Published online Jan 9, 2022. doi: 10.5492/wjccm.v11.i1.22
Peer-review started: March 14, 2021
First decision: July 18, 2021
Revised: August 4, 2021
Accepted: November 15, 2021
Article in press: November 15, 2021
Published online: January 9, 2022
Processing time: 301 Days and 12.2 Hours
Acute exacerbations of interstitial lung disease (AE-ILD) represent an acute, frequent and often highly morbid event in the disease course of ILD patients. Admission in the intensive care unit (ICU) is very common and the need for mechanical ventilation arises early. While non-invasive ventilation has shown promise in staving off intubation in selected patients, it is unclear whether mechanical ventilation can alter the exacerbation course unless it is a bridge to lung transplantation. Risk stratification using clinical and radiographic findings, and early palliative care involvement, are important in ICU care. In this review, we discuss many of the pathophysiological aspects of AE-ILD and raise the hypothesis that ventilation strategies used in acute respiratory distress syndrome might be implemented in AE-ILD. We present possible decision-making and management algorithms that can be used by the intensivist when caring for these patients.
Core Tip: During the acute and morbid event of acute exacerbation of interstitial lung disease, an intensivist needs to understand the pathophysiology and reversible causes of acute exacerbations, the diagnostics and treatments that are usually recommended, and the experimental therapies on the horizon. More importantly, the intensivist needs to be able to risk stratify the patients, selectively pursue mechanical ventilation, minimize ventilator induced lung injury, and involve palliative care early in non-lung transplant candidates.
- Citation: Charokopos A, Moua T, Ryu JH, Smischney NJ. Acute exacerbation of interstitial lung disease in the intensive care unit. World J Crit Care Med 2022; 11(1): 22-32
- URL: https://www.wjgnet.com/2220-3141/full/v11/i1/22.htm
- DOI: https://dx.doi.org/10.5492/wjccm.v11.i1.22
Acute exacerbations in interstitial lung diseases (AE-ILD) represent an acute, and frequently morbid, deterioration of the patients’ respiratory function, often leading to hospital admission. Intensivists are at the forefront of care for these patients, and often need to make critical decisions about treatment and whether mechanical ventilation will be beneficial. While originally and most thoroughly described in idiopathic pulmonary fibrosis (IPF), acute exacerbations are increasingly recognized in other types of fibrotic interstitial lung disease (ILD) such as fibrotic (chronic) hypersensitivity pneumonitis[1,2] and connective-tissue disease related ILD[3-5]. To distinguish between the two entities, we will refer to i) acute exacerbations of IPF (AE-IPF) and ii) acute exacerbations of non-IPF interstitial lung disease (AE-nonIPF), grouped together as AE-ILD.
The definition of AE-IPF has shifted between 2007 (Idiopathic Pulmonary Fibrosis network, IPFnet)[6] and 2016 (revised criteria by international working group)[7]. The definition currently includes: (1) Known diagnosis of IPF; (2) Worsening dyspnea within the last 30 d; and (3) New bilateral ground glass opacities and/or consolidation upon a background of usual interstitial pneumonia (UIP); the previous requirement for exclusion of concurrent pulmonary embolism (PE) and identifiable infection has been eliminated[7].
The incidence rate of AE-IPF has been estimated to be 41 cases per 1000 person-years[8] with approximately 10% of IPF patients experiencing an acute exacerbation in the two years following their diagnosis[9]. AE-IPF tends to be more prevalent in those with more advanced disease, as measured by worse pulmonary function (especially forced vital capacity, and diffusing capacity for carbon monoxide), shorter 6 min walking distance, and lower baseline oxygenation[10-14].
An acute exacerbation occurring in patients with IPF and other fibrotic ILDs is often unpredictable, but specific intrinsic and extrinsic factors have been hypothesized to trigger the event. Intrinsic factors, such as epithelial homeostatic imbalance affecting fibrocyte differentiation, macrophage immune polarization, and possibly autoimmunity emergence against heat-shock proteins and phospholipid-binding proteins[15-18], have been identified in patients with AE-IPF. Several other factors, such as air pollution[19] and micro-aspiration[20,21], have also been identified. Interestingly, in a retrospective analysis of three well-known IPF placebo controlled clinical trials, none of the patients who developed AE-IPF were on anti-acid treatment[22,23]. A higher eosinophil percentage in bronchoalveolar lavage (BAL) has been associated with the onset of AE-IPF[24].
When an identifiable extrinsic trigger for AE-ILD is lacking, then the AE-ILD is considered idiopathic. On the contrary, infection, aspiration and drug toxicity are common extrinsic triggers of AE-ILD. Infection has been identified in 10% to 30% of patients with AE-ILD[25-27]. Furthermore, post-procedural AE-ILD has also been reported, including video-assisted thoracoscopic procedures and bronchoscopy with lavage[28-30]. The underlying mechanism is thought to be due to possible ventilator-induced injury (including hyperoxia or barotrauma), perioperative mechanical stretch, or fluid balance[7,31]. In a large study of acute exacerbations in all types of ILD, 52% of admissions for acute respiratory worsening were considered idiopathic, 20% due to infection, 15% due to subacute progression or end-stage disease, 6% due to heart failure or severe pulmonary hypertension, 4% due to venous thromboembolic disease, and 2% from diffuse alveolar hemorrhage or peri-procedural exacerbation[25].
Both AE-ILD and acute respiratory distress syndrome (ARDS) have bilateral ground glass opacities and/or consolidations on imaging and often refractory hypoxemia. Similar to ARDS, the most frequent histopathologic finding on lung biopsy seen in AE-ILD is diffuse alveolar damage[3,32], which involves an acute exudative phase followed by an organizing-proliferative phase[33]. It is likely that both patients with AE-ILD and ARDS have an aberrant and defective healing response to lung injury, that involves a pro-fibrotic positive-feedback loop[34-36].
When a patient with ILD, or specifically IPF, is admitted for acute respiratory worsening, it is up to the inpatient physician, or more often the intensivist, to distinguish between idiopathic acute exacerbation vs acute exacerbation secondary to a specific “treatable” trigger such as infection. In-hospital survival is worse in those with idiopathic AE-ILD compared to those stemming from a known-trigger[25], possibly due to lack of targeted treatment.
Interestingly, acute exacerbation may be the first presentation of previously undiagnosed ILD, with such patients comprising 29% of one large academic cohort[25]. Radiologic findings of fibrotic disease including reticulation and traction bronchiectasis, in a patient without known pulmonary disease suggests undiagnosed ILD. Surgical lung biopsy is often avoided during AE-IPF as its results often do not alter the course of acute exacerbation[32], and have increased peri/post-operative morbidity[37].
If the patient has previously undiagnosed ILD as noted above, then autoimmune serologies, including evaluation for pulmonary vasculitis with antineutrophil cytoplasmic antibodies, would be indicated to further clarify any potential autoimmunity that would suggest a related connective-tissue disease or interstitial pne
Infection can be evaluated by various sources, including laboratory findings (white cell count, urine Legionella or Streptococcus pneumoniae antigens, procalcitonin[39], nasal or sputum viral polymerase chain reaction [PCR] tests), vital signs, and of course blood or respiratory cultures[40]. The yield of bronchoscopy has been found to be relatively low; only 13% of bronchoscopies in AE-ILD yielded abnormal results according to a major study[27], with 25% of patients having bronchoscopy on the general floor necessitating post-procedural ICU transfer. When bronchoscopy is performed, BAL specimens should be sent for bacterial, fungal and mycobacterial cultures, including viral PCR tests. Since AE-non-IPF patients are often immunocompromised, an intensivist should consider pneumocystis jirovecii and herpesvirus infections, which represented 25% and 18% of positive bronchoscopies in one study, respectively[27].
High-resolution computed tomography (CT) is critical in clarifying the extent of underlying fibrotic interstitial disease and suspected new or superimposed ground glass or consolidative abnormalities. The extent and pattern of superimposed infiltrates on high-resolution CT have been found to be predictive of survival in AE-IPF[41,42]. The separation of the Kaplan-Meier survival curves depending on 3 different types of CT findings (peripheral, multifocal, or diffuse pattern) was found to be quite striking[41]. A protocol assessing for pulmonary embolism - or a ventilation-perfusion and lower extremity doppler scan in patients with renal impairment - may be reasonable to exclude thromboembolic disease. However, a PE protocol study was performed in only 43% of admissions for acute respiratory worsening in ILD patients[25]. Interestingly, a link between a profibrotic and a prothrombotic state has been found[43], with studies reporting higher risk of venous thromboembolism (VTE) in IPF patients[44,45]. Physical examination, serum brain natriuretic peptide concentrations, and echocardiography are used to evaluate for any component of heart failure and pulmonary hypertension[7].
When an intensivist encounters a deteriorating patient with AE-ILD, the decision for invasive mechanical ventilation (IMV) must be balanced with the prognosis and reversibility of the patient’s condition. Multiple studies have shown poor outcomes in this population, including studies that analyzed admissions before[46-48] and after[25,49] changes in lung protective ventilation following the publication of the ARDSnet trial in 2000. In-hospital mortality may reach 50% with 1-year mortality at 70%. In the years before lung protective ventilation strategies, studies identified that 85% mechanically ventilated patients with AE-IPF died while ventilated, and proposed that ICU admission and intubation may be futile[46]. Nevertheless, both due to: (1) the acceptance of lower tidal volumes in ICUs; and (2) Changes in the definition of AE-IPF to include potentially reversible causes, the outcomes of ventilated patients with AE-IPF have improved, but still remain poor. In a nationwide cohort from 2006-2012, in-hospital mortality of AE-IPF patients who received mechanical ventilation was 51.6% (although improved from 58.4% in 2006 to 49.3% in 2012) and of patients who received non-invasive ventilation (NIV) was 30.9%[49]. In another study of patients in French ICUs from 2002 to 2009, only 30% of those mechanically ventilated were successfully weaned[50]. As expected, in-hospital mortality varies according to ventilation type, being higher in patients requiring IMV compared to patients requiring NIV or no ventilation support in a large multicenter ICU database study[51]. NIV is a reasonable therapeutic option which may allow certain patients to avoid the morbidity of IMV[51,52].
In general, mortality is affected by disease type, with IPF for example having worse outcomes compared to other fibrotic ILD associated with autoimmune disorders or hypersensitivity pneumonitis. In a landmark study that explored admissions for acute respiratory worsening in patients with chronic fibrotic lung disease, in-hospital mortality was the same between IPF and patients without IPF (55% vs 45%, P > 0.05)[25], although other studies found nonspecific interstitial pneumonia to be associated with a relatively good discharge rate and long-term prognosis[4]. In a different study, 90-day mortality was found to be significantly higher in AE-IPF than AE-non-IPF (69% vs 34%)[53]. One-year mortality after hospitalization for acute exacerbation was worse in IPF than non-IPF (87% vs 71%), yet still very high in both groups[25]. Furthermore, while infection accounted for a third of AE-ILD cases in another United States cohort, outcomes did not differ between those with infection and those without[26]. However, post-operative exacerbation and respiratory failure in ILD patients is associated with a better prognosis[54]. Specific findings on high-resolution CT at admission in AE-IPF patients have been correlated with prognosis[41,42]. Artificial intelligence software is increasingly showing application and promise in the analysis of CT scans in ILD patients, and may potentially be used for prognostication[55].
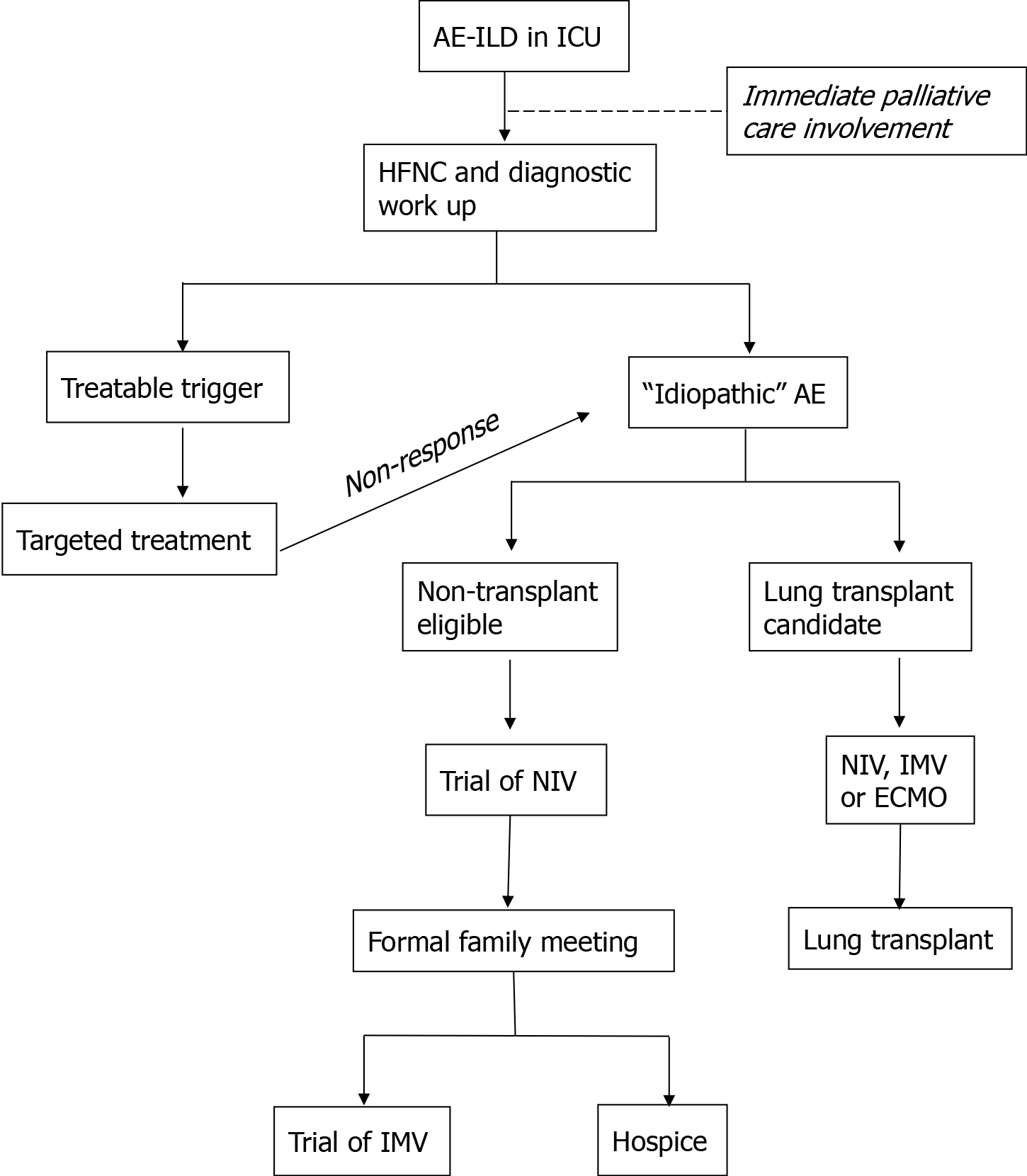
In the authors’ opinion, risk stratification and goals of care discussion need to take place early on when a patient with AE-ILD is admitted to the ICU. Studies have shown that a subset of patients can be weaned from mechanical ventilation and discharged, suggesting that IMV should not be systematically denied to these patients but considered individually[50]. Risk stratification certainly depends on clinical judgement, but can also be assisted by other published insights, including the aforementioned CT characteristics[41,42]. On admission to the hospital for respiratory worsening, only 20% of patients with fibrotic lung disease have a “do not resuscitate, do not intubate” code status[25]. Palliative care should be consulted early in the patients’ admission, and eligibility (or pre-existing enrollment with previous work-up completion) of patients for lung transplant should play important roles in the management decision tree (Figure 1). While the poor outcomes of mechanical ventilation place it in the role of “bridge therapy”, lung transplant is a potential “destination therapy” even for patients with severe acute exacerbations and deteriorating oxygenation. In non-transplant candidates who are deemed high risk for poor outcome, hospice should be brought up early in family discussions and goals of patient comfort and wishes for end-of-life strongly taken into consideration.
While the outcomes of AE-ILD patients have been well described, well-designed prospective clinical research in the management of these patients is lacking. It is unclear if the high morbidity and mortality of acute exacerbations creates a fertile environment for research as accepted by distressed patients and their families. International guidelines for AE-IPF make a weak recommendation for the use of corticosteroids, namely that corticosteroids should be used in the majority of patients with acute exacerbation of IPF, but not using may be reasonable in a minority[56]. This weak recommendation is based on expert opinion and retrospective reports[41,46,53]. No particular corticosteroid formulation has been found preferrable over another in AE-ILD, despite good outcomes with dexamethasone in ARDS and Coronavirus disease 2019 (Covid-19) associated lung injury[57,58]. Doses ranging from 1mg/kg of prednisone to pulse steroids (methylprednisolone 1 g daily for 3 d) have been used, depending on institutional preference and severity of presentation. In studies comparing corticosteroid treatment in acute exacerbations in idiopathic interstitial pneumonias vs connective tissue disease-associated ILD, both groups were observed to be treated with corticosteroids[53]. While others have argued for a steroid-free approach in AE-IPF[59,60], the frequent misdiagnosis of fibrotic hypersensitivity pneumonitis as IPF may be confounding[61]. The uncertainty but routine use of corticosteroids in AE-ILD supports a need for a prospective clinical trial.
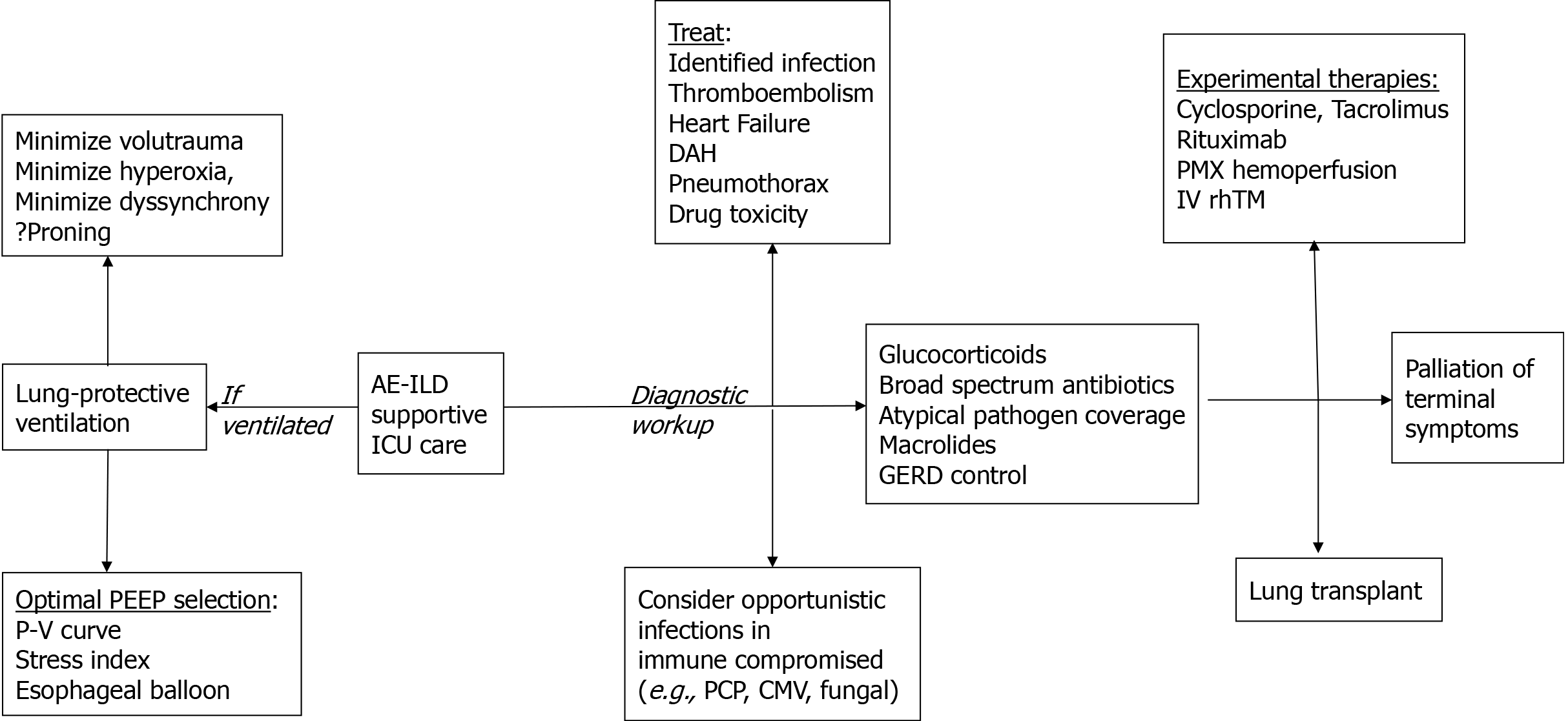
Antibiotics are routinely used in AE-ILD, accompanied by appropriate work up to evaluate underlying infection. Both broad spectrum and coverage for atypical pathogens should be considered. Azithromycin, which has been reported to improve outcomes in acute lung injury[62], has also shown particular promise in AE-ILD[63]. This is thought to a result of azithromycin’s anti-inflammatory and immune-modulating effects rather than antimicrobial activity, as it has been compared to fluoroquinolones which also cover atypical bacteria[63]. If no underlying infection is found, a routine 7 to 10 day course is reasonable. In a randomized trial, use of procalcitonin to guide antibiotic therapy in patients with AE-IPF resulted in reduced exposure to antibiotics without adversely affecting patient outcomes[39]. Since AE-non-IPF patients are often immunocompromised prior to admission, search for opportunistic pathogens and targeted treatment is prudent (Figure 2).
Key treatments that have been shown to partially prevent AE-IPF or AE-ILD in the outpatient setting - such as antacid therapy[22] and nintentanib[64] - have not been evaluated clinically during acute exacerbation. From the authors’ point of view, it is reasonable to continue inpatient use of both antacids and antifibrotics in patients previously treated with them. While there is no peer-reviewed evidence for benefit in initiating antifibrotics in the acute setting except rare case reports[65], antacid therapy should be easily and already instituted in AE-ILD patients treated with corticosteroids and/or mechanical ventilation.
AE-ILD has some parallels with ARDS both from a clinical (ground glass infiltrates and severe hypoxemia) and histological (diffuse alveolar damage on pathology) perspective. Similar to ARDS, patients with AE-ILD are prone to ventilator induced injury. Thus, mechanical ventilation strategies used in ARDS should be reasonably utilized in patients with AE-ILD[66]. Avoidance of ventilator-patient dyssynchrony (causing stacked inspired tidal volumes) and prevention of ventilator induced lung injury are of particular importance. Notably 42% of AE-ILD patients required paralytics in a large cohort, although paralytic use was associated with higher mortality in unadjusted analysis and possibly reflective of underlying disease severity[67]. Optimization of positive end-expiratory pressure (PEEP) and lung recruitment using pressure-volume hysteresis curves, stress index, or calculation of transpulmonary pressure with esophageal balloons present an opportunity to at least prevent iatrogenic contribution to a patient's already difficult prognosis. While prone posi
Only two studies have examined the effect of ventilator parameters on mortality in patients with AE-ILD[54,67]. The largest study examined 114 admissions for AE-ILD, of which 34% were AE-IPF and 66% were AE-nonIPF[67]. Only 50% of patients in this study achieved a low tidal volume strategy (plateau pressure ≤ 30 cm H2O) within 3 h of intubation. A variety of modifiable and nonmodifiable parameters - including increased time to intubation, higher initial fraction of inspired oxygen or PEEP, higher mean airway pressures, vasopressor use and right ventricular systolic pressure - were associated with in-hospital mortality. In the second retrospective study, step changes in positive end-expiratory pressure > 10 cm of water were found to have been attempted in 20 patients and resulted in increased airway pressures and decrease in respiratory system compliance suggestive of overdistension[54].
The importance of fluid management - with a goal of net-neutral or net-negative fluid balance - has been increasingly recognized[70], similarly to the management of ARDS. A retrospective study of postoperative AE-IPF patients surgically treated for lung cancer, a common finding in the IPF population[71], showed that more intraoperative fluid administration was associated with higher probability of AE-IPF[31]. Total net fluid status was also an important adjusted risk predictor for mortality in a large study of mechanical ventilation in AE-ILD[67].
In light of currently limited therapeutic options and the high mortality of patients with AE-ILD, experimental therapies have been tested in only a few small studies. Based on the premise of immune dysregulation being a primary driver of AE-IPF and/or AE-nonIPF[72], studies have focused on alternative immunosuppressants or cytokine filtration removal, often in conjunction with corticosteroids (Figure 2). Cyclophosphamide has not been studied using matched controls, but in one single-institution study administration of 1 g daily of methylprednisolone for 3 d followed by monthly cyclophosphamide administration for up to 6 doses showed a favorable overall survival at 3 mo (73%), 6 mo (63%) and 12 mo (55%) compared to the general literature[73]. Calcineurin inhibitors, such as tacrolimus and cyclosporine, have shown some benefit but have only been evaluated in small retrospective studies of 15-45 patients[74-76]. Due to possible autoantibodies in AE-IPF[18], rituximab and plasma exchange were studied in 11 patients with AE-IPF and compared to 20 controls, showing 82% of treated patients improved in terms of oxygenation with some sustaining a relapse-free response[77]. Polymyxin-B immobilized fiber (PMX) hemoperfusion is an alternative approach mostly studied in removing bacterial toxins, but has also been postulated for removing proinflammatory cytokines[78,79] and promoting antifibrotic cytokines[80]. Retrospective studies have shown notable survival benefit from PMX treatment in AE-IPF (12-month survival 41.7% in the PMX group vs 9.8% in the non-PMX group)[81,82], although this has not been confirmed in randomized trials. Disordered hypercoagulation has also been implicated in AE-IPF pathophysiology. Recombinant human thrombomodulin (rhTM), a cofactor for thrombin and anti-coagulant molecule, was recently evaluated as add-on therapy to routine corticosteroid-treated AE-IPF patients decreasing 3 mo mortality to 30%-40 from control levels of 65%-70%[83-85].
Despite the relatively common occurrence of AE-IPF and AE-ILD in general[8,9], randomized clinical trials of interventions in acute exacerbations are lacking. As noted in a recent International Working Group report, the optimal management of AE-IPF represents an area of major unmet medical need[7]. Robust prospective clinical studies and randomized trials of therapeutics and maybe ventilation strategies are critical to advance the field and improve the grim prognosis of these patients.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Critical care medicine
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Singh A, Xu J S-Editor: Wang LL L-Editor: A P-Editor: Wang LL
| 1. | Miyazaki Y, Tateishi T, Akashi T, Ohtani Y, Inase N, Yoshizawa Y. Clinical predictors and histologic appearance of acute exacerbations in chronic hypersensitivity pneumonitis. Chest. 2008;134:1265-1270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 108] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 2. | Olson AL, Huie TJ, Groshong SD, Cosgrove GP, Janssen WJ, Schwarz MI, Brown KK, Frankel SK. Acute exacerbations of fibrotic hypersensitivity pneumonitis: a case series. Chest. 2008;134:844-850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 66] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 3. | Rice AJ, Wells AU, Bouros D, du Bois RM, Hansell DM, Polychronopoulos V, Vassilakis D, Kerr JR, Evans TW, Nicholson AG. Terminal diffuse alveolar damage in relation to interstitial pneumonias. An autopsy study. Am J Clin Pathol. 2003;119:709-714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 36] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 4. | Park IN, Kim DS, Shim TS, Lim CM, Lee SD, Koh Y, Kim WS, Kim WD, Jang SJ, Colby TV. Acute exacerbation of interstitial pneumonia other than idiopathic pulmonary fibrosis. Chest. 2007;132:214-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 247] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 5. | Suda T, Kaida Y, Nakamura Y, Enomoto N, Fujisawa T, Imokawa S, Hashizume H, Naito T, Hashimoto D, Takehara Y, Inui N, Nakamura H, Colby TV, Chida K. Acute exacerbation of interstitial pneumonia associated with collagen vascular diseases. Respir Med. 2009;103:846-853. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 160] [Cited by in RCA: 163] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 6. | Collard HR, Moore BB, Flaherty KR, Brown KK, Kaner RJ, King TE Jr, Lasky JA, Loyd JE, Noth I, Olman MA, Raghu G, Roman J, Ryu JH, Zisman DA, Hunninghake GW, Colby TV, Egan JJ, Hansell DM, Johkoh T, Kaminski N, Kim DS, Kondoh Y, Lynch DA, Müller-Quernheim J, Myers JL, Nicholson AG, Selman M, Toews GB, Wells AU, Martinez FJ; Idiopathic Pulmonary Fibrosis Clinical Research Network Investigators. Acute exacerbations of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2007;176:636-643. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 917] [Cited by in RCA: 815] [Article Influence: 45.3] [Reference Citation Analysis (0)] |
| 7. | Collard HR, Ryerson CJ, Corte TJ, Jenkins G, Kondoh Y, Lederer DJ, Lee JS, Maher TM, Wells AU, Antoniou KM, Behr J, Brown KK, Cottin V, Flaherty KR, Fukuoka J, Hansell DM, Johkoh T, Kaminski N, Kim DS, Kolb M, Lynch DA, Myers JL, Raghu G, Richeldi L, Taniguchi H, Martinez FJ. Acute Exacerbation of Idiopathic Pulmonary Fibrosis. An International Working Group Report. Am J Respir Crit Care Med. 2016;194:265-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 687] [Cited by in RCA: 992] [Article Influence: 110.2] [Reference Citation Analysis (0)] |
| 8. | Atkins CP, Loke YK, Wilson AM. Outcomes in idiopathic pulmonary fibrosis: a meta-analysis from placebo controlled trials. Respir Med. 2014;108:376-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 59] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 9. | Kim DS, Park JH, Park BK, Lee JS, Nicholson AG, Colby T. Acute exacerbation of idiopathic pulmonary fibrosis: frequency and clinical features. Eur Respir J. 2006;27:143-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 345] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 10. | Collard HR, Yow E, Richeldi L, Anstrom KJ, Glazer C; IPFnet investigators. Suspected acute exacerbation of idiopathic pulmonary fibrosis as an outcome measure in clinical trials. Respir Res. 2013;14:73. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 139] [Cited by in RCA: 161] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 11. | Song JW, Hong SB, Lim CM, Koh Y, Kim DS. Acute exacerbation of idiopathic pulmonary fibrosis: incidence, risk factors and outcome. Eur Respir J. 2011;37:356-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 490] [Cited by in RCA: 567] [Article Influence: 37.8] [Reference Citation Analysis (0)] |
| 12. | Kondoh Y, Taniguchi H, Ebina M, Azuma A, Ogura T, Taguchi Y, Suga M, Takahashi H, Nakata K, Sugiyama Y, Kudoh S, Nukiwa T. Risk factors for acute exacerbation of idiopathic pulmonary fibrosis--Extended analysis of pirfenidone trial in Japan. Respir Investig. 2015;53:271-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 61] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 13. | Kondoh Y, Taniguchi H, Katsuta T, Kataoka K, Kimura T, Nishiyama O, Sakamoto K, Johkoh T, Nishimura M, Ono K, Kitaichi M. Risk factors of acute exacerbation of idiopathic pulmonary fibrosis. Sarcoidosis Vasc Diffuse Lung Dis Off J WASOG. 2010;27:103-110. |
| 14. | Simon-Blancal V, Freynet O, Nunes H, Bouvry D, Naggara N, Brillet PY, Denis D, Cohen Y, Vincent F, Valeyre D, Naccache JM. Acute exacerbation of idiopathic pulmonary fibrosis: outcome and prognostic factors. Respiration. 2012;83:28-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 110] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 15. | Moeller A, Gilpin SE, Ask K, Cox G, Cook D, Gauldie J, Margetts PJ, Farkas L, Dobranowski J, Boylan C, O'Byrne PM, Strieter RM, Kolb M. Circulating fibrocytes are an indicator of poor prognosis in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2009;179:588-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 391] [Cited by in RCA: 383] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 16. | Schupp JC, Binder H, Jäger B, Cillis G, Zissel G, Müller-Quernheim J, Prasse A. Macrophage activation in acute exacerbation of idiopathic pulmonary fibrosis. PLoS One. 2015;10:e0116775. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 125] [Cited by in RCA: 159] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 17. | Kahloon RA, Xue J, Bhargava A, Csizmadia E, Otterbein L, Kass DJ, Bon J, Soejima M, Levesque MC, Lindell KO, Gibson KF, Kaminski N, Banga G, Oddis CV, Pilewski JM, Sciurba FC, Donahoe M, Zhang Y, Duncan SR. Patients with idiopathic pulmonary fibrosis with antibodies to heat shock protein 70 have poor prognoses. Am J Respir Crit Care Med. 2013;187:768-775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 147] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 18. | Kurosu K, Takiguchi Y, Okada O, Yumoto N, Sakao S, Tada Y, Kasahara Y, Tanabe N, Tatsumi K, Weiden M, Rom WN, Kuriyama T. Identification of annexin 1 as a novel autoantigen in acute exacerbation of idiopathic pulmonary fibrosis. J Immunol. 2008;181:756-767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 80] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 19. | Johannson KA, Vittinghoff E, Lee K, Balmes JR, Ji W, Kaplan GG, Kim DS, Collard HR. Acute exacerbation of idiopathic pulmonary fibrosis associated with air pollution exposure. Eur Respir J. 2014;43:1124-1131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 204] [Article Influence: 17.0] [Reference Citation Analysis (1)] |
| 20. | Lee JS, Ryu JH, Elicker BM, Lydell CP, Jones KD, Wolters PJ, King TE Jr, Collard HR. Gastroesophageal reflux therapy is associated with longer survival in patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2011;184:1390-1394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 307] [Cited by in RCA: 302] [Article Influence: 21.6] [Reference Citation Analysis (1)] |
| 21. | Lee AS, Lee JS, He Z, Ryu JH. Reflux-Aspiration in Chronic Lung Disease. Ann Am Thorac Soc. 2020;17:155-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 38] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 22. | Lee JS, Collard HR, Anstrom KJ, Martinez FJ, Noth I, Roberts RS, Yow E, Raghu G; IPFnet Investigators. Anti-acid treatment and disease progression in idiopathic pulmonary fibrosis: an analysis of data from three randomised controlled trials. Lancet Respir Med. 2013;1:369-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 293] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 23. | Leuschner G, Behr J. Acute Exacerbation in Interstitial Lung Disease. Front Med (Lausanne). 2017;4:176. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 112] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 24. | Kakugawa T, Sakamoto N, Sato S, Yura H, Harada T, Nakashima S, Hara A, Oda K, Ishimoto H, Yatera K, Ishimatsu Y, Obase Y, Kohno S, Mukae H. Risk factors for an acute exacerbation of idiopathic pulmonary fibrosis. Respir Res. 2016;17:79. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 57] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 25. | Moua T, Westerly BD, Dulohery MM, Daniels CE, Ryu JH, Lim KG. Patients With Fibrotic Interstitial Lung Disease Hospitalized for Acute Respiratory Worsening: A Large Cohort Analysis. Chest. 2016;149:1205-1214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 81] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 26. | Huie TJ, Olson AL, Cosgrove GP, Janssen WJ, Lara AR, Lynch DA, Groshong SD, Moss M, Schwarz MI, Brown KK, Frankel SK. A detailed evaluation of acute respiratory decline in patients with fibrotic lung disease: aetiology and outcomes. Respirology. 2010;15:909-917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 88] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 27. | Arcadu A, Moua T. Bronchoscopy assessment of acute respiratory failure in interstitial lung disease. Respirology. 2017;22:352-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 28. | Bando M, Ohno S, Hosono T, Yanase K, Sato Y, Sohara Y, Hironaka M, Sugiyama Y. Risk of Acute Exacerbation After Video-assisted Thoracoscopic Lung Biopsy for Interstitial Lung Disease. J Bronchology Interv Pulmonol. 2009;16:229-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 29. | Sakamoto K, Taniguchi H, Kondoh Y, Wakai K, Kimura T, Kataoka K, Hashimoto N, Nishiyama O, Hasegawa Y. Acute exacerbation of IPF following diagnostic bronchoalveolar lavage procedures. Respir Med. 2012;106:436-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 30. | Suzuki H, Sekine Y, Yoshida S, Suzuki M, Shibuya K, Yonemori Y, Hiroshima K, Nakatani Y, Mizuno S, Takiguchi Y, Yoshino I. Risk of acute exacerbation of interstitial pneumonia after pulmonary resection for lung cancer in patients with idiopathic pulmonary fibrosis based on preoperative high-resolution computed tomography. Surg Today. 2011;41:914-921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 60] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 31. | Mizuno Y, Iwata H, Shirahashi K, Takamochi K, Oh S, Suzuki K, Takemura H. The importance of intraoperative fluid balance for the prevention of postoperative acute exacerbation of idiopathic pulmonary fibrosis after pulmonary resection for primary lung cancer. Eur J Cardiothorac Surg. 2012;41:e161-e165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 77] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 32. | Parambil JG, Myers JL, Ryu JH. Histopathologic features and outcome of patients with acute exacerbation of idiopathic pulmonary fibrosis undergoing surgical lung biopsy. Chest. 2005;128:3310-3315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 181] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 33. | Faverio P, De Giacomi F, Sardella L, Fiorentino G, Carone M, Salerno F, Ora J, Rogliani P, Pellegrino G, Sferrazza Papa GF, Bini F, Bodini BD, Messinesi G, Pesci A, Esquinas A. Management of acute respiratory failure in interstitial lung diseases: overview and clinical insights. BMC Pulm Med. 2018;18:70. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 40] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 34. | Parker MW, Rossi D, Peterson M, Smith K, Sikström K, White ES, Connett JE, Henke CA, Larsson O, Bitterman PB. Fibrotic extracellular matrix activates a profibrotic positive feedback loop. J Clin Invest. 2014;124:1622-1635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 348] [Cited by in RCA: 426] [Article Influence: 38.7] [Reference Citation Analysis (0)] |
| 35. | Marshall RP, Bellingan G, Webb S, Puddicombe A, Goldsack N, McAnulty RJ, Laurent GJ. Fibroproliferation occurs early in the acute respiratory distress syndrome and impacts on outcome. Am J Respir Crit Care Med. 2000;162:1783-1788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 194] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 36. | Rocco PRM, Dos Santos C, Pelosi P. Lung parenchyma remodeling in acute respiratory distress syndrome. Minerva Anestesiol. 2009;75:730-740. |
| 37. | Hutchinson JP, Fogarty AW, McKeever TM, Hubbard RB. In-Hospital Mortality after Surgical Lung Biopsy for Interstitial Lung Disease in the United States. 2000 to 2011. Am J Respir Crit Care Med. 2016;193:1161-1167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 310] [Article Influence: 38.8] [Reference Citation Analysis (0)] |
| 38. | Idiopathic Pulmonary Fibrosis Clinical Research Network. , Raghu G, Anstrom KJ, King TE Jr, Lasky JA, Martinez FJ. Prednisone, azathioprine, and N-acetylcysteine for pulmonary fibrosis. N Engl J Med. 2012;366:1968-1977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1082] [Cited by in RCA: 1177] [Article Influence: 90.5] [Reference Citation Analysis (0)] |
| 39. | Ding J, Chen Z, Feng K. Procalcitonin-guided antibiotic use in acute exacerbations of idiopathic pulmonary fibrosis. Int J Med Sci. 2013;10:903-907. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 40. | Azadeh N, Limper AH, Carmona EM, Ryu JH. The Role of Infection in Interstitial Lung Diseases: A Review. Chest. 2017;152:842-852. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 75] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 41. | Akira M, Kozuka T, Yamamoto S, Sakatani M. Computed tomography findings in acute exacerbation of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2008;178:372-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 160] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 42. | Fujimoto K, Taniguchi H, Johkoh T, Kondoh Y, Ichikado K, Sumikawa H, Ogura T, Kataoka K, Endo T, Kawaguchi A, Müller NL. Acute exacerbation of idiopathic pulmonary fibrosis: high-resolution CT scores predict mortality. Eur Radiol. 2012;22:83-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 82] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 43. | Sprunger DB, Olson AL, Huie TJ, Fernandez-Perez ER, Fischer A, Solomon JJ, Brown KK, Swigris JJ. Pulmonary fibrosis is associated with an elevated risk of thromboembolic disease. Eur Respir J. 2012;39:125-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 93] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 44. | Hubbard RB, Smith C, Le Jeune I, Gribbin J, Fogarty AW. The association between idiopathic pulmonary fibrosis and vascular disease: a population-based study. Am J Respir Crit Care Med. 2008;178:1257-1261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 174] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 45. | Sode BF, Dahl M, Nielsen SF, Nordestgaard BG. Venous thromboembolism and risk of idiopathic interstitial pneumonia: a nationwide study. Am J Respir Crit Care Med. 2010;181:1085-1092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 75] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 46. | Al-Hameed FM, Sharma S. Outcome of patients admitted to the intensive care unit for acute exacerbation of idiopathic pulmonary fibrosis. Can Respir J. 2004;11:117-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 113] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 47. | Rangappa P, Moran JL. Outcomes of patients admitted to the intensive care unit with idiopathic pulmonary fibrosis. Crit Care Resusc J Australas Acad Crit Care Med. 2009;11:102-109. |
| 48. | Saydain G, Islam A, Afessa B, Ryu JH, Scott JP, Peters SG. Outcome of patients with idiopathic pulmonary fibrosis admitted to the intensive care unit. Am J Respir Crit Care Med. 2002;166:839-842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 124] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 49. | Rush B, Wiskar K, Berger L, Griesdale D. The use of mechanical ventilation in patients with idiopathic pulmonary fibrosis in the United States: A nationwide retrospective cohort analysis. Respir Med. 2016;111:72-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 61] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 50. | Gaudry S, Vincent F, Rabbat A, Nunes H, Crestani B, Naccache JM, Wolff M, Thabut G, Valeyre D, Cohen Y, Mal H. Invasive mechanical ventilation in patients with fibrosing interstitial pneumonia. J Thorac Cardiovasc Surg. 2014;147:47-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 68] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 51. | Schrader M, Sathananthan M, Jeganathan N. Patients With Idiopathic Pulmonary Fibrosis Admitted to the ICU With Acute Respiratory Failure-A Reevaluation of the Risk Factors and Outcomes. J Intensive Care Med. 2021;885066621989244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 52. | Yokoyama T, Kondoh Y, Taniguchi H, Kataoka K, Kato K, Nishiyama O, Kimura T, Hasegawa R, Kubo K. Noninvasive ventilation in acute exacerbation of idiopathic pulmonary fibrosis. Intern Med. 2010;49:1509-1514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 72] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 53. | Tachikawa R, Tomii K, Ueda H, Nagata K, Nanjo S, Sakurai A, Otsuka K, Kaji R, Hayashi M, Katakami N, Imai Y. Clinical features and outcome of acute exacerbation of interstitial pneumonia: collagen vascular diseases-related vs idiopathic. Respiration. 2012;83:20-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 102] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 54. | Fernández-Pérez ER, Yilmaz M, Jenad H, Daniels CE, Ryu JH, Hubmayr RD, Gajic O. Ventilator settings and outcome of respiratory failure in chronic interstitial lung disease. Chest. 2008;133:1113-1119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 94] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 55. | Jacob J, Bartholmai BJ, Rajagopalan S, Kokosi M, Nair A, Karwoski R, Walsh SL, Wells AU, Hansell DM. Mortality prediction in idiopathic pulmonary fibrosis: evaluation of computer-based CT analysis with conventional severity measures. Eur Respir J. 2017;49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 210] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 56. | Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, Colby TV, Cordier JF, Flaherty KR, Lasky JA, Lynch DA, Ryu JH, Swigris JJ, Wells AU, Ancochea J, Bouros D, Carvalho C, Costabel U, Ebina M, Hansell DM, Johkoh T, Kim DS, King TE Jr, Kondoh Y, Myers J, Müller NL, Nicholson AG, Richeldi L, Selman M, Dudden RF, Griss BS, Protzko SL, Schünemann HJ; ATS/ERS/JRS/ALAT Committee on Idiopathic Pulmonary Fibrosis. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183:788-824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5557] [Cited by in RCA: 5294] [Article Influence: 378.1] [Reference Citation Analysis (0)] |
| 57. | Villar J, Ferrando C, Martínez D, Ambrós A, Muñoz T, Soler JA, Aguilar G, Alba F, González-Higueras E, Conesa LA, Martín-Rodríguez C, Díaz-Domínguez FJ, Serna-Grande P, Rivas R, Ferreres J, Belda J, Capilla L, Tallet A, Añón JM, Fernández RL, González-Martín JM; dexamethasone in ARDS network. Dexamethasone treatment for the acute respiratory distress syndrome: a multicentre, randomised controlled trial. Lancet Respir Med. 2020;8:267-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 529] [Cited by in RCA: 766] [Article Influence: 153.2] [Reference Citation Analysis (0)] |
| 58. | RECOVERY Collaborative Group; Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, Linsell L, Staplin N, Brightling C, Ustianowski A, Elmahi E, Prudon B, Green C, Felton T, Chadwick D, Rege K, Fegan C, Chappell LC, Faust SN, Jaki T, Jeffery K, Montgomery A, Rowan K, Juszczak E, Baillie JK, Haynes R, Landray MJ. Dexamethasone in Hospitalized Patients with Covid-19 - Preliminary Report. N Engl J Med. (e-pub ahead of print 17 July 2020). [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6762] [Cited by in RCA: 7376] [Article Influence: 1844.0] [Reference Citation Analysis (1)] |
| 59. | Papiris SA, Kagouridis K, Kolilekas L, Papaioannou AI, Roussou A, Triantafillidou C, Baou K, Malagari K, Argentos S, Kotanidou A, Karakatsani A, Manali ED. Survival in Idiopathic pulmonary fibrosis acute exacerbations: the non-steroid approach. BMC Pulm Med. 2015;15:162. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 58] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 60. | Farrand E, Vittinghoff E, Ley B, Butte AJ, Collard HR. Corticosteroid use is not associated with improved outcomes in acute exacerbation of IPF. Respirology. 2020;25:629-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 52] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 61. | Morell F, Villar A, Montero MÁ, Muñoz X, Colby TV, Pipvath S, Cruz MJ, Raghu G. Chronic hypersensitivity pneumonitis in patients diagnosed with idiopathic pulmonary fibrosis: a prospective case-cohort study. Lancet Respir Med. 2013;1:685-694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 273] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 62. | Walkey AJ, Wiener RS. Macrolide antibiotics and survival in patients with acute lung injury. Chest. 2012;141:1153-1159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 78] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 63. | Kawamura K, Ichikado K, Suga M, Yoshioka M. Efficacy of azithromycin for treatment of acute exacerbation of chronic fibrosing interstitial pneumonia: a prospective, open-label study with historical controls. Respiration. 2014;87:478-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 64. | Richeldi L, Costabel U, Selman M, Kim DS, Hansell DM, Nicholson AG, Brown KK, Flaherty KR, Noble PW, Raghu G, Brun M, Gupta A, Juhel N, Klüglich M, du Bois RM. Efficacy of a tyrosine kinase inhibitor in idiopathic pulmonary fibrosis. N Engl J Med. 2011;365:1079-1087. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 768] [Cited by in RCA: 817] [Article Influence: 58.4] [Reference Citation Analysis (0)] |
| 65. | Briones Claudett KH, Briones Claudett MH, Vargas Domenica E, Rodriguez Garcia S, Benites Solis J, Andrade Cabrera C, Grunauer Andrade M. Volume-assured pressure support mode plus pirfenidone as resuscitation therapy in patients with exacerbation of idiopathic pulmonary fibrosis. Adv Respir Med. 2020;88:147-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 66. | Marchioni A, Tonelli R, Ball L, Fantini R, Castaniere I, Cerri S, Luppi F, Malerba M, Pelosi P, Clini E. Acute exacerbation of idiopathic pulmonary fibrosis: lessons learned from acute respiratory distress syndrome? Crit Care. 2018;22:80. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 57] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 67. | Martin MJ, Moua T. Mechanical Ventilation and Predictors of In-Hospital Mortality in Fibrotic Interstitial Lung Disease With Acute Respiratory Failure: A Cohort Analysis Through the Paradigm of Acute Respiratory Distress Syndrome. Crit Care Med. 2020;48:993-1000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 68. | Guérin C, Reignier J, Richard JC, Beuret P, Gacouin A, Boulain T, Mercier E, Badet M, Mercat A, Baudin O, Clavel M, Chatellier D, Jaber S, Rosselli S, Mancebo J, Sirodot M, Hilbert G, Bengler C, Richecoeur J, Gainnier M, Bayle F, Bourdin G, Leray V, Girard R, Baboi L, Ayzac L; PROSEVA Study Group. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med. 2013;368:2159-2168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2391] [Cited by in RCA: 2544] [Article Influence: 212.0] [Reference Citation Analysis (0)] |
| 69. | Nakos G, Tsangaris I, Kostanti E, Nathanail C, Lachana A, Koulouras V, Kastani D. Effect of the prone position on patients with hydrostatic pulmonary edema compared with patients with acute respiratory distress syndrome and pulmonary fibrosis. Am J Respir Crit Care Med. 2000;161:360-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 83] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 70. | Azadeh N, Moua T, Baqir M, Ryu JH. Treatment of acute exacerbations of interstitial lung disease. Expert Rev Respir Med. 2018;12:309-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 71. | Hubbard R, Venn A, Lewis S, Britton J. Lung cancer and cryptogenic fibrosing alveolitis. A population-based cohort study. Am J Respir Crit Care Med. 2000;161:5-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 442] [Cited by in RCA: 447] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 72. | Shenderov K, Collins SL, Powell JD, Horton MR. Immune dysregulation as a driver of idiopathic pulmonary fibrosis. J Clin Invest. 2021;131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 173] [Article Influence: 43.3] [Reference Citation Analysis (0)] |
| 73. | Novelli L, Ruggiero R, De Giacomi F, Biffi A, Faverio P, Bilucaglia L, Gamberini S, Messinesi G, Pesci A. Corticosteroid and cyclophosphamide in acute exacerbation of idiopathic pulmonary fibrosis: a single center experience and literature review. Sarcoidosis Vasc Diffuse Lung Dis Off J WASOG. 2016;33:385-391. |
| 74. | Horita N, Akahane M, Okada Y, Kobayashi Y, Arai T, Amano I, Takezawa T, To M, To Y. Tacrolimus and steroid treatment for acute exacerbation of idiopathic pulmonary fibrosis. Intern Med. 2011;50:189-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 62] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 75. | Homma S, Sakamoto S, Kawabata M, Kishi K, Tsuboi E, Motoi N, Yoshimura K. Cyclosporin treatment in steroid-resistant and acutely exacerbated interstitial pneumonia. Intern Med. 2005;44:1144-1150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 62] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 76. | Inase N, Sawada M, Ohtani Y, Miyake S, Isogai S, Sakashita H, Miyazaki Y, Yoshizawa Y. Cyclosporin A followed by the treatment of acute exacerbation of idiopathic pulmonary fibrosis with corticosteroid. Intern Med. 2003;42:565-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 70] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 77. | Donahoe M, Valentine VG, Chien N, Gibson KF, Raval JS, Saul M, Xue J, Zhang Y, Duncan SR. Autoantibody-Targeted Treatments for Acute Exacerbations of Idiopathic Pulmonary Fibrosis. PLoS One. 2015;10:e0127771. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 84] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 78. | Abe S, Hayashi H, Seo Y, Matsuda K, Kamio K, Saito Y, Usuki J, Azuma A, Kudo S, Gemma A. Reduction in serum high mobility group box-1 Level by polymyxin B-immobilized fiber column in patients with idiopathic pulmonary fibrosis with acute exacerbation. Blood Purif. 2011;32:310-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 61] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 79. | Oishi K, Mimura-Kimura Y, Miyasho T, Aoe K, Ogata Y, Katayama H, Murata Y, Ueoka H, Matsumoto T, Mimura Y. Association between cytokine removal by polymyxin B hemoperfusion and improved pulmonary oxygenation in patients with acute exacerbation of idiopathic pulmonary fibrosis. Cytokine. 2013;61:84-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 68] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 80. | Tachibana K, Inoue Y, Nishiyama A, Sugimoto C, Matsumuro A, Hirose M, Kitaichi M, Akira M, Arai T, Hayashi S. Polymyxin-B hemoperfusion for acute exacerbation of idiopathic pulmonary fibrosis: serum IL-7 as a prognostic marker. Sarcoidosis Vasc Diffuse Lung Dis Off J WASOG. 2011;28:113-122. |
| 81. | Oishi K, Aoe K, Mimura Y, Murata Y, Sakamoto K, Koutoku W, Matsumoto T, Ueoka H, Yano M. Survival from an Acute Exacerbation of Idiopathic Pulmonary Fibrosis with or without Direct Hemoperfusion with a Polymyxin B-immobilized Fiber Column: A Retrospective Analysis. Intern Med. 2016;55:3551-3559. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 82. | Abe S, Azuma A, Mukae H, Ogura T, Taniguchi H, Bando M, Sugiyama Y. Polymyxin B-immobilized fiber column (PMX) treatment for idiopathic pulmonary fibrosis with acute exacerbation: a multicenter retrospective analysis. Intern Med. 2012;51:1487-1491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 85] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 83. | Isshiki T, Sakamoto S, Kinoshita A, Sugino K, Kurosaki A, Homma S. Recombinant human soluble thrombomodulin treatment for acute exacerbation of idiopathic pulmonary fibrosis: a retrospective study. Respiration. 2015;89:201-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 62] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 84. | Kataoka K, Taniguchi H, Kondoh Y, Nishiyama O, Kimura T, Matsuda T, Yokoyama T, Sakamoto K, Ando M. Recombinant Human Thrombomodulin in Acute Exacerbation of Idiopathic Pulmonary Fibrosis. Chest. 2015;148:436-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 88] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 85. | Tsushima K, Yamaguchi K, Kono Y, Yokoyama T, Kubo K, Matsumura T, Ichimura Y, Abe M, Terada J, Tatsumi K. Thrombomodulin for acute exacerbations of idiopathic pulmonary fibrosis: a proof of concept study. Pulm Pharmacol Ther. 2014;29:233-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 55] [Article Influence: 5.0] [Reference Citation Analysis (0)] |