ampoule of 1000 μg of vitamin B12. This treatment was effective for vitamin B12 nutritional deficiency[96]. Another study that evaluated 47 individuals aged 1 mo to 17 years with serum vitamin B12 levels less than 200 pg/mL treated for 120 d with 1000 μg of this oral vitamin showed improvement in cobalamin levels. However, despite the high dose, reduced results were achieved in older children, indicating the need for dose adjustment according to weight[97]. Yet, patients ≥ 6 or ≥ 18 years who reported gastrointestinal abnormalities or restricted diet received 1000 μg of oral vitamin B12 or 1000 μg intramuscularly in nine injections for 3 mo and both administrations restored the cobalamin levels of all patients[98].
A study performed joint supplementation of magnesium and vitamins B12 and D3 in individuals aged over 50 years with COVID-19 and observed less need for supplemental oxygen and ICU admission[99]. Therefore, these findings suggest the potential role of vitamin B12 in limiting disorders and complications related to SARS-CoV-2 infection, and further studies are needed to more reliably establish whether vitamin B12 alone is able to show statistically significant results in these patients[100,101].
Although the level of vitamin D has been widely studied in patients infected with SARS-CoV-2, other previous studies have evaluated the role of this vitamin in patients with acute respiratory infections (ARI), mainly in the upper airways[102,103].
The role of vitamin D in bone health, through calcium and phosphorus maintenance, is well established[104], but its role in respiratory infections appears to be related to the production of antimicrobial peptides in the respiratory epithelium and in the response of the inflammatory cascade against the virus[105-107]. In addition, vitamin D helps maintain cell junctions and gaps, decreasing the cytokine storm caused by the infection[108], and inhibiting type 1 T helper cell response and T cell induction[109]. Furthermore, vitamin D deficiency causes deprivation in macrophage production and performance, interfering with the innate immune response and favoring the establishment of infection[110]. Thus, although the levels of this macronutrient do not represent a great impact in reducing the risk of contracting the disease, studies show that there is a great impact on the modulation of the innate and adaptive immune response and, consequently, on the severity of the disease[111].
The pathophysiology of SARS-CoV-2 infection is favored by high expression of ACE2, a receptor through which the virus enters cells of the lung epithelium and other organs, triggering activation of the pro-inflammatory cascade and viral replication[112]. Increased storage of the inactive form of vitamin D (calciferol) increases the risk of virus infection because it stimulates ACE2 production[110]. However, as this is one of the factors for the manifestation of more critical forms of COVID-19, at the experimental level, vitamin D helps to reduce ACE2 expression and viral load by reducing the inflammatory cascade[111,112].
Despite such evidence, studies involving this vitamin and the prognosis of patients with COVID-19 are inconclusive, and most of them are observational or retrospective studies with a small, usually single-center sample. Therefore, the medical recommendation for vitamin D supplementation is based on the observation of increased mortality from COVID-19 in those with low vitamin D levels, even with adjustment for patient age[111]. Studies indicate that vitamin supplementation is relevant only in patients who are vitamin-deficient or at risk for immune system deficiency, such as patients with chronic diseases[113]. In a study that looked at different doses of vitamin D in patients with COVID-19, the recommendation for people at risk of influenza and/or COVID-19 was supplementation of 10000 IU/d of vitamin D3 for a few weeks and then 5000 IU/d, without describing the variation for the patients’ age group. The ultimate goal would be to rapidly increase 25(OH)D concentrations and reach concentrations between 40-60 ng/mL (100-150 nmol/L). For the treatment of patients with COVID-19, higher doses, depending on the reference protocol, may be useful[108].
There are studies that have shown lower vitamin D levels in critically ill patients with COVID-19[78,114] and in addition, a 15% reduction in the number of severe COVID-19 cases with normal vitamin D status was found in a population[115]. However, after removing confounding variables, the results are still inconclusive. Other studies that have found increased mortality from infection in countries with vitamin D deficient populations, such as Italy, point to overlapping risk factors related to old age, obesity, and diabetes[104].
Meanwhile, high level supplementation may be recommended for patients at risk. A randomized controlled trial indicates that high doses of vitamin D supplementation are a successful treatment for high-risk elderly patients, and that this type of treatment would not pose risks to patients. Still, it is clear that further prospective, randomized, controlled, large-scale studies on vitamin D supplementation related to mortality and severity of COVID-19 are needed to conclude[113].
Hypocalcemia is quite common in viral diseases, which overcomes the fact that studies report its presence in more than 60% of patients hospitalized for SARS-CoV-2 infection[116]. The calcium ion is involved in two important parts of the development of COVID-19. It is of paramount importance for the life cycle of the virus, but it is also related to the inflammatory response and its regulation[117].
Some hypotheses are raised to explain this condition. Among them, we can mention some degree of malnutrition that causes hypovitaminosis D and hypoalbuminemia in COVID-19, given that the calcium ion is primarily linked to albumin, the high degree of inflammation in the infected patient, as well as a consequence of this, alterations in the receptors and in the hormonal axis of calcium, which causes it to be mobilized from the bones. Furthermore, it is possible to mention the fact that patients with hypocalcemia have fewer lymphocytes and higher levels of D-dimer, justifying the more intense inflammatory response, as well as greater chances of developing coagulopathies. Regarding lymphopenia, it can be justified by mechanisms of bone marrow suppression that may have been caused by the virus and/or by direct destruction of these lymphocytes, due to all the toxic substances that are produced during the SARS-CoV-2 infection, mainly the cytokines[118]. D-dimer is related to the cytokine storm that is caused in more severe cases of infection. This is because this intense immune reaction activates the coagulation cascade, favoring the occurrence of thrombotic events. As D-dimer is involved in blood clotting events, its detection in examinations is favorable to attest to a possible state of thrombosis in the patient, which increases the chances of pulmonary complications and thromboembolism[118]. However, the increase in unsaturated lipids can also contribute to hypocalcemia, due to the link established with the ion[116,119,120].
Given this and studies that have evaluated calcemia in hospitalized patients, it was possible to establish an important risk factor between low calcium levels and increased risk of developing serious diseases, complications in the cardiovascular system, nervous system, and muscle, and mortality[116,120,121].
In regard to the viral life cycle, much has been studied about the role of calcium. SARS-CoV-2 needs to release its genetic material inside the host cell and to do so, it needs to penetrate the host cell membrane and fuse its membrane with the viral membrane[122].
The key and initial point lies with the spike (S) protein, which is composed of two subunits, S1 and S2, containing a region called fusion peptide (FP) that is crucial in the cell invasion process, along with the help of calcium, which binds to two negatively charged FP residues located in the S2 subunit to allow viral fusion. In this sense, calcium acts directly on the proteins responsible for mediating fusion, playing an activating role and increasing the binding of the S protein to host cells, favoring viral penetration[122-124]. Importantly, PF interacts with the host cell membrane, changing its structure and allowing membrane fusion[124].
Despite the use of calcium in the process of virus entry into the host cell, what may account for the hypocalcemia is the lack of the viral envelope protein E that alters intracellular calcium metabolism, favoring the increase of IL-1B. This cytokine is responsible for regulating the expression of a calcium-sensitive receptor. With the action of the cytokine, the set point of calcium suppression by PTH is reduced. Thus, even though calcium is in lesser amounts, it is able to decrease PTH secretion and corroborate an even greater decrease in serum calcium[117].
Early use of calcium and albumin supplementation is reported to lead to reduced toxicity from free fatty acids, which are then carried by albumin, and to decrease the degree of mitochondrial metabolic dysfunction and organ damage[125,126]. Also, a meta-analysis of 199298 patients demonstrated that the use of calcium channel blockers (CCBs) in hypertensive patients reduced mortality rates in hypertensive patients with COVID-19. This may be explained by the action of CCBs blocking the virus replication cycle through ion-dependent pathways, although the use of CCBs has not been shown to interfere with the severity of disease presentation[126]. Some studies, which used a smaller sample of patients, are against the use of CCBs in the treatment of patients with COVID-19 and found an increased risk of respiratory failure, intubation, and death in patients taking this medication[127].
Further studies are needed, but vitamin D supplementation is hypothesized to prevent hypocalcemia, severe disease, and other complications[120,128].
Hemoglobin, iron, and saturated transferrin levels were lower in patients with COVID-19 compared to individuals without the disease, while ferritin levels were higher in SARS-CoV-2 infected patients[129]. Correspondingly, Zhou et al[130] reported that serum hepcidin and ferritin levels contribute independently to the severity of COVID-19. Another study points out that the relationship between iron levels and disease severity is U-shaped, considering that patients with mild respiratory failure had significantly lower serum iron levels compared to individuals without respiratory failure, while no significant differences in iron levels were observed between the group without respiratory failure and those with severe respiratory failure[131]. Hippchen et al[132] identified an iron concentration
In order to decrease viral replication, the innate immune system stimulates the reduction of iron bioavailability, so hepcidin levels tend to increase and block ferroportin activity, which results in cellular accumulation of the metal, mainly inside macrophages, hepatocytes, and enterocytes[135]. The increase in intracellular iron stimulates the expression of inflammatory cytokines, such as IL-6, IL-8, and TNF-α, which worsen the accumulation of iron in cells, generating a cycle that contributes to the “cytokine storm” in patients with COVID-19[133].
In general, adequate levels of iron are obtained through diet. However, supplementation of this mineral can be used in patients with challenges in meeting dietary requirements[136]. The usual dosage for therapeutic iron supplementation is 325 mg (equivalent to 65 mg of elemental iron), three times a day[137]. Iron supplementation therapy has been considered a more promising approach than transfusion to promote erythropoiesis in pregnant women and cancer patients with anemia and COVID-19[138].
Skalny et al[139] reported that the copper/zinc ratio, besides being increased in patients with COVID-19 compared to healthy individuals, presents a gradual increase according to the severity of the cases and was considered as a predictor of lower O2 saturation. A cohort of 306 patients with COVID-19 in Wuhan also identified an increase in copper levels in severe cases compared to non-severe patients[140]. On the other hand, Hackler et al[141] reported that patients surviving COVID-19 had higher mean serum copper levels compared to non-surviving patients. Arrieta et al[142], in turn, carried out a study with patients with severe COVID-19 on parental nutrition and supplemented with zinc, revealing that serum copper concentrations were lower in critically ill participants. However, it should be considered that copper and zinc are competitively absorbed in the small intestine, which may justify the reduction of copper in these patients[143].
SARS-CoV-2 infection involves the induction of an inducible transcription factor (NF-κB), responsible for triggering an inflammatory process. Copper, in turn, acts by preventing inflammatory events, through several mechanisms, such as the generation of reactive oxygen species, which act in the destruction of viral morphology and genomes[143]. Despite a favorable theoretical approach to complementary therapy with copper supplementation, there is still no evidence to support its use in cases of patients with COVID-19[142].
Zinc plays an important role in modulating the immune system, including roles in antiviral and antibacterial responses[144]. Zinc is essential for the recruitment of neutrophil granulocytes and chemotaxis process and positively influences NK cells, phagocytosis, oxidative burst generation, and CD4+ and CD8+ T cells[145]. It has already been clarified that acute zinc deficiency has the potential to interfere with both innate immunity and T cell-mediated immunity by impairing those defenses, whereas chronic deficiency of that metal is associated with an increase in pro-inflammatory cytokines[146]. In addition, previous studies have already suggested the use of zinc in order to reduce the duration of acute respiratory tract viral infections and to prevent symptoms[147].
A possible therapeutic role of the mineral in respiratory tract infections was the demonstration that zinc gluconate supplementation inhibits the NF-κB-dependent transcription of inflammatory genes, contributing to a reduction of neutrophilic infiltration and TNF-α release in the airways[148]. In that context, it was hypothesized that zinc could inhibit SARS-CoV-2 viral replication since it inhibits RNA-dependent RNA polymerase (RdRp) activity in vitro by inhibiting SARS-CoV-2 RdRp elongation and binding of model[149,150]. Furthermore, it is possible that zinc has the potential to restrict SARS-CoV-2 access in host cells by inhibiting ACE2 activity[151,152]. Therefore, during the COVID-19 pandemic, the possibility of reducing infection severity through zinc administration led scientists to research this metal. Among these studies, a non-randomized clinical trial including 113 patients compared the use of combined nitazoxanide, ribavirin, ivermectin, and zinc along with routine supportive treatment and the results showed that the combination effectively cleared SARS-CoV-2 from the nasopharynx faster than supportive therapy; however, patients experienced some side effects such as gastrointestinal disturbances[153]. In contrast, a randomized clinical trial including 214 patients looked at whether high-dose zinc, high-dose ascorbic acid, or both substances were able to reduce the severity or duration of symptoms caused by SARS-CoV-2 infection compared to standard care. The results of the study concluded that there was no significant difference between groups and that treatment with zinc, ascorbic acid, or both did not interfere with the symptoms of the disease[154]. In addition, a prospective clinical trial with 134 patients analyzed the serum zinc levels of patients positive for COVID-19 at various severity levels, with and without olfactory alterations, in order to assess the therapeutic potential of zinc supplementation. The authors concluded that there were no significant differences between the subgroups regarding severity, recovery time, or the presence or absence of olfactory and taste dysfunction. However, olfactory and taste functions recovered more quickly in patients who underwent zinc therapy (
Magnesium ion is one of the most relevant elements in the homeostasis of several body systems such as the respiratory, neurological, cardiovascular, and digestive systems. It has anti-oxidant and anti-inflammatory functions and integrates several biochemical and metabolic reactions, such as transport of other ions and activation of vitamin D, and it is involved in energy metabolism[159]; considering the role of magnesium in body homeostasis, this element is involved in the context of the organic disorders caused by COVID-19.
When there is a cytokine storm and an increase in the generalized inflammatory status, there is a functional imbalance between the cells of the immune system and higher energy depletion[160,161].
A good part of the population already has low serum magnesium levels and, with the infection, food intake is reduced in more critical cases. As a result, the organism uses other means of obtaining this ion which, along with phosphate, is removed from its natural reservoirs, mainly the musculoskeletal system, catabolizing it[159,161].
Besides the muscle tissue involved, which may evolve to kidney injury and rhabdomyolysis, low magnesium levels may favor the development of respiratory complications by integrating membrane proteins involved in energy metabolism. Nevertheless, hypomagnesemia can contribute to endothelial dysfunction, favoring, as the calcium mentioned above, prothrombotic situations[159,160].
To date, little is known about magnesium homeostasis during COVID-19, as it is not a commonly assessed parameter, even though many patients have low Mg levels during the disease. However, in addition to all the inflammatory and metabolic issues involved with hypomagnesemia, SARS-CoV-2 has magnesium in its structure. In this sense, the virus would need the ion to remain structurally and functionally active[162-165].
The use of magnesium, vitamin D, and vitamin B12 supplementation was positive in the devel
To summarize the influence of nutrients on the immune system, Table 1 brings the macro- and micro-nutrients above cited, relating it to the modulation in cells and cytokines and to clinical outcomes.
| Micro-/macro-nutrient | Clinical outcomes | Affected cells and cytokines | Immunological outcomes | Ref. |
| Proteins | Whey protein has antiviral properties; supplementation facilitates the patients' recovery in viral infections | DNA or RNA polymerases, reverse transcriptase, integrase, etc. | Antiviral activities against enveloped and non-enveloped viruses; inhibit the entrance of the virus into the cell; inhibit the virus enzymes activity; prevent virus replication | Siqueiros et al[19], 2014; Nejati et al[20], 2021; Ng et al[21], 2001; Ng et al[43], 2015; Olsen et al[44], 2014 |
| Lipids/omega-3 | Improvement of oxygenation and reduced length of stay after omega-3 administration; normalization of blood pH, reducing base excess; improves renal function | IL-6, IL-8, IL-1beta, free radicals | Altering the composition of cell membranes and modulating cell signaling; decrease the pro-inflammatory response by reducing the levels of proinflammatory cytokines IL-6, IL-8, IL-1beta, and free radicals | Hawryłkowicz et al[62], 2021; Romano et al[73], 2020; McClave et al[74], 2016; Vivar-Sierra et al[79], 2021; Asher et al[81], 2021; Doaei et al[83], 2021 |
| Vitamin B12 | Combined supplementation resulted in lower necessity of oxygen and ICU admission; increased levels of B12 are correlated to higher risk of ICU admission, intubation, and death | T and B lymphocytes, NK cells; antibodies | Cell differentiation and proliferation; maintenance of the ratio between T helper and cytotoxic cells; influence on NK cell activity; in association with folate and production of antibodies | Gombart et al[2], 2020; Chaari et al[86], 2021; Ersöz et al[92], 2021; Tan et al[99], 2020 |
| Vitamin D | Increased mortality in patients with low vitamin D levels; high dose supplementation is related to successful treatment of high risk elderly patients | Antimicrobial peptides; T cells, macrophages | Production of antimicrobial peptides in the respiratory epithelium; helps maintain cell junctions and gaps; decreasing the cytokine storm; inhibiting type 1 T helper cell response and T cell induction; its deficiency causes deprivation in the production and performance of macrophages | Dankers et al[105], 2016; Gombart et al[106], 2005; Greiller and Martineau[107], 2015; Grant et al[108], 2020; Cantorna et al[109], 2015; Ilie et al[110], 2020; Rhodes et al[111], 2021; Annweiler et al[113], 2020 |
| Calcium | Calcium associated with albumin is capable of decreasing metabolic dysfunctions and organ damage during the COVID-19 infection | Cytotoxic T lymphocytes; IL-1, IL-6 | Hypocalcemia as a result of hypoalbuminemia; increased pro-inflammatory cytokines IL-1 and IL-6 interfere with calcium metabolism; lower levels of lymphocyte counts related to higher levels of D-dimer in critically ill patients | Alemzadeh et al[116], 2021; Alsagaff et al[126], 2021; Mendez et al[127], 2021 |
| Iron | Maintaining adequate levels of iron is related to lower levels of respiratory failure | T cells, B cells, macrophages | Chelation/deficiency: Enhances IFN-γ signaling and STAT1 activation which may stabilize the TH1 phenotype in early TH polarization; activates the transcription factors hypoxia-inducible factor (HIF)-1α and nuclear factor (NF)-IL6 in macrophages. Supplementation/overload: in TH1 cells, stimulates the production of GM-CS, and reduces expression of the T-box transcription factor T-BET; inhibits ICAM1 and MHC-II expression in macrophages, impairing TH1 immunity; in B cells, counteracts the Ig class switch towards IgG; may promote TH2 polarization | Tojo et al[131], 2021; Sonnweber et al[133], 2020; Akhtar et al[138], 2021; Nairz and Weiss[164], 2020 |
| Copper | There is still no evidence to support the supplementation of copper in COVID-19 patients | Macrophages, neutrophils, NK cells; IL-2 | Participates in the functioning of innate immune cells (e.g., it accumulates in macrophage phagolysosomes to combat pathogens); has intrinsic antimicrobial properties; acts in defense against reactive oxygen species; has a role in IL-2 production and response; maintains intracellular antioxidant balance; has a role in differentiation and proliferation of T cells | Zhou et al[130], 2020; Zeng et al[140], 2021; Rani et al[143], 2021 |
| Zinc | Currently there is no evidence of interferences of this element regarding severe cases | Th1 cells; IL-2, IL-1β, IL-6, IL-8 | Acute zinc deficiency promotes the adhesion of monocytes to endothelial cells in vitro and reduces the production of TH1 profile cytokines including IFN-γ, IL-2, and TNF-α; it has the potential to inhibit the inflammatory process by stimulating the release of IL-1-β depending on the transcription factor NF-κB; low levels of zinc are associated with an increase in IL-6, IL-8, and TNF-α which contributes to inflammation | Gammoh et al[144], 2017; Elalfy et al[153], 2021; Thomas et al[154], 2021; Abdelmaksoud et al[155], 2021; Mariani et al [165], 2006 |
| Magnesium | This nutrient is capable of reducing the necessity of oxygen and intensive care unit admission | Natural killer cells, CD8 killer T cells, monocytes, macrophages, leukocytes | Reduction of immune cell toxicity; cytokine storm favoring; decreased anti-oxidant and anti-inflammatory action, energy depletion, muscle catabolism, and prothrombotic conditions | Tang et al[159], 2020; DiNicolantonio and O’keefe[160], 2021; van Niekerk et al[161], 2018; Zhu et al[162], 2021; Iotti et al[163], 2020; Nairz and Weiss[164], 2020 |
The relationship between COVID-19 and nutrients is controversial. The expression of pro-inflammatory compounds and the individual's dysregulated immune response are the main causes of modulation in critically ill patients infected with the virus. In view of this, correct modulation is essential to avoid mild or exaggerated responses. The macro- and micro-nutrients mentioned are directly involved in the basic structure of the immune system, participating in the development of cells, cytokines, and antibodies. Some nutrients such as vitamin B12 and copper are contradictory as to the beneficial effects of their bioavailability, and their overstocking is predictive of a worse prognosis. The lack of studies with this isolated micronutrient requires further analysis to guide medical professionals in prescribing vitamin B12 supplementation. Furthermore, supplementation of vitamin D, calcium, iron, and magnesium is beneficial, especially in patients with comorbidities, whose risk of developing the most severe forms of the disease is greater. The action of these elements, promoting anti-inflammatory and antioxidant functions, is essential to control the aggressive COVID-19 response. Vitamin D, calcium, and magnesium supplementation is important for patients at risk and with deficiency. In addition, early use of calcium associated with albumin has shown benefits in preventing toxicity and organ damages that can lead to severe cases of COVID-19. Those findings are alien to what is found in ESPEN expert statements and practical guidance for nutritional management of individuals with SARS-CoV-2 infection, which points that vitamins D and B, zinc, iron, and omega-3 PUFAs should be considered in COVID-19 patients for nutritional support. It is also suggested that the daily supply of these micronutrients should be ensured in malnourished patients with SARS-CoV-2 infection.
Regarding the high consumption of proteins, carbohydrates, and lipids, there is influence of the excess of these in the diets of prehospital patients, considering the connection with the acquisition of pneumonia. Moreover, these nutrients influence the function of adipose tissue by stimulating the inflammatory response, worsening the patient's condition. Meanwhile, omega 3 PUFA supplementation is recommended to improve oxygenation, contributing additionally to the regulation of laboratory tests and renal function. However, further randomized controlled trials are needed to complement and confirm the information on the influence of vitamins and other nutrients on immunomodulation of the COVID-19 response, in order to determine which nutrients are beneficially administered and select the correct doses for the treatment of critically ill patients.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Critical care medicine
Country/Territory of origin: Brazil
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Bittner EA, United States; Wong YP, Malaysia A-Editor: Liu X S-Editor: Wang LL L-Editor: Wang TQ P-Editor: Wang LL
| 1. | Zhang L, Liu Y. Potential interventions for novel coronavirus in China: A systematic review. J Med Virol. 2020;92:479-490. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 674] [Cited by in RCA: 725] [Article Influence: 145.0] [Reference Citation Analysis (1)] |
| 2. | Gombart AF, Pierre A, Maggini S. A Review of Micronutrients and the Immune System-Working in Harmony to Reduce the Risk of Infection. Nutrients. 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 715] [Cited by in RCA: 676] [Article Influence: 135.2] [Reference Citation Analysis (0)] |
| 3. | Calder PC, Carr AC, Gombart AF, Eggersdorfer M. Optimal Nutritional Status for a Well-Functioning Immune System Is an Important Factor to Protect against Viral Infections. Nutrients. 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 565] [Cited by in RCA: 493] [Article Influence: 98.6] [Reference Citation Analysis (0)] |
| 4. | Kumar P, Kumar M, Bedi O, Gupta M, Kumar S, Jaiswal G, Rahi V, Yedke NG, Bijalwan A, Sharma S, Jamwal S. Role of vitamins and minerals as immunity boosters in COVID-19. Inflammopharmacology. 2021;29:1001-1016. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 77] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 5. | Singer P, Blaser AR, Berger MM, Alhazzani W, Calder PC, Casaer MP, Hiesmayr M, Mayer K, Montejo JC, Pichard C, Preiser JC, van Zanten ARH, Oczkowski S, Szczeklik W, Bischoff SC. ESPEN guideline on clinical nutrition in the intensive care unit. Clin Nutr. 2019;38:48-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 989] [Cited by in RCA: 1530] [Article Influence: 218.6] [Reference Citation Analysis (0)] |
| 6. | Fantacone ML, Lowry MB, Uesugi SL, Michels AJ, Choi J, Leonard SW, Gombart SK, Gombart JS, Bobe G, Gombart AF. The Effect of a Multivitamin and Mineral Supplement on Immune Function in Healthy Older Adults: A Double-Blind, Randomized, Controlled Trial. Nutrients. 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 7. | Payen D, Cravat M, Maadadi H, Didelot C, Prosic L, Dupuis C, Losser MR, De Carvalho Bittencourt M. A Longitudinal Study of Immune Cells in Severe COVID-19 Patients. Front Immunol. 2020;11:580250. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 52] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 8. | Kared H, Redd AD, Bloch EM, Bonny TS, Sumatoh H, Kairi F, Carbajo D, Abel B, Newell EW, Bettinotti MP, Benner SE, Patel EU, Littlefield K, Laeyendecker O, Shoham S, Sullivan D, Casadevall A, Pekosz A, Nardin A, Fehlings M, Tobian AA, Quinn TC. SARS-CoV-2-specific CD8+ T cell responses in convalescent COVID-19 individuals. J Clin Invest. 2021;131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 195] [Article Influence: 48.8] [Reference Citation Analysis (0)] |
| 9. | Sattler A, Angermair S, Stockmann H, Heim KM, Khadzhynov D, Treskatsch S, Halleck F, Kreis ME, Kotsch K. SARS-CoV-2-specific T cell responses and correlations with COVID-19 patient predisposition. J Clin Invest. 2020;130:6477-6489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 158] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 10. | Secchi M, Bazzigaluppi E, Brigatti C, Marzinotto I, Tresoldi C, Rovere-Querini P, Poli A, Castagna A, Scarlatti G, Zangrillo A, Ciceri F, Piemonti L, Lampasona V. COVID-19 survival associates with the immunoglobulin response to the SARS-CoV-2 spike receptor binding domain. J Clin Invest. 2020;130:6366-6378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 84] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 11. | Bassaganya-Riera J, Hontecillas R. CLA and n-3 PUFA differentially modulate clinical activity and colonic PPAR-responsive gene expression in a pig model of experimental IBD. Clin Nutr. 2006;25:454-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 157] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 12. | Bendich A. Physiological role of antioxidants in the immune system. J Dairy Sci. 1993;76:2789-2794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 90] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 13. | Rocha J, Basra T, El Kurdi B, Venegas-Borsellino C. Effects of Potential Micro- and Macro-nutrients in Combatting COVID-19. Curr Surg Rep. 2021;9:24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 14. | Fernandes AC, Bezerra OMPA. Terapia nutricional na doença pulmonar obstrutivacrônica e suas complicações nutricionais. J Bras Pneumol. 2006;32:461-471. [RCA] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 15. | Mente A, Chalcraft K, Ak H, Davis AD, Lonn E, Miller R, Potter MA, Yusuf S, Anand SS, McQueen MJ. The Relationship Between Trimethylamine-N-Oxide and Prevalent Cardiovascular Disease in a Multiethnic Population Living in Canada. Can J Cardiol. 2015;31:1189-1194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 102] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 16. | Banerjee A, Kulcsar K, Misra V, Frieman M, Mossman K. Bats and Coronaviruses. Viruses. 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 273] [Cited by in RCA: 289] [Article Influence: 48.2] [Reference Citation Analysis (0)] |
| 17. | Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell. 2002;10:417-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4047] [Cited by in RCA: 4691] [Article Influence: 204.0] [Reference Citation Analysis (0)] |
| 18. | Childs CE, Calder PC, Miles EA. Diet and Immune Function. Nutrients. 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 169] [Cited by in RCA: 252] [Article Influence: 42.0] [Reference Citation Analysis (0)] |
| 19. | Siqueiros-Cendón T, Arévalo-Gallegos S, Iglesias-Figueroa BF, García-Montoya IA, Salazar-Martínez J, Rascón-Cruz Q. Immunomodulatory effects of lactoferrin. Acta Pharmacol Sin. 2014;35:557-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 216] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 20. | Nejati M, Dehghan P, Hashempour-Baltork F, Alizadeh AM, Farshi P, Khosravi-Darani K. Potential Dietary Interventions for COVID-19 Infection Based on the Gut-Immune Axis: An Update Review on Bioactive Component of Macronutrients. Int J Prev Med. 2021;12:105. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 21. | Ng TB, Lam TL, Au TK, Ye XY, Wan CC. Inhibition of human immunodeficiency virus type 1 reverse transcriptase, protease and integrase by bovine milk proteins. Life Sci. 2001;69:2217-2223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 22. | Thorburn AN, Macia L, Mackay CR. Diet, metabolites, and "western-lifestyle" inflammatory diseases. Immunity. 2014;40:833-842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 573] [Cited by in RCA: 667] [Article Influence: 60.6] [Reference Citation Analysis (0)] |
| 23. | Chemudupati M, Kenney AD, Smith AC, Fillinger RJ, Zhang L, Zani A, Liu SL, Anderson MZ, Sharma A, Yount JS. Butyrate Reprograms Expression of Specific Interferon-Stimulated Genes. J Virol. 2020;94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 53] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 24. | Simonnet A, Chetboun M, Poissy J, Raverdy V, Noulette J, Duhamel A, Labreuche J, Mathieu D, Pattou F, Jourdain M; LICORN and the Lille COVID-19 and Obesity study group. High Prevalence of Obesity in Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) Requiring Invasive Mechanical Ventilation. Obesity (Silver Spring). 2020;28:1195-1199. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1263] [Cited by in RCA: 1577] [Article Influence: 315.4] [Reference Citation Analysis (0)] |
| 25. | Di Renzo L, Gualtieri P, Romano L, Marrone G, Noce A, Pujia A, Perrone MA, Aiello V, Colica C, De Lorenzo A. Role of Personalized Nutrition in Chronic-Degenerative Diseases. Nutrients. 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 94] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 26. | Yang Y, Wang L, Liu J, Fu S, Zhou L, Wang Y. Obesity or increased body mass index and the risk of severe outcomes in patients with COVID-19: A protocol for systematic review and meta-analysis. Medicine (Baltimore). 2022;101:e28499. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 19] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 27. | de Heredia FP, Gómez-Martínez S, Marcos A. Obesity, inflammation and the immune system. Proc Nutr Soc. 2012;71:332-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 410] [Cited by in RCA: 557] [Article Influence: 42.8] [Reference Citation Analysis (0)] |
| 28. | Wu T, Jia X, Shi H, Niu J, Yin X, Xie J, Wang X. Prevalence of mental health problems during the COVID-19 pandemic: A systematic review and meta-analysis. J Affect Disord. 2021;281:91-98. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 812] [Cited by in RCA: 724] [Article Influence: 181.0] [Reference Citation Analysis (0)] |
| 29. | Lei L, Huang X, Zhang S, Yang J, Yang L, Xu M. Comparison of Prevalence and Associated Factors of Anxiety and Depression Among People Affected by vs People Unaffected by Quarantine During the COVID-19 Epidemic in Southwestern China. Med Sci Monit. 2020;26:e924609. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 463] [Cited by in RCA: 419] [Article Influence: 83.8] [Reference Citation Analysis (0)] |
| 30. | Qiu J, Shen B, Zhao M, Wang Z, Xie B, Xu Y. A nationwide survey of psychological distress among Chinese people in the COVID-19 epidemic: implications and policy recommendations. Gen Psychiatr. 2020;33:e100213. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2636] [Cited by in RCA: 2201] [Article Influence: 440.2] [Reference Citation Analysis (0)] |
| 31. | Isasi CR, Parrinello CM, Jung MM, Carnethon MR, Birnbaum-Weitzman O, Espinoza RA, Penedo FJ, Perreira KM, Schneiderman N, Sotres-Alvarez D, Van Horn L, Gallo LC. Psychosocial stress is associated with obesity and diet quality in Hispanic/Latino adults. Ann Epidemiol. 2015;25:84-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 110] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 32. | Rodríguez MÁ, Crespo I, Olmedillas H. Exercising in times of COVID-19: what do experts recommend doing within four walls? Rev Esp Cardiol (Engl Ed). 2020;73:527-529. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 33. | Zhu L, She ZG, Cheng X, Qin JJ, Zhang XJ, Cai J, Lei F, Wang H, Xie J, Wang W, Li H, Zhang P, Song X, Chen X, Xiang M, Zhang C, Bai L, Xiang D, Chen MM, Liu Y, Yan Y, Liu M, Mao W, Zou J, Liu L, Chen G, Luo P, Xiao B, Zhang Z, Lu Z, Wang J, Lu H, Xia X, Wang D, Liao X, Peng G, Ye P, Yang J, Yuan Y, Huang X, Guo J, Zhang BH. Association of Blood Glucose Control and Outcomes in Patients with COVID-19 and Pre-existing Type 2 Diabetes. Cell Metab. 2020;31:1068-1077.e3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1198] [Cited by in RCA: 1099] [Article Influence: 219.8] [Reference Citation Analysis (0)] |
| 34. | Muniyappa R, Gubbi S. COVID-19 pandemic, coronaviruses, and diabetes mellitus. Am J Physiol Endocrinol Metab. 2020;318:E736-E741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 526] [Cited by in RCA: 503] [Article Influence: 100.6] [Reference Citation Analysis (0)] |
| 35. | Katsiki N, Gómez-Huelgas R, Mikhailidis DP, Pérez-Martínez P. Narrative review on clinical considerations for patients with diabetes and COVID-19: More questions than answers. Int J Clin Pract. 2021;75:e14833. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 36. | Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S, Huang H, Zhang L, Du C, Zhang Y, Song J, Wang S, Chao Y, Yang Z, Xu J, Chen D, Xiong W, Xu L, Zhou F, Jiang J, Bai C, Zheng J, Song Y. Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern Med. 2020;180:934-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4960] [Cited by in RCA: 5518] [Article Influence: 1103.6] [Reference Citation Analysis (1)] |
| 37. | Jin YH, Cai L, Cheng ZS, Cheng H, Deng T, Fan YP, Fang C, Huang D, Huang LQ, Huang Q, Han Y, Hu B, Hu F, Li BH, Li YR, Liang K, Lin LK, Luo LS, Ma J, Ma LL, Peng ZY, Pan YB, Pan ZY, Ren XQ, Sun HM, Wang Y, Wang YY, Weng H, Wei CJ, Wu DF, Xia J, Xiong Y, Xu HB, Yao XM, Yuan YF, Ye TS, Zhang XC, Zhang YW, Zhang YG, Zhang HM, Zhao Y, Zhao MJ, Zi H, Zeng XT, Wang XH; , for the Zhongnan Hospital of Wuhan University Novel Coronavirus Management and Research Team, Evidence-Based Medicine Chapter of China International Exchange and Promotive Association for Medical and Health Care (CPAM). A rapid advice guideline for the diagnosis and treatment of 2019 novel coronavirus (2019-nCoV) infected pneumonia (standard version). Mil Med Res. 2020;7:4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 881] [Cited by in RCA: 1137] [Article Influence: 227.4] [Reference Citation Analysis (0)] |
| 38. | McPherson RA, Hardy G. Clinical and nutritional benefits of cysteine-enriched protein supplements. Curr Opin Clin Nutr Metab Care. 2011;14:562-568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 48] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 39. | Rondanelli M, Faliva MA, Gasparri C, Peroni G, Spadaccini D, Maugeri R, Nichetti M, Infantino V, Perna S. Current opinion on dietary advice in order to preserve fat-free mass during a low-calorie diet. Nutrition. 2020;72:110667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 40. | Teixeira FJ, Santos HO, Howell SL, Pimentel GD. Whey protein in cancer therapy: A narrative review. Pharmacol Res. 2019;144:245-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 60] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 41. | Cereda E, Turri A, Klersy C, Cappello S, Ferrari A, Filippi AR, Brugnatelli S, Caraccia M, Chiellino S, Borioli V, Monaco T, Stella GM, Arcaini L, Benazzo M, Grugnetti G, Pedrazzoli P, Caccialanza R. Whey protein isolate supplementation improves body composition, muscle strength, and treatment tolerance in malnourished advanced cancer patients undergoing chemotherapy. Cancer Med. 2019;8:6923-6932. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 78] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 42. | Cross ML, Gill HS. Immunomodulatory properties of milk. Br J Nutr. 2000;84 Suppl 1:S81-S89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 63] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 43. | Ng TB, Cheung RC, Wong JH, Wang Y, Ip DT, Wan DC, Xia J. Antiviral activities of whey proteins. Appl Microbiol Biotechnol. 2015;99:6997-7008. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 62] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 44. | Olsen MF, Abdissa A, Kæstel P, Tesfaye M, Yilma D, Girma T Wells J CK, Ritz C, Mølgaard C, Michaelsen KF, Zerfu D, Brage S, B Andersen A, Friis H. Effects of nutritional supplementation for HIV patients starting antiretroviral treatment: randomized controlled trial in Ethiopia. BMJ. 2014;348:g3187. [RCA] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 45. | Paulsrud JR, Pensler L, Whitten CF, Stewart S, Holman RT. Essential fatty acid deficiency in infants induced by fat-free intravenous feeding. Am J Clin Nutr. 1972;25:897-904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 158] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 46. | Calder PC, Adolph M, Deutz NE, Grau T, Innes JK, Klek S, Lev S, Mayer K, Michael-Titus AT, Pradelli L, Puder M, Vlaardingerbroek H, Singer P. Lipids in the intensive care unit: Recommendations from the ESPEN Expert Group. Clin Nutr. 2018;37:1-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 95] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 47. | Calder PC. Functional Roles of Fatty Acids and Their Effects on Human Health. JPEN J Parenter Enteral Nutr. 2015;39:18S-32S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 452] [Cited by in RCA: 642] [Article Influence: 64.2] [Reference Citation Analysis (0)] |
| 48. | Su H, Liu R, Chang M, Huang J, Wang X. Dietary linoleic acid intake and blood inflammatory markers: a systematic review and meta-analysis of randomized controlled trials. Food Funct. 2017;8:3091-3103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 49. | Talasaz AH, Sadeghipour P, Aghakouchakzadeh M, Dreyfus I, Kakavand H, Ariannejad H, Gupta A, Madhavan MV, Van Tassell BW, Jimenez D, Monreal M, Vaduganathan M, Fanikos J, Dixon DL, Piazza G, Parikh SA, Bhatt DL, Lip GYH, Stone GW, Krumholz HM, Libby P, Goldhaber SZ, Bikdeli B. Investigating Lipid-Modulating Agents for Prevention or Treatment of COVID-19: JACC State-of-the-Art Review. J Am Coll Cardiol. 2021;78:1635-1654. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 42] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 50. | Innes JK, Calder PC. Omega-6 fatty acids and inflammation. Prostaglandins Leukot Essent Fatty Acids. 2018;132:41-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 427] [Cited by in RCA: 595] [Article Influence: 85.0] [Reference Citation Analysis (0)] |
| 51. | Calder PC. Fatty acids and inflammation: the cutting edge between food and pharma. Eur J Pharmacol. 2011;668 Suppl 1:S50-S58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 356] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 52. | Saini RK, Keum YS. Omega-3 and omega-6 polyunsaturated fatty acids: Dietary sources, metabolism, and significance - A review. Life Sci. 2018;203:255-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 462] [Cited by in RCA: 684] [Article Influence: 97.7] [Reference Citation Analysis (0)] |
| 53. | D'Angelo S, Motti ML, Meccariello R. ω-3 and ω-6 Polyunsaturated Fatty Acids, Obesity and Cancer. Nutrients. 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 151] [Article Influence: 30.2] [Reference Citation Analysis (0)] |
| 54. | DiNicolantonio JJ, O'Keefe JH. Importance of maintaining a low omega-6/omega-3 ratio for reducing inflammation. Open Heart. 2018;5:e000946. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 121] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 55. | Martinez RM, Fattori V, Saito P, Melo CBP, Borghi SM, Pinto IC, Bussmann AJC, Baracat MM, Georgetti SR, Verri WA Jr, Casagrande R. Lipoxin A4 inhibits UV radiation-induced skin inflammation and oxidative stress in mice. J Dermatol Sci. 2018;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 56. | Luo CL, Li QQ, Chen XP, Zhang XM, Li LL, Li BX, Zhao ZQ, Tao LY. Lipoxin A4 attenuates brain damage and downregulates the production of pro-inflammatory cytokines and phosphorylated mitogen-activated protein kinases in a mouse model of traumatic brain injury. Brain Res. 2013;1502:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 57. | Pirault J, Bäck M. Lipoxin and Resolvin Receptors Transducing the Resolution of Inflammation in Cardiovascular Disease. Front Pharmacol. 2018;9:1273. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 102] [Cited by in RCA: 119] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 58. | Serhan CN, Chiang N, Van Dyke TE. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat Rev Immunol. 2008;8:349-361. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2350] [Cited by in RCA: 2168] [Article Influence: 127.5] [Reference Citation Analysis (0)] |
| 59. | Liu X, Wang X, Duan X, Poorun D, Xu J, Zhang S, Gan L, He M, Zhu K, Ming Z, Hu F, Chen H. Lipoxin A4 and its analog suppress inflammation by modulating HMGB1 translocation and expression in psoriasis. Sci Rep. 2017;7:7100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 42] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 60. | Karra L, Haworth O, Priluck R, Levy BD, Levi-Schaffer F. Lipoxin B₄ promotes the resolution of allergic inflammation in the upper and lower airways of mice. Mucosal Immunol. 2015;8:852-862. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 59] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 61. | Jeyakumar SM, Vajreswari A. Pharmaconutrition strategy to resolve SARS-CoV-2-induced inflammatory cytokine storm in non-alcoholic fatty liver disease: Omega-3 Long-chain polyunsaturated fatty acids. World J Clin Cases. 2021;9:9333-9349. [PubMed] |
| 62. | Hawryłkowicz V, Lietz-Kijak D, Kaźmierczak-Siedlecka K, Sołek-Pastuszka J, Stachowska L, Folwarski M, Parczewski M, Stachowska E. Patient Nutrition and Probiotic Therapy in COVID-19: What Do We Know in 2021? Nutrients. 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 63. | Harvey SE, Parrott F, Harrison DA, Bear DE, Segaran E, Beale R, Bellingan G, Leonard R, Mythen MG, Rowan KM; CALORIES Trial Investigators. Trial of the route of early nutritional support in critically ill adults. N Engl J Med. 2014;371:1673-1684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 381] [Cited by in RCA: 386] [Article Influence: 35.1] [Reference Citation Analysis (0)] |
| 64. | Serhan CN, Petasis NA. Resolvins and protectins in inflammation resolution. Chem Rev. 2011;111:5922-5943. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 819] [Cited by in RCA: 760] [Article Influence: 54.3] [Reference Citation Analysis (0)] |
| 65. | Regidor PA, Santos FG, Rizo JM, Egea FM. Pro resolving inflammatory effects of the lipid mediators of omega 3 fatty acids and its implication in SARS COVID-19. Med Hypotheses. 2020;145:110340. [PubMed] |
| 66. | Russell CD, Schwarze J. The role of pro-resolution lipid mediators in infectious disease. Immunology. 2014;141:166-173. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 86] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 67. | Yang A, Wu Y, Yu G, Wang H. Role of specialized pro-resolving lipid mediators in pulmonary inflammation diseases: mechanisms and development. Respir Res. 2021;22:204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 68. | Balta MG, Papathanasiou E, Christopoulos PF. Specialized Pro-Resolving Mediators as Potential Regulators of Inflammatory Macrophage Responses in COVID-19. Front Immunol. 2021;12:632238. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 69. | Regidor PA, De La Rosa X, Santos FG, Rizo JM, Gracia Banzo R, Silva RS. Acute severe SARS COVID-19 patients produce pro-resolving lipids mediators and eicosanoids. Eur Rev Med Pharmacol Sci. 2021;25:6782-6796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 70. | Wang Q, Zheng X, Cheng Y, Zhang YL, Wen HX, Tao Z, Li H, Hao Y, Gao Y, Yang LM, Smith FG, Huang CJ, Jin SW. Resolvin D1 stimulates alveolar fluid clearance through alveolar epithelial sodium channel, Na,K-ATPase via ALX/cAMP/PI3K pathway in lipopolysaccharide-induced acute lung injury. J Immunol. 2014;192:3765-3777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 95] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 71. | Fullerton JN, Gilroy DW. Resolution of inflammation: a new therapeutic frontier. Nat Rev Drug Discov. 2016;15:551-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 469] [Cited by in RCA: 648] [Article Influence: 72.0] [Reference Citation Analysis (0)] |
| 72. | Saeed H, Osama H, Abdelrahman MA, Madney YM, Harb HS, Abdelrahim MEA, Ali F. Vitamins and other immune-supportive elements as cofactors for passing the COVID-19 pandemic. Beni Suef Univ J Basic Appl Sci. 2021;10:71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 73. | Romano L, Bilotta F, Dauri M, Macheda S, Pujia A, De Santis GL, Tarsitano MG, Merra G, Di Renzo L, Esposito E, De Lorenzo A. Short Report - Medical nutrition therapy for critically ill patients with COVID-19. Eur Rev Med Pharmacol Sci. 2020;24:4035-4039. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 23] [Reference Citation Analysis (0)] |
| 74. | McClave SA, Taylor BE, Martindale RG, Warren MM, Johnson DR, Braunschweig C, McCarthy MS, Davanos E, Rice TW, Cresci GA, Gervasio JM, Sacks GS, Roberts PR, Compher C; Society of Critical Care Medicine; American Society for Parenteral and Enteral Nutrition. Guidelines for the Provision and Assessment of Nutrition Support Therapy in the Adult Critically Ill Patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.). JPEN J Parenter Enteral Nutr. 2016;40:159-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1695] [Cited by in RCA: 1869] [Article Influence: 207.7] [Reference Citation Analysis (3)] |
| 75. | Zakrzewska K, Oszajca K, Zep W, Piekarska A, Sidorkiewicz M. The Impact of Short-Term Shark Liver Oil Supplementation on the Fatty Acid Composition of Erythrocyte Membranes. Nutrients. 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 76. | Goc A, Niedzwiecki A, Rath M. Polyunsaturated ω-3 fatty acids inhibit ACE2-controlled SARS-CoV-2 binding and cellular entry. Sci Rep. 2021;11:5207. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 76] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 77. | Ling V, Zabetakis I. The Role of an Anti-Inflammatory Diet in Conjunction to COVID-19. Diseases. 2021;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 78. | Weill P, Plissonneau C, Legrand P, Rioux V, Thibault R. May omega-3 fatty acid dietary supplementation help reduce severe complications in Covid-19 patients? Biochimie. 2020;179:275-280. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 91] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 79. | Vivar-Sierra A, Araiza-Macías MJ, Hernández-Contreras JP, Vergara-Castañeda A, Ramírez-Vélez G, Pinto-Almazán R, Salazar JR, Loza-Mejía MA. In Silico Study of Polyunsaturated Fatty Acids as Potential SARS-CoV-2 Spike Protein Closed Conformation Stabilizers: Epidemiological and Computational Approaches. Molecules. 2021;26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 80. | Clemente-Suárez VJ, Ramos-Campo DJ, Mielgo-Ayuso J, Dalamitros AA, Nikolaidis PA, Hormeño-Holgado A, Tornero-Aguilera JF. Nutrition in the Actual COVID-19 Pandemic. A Narrative Review. Nutrients. 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 73] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 81. | Asher A, Tintle NL, Myers M, Lockshon L, Bacareza H, Harris WS. Blood omega-3 fatty acids and death from COVID-19: A pilot study. Prostaglandins Leukot Essent Fatty Acids. 2021;166:102250. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 73] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 82. | Zapata B R, Müller JM, Vásquez JE, Ravera F, Lago G, Cañón E, Castañeda D, Pradenas M, Ramírez-Santana M. Omega-3 Index and Clinical Outcomes of Severe COVID-19: Preliminary Results of a Cross-Sectional Study. Int J Environ Res Public Health. 2021;18. [PubMed] [DOI] [Full Text] |
| 83. | Doaei S, Gholami S, Rastgoo S, Gholamalizadeh M, Bourbour F, Bagheri SE, Samipoor F, Akbari ME, Shadnoush M, Ghorat F, Mosavi Jarrahi SA, Ashouri Mirsadeghi N, Hajipour A, Joola P, Moslem A, Goodarzi MO. The effect of omega-3 fatty acid supplementation on clinical and biochemical parameters of critically ill patients with COVID-19: a randomized clinical trial. J Transl Med. 2021;19:128. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 125] [Cited by in RCA: 120] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 84. | Rizzo G, Laganà AS, Rapisarda AM, La Ferrera GM, Buscema M, Rossetti P, Nigro A, Muscia V, Valenti G, Sapia F, Sarpietro G, Zigarelli M, Vitale SG. Vitamin B12 among Vegetarians: Status, Assessment and Supplementation. Nutrients. 2016;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 215] [Cited by in RCA: 202] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 85. | Shakoor H, Feehan J, Mikkelsen K, Al Dhaheri AS, Ali HI, Platat C, Ismail LC, Stojanovska L, Apostolopoulos V. Be well: A potential role for vitamin B in COVID-19. Maturitas. 2021;144:108-111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 89] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 86. | Chaari A, Bendriss G, Zakaria D, McVeigh C. Importance of Dietary Changes During the Coronavirus Pandemic: How to Upgrade Your Immune Response. Front Public Health. 2020;8:476. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 87. | Maggini S, Wintergerst ES, Beveridge S, Hornig DH. Selected vitamins and trace elements support immune function by strengthening epithelial barriers and cellular and humoral immune responses. Br J Nutr. 2007;98 Suppl 1:S29-S35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 333] [Cited by in RCA: 349] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 88. | Im JH, Je YS, Baek J, Chung MH, Kwon HY, Lee JS. Nutritional status of patients with COVID-19. Int J Infect Dis. 2020;100:390-393. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 193] [Cited by in RCA: 176] [Article Influence: 35.2] [Reference Citation Analysis (1)] |
| 89. | Wee AKH. COVID-19's toll on the elderly and those with diabetes mellitus - Is vitamin B12 deficiency an accomplice? Med Hypotheses. 2021;146:110374. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 90. | Shakeri H, Azimian A, Ghasemzadeh-Moghaddam H, Safdari M, Haresabadi M, Daneshmand T, Namdar Ahmadabad H. Evaluation of the relationship between serum levels of zinc, vitamin B12, vitamin D, and clinical outcomes in patients with COVID-19. J Med Virol. 2022;94:141-146. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 30] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 91. | Beigmohammadi MT, Bitarafan S, Abdollahi A, Amoozadeh L, Salahshour F, Mahmoodi Ali Abadi M, Soltani D, Motallebnejad ZA. The association between serum levels of micronutrients and the severity of disease in patients with COVID-19. Nutrition. 2021;91-92:111400. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 92. | Ersöz A, Yılmaz TE. The association between micronutrient and hemogram values and prognostic factors in COVID-19 patients: A single-center experience from Turkey. Int J Clin Pract. 2021;75:e14078. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 93. | Itelman E, Wasserstrum Y, Segev A, Avaky C, Negru L, Cohen D, Turpashvili N, Anani S, Zilber E, Lasman N, Athamna A, Segal O, Halevy T, Sabiner Y, Donin Y, Abraham L, Berdugo E, Zarka A, Greidinger D, Agbaria M, Kitany N, Katorza E, Shenhav-Saltzman G, Segal G. Clinical Characterization of 162 COVID-19 patients in Israel: Preliminary Report from a Large Tertiary Center. Isr Med Assoc J. 2020;22:271-274. [PubMed] |
| 94. | Ermens AA, Vlasveld LT, Lindemans J. Significance of elevated cobalamin (vitamin B12) levels in blood. Clin Biochem. 2003;36:585-590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 165] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 95. | Stabler SP. Clinical practice. Vitamin B12 deficiency. N Engl J Med. 2013;368:149-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 732] [Cited by in RCA: 754] [Article Influence: 62.8] [Reference Citation Analysis (0)] |
| 96. | Sezer RG, Bozaykut A, Akoğlu HA, Özdemir GN. The Efficacy of Oral Vitamin B12 Replacement for Nutritional Vitamin B12 Deficiency. J Pediatr Hematol Oncol. 2018;40:e69-e72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 97. | Bahadir A, Reis PG, Erduran E. Oral vitamin B12 treatment is effective for children with nutritional vitamin B12 deficiency. J Paediatr Child Health. 2014;50:721-725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 98. | Castelli MC, Friedman K, Sherry J, Brazzillo K, Genoble L, Bhargava P, Riley MG. Comparing the efficacy and tolerability of a new daily oral vitamin B12 formulation and intermittent intramuscular vitamin B12 in normalizing low cobalamin levels: a randomized, open-label, parallel-group study. Clin Ther. 2011;33:358-371.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 52] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 99. | Tan CW, Ho LP, Kalimuddin S, Cherng BPZ, Teh YE, Thien SY, Wong HM, Tern PJW, Chandran M, Chay JWM, Nagarajan C, Sultana R, Low JGH, Ng HJ. Cohort study to evaluate the effect of vitamin D, magnesium, and vitamin B12 in combination on progression to severe outcomes in older patients with coronavirus (COVID-19). Nutrition. 2020;79-80:111017. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 111] [Cited by in RCA: 172] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 100. | Junaid K, Ejaz H, Abdalla AE, Abosalif KOA, Ullah MI, Yasmeen H, Younas S, Hamam SSM, Rehman A. Effective Immune Functions of Micronutrients against SARS-CoV-2. Nutrients. 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 101. | Jovic TH, Ali SR, Ibrahim N, Jessop ZM, Tarassoli SP, Dobbs TD, Holford P, Thornton CA, Whitaker IS. Could Vitamins Help in the Fight Against COVID-19? Nutrients. 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 145] [Cited by in RCA: 125] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 102. | Bergman P. The link between vitamin D and COVID-19: distinguishing facts from fiction. J Intern Med. 2021;289:131-133. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 103. | Hyppönen E, Power C. Hypovitaminosis D in British adults at age 45 y: nationwide cohort study of dietary and lifestyle predictors. Am J Clin Nutr. 2007;85:860-868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 589] [Cited by in RCA: 553] [Article Influence: 30.7] [Reference Citation Analysis (0)] |
| 104. | Mitchell F. Vitamin-D and COVID-19: do deficient risk a poorer outcome? Lancet Diabetes Endocrinol. 2020;8:570. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 159] [Cited by in RCA: 161] [Article Influence: 32.2] [Reference Citation Analysis (0)] |
| 105. | Dankers W, Colin EM, van Hamburg JP, Lubberts E. Vitamin D in Autoimmunity: Molecular Mechanisms and Therapeutic Potential. Front Immunol. 2016;7:697. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 146] [Cited by in RCA: 214] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 106. | Gombart AF, Borregaard N, Koeffler HP. Human cathelicidin antimicrobial peptide (CAMP) gene is a direct target of the vitamin D receptor and is strongly up-regulated in myeloid cells by 1,25-dihydroxyvitamin D3. FASEB J. 2005;19:1067-1077. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 781] [Cited by in RCA: 832] [Article Influence: 43.8] [Reference Citation Analysis (0)] |
| 107. | Greiller CL, Martineau AR. Modulation of the immune response to respiratory viruses by vitamin D. Nutrients. 2015;7:4240-4270. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 250] [Cited by in RCA: 306] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 108. | Grant WB, Lahore H, McDonnell SL, Baggerly CA, French CB, Aliano JL, Bhattoa HP. Evidence that Vitamin D Supplementation Could Reduce Risk of Influenza and COVID-19 Infections and Deaths. Nutrients. 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1018] [Cited by in RCA: 1095] [Article Influence: 219.0] [Reference Citation Analysis (0)] |
| 109. | Cantorna MT, Snyder L, Lin YD, Yang L. Vitamin D and 1,25(OH)2D regulation of T cells. Nutrients. 2015;7:3011-3021. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 284] [Cited by in RCA: 379] [Article Influence: 37.9] [Reference Citation Analysis (0)] |
| 110. | Ilie PC, Stefanescu S, Smith L. The role of vitamin D in the prevention of coronavirus disease 2019 infection and mortality. Aging Clin Exp Res. 2020;32:1195-1198. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 434] [Cited by in RCA: 467] [Article Influence: 93.4] [Reference Citation Analysis (0)] |
| 111. | Rhodes JM, Subramanian S, Laird E, Griffin G, Kenny RA. Perspective: Vitamin D deficiency and COVID-19 severity - plausibly linked by latitude, ethnicity, impacts on cytokines, ACE2 and thrombosis. J Intern Med. 2021;289:97-115. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 167] [Cited by in RCA: 152] [Article Influence: 38.0] [Reference Citation Analysis (0)] |
| 112. | Kumar R, Rathi H, Haq A, Wimalawansa SJ, Sharma A. Putative roles of vitamin D in modulating immune response and immunopathology associated with COVID-19. Virus Res. 2021;292:198235. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 92] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 113. | Annweiler C, Beaudenon M, Gautier J, Simon R, Dubée V, Gonsard J, Parot-Schinkel E; COVIT-TRIAL study group. COvid-19 and high-dose VITamin D supplementation TRIAL in high-risk older patients (COVIT-TRIAL): study protocol for a randomized controlled trial. Trials. 2020;21:1031. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 59] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 114. | Munshi R, Hussein MH, Toraih EA, Elshazli RM, Jardak C, Sultana N, Youssef MR, Omar M, Attia AS, Fawzy MS, Killackey M, Kandil E, Duchesne J. Vitamin D insufficiency as a potential culprit in critical COVID-19 patients. J Med Virol. 2021;93:733-740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 123] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 115. | Ali N. Role of vitamin D in preventing of COVID-19 infection, progression and severity. J Infect Public Health. 2020;13:1373-1380. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 310] [Cited by in RCA: 288] [Article Influence: 57.6] [Reference Citation Analysis (0)] |
| 116. | Alemzadeh E, Alemzadeh E, Ziaee M, Abedi A, Salehiniya H. The effect of low serum calcium level on the severity and mortality of Covid patients: A systematic review and meta-analysis. Immun Inflamm Dis. 2021;9:1219-1228. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 117. | Torres B, Alcubilla P, González-Cordón A, Inciarte A, Chumbita M, Cardozo C, Meira F, Giménez M, de Hollanda A, Soriano A; COVID19 Hospital Clínic Infectious Diseases Research Group. Impact of low serum calcium at hospital admission on SARS-CoV-2 infection outcome. Int J Infect Dis. 2021;104:164-168. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 118. | Paliogiannis P, Mangoni AA, Dettori P, Nasrallah GK, Pintus G, Zinellu A. D-Dimer Concentrations and COVID-19 Severity: A Systematic Review and Meta-Analysis. Front Public Health. 2020;8:432. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 80] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 119. | di Filippo L, Allora A, Locatelli M, Rovere Querini P, Frara S, Banfi G, Giustina A. Hypocalcemia in COVID-19 is associated with low vitamin D levels and impaired compensatory PTH response. Endocrine. 2021;74:219-225. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 120. | di Filippo L, Doga M, Frara S, Giustina A. Hypocalcemia in COVID-19: Prevalence, clinical significance and therapeutic implications. Rev Endocr Metab Disord. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 58] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 121. | Osman W, Al Fahdi F, Al Salmi I, Al Khalili H, Gokhale A, Khamis F. Serum Calcium and Vitamin D levels: Correlation with severity of COVID-19 in hospitalized patients in Royal Hospital, Oman. Int J Infect Dis. 2021;107:153-163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 122. | Khelashvili G, Plante A, Doktorova M, Weinstein H. Ca2+-dependent mechanism of membrane insertion and destabilization by the SARS-CoV-2 fusion peptide. Biophys J. 2021;120:1105-1119. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 50] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 123. | Dakal TC. SARS-CoV-2 attachment to host cells is possibly mediated via RGD-integrin interaction in a calcium-dependent manner and suggests pulmonary EDTA chelation therapy as a novel treatment for COVID 19. Immunobiology. 2021;226:152021. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 124. | Lai AL, Freed JH. SARS-CoV-2 Fusion Peptide has a Greater Membrane Perturbating Effect than SARS-CoV with Highly Specific Dependence on Ca2. J Mol Biol. 2021;433:166946. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 55] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 125. | Berlansky S, Sallinger M, Grabmayr H, Humer C, Bernhard A, Fahrner M, Frischauf I. Calcium Signals during SARS-CoV-2 Infection: Assessing the Potential of Emerging Therapies. Cells. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 29] [Article Influence: 9.7] [Reference Citation Analysis (1)] |
| 126. | Alsagaff MY, Mulia EPB, Maghfirah I, Luke K, Nugraha D, Rachmi DA, Septianda I, A'yun MQ. Association of calcium channel blocker use with clinical outcome of COVID-19: A meta-analysis. Diabetes Metab Syndr. 2021;15:102210. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 127. | Mendez SR, Frank RC, Stevenson EK, Chung M, Silverman MG. Dihydropyridine Calcium Channel Blockers and the Risk of Severe COVID-19. Chest. 2021;160:89-93. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 128. | Bennouar S, Cherif AB, Kessira A, Bennouar DE, Abdi S. Lower plasma calcium associated with COVID-19, but not with disease severity: a two-centre retrospective cohort study. Journal of the American College of Nutrition. 2021;40:104-110. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 60] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 129. | Bastin A, Shiri H, Zanganeh S, Fooladi S, Momeni Moghaddam MA, Mehrabani M, Nematollahi MH. Iron Chelator or Iron Supplement Consumption in COVID-19? Biol Trace Elem Res. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 16] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 130. | Zhou C, Chen Y, Ji Y, He X, Xue D. Increased Serum Levels of Hepcidin and Ferritin Are Associated with Severity of COVID-19. Med Sci Monit. 2020;26:e926178. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 81] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 131. | Tojo K, Sugawara Y, Oi Y, Ogawa F, Higurashi T, Yoshimura Y, Miyata N, Hayami H, Yamaguchi Y, Ishikawa Y, Takeuchi I, Tachikawa N, Goto T. The U-shaped association of serum iron level with disease severity in adult hospitalized patients with COVID-19. Sci Rep. 2021;11:13431. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 132. | Hippchen T, Altamura S, Muckenthaler MU, Merle U. Hypoferremia is Associated With Increased Hospitalization and Oxygen Demand in COVID-19 Patients. Hemasphere. 2020;4:e492. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 58] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 133. | Sonnweber T, Boehm A, Sahanic S, Pizzini A, Aichner M, Sonnweber B, Kurz K, Koppelstätter S, Haschka D, Petzer V, Hilbe R, Theurl M, Lehner D, Nairz M, Puchner B, Luger A, Schwabl C, Bellmann-Weiler R, Wöll E, Widmann G, Tancevski I, Judith-Löffler-Ragg, Weiss G. Persisting alterations of iron homeostasis in COVID-19 are associated with non-resolving lung pathologies and poor patients' performance: a prospective observational cohort study. Respir Res. 2020;21:276. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 122] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 134. | Zhao K, Huang J, Dai D, Feng Y, Liu L, Nie S. Serum Iron Level as a Potential Predictor of Coronavirus Disease 2019 Severity and Mortality: A Retrospective Study. Open Forum Infect Dis. 2020;7:ofaa250. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 102] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 135. | Taneri PE, Gómez-Ochoa SA, Llanaj E, Raguindin PF, Rojas LZ, Roa-Díaz ZM, Salvador D Jr, Groothof D, Minder B, Kopp-Heim D, Hautz WE, Eisenga MF, Franco OH, Glisic M, Muka T. Anemia and iron metabolism in COVID-19: a systematic review and meta-analysis. Eur J Epidemiol. 2020;35:763-773. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 149] [Cited by in RCA: 237] [Article Influence: 47.4] [Reference Citation Analysis (0)] |
| 136. | de Faria Coelho-Ravagnani C, Corgosinho FC, Sanches FFZ, Prado CMM, Laviano A, Mota JF. Dietary recommendations during the COVID-19 pandemic. Nutr Rev. 2021;79:382-393. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 126] [Cited by in RCA: 119] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 137. | Lopez A, Cacoub P, Macdougall IC, Peyrin-Biroulet L. Iron deficiency anaemia. Lancet. 2016;387:907-916. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 732] [Cited by in RCA: 848] [Article Influence: 94.2] [Reference Citation Analysis (4)] |
| 138. | Akhtar S, Das JK, Ismail T, Wahid M, Saeed W, Bhutta ZA. Nutritional perspectives for the prevention and mitigation of COVID-19. Nutr Rev. 2021;79:289-300. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 45] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 139. | Skalny AV, Timashev PS, Aschner M, Aaseth J, Chernova LN, Belyaev VE, Grabeklis AR, Notova SV, Lobinski R, Tsatsakis A, Svistunov AA, Fomin VV, Tinkov AA, Glybochko PV. Serum Zinc, Copper, and Other Biometals Are Associated with COVID-19 Severity Markers. Metabolites. 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 53] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 140. | Zeng HL, Yang Q, Yuan P, Wang X, Cheng L. Associations of essential and toxic metals/metalloids in whole blood with both disease severity and mortality in patients with COVID-19. FASEB J. 2021;35:e21392. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 62] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 141. | Hackler J, Heller RA, Sun Q, Schwarzer M, Diegmann J, Bachmann M, Moghaddam A, Schomburg L. Relation of Serum Copper Status to Survival in COVID-19. Nutrients. 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 53] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 142. | Arrieta F, Martinez-Vaello V, Bengoa N, Jiménez-Mendiguchia L, Rosillo M, de Pablo A, Voguel C, Martinez-Barros H, Pintor R, Belanger-Quintana A, Mateo R, Candela A, Botella-Carretero JI. Serum zinc and copper in people with COVID-19 and zinc supplementation in parenteral nutrition. Nutrition. 2021;91-92:111467. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 143. | Rani I, Goyal A, Bhatnagar M, Manhas S, Goel P, Pal A, Prasad R. Potential molecular mechanisms of zinc- and copper-mediated antiviral activity on COVID-19. Nutr Res. 2021;92:109-128. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 46] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 144. | Gammoh NZ, Rink L. Zinc in Infection and Inflammation. Nutrients. 2017;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 433] [Cited by in RCA: 458] [Article Influence: 57.3] [Reference Citation Analysis (0)] |
| 145. | Shakoor H, Feehan J, Al Dhaheri AS, Ali HI, Platat C, Ismail LC, Apostolopoulos V, Stojanovska L. Immune-boosting role of vitamins D, C, E, zinc, selenium and omega-3 fatty acids: Could they help against COVID-19? Maturitas. 2021;143:1-9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 269] [Cited by in RCA: 220] [Article Influence: 55.0] [Reference Citation Analysis (0)] |
| 146. | Bonaventura P, Benedetti G, Albarède F, Miossec P. Zinc and its role in immunity and inflammation. Autoimmun Rev. 2015;14:277-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 378] [Cited by in RCA: 482] [Article Influence: 43.8] [Reference Citation Analysis (0)] |
| 147. | Hunter J, Arentz S, Goldenberg J, Yang G, Beardsley J, Myers SP, Mertz D, Leeder S. Zinc for the prevention or treatment of acute viral respiratory tract infections in adults: a rapid systematic review and meta-analysis of randomised controlled trials. BMJ Open. 2021;11:e047474. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 148. | Morgan CI, Ledford JR, Zhou P, Page K. Zinc supplementation alters airway inflammation and airway hyperresponsiveness to a common allergen. J Inflamm (Lond). 2011;8:36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 149. | Karim MM, Sultana S, Sultana R, Rahman MT. Possible Benefits of Zinc supplement in CVD and COVID-19 Comorbidity. J Infect Public Health. 2021;14:1686-1692. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 150. | te Velthuis AJ, van den Worm SH, Sims AC, Baric RS, Snijder EJ, van Hemert MJ. Zn(2+) inhibits coronavirus and arterivirus RNA polymerase activity in vitro and zinc ionophores block the replication of these viruses in cell culture. PLoS Pathog. 2010;6:e1001176. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 513] [Cited by in RCA: 576] [Article Influence: 38.4] [Reference Citation Analysis (0)] |
| 151. | Bagheri-Hosseinabadi Z, Pirsadeghi A, Rahnama A, Bahrehmand F, Abbasifard M. Is there any relationship between serum zinc levels and angiotensin-converting enzyme 2 gene expression in patients with coronavirus disease 2019? Meta Gene. 2022;31:100991. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 152. | McPherson SW, Keunen JE, Bird AC, Chew EY, van Kuijk FJ. Investigate Oral Zinc as a Prophylactic Treatment for Those at Risk for COVID-19. Am J Ophthalmol. 2020;216:A5-A6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 153. | Elalfy H, Besheer T, El-Mesery A, El-Gilany AH, Soliman MA, Alhawarey A, Alegezy M, Elhadidy T, Hewidy AA, Zaghloul H, Neamatallah MAM, Raafat D, El-Emshaty WM, Abo El Kheir NY, El-Bendary M. Effect of a combination of nitazoxanide, ribavirin, and ivermectin plus zinc supplement (MANS.NRIZ study) on the clearance of mild COVID-19. J Med Virol. 2021;93:3176-3183. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 47] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 154. | Thomas S, Patel D, Bittel B, Wolski K, Wang Q, Kumar A, Il'Giovine ZJ, Mehra R, McWilliams C, Nissen SE, Desai MY. Effect of High-Dose Zinc and Ascorbic Acid Supplementation vs Usual Care on Symptom Length and Reduction Among Ambulatory Patients With SARS-CoV-2 Infection: The COVID A to Z Randomized Clinical Trial. JAMA Netw Open. 2021;4:e210369. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 193] [Cited by in RCA: 248] [Article Influence: 62.0] [Reference Citation Analysis (0)] |
| 155. | Abdelmaksoud AA, Ghweil AA, Hassan MH, Rashad A, Khodeary A, Aref ZF, Sayed MAA, Elsamman MK, Bazeed SES. Olfactory Disturbances as Presenting Manifestation Among Egyptian Patients with COVID-19: Possible Role of Zinc. Biol Trace Elem Res. 2021;199:4101-4108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 45] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 156. | Muhamed PK, Vadstrup S. Zinc is the most important trace element. Ugeskr Laeger. 2014;176. [PubMed] |
| 157. | Santos HO, Teixeira FJ, Schoenfeld BJ. Dietary vs. pharmacological doses of zinc: A clinical review. Clin Nutr. 2020;39:1345-1353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 44] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 158. | Carlucci PM, Ahuja T, Petrilli C, Rajagopalan H, Jones S, Rahimian J. Zinc sulfate in combination with a zinc ionophore may improve outcomes in hospitalized COVID-19 patients. J Med Microbiol. 2020;69:1228-1234. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 109] [Cited by in RCA: 101] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 159. | Tang CF, Ding H, Jiao RQ, Wu XX, Kong LD. Possibility of magnesium supplementation for supportive treatment in patients with COVID-19. Eur J Pharmacol. 2020;886:173546. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 70] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 160. | DiNicolantonio JJ, O'Keefe JH. Magnesium and Vitamin D Deficiency as a Potential Cause of Immune Dysfunction, Cytokine Storm and Disseminated Intravascular Coagulation in covid-19 patients. Mo Med. 2021;118:68-73. [PubMed] |
| 161. | van Niekerk G, Mitchell M, Engelbrecht AM. Bone resorption: supporting immunometabolism. Biol Lett. 2018;14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 162. | Zhu L, Bao X, Bi J, Lin Y, Shan C, Fan X, Bian J, Wang X. Serum magnesium in patients with severe acute respiratory syndrome coronavirus 2 from Wuhan, China. Magnes Res. 2021;34:103-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 163. | Iotti S, Wolf F, Mazur A, Maier JA. The COVID-19 pandemic: is there a role for magnesium? Magnes Res. 2020;33:21-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 47] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 164. | Nairz M, Weiss G. Iron in infection and immunity. Mol Aspects Med. 2020;75:100864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 212] [Article Influence: 42.4] [Reference Citation Analysis (0)] |
| 165. | Mariani E, Cattini L, Neri S, Malavolta M, Mocchegiani E, Ravaglia G, Facchini A. Simultaneous evaluation of circulating chemokine and cytokine profiles in elderly subjects by multiplex technology: relationship with zinc status. Biogerontology. 2006;7:449-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 71] [Article Influence: 3.9] [Reference Citation Analysis (0)] |