Published online Mar 9, 2022. doi: 10.5492/wjccm.v11.i2.85
Peer-review started: April 7, 2021
First decision: July 27, 2021
Revised: September 4, 2021
Accepted: February 25, 2022
Article in press: February 25, 2022
Published online: March 9, 2022
Processing time: 329 Days and 7.7 Hours
Despite major advances in pharmacologic treatment, patients with pulmonary arterial hypertension (PAH) still have a considerably reduced life expectancy. In this context, chronic hyperactivity of the neurohormonal axis has been shown to be detrimental in PAH, thus providing novel insights on the role of neuroho
To evaluate the application and prognostic effect of neurohormonal inhibitors (NEUi) in a single-center sample of patients with idiopathic PAH and risk factors for left heart disease.
We analyzed data retrospectively collected from our register of right heart catheterizations performed consecutively from January 1, 2005 to October 31, 2018. Patients on beta-blocker, angiotensin-converting enzyme inhibitor, angiotensin receptor blocker or mineralocorticoid receptor antagonist at the time of right heart catheterization were classified as NEUi users and compared to NEUi non-recipients.
Complete data were available for 57 PAH subjects: 27 of those (47.4%) were taking at least one NEUi at the time of right heart catheterization and were compared with the remaining 36 NEUi non-recipients. NEUi users were older and had a higher cardiovascular risk profile compared to non-recipients. Additionally, NEUi non-users had a higher probability of dying during the course of follow-up than NEUi recipients (56.7% vs 25.9%, log-rank P = 0.020).
The above data highlighted a subgroup of patients with PAH and comorbidities for left heart disease in which NEUi use has shown to be associated with improved survival. Future prospective studies are needed to identify the most appropriate therapeutic strategies in this subset population.
Core Tip: In this observational study we underscored an increase in risk predictors for left heart disease among patients with idiopathic pulmonary arterial hypertension. Data were retrospectively collected from a single-center sample of patients with idiopathic pulmonary arterial hypertension who underwent right heart catheterization from January 1, 2005 to October 31, 2018. Among them, subjects treated with neurohormonal inhibitors showed a significantly better prognostic outcome during the course of follow-up as compared to neurohormonal inhibitor non-recipients.
- Citation: Scagliola R, Brunelli C, Balbi M. Treatment with neurohormonal inhibitors and prognostic outcome in pulmonary arterial hypertension with risk factors for left heart disease. World J Crit Care Med 2022; 11(2): 85-91
- URL: https://www.wjgnet.com/2220-3141/full/v11/i2/85.htm
- DOI: https://dx.doi.org/10.5492/wjccm.v11.i2.85
Pulmonary arterial hypertension (PAH) is a life-threatening cause of right ventricular failure, characterized by endothelial dysfunction and pulmonary vascular remodeling[1]. Despite major advances in pharmacologic treatment, patients with PAH still have a considerably reduced life expectancy. In this context, chronic hyperactivity of the neurohormonal axis has been shown to be detrimental in PAH, thus providing novel insights on the role of neurohormonal blockade as a potential therapeutic target[2]. To date, neurohormonal inhibitors (NEUi) are not currently labelled in PAH by contemporary guidelines, while they are used to treat PAH subjects with concomitant risk factors for left heart disease (LHD), for which they are instead scheduled for[3,4].
In recent years, further investigations have challenged the paradigm according to which PAH and pulmonary hypertension (PH) due to LHD are considered two separate pathophysiological entities. The AMBITION (Ambrisentan and Tadalafil in Patients with Pulmonary Arterial Hypertension) trial found a higher than expected prevalence of risk predictors for LHD among PAH patients[5]. In the same way, data from the COMPERA (Comparative, Prospective Registry of Newly Initiated Therapies for Pulmonary Arterial Hypertension) and other registry reports showed a significant trend towards an increased age and a higher percentage of cardiovascular comorbidities at diagnosis of PAH, together with a weaker response to targeted PAH therapy[6,7]. So the emerging definition of ‘atypical PAH’ or ‘PAH with comorbidities’ has been coined to identify such a hybrid PH phenotype with a purely precapillary hemodynamic profile and risk predictors for LHD, in which a concealed post-capillary involvement may be supposed[8,9]. In this way, the favorable impact of NEUi in this subset population has been hypothesized, by targeting cardiovascular risk factors and hidden LHD.
We evaluated retrospectively collected data of subjects who underwent right heart catheterization (RHC) in a single-center cohort followed in the Cardiology Unit of University Hospital San Martino in Genoa, Italy from January 1, 2005 up to October 31, 2018. Following the current European Society of Cardiology and European Respiratory Society guidelines for the diagnosis and treatment of pulmonary hypertension[3], PAH was defined hemodynamically by mean pulmonary arterial pressure ≥ 25 mmHg, together with pulmonary artery wedge pressure ≤ 15 mmHg and pulmonary vascular resistance > 3 Wood units, in the absence of other identifiable etiologies of precapillary PH.
We selected patients with idiopathic PAH and complete information about demographics, biochemical data and drug therapy at the time of RHC. Patients with PAH and associated clinical conditions, such as PH due to lung disease and/or hypoxia, chronic thromboembolic PH or PH related to unclear or multifactorial mechanisms, were ruled out of the observational analysis. Subjects with a diagnosis of LHD (defined by instrumental signs of left ventricular systolic or diastolic dysfunction or left heart valvular disease) did not undergo hemodynamic assessment by RHC and were excluded from the study population, according to our guidelines recommended study protocol[3,10].
In order to rule out occult post-capillary PH in patients suspected of having PAH, rapid fluid administration of 500 mL 0.9% NaCl solution within 5 min (by pressure cuff, C-fusor 500, Smiths Medical, Minneapolis, MN, United States) was performed, and the response of pulmonary artery wedge pressure to shifts in volume status was recorded within 2 min after the fluid challenge[11,12].
Patients on beta-blocker, angiotensin-converting enzyme inhibitor, angiotensin receptor blocker or mineralocorticoid receptor antagonist at the time of RHC were classified as NEUi users and compared with NEUi non-recipients. Comparisons between NEUi users and NEUi non-users were performed in terms of demographics, cardiovascular risk factors, biochemical samples, hemodynamic parameters and prognostic outcome.
This study was conducted in accordance with the principles of the Declaration of Helsinki, and the ethics committee of the Medical University of Genoa approved the protocol. Due to the retrospective design, written informed consent to participate in the study was not applicable.
Statistical analysis was carried out using the Statistica 13.1 software for Windows (StatSoft, Inc., Tulsa, OK, United States). Quantitative variables were expressed either as number (percentage of total) or mean ± standard deviation. The statistical significance of the results between the two groups was determined by means of either χ2 test or t-test, as appropriate. Death from any cause was assessed by Kaplan-Meyer survival analysis. A P value < 0.05 was considered statistically significant.
Complete data were available for 57 patients affected by idiopathic PAH. The majority of them were female (64.9%), mean age was 63.6 ± 10.6 years and mean follow-up period was 4.2 ± 3.0 years. Mean pulmonary arterial pressure, pulmonary artery wedge pressure and pulmonary vascular resistance were 45.0 ± 14.9 mmHg, 11.0 ± 2.8 mmHg and 8.8 ± 5.0 Wood units, respectively. Twenty-seven patients (47.4%) were under treatment with at least one NEUi at the time of RHC and constituted the NEUi user group: 15 (26.3%) were taking angiotensin-converting enzyme inhibitor/angiotensin receptor blocker and 12 (21.1%) beta-blockers, while 6 (10.5%) were taking mineralocorticoid receptor antagonists. The remaining 36 subjects of the study population belonged to the NEUi non-recipients.
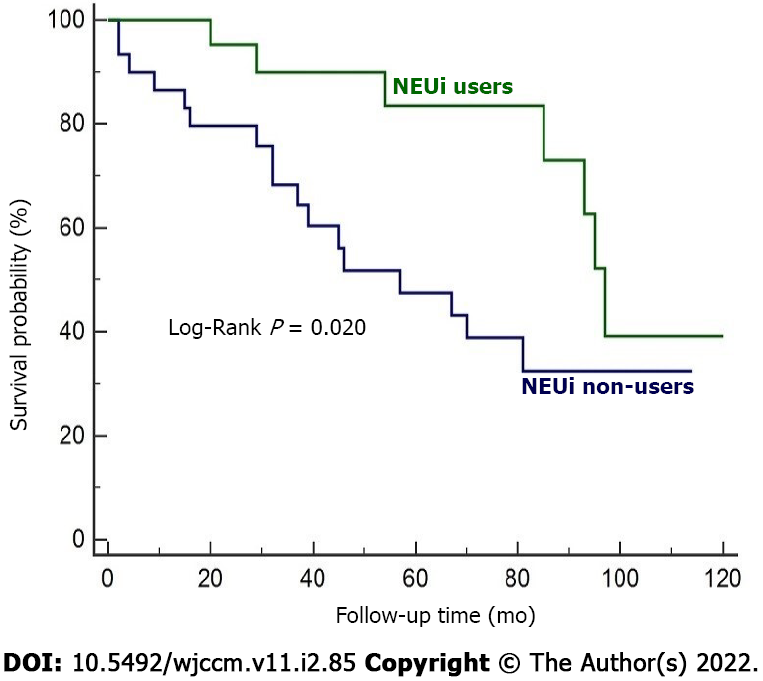
The two groups were comparable in terms of PAH-specific drugs taken during the follow-up period, as well as of prognostic determinants for PAH provided by the current European guidelines, including World Health Organization functional class, 6-min walking distance, right atrial pressure, cardiac index and N-terminal pro-brain natriuretic peptide plasmatic levels (P = not significant). NEUi users were significantly older (67.6 ± 11.9 years vs 60.1 ± 14.5 years, P = 0.039), had a lower glomerular filtration rate (58.7 ± 22.7 mL/min/1.73 m2vs 73.7 ± 24.7 mL/min/1.73 m2, P = 0.022), a higher body mass index (25.9 ± 4.4 vs 23.5 ± 3.5, P = 0.025), an increased prevalence of smoking habits (51.9% vs 20.0%, P = 0.025) and increased systemic arterial hypertension (74.1% vs 40.0%, P = 0.020) compared to non-recipients. Additionally, 5 NEUi recipients (18.5%) underwent coronary artery revascularization compared to NEUi non-users (P = 0.046). Baseline characteristics and statistical results are summarized in Table 1. NEUi non-users had a higher probability of dying during the course of follow-up than NEUi recipients (56.7% vs 25.9%, log-rank P = 0.020) (Figure 1).
| Variable | NEUi non-users, n = 30 | NEUi users, n = 27 | P |
| Age in yr | 60.1 ± 14.5 | 67.6 ± 11.9 | 0.039 |
| Men/Women, n (%) | 11 (36.7)/19 (63.3) | 9 (33.3)/18 (66.7) | 0.988 |
| Follow-up in yr | 4.0 ± 2.7 | 4.5 ± 3.3 | 0.504 |
| Dead at follow-up, n (%) | 17 (56.7) | 7 (25.9) | 0.038 |
| BMI in kg/m2 | 23.5 ± 3.5 | 25.9 ± 4.4 | 0.025 |
| Arterial hypertension, n (%) | 12 (40.0) | 20 (74.1) | 0.020 |
| Smoking habits, n (%) | 6 (20.0) | 14 (51.9) | 0.025 |
| Dyslipidemia, n (%) | 7 (23.3) | 12 (44.4) | 0.160 |
| Diabetes mellitus, n (%) | 2 (6.7) | 5 (18.5) | 0.339 |
| Supraventricular arrhythmias, n (%) | 4 (13.3) | 7 (25.9) | 0.386 |
| Coronary artery disease, n (%) | 0 (0) | 5 (18.5) | 0.046 |
| eGFR in mL/min/1.73 m2 [CKD-EPI] | 73.7 ± 24.7 | 58.7 ± 22.7 | 0.022 |
| WHO-FC | 2.2 ± 0.76 | 2.3 ± 0.47 | 0.572 |
| 6MWD in m | 383.9 ± 129.7 | 374.3 ± 145.1 | 0.845 |
| NT-proBNP in ng/mL | 714.9 ± 692.4 | 808.7 ± 617.9 | 0.593 |
| Systolic PAP in mmHg | 74.7 ± 26.3 | 71.0 ± 21.3 | 0.569 |
| Diastolic PAP in mmHg | 27.5 ± 11.6 | 26.3 ± 9.6 | 0.681 |
| Mean PAP in mmHg | 46.2 ± 16.1 | 43.6 ± 13.6 | 0.509 |
| Right atrial pressure in mmHg | 8.3 ± 3.9 | 10.5 ± 5.0 | 0.063 |
| PAWP in mmHg | 10.5 ± 2.9 | 11.7 ± 2.5 | 0.105 |
| PVR in Wood unit | 9.0 ± 5.4 | 8.6 ± 4.6 | 0.789 |
| Cardiac index in L/min/m2 | 2.6 ± 0.9 | 2.4 ± 0.6 | 0.258 |
The reported data detected a significantly higher cardiovascular risk profile in the study population, encountering more than 50% of subjects with arterial hypertension and more than 30% with smoking habits and dyslipidemia. Albeit limited by the retrospective nature of the investigation, the small size and the single-center origin of the sample examined, these findings are in agreement with the results from the AMBITION trial and substantiated by registry data supporting that PAH with cardiovascular comorbidities is a codified PH entity in clinical practice[5,7]. However, to date these data have not been acknowledged by the current international guidelines on PH, which still fail to consider patients with PAH and cardiovascular comorbidities as belonging to a defined clinical subset[3,13]. This lack in the current state of regard for PH has limited further speculation on the potential therapeutic effects of NEUi in these kinds of patients. In this regard, the analysis of the two patient populations studied herein showed a significantly higher cardiovascular risk profile for LHD among NEUi users, in whom a better prognostic outcome has been observed compared to NEUi non-recipients.
A plausible explanation to these observations comes from the beneficial effects of NEUi use on cardiovascular comorbidities, which tended to cluster in the NEUi users group acting mainly on systemic inflammation and microvascular circulation, with consequent worsening of right ventricular impairment and survival[14,15]. In the same line, data from the literature pointed out a plausible overlap between idiopathic PAH and PH due to LHD in terms of pathophysiologic mechanisms, prognostic outcomes and response to targeted PAH-specific treatment[11,14]. In the analysis conducted by Obokata et al[16], the activation of the endothelin signaling pathway seemed to contribute to right ventricular functional impairment in subjects with heart failure with preserved ejection fraction, while endothelin-1 is also historically known for its pathogenic role in developing PAH by pulmonary vasoconstriction, smooth muscle cell proliferation and pulmonary vascular remodeling.
Several studies emphasized a proposed paradigm whereby metabolic syndrome and cardiovascular comorbidities could reinforce PH in patients with LHD by exploiting molecular pathways actively involved in developing PAH, like a deranged interplay between decreased microvascular nitric oxide availability and enhanced endothelin expression[17-20]. Therefore, the close relationship between these two PH phenotypes raised the hypothesis of a potential continuum disease, in which PAH with risk factor for LHD lies in-between. For these reasons, it is possible to assume that the better prognostic outcome observed in NEUi recipients of our study population could also be intrinsically related to an intermediate pathophysiologic standpoint in the spectrum of disease (phenotypically closer to PH due to LHD albeit with a hemodynamic profile comparable with precapillary PH) rather than solely ascribed to the therapeutic properties of neurohormonal axis blockers on cardiovascular comorbidities.
Finally, considering the aforementioned upregulation of the neurohormonal axis in PAH and its deleterious properties on worsening right heart failure in the long-run, a direct favorable implication of NEUi on right ventricular function and pulmonary circulation in this study population may be also taken into account[2,21].
In conclusion, our data highlighted a codified subset of patients with PAH and a comorbidity profile for LHD, lying between the extremes of a pathophysiological continuum, in which NEUi use has been shown to be associated with a better prognostic outcome. Further investigation is required to define the proper pharmacological treatment in patients with PAH and hidden LHD.
Despite new insights in pharmacological treatment, patents with pulmonary arterial hypertension (PAH) still have a considerably reduced life expectancy.
Chronic hyperactivity of the neurohormonal axis has been shown to be detrimental in PAH, thus providing novel insights on the role of neurohormonal inhibitors (NEUi) as a new potential therapeutic target.
To assess the use and prognostic impact of NEUi in a single-center cohort of subjects with idiopathic PAH and risk factors for left heart disease.
This was a single-center, retrospective observational study, involving 57 subjects with idiopathic PAH, confirmed by right heart catheterization. Patients on beta-blocker, angiotensin-converting enzyme inhibitor, angiotensin receptor blocker or mineralocorticoid receptor antagonist at the time of right heart catheterization were classified as NEUi users and compared to NEUi non-recipients.
NEUi users were significantly older (67.6 ± 11.9 years vs 60.1 ± 14.5 years, P = 0.039), had a higher body mass index (25.9 ± 4.4 vs 23.5 ± 3.5, P = 0.025), a lower estimated glomerular filtration rate (58.7 ± 22.7 mL/min/1.73 m2vs 73.7 ± 24.7 mL/min/1.73 m2, P = 0.022) and more frequent systemic arterial hypertension (74.1% vs 40.0%, P = 0.020) and smoking habits (51.9% vs 20.0%, P = 0.025) compared to non-recipients. Mortality rate was significantly higher among NEUi non-users than in NEUi users (56.7% vs 25.9%, P = 0.038). NEUi non-users were more likely to die over the course of follow-up (log-rank P = 0.020).
Our analysis highlighted a subset of patients with PAH and cardiovascular comorbidities in which NEUi use has been shown to be associated with improved survival.
Future prospective studies are needed to identify the most appropriate therapeutic strategies in this subset population.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cardiac and cardiovascular systems
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Li P, China S-Editor: Wu YXJ L-Editor: Filipodia P-Editor: Wu YXJ
| 1. | Handoko ML, de Man FS, Allaart CP, Paulus WJ, Westerhof N, Vonk-Noordegraaf A. Perspectives on novel therapeutic strategies for right heart failure in pulmonary arterial hypertension: lessons from the left heart. Eur Respir Rev. 2010;19:72-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 97] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 2. | de Man FS, Handoko ML, Guignabert C, Bogaard HJ, Vonk-Noordegraaf A. Neurohormonal axis in patients with pulmonary arterial hypertension: friend or foe? Am J Respir Crit Care Med. 2013;187:14-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 73] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 3. | Galiè N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, Simonneau G, Peacock A, Vonk Noordegraaf A, Beghetti M, Ghofrani A, Gomez Sanchez MA, Hansmann G, Klepetko W, Lancellotti P, Matucci M, McDonagh T, Pierard LA, Trindade PT, Zompatori M, Hoeper M; ESC Scientific Document Group. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J. 2016;37:67-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3636] [Cited by in RCA: 3916] [Article Influence: 391.6] [Reference Citation Analysis (0)] |
| 4. | Thenappan T, Roy SS, Duval S, Glassner-Kolmin C, Gomberg-Maitland M. β-blocker therapy is not associated with adverse outcomes in patients with pulmonary arterial hypertension: a propensity score analysis. Circ Heart Fail. 2014;7:903-910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 5. | McLaughlin VV, Vachiery JL, Oudiz RJ, Rosenkranz S, Galiè N, Barberà JA, Frost AE, Ghofrani HA, Peacock AJ, Simonneau G, Rubin LJ, Blair C, Langley J, Hoeper MM; AMBITION Study Group. Patients with pulmonary arterial hypertension with and without cardiovascular risk factors: Results from the AMBITION trial. J Heart Lung Transplant. 2019;38:1286-1295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 78] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 6. | Hoeper MM, Huscher D, Ghofrani HA, Delcroix M, Distler O, Schweiger C, Grunig E, Staehler G, Rosenkranz S, Halank M, Held M, Grohé C, Lange TJ, Behr J, Klose H, Wilkens H, Filusch A, Germann M, Ewert R, Seyfarth HJ, Olsson KM, Opitz CF, Gaine SP, Vizza CD, Vonk-Noordegraaf A, Kaemmerer H, Gibbs JS, Pittrow D. Elderly patients diagnosed with idiopathic pulmonary arterial hypertension: results from the COMPERA registry. Int J Cardiol. 2013;168:871-880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 311] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 7. | Charalampopoulos A, Howard LS, Tzoulaki I, Gin-Sing W, Grapsa J, Wilkins MR, Davies RJ, Nihoyannopoulos P, Connolly SB, Gibbs JS. Response to pulmonary arterial hypertension drug therapies in patients with pulmonary arterial hypertension and cardiovascular risk factors. Pulm Circ. 2014;4:669-678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 8. | Opitz CF, Hoeper MM, Gibbs JS, Kaemmerer H, Pepke-Zaba J, Coghlan JG, Scelsi L, D'Alto M, Olsson KM, Ulrich S, Scholtz W, Schulz U, Grünig E, Vizza CD, Staehler G, Bruch L, Huscher D, Pittrow D, Rosenkranz S. Pre-Capillary, Combined, and Post-Capillary Pulmonary Hypertension: A Pathophysiological Continuum. J Am Coll Cardiol. 2016;68:368-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 233] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 9. | Kovacs G, Dumitrescu D, Barner A, Greiner S, Grünig E, Hager A, Köhler T, Kozlik-Feldmann R, Kruck I, Lammers AE, Mereles D, Meyer A, Meyer J, Pabst S, Seyfarth HJ, Sinning C, Sorichter S, Stähler G, Wilkens H, Held M. Definition, clinical classification and initial diagnosis of pulmonary hypertension: Updated recommendations from the Cologne Consensus Conference 2018. Int J Cardiol. 2018;272S:11-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 10. | Pieske B, Tschöpe C, de Boer RA, Fraser AG, Anker SD, Donal E, Edelmann F, Fu M, Guazzi M, Lam CSP, Lancellotti P, Melenovsky V, Morris DA, Nagel E, Pieske-Kraigher E, Ponikowski P, Solomon SD, Vasan RS, Rutten FH, Voors AA, Ruschitzka F, Paulus WJ, Seferovic P, Filippatos G. How to diagnose heart failure with preserved ejection fraction: the HFA-PEFF diagnostic algorithm: a consensus recommendation from the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur J Heart Fail. 2020;22:391-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 213] [Article Influence: 42.6] [Reference Citation Analysis (0)] |
| 11. | Robbins IM, Hemnes AR, Pugh ME, Brittain EL, Zhao DX, Piana RN, Fong PP, Newman JH. High prevalence of occult pulmonary venous hypertension revealed by fluid challenge in pulmonary hypertension. Circ Heart Fail. 2014;7:116-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 131] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 12. | Fujimoto N, Borlaug BA, Lewis GD, Hastings JL, Shafer KM, Bhella PS, Carrick-Ranson G, Levine BD. Hemodynamic responses to rapid saline loading: the impact of age, sex, and heart failure. Circulation. 2013;127:55-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 146] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 13. | Yaghi S, Novikov A, Trandafirescu T. Clinical update on pulmonary hypertension. J Investig Med. 2020;68:821-827. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 14. | Scagliola R. Pulmonary arterial hypertension and pulmonary hypertension due to left heart disease: so near and yet so far. Pol Arch Intern Med. 2020;130:349-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 15. | Paulus WJ, Tschöpe C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol. 2013;62:263-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1983] [Cited by in RCA: 2559] [Article Influence: 213.3] [Reference Citation Analysis (0)] |
| 16. | Obokata M, Kane GC, Reddy YNV, Melenovsky V, Olson TP, Jarolim P, Borlaug BA. The neurohormonal basis of pulmonary hypertension in heart failure with preserved ejection fraction. Eur Heart J. 2019;40:3707-3717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 53] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 17. | Franssen C, Paulus WJ. Normal resting pulmonary artery wedge pressure: a diagnostic trap for heart failure with preserved ejection fraction. Eur J Heart Fail. 2015;17:132-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 18. | Robbins IM, Newman JH, Johnson RF, Hemnes AR, Fremont RD, Piana RN, Zhao DX, Byrne DW. Association of the metabolic syndrome with pulmonary venous hypertension. Chest. 2009;136:31-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 130] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 19. | Rocha NG, Templeton DL, Greiner JJ, Stauffer BL, DeSouza CA. Metabolic syndrome and endothelin-1 mediated vasoconstrictor tone in overweight/obese adults. Metabolism. 2014;63:951-956. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 20. | van Heerebeek L, Hamdani N, Falcão-Pires I, Leite-Moreira AF, Begieneman MP, Bronzwaer JG, van der Velden J, Stienen GJ, Laarman GJ, Somsen A, Verheugt FW, Niessen HW, Paulus WJ. Low myocardial protein kinase G activity in heart failure with preserved ejection fraction. Circulation. 2012;126:830-839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 337] [Cited by in RCA: 398] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 21. | Emanuel R, Chichra A, Patel N, Le Jemtel TH, Jaiswal A. Neurohormonal modulation as therapeutic avenue for right ventricular dysfunction in pulmonary artery hypertension: till the dawn, waiting. Ann Transl Med. 2018;6:301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |