Published online Jan 9, 2021. doi: 10.5492/wjccm.v10.i1.1
Peer-review started: September 16, 2020
First decision: December 1, 2020
Revised: December 7, 2020
Accepted: December 23, 2020
Article in press: December 23, 2020
Published online: January 9, 2021
Processing time: 111 Days and 12.4 Hours
The ongoing outbreak of severe acute respiratory syndrome coronavirus-2 [SARS-CoV-2, or coronavirus disease 2019 (COVID-19)] was declared a pandemic by the World Health Organization on March 11, 2020. Worldwide, more than 65 million people have been infected with this SARS-CoV-2 virus, and over 1.5 million people have died due to the viral illness. Although a tremendous amount of medical progress has been made since its inception, there continues to be ongoing research regarding the pathophysiology, treatments, and vaccines. While a vast majority of those infected develop only mild to moderate symptoms, about 5% of people have severe forms of infection resulting in respiratory failure, myocarditis, septic shock, or multi-organ failure. Despite maximal cardiopulmonary support and invasive mechanical ventilation, mortality remains high. Extracorporeal membrane oxygenation (ECMO) remains a valid treatment option when maximal conventional strategies fail. Utilization of ECMO in the pandemic is challenging from both resource allocation and ethical standpoints. This article reviews the rationale behind its use, current status of utilization, and future considerations for ECMO in critically ill COVID-19 patients.
Core Tip: This article aims to provide a review of the rationale for the use of extracorporeal membrane oxygenation (ECMO) in patients suffering from severe coronavirus disease 2019 (COVID-19) infection, including a discussion of current utilization practices, and ends with important future considerations for ECMO in critically ill COVID-19 patients as we progress during the current pandemic.
- Citation: Kichloo A, Kumar A, Amir R, Aljadah M, Farooqi N, Albosta M, Singh J, Jamal S, El-Amir Z, Kichloo A, Lone N. Utilization of extracorporeal membrane oxygenation during the COVID-19 pandemic. World J Crit Care Med 2021; 10(1): 1-11
- URL: https://www.wjgnet.com/2220-3141/full/v10/i1/1.htm
- DOI: https://dx.doi.org/10.5492/wjccm.v10.i1.1
On December 31, 2019, the World Health Organization (WHO) was alerted of cases of pneumonia with an unknown etiology detected in Wuhan City, Hubei Provence, China. With rising fear of a potential endemic in the overpopulated city of Wuhan, Chinese national authorities along with the Wuhan Municipal Health Commission began a quest to identify all cases, amongst the 19 million occupants, as early as possible, as well as to trace potential sources through retrospective investigation. Initial investigations revealed the source of the first 27 confirmed cases of the novel coronavirus, severe acute respiratory syndrome coronavirus-2 [SARS-CoV-2, coronavirus disease 2019 (COVID-19)], was the Huanan seafood market[1]. The market was immediately shut down, but the virus had already spread beyond what was anticipated. Not long after, reports of human-to-human transmission were documented and surrounding areas including Hong Kong, Taiwan and Macau took the drastic step of shutting down borders with their long-time allies.
Chinese scientists continued to study this unidentified pathogen until, finally, on the 7th of January 2020, the novel coronavirus was isolated from a single patient and gene sequencing was successfully performed and made available to the WHO five days later. This facilitated the ability for laboratories worldwide to produce diagnostic PCR tests to detect this new virus.
The novel coronavirus continued to spread to neighboring countries despite valiant efforts to subdue the spread. Today, COVID-19 has spread to over 200 countries, spread over six continents, infected over 65.8 million, and taken the lives of 1.5 million people worldwide to date[2]. On the 11th of March 2020, the WHO officially declared the COVID-19 outbreak a global pandemic, as what began as a simple case of viral pneumonia subsequently became one of the most devastating pandemics of the twenty-first century.
The main method of person-person transmission of SARS-CoV-2 is by respiratory droplets, which is similar to the spread of influenza[3]. With droplet transmission, the virus can be spread by coughing, sneezing, or conversing up to six feet away[3]. The virus has also been shown to be able to linger on surfaces for hours and in the air under experimental conditions[3]. Upon exposure to the virus, the incubation period has been shown to be within 14 d, with most cases presenting 4-5 d after exposure[3].
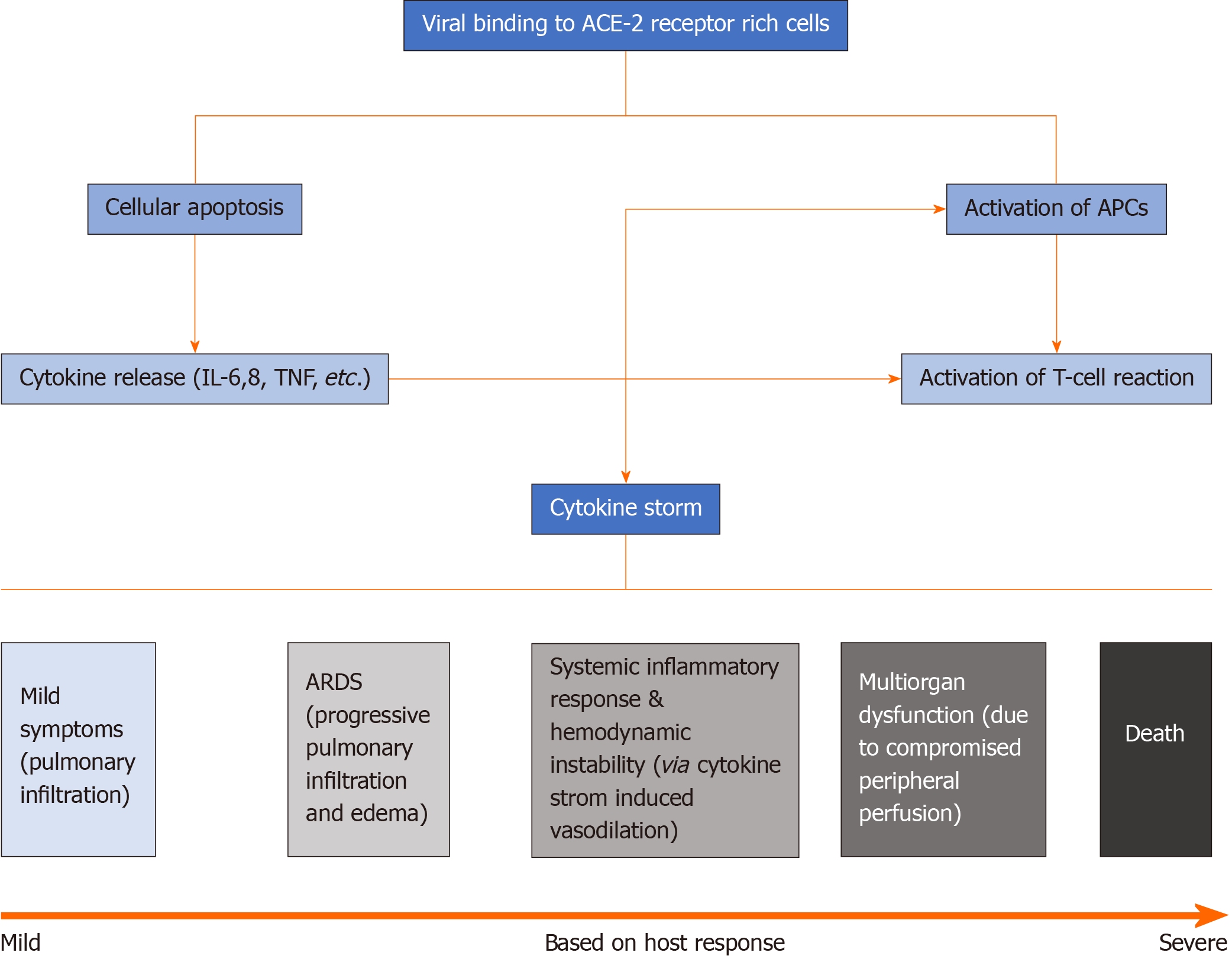
In order to fully comprehend the pathophysiology of SARS-CoV-2, the basic viral structure must first be understood. Coronaviruses, including SARS-CoV-2, are positive single stranded RNA viruses of approximately 30 kb in length[4]. They are composed of four main structural proteins: Membrane (M), spike (S), envelope (E) and nucleocapsid (N)[5]. The spike protein dictates host tropism and has been found to have an affinity to angiotensin converting enzyme-2 (ACE-2) receptors[5]. Epithelial cells in the lungs have a high concentration of ACE-2 receptors likely explaining the high incidence of respiratory symptoms associated with SARS-CoV-2[5]. Other organs with increased ACE-2 expression include the heart, ileum, kidneys and urinary bladder. Following virus binding to host cells via previously mentioned receptors, spike protein is cleaved and subsequently activated leading to irreversible membrane fusion[5]. After cell invasion, the positive stranded RNA released by the virus leads to the production of peptides and proteins by translation in the host, and RNA-dependent RNA polymerase which can further replicate viral RNA[5].
Based on degree of invasion and inflammatory response, symptoms can range from mild to severe respiratory distress and multiorgan failure[4]. Typically, initial symptoms are respiratory in nature given that the inhaled droplets easily invade lung epithelial cells expressing ACE-2 receptors. Surrounding the epithelial cells of the lungs are dendritic cells and macrophages, also known as antigen presenting cells, which present viral antigen to neighboring T-cells initiating a T-cell mediated response[4]. Cytotoxic T-cells (CD8+) play a role in killing the viral antigen while helper T-cells (CD4+) activate B-lymphocytes promoting antibody formation. However, with time, T-cell exhaustion is being observed in patients infected with SARS-CoV-2 which can partially explain clinical deterioration over time[4].
Activation of lymphocytes along with destruction of infected cells leads to the release of cytokines and inflammatory mediators, leading to what is commonly known as the cytokine storm[4]. The most important cytokines released include interleukins 6 and 8 (IL-6, IL-8). IL-6 interacts with the hypothalamus leading to high grade fever; IL-8 is a well-known chemoattractant for T-cells and neutrophils leading to an influx of inflammatory cells into the lungs, or other infected areas, and subsequent diffuse alveolar damage and pulmonary infiltration[4]. These cytokines can also cause vasodilation and increased vessel permeability that leads to hypoxemia, increased work of breathing, and acute respiratory distress syndrome (ARDS)[4]. This increased inflammatory response has also been found to cause significant endothelial damage, creating a hypercoagulable status with the end result ranging from capillary microthrombi to diffuse pulmonary emboli[4].
Unfortunately, the destructive effects of SARS-CoV-2 are not limited only to the pulmonary system. The inflammatory cascade it triggers can involve other organ systems as well, most prominently the cardiovascular system, as evidenced by increasing reports of myocarditis in the younger population[4]. This overwhelming inflammatory state can progress into critical disease, multiorgan failure, and eventually death. Most often, cytokine storm causes systemic vasodilation, which in turn leads to hemodynamic instability and suboptimal peripheral perfusion[4]. Compromised perfusion can cause renal failure, seen by elevation in blood urea nitrogen and creatinine, liver damage, noted by increased liver enzymes, myocardial infarction, and other organ dysfunction. Endothelial injury can also lead to prothrombotic states[4]. It is any combination of ARDS, multi-organ dysfunction from poor perfusion, hemodynamic cardiovascular collapse, and hypercoagulability that puts a patient at risk of shock and ultimately death[4] (Figure 1).
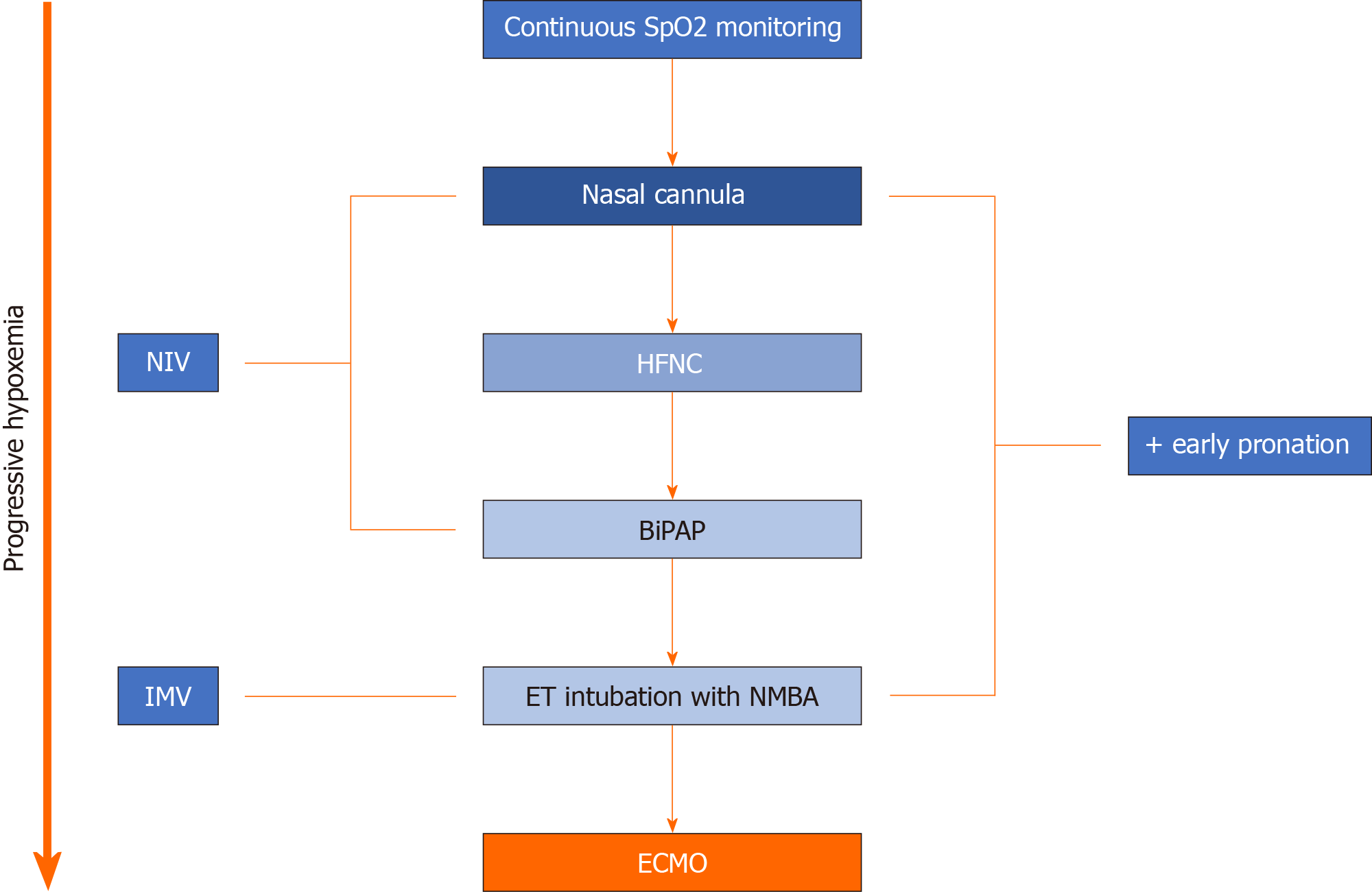
Extracorporeal membrane oxygenation (ECMO) is often used as a last resort in patients with critical pulmonary or cardiovascular compromise, requiring mechanical support (Figure 2). It has various configurations based on the patient’s initial requirement (pulmonary support, cardiovascular support, or both) and can be adjusted according to complication. Cardiac indications for ECMO include cardiogenic shock from a myocardial infarction, arrhythmia, pulmonary embolism, etc., as well as post heart transplant, or as a bridge to longer term ventricular assist device (VAD) placement[6]. Respiratory indications for ECMO include ARDS secondary to pneumonia, aspiration, etc., in addition to lung transplant (as a bridge before the procedure or after if evidence of graft failure), and pulmonary hemorrhage[6]. The potential for ECMO use in COVID-19 patients has been a topic of discussion recently. Previous success with ECMO in critically-ill patients diagnosed with Middle Eastern respiratory syndrome (MERS) encouraged physicians to try ECMO in an attempt to treat COVID-19 patients[7].
The most common clinical scenario in which patients with COVID-19 require ECMO is ARDS refractory to standard lung-protective ventilation and pronation[8,9]. In this situation, gas exchange is compromised given the underlying alveolar inflammation and edema; hence, patients require assistance with oxygenation. Veno-venous ECMO (V-V ECMO) is the modality used in such cases where blood is typically drained from a large peripheral vein, oxygenated via a synthetic lung, and returned to the circulation via a large peripheral vein[8]. Afterwards, newly oxygenated blood flows through the normal circulatory pathway to provide oxygenation to the remainder of the organ systems. With these ECMO settings, the native heart is required to function appropriately to ensure adequate blood distribution[8]. Initiation of ECMO in COVID-19 patients presents unique challenges as these patients are on maximal ventilatory support and are often in a prone position. Therefore, very specific criteria for ECMO initiation have been suggested: PaO2/FiO2 < 60 mmHg for > 6 h, PaO2/FiO2 < 50 mmHg for > 3 h or PCO2 > 80 mmHg for > 6 h and arterial pH < 7.2[8].
In situations where the patient’s cardiovascular function may be severely compromised in addition to respiratory compromise, such as in severe myocarditis, veno-arterial ECMO (V-A ECMO) is the optimal configuration used. In V-A ECMO, venous blood is drained, oxygenated via synthetic lung, then returned with force via a large peripheral artery towards the aorta. This increase in aortic blood flow enhances peripheral perfusion[10].
Superimposed sepsis or multi-organ dysfunction may develop in patients on V-V/V-A ECMO, requiring further calibration of ECMO settings to enhance cardiac output and support bodily functions. The main concept behind modifying V-V/V-A ECMO in such difficult circumstances is through the addition of an extra lumen, converting double lumen to triple lumen ECMO. The addition of this third lumen can help optimize settings based on a patient’s requirements. For instance, if a patient on V-V ECMO (pulmonary support only) develops cardiac complications leading to compromise of cardiovascular function, the addition of an arterial output lumen, veno-venoarterial ECMO (V-VA ECMO), will allow for the addition of cardiac support to pre-existing pulmonary support[11]. In other circumstances, patients with both pulmonary and cardiovascular compromise may be inadequately oxygenating despite V-A ECMO; this is typically seen in larger patients or if a lumen with a small diameter is used[11]. The addition of a venous drainage lumen, venovenous-Arterial ECMO (VV-A ECMO), will allow more blood to be drained and oxygenated at a faster rate, thus improving oxygen supply[11]. Furthermore, it is not uncommon for patients on ECMO to develop acute kidney injuries within the first 48 h. The ECMO circuit itself can create an inflammatory reaction leading to capillary leak and subsequent pre-renal azotemia or even acute tubular necrosis[6]. In these instances, if pre-renal oliguria does not resolve after 72 h, continuous renal replacement therapy simultaneously with ECMO can be used to manage fluid status and maintain renal function[6]. Lastly, electrolytes and blood counts should be monitored very closely, as platelet consumption and potassium, magnesium, and phosphorous shifts have been observed in patients on ECMO and should be replaced accordingly[6].
Given the above, it would theoretically be rational to use ECMO for pulmonary and/or cardiovascular support in patients with COVID-19 refractory ARDS and certain other COVID-19-related complications; yet given the lack of clinical trials and prospective studies, questions regarding the true validity in the clinical setting remain unanswered. The two main factors that should be taken into consideration are its effectiveness and feasibility.
With regards to effectiveness, proof of ECMO success in patients with COVID-19 is scarce. Even prior to COVID-19, ECMO was shown to not lower 60-d mortality in patients with severe ARDS (from other non-COVID-19 conditions) vs other invasive ventilation techniques[9]. While trialing of ECMO in COVID-19 patients has increased during the pandemic, there are very limited reports of clinical outcomes. Furthermore, the handful of cases that have been published report inconsistent results. In a retrospective multicenter study by Ruan et al[12] that included 137 patients with COVID-19, seven patients required ECMO and there was 100% mortality despite ECMO use. These findings were supported by Yang et al[13] and Zhou et al[14], who reported 83% (5 out of 6) and 100% (3 out of 3) mortality rates in patients with COVID-19 who required ECMO at their respective centers. However, Wu et al[15] and Shen et al[16] each reported one patient on ECMO who survived. Although there are not any other official publications regarding ECMO support, the Extracorporeal Life Support Organization (ELSO) is performing real-time tracking of all COVID-19 cases on ECMO worldwide, and there is currently insufficient data for the ELSO to recommend either for or against ECMO in patients with COVID-19[17].
With regards to feasibility, ECMO is complex, especially when designing a referral system. ECMO is expensive to incorporate, and there is a complexity of management associated with its use that requires an individually trained critical care team, often only available in highly specialized centers. In addition, increasing healthcare worker exposure with such a high-risk procedure, particularly with lack of clinical trial evidence to prove its efficacy, raises ethical concerns. Most smaller healthcare centers, both inside and outside of the United States, lack access to ECMO devices and the training required to operate them. Therefore, most authors are supportive of ECMO use in critically ill patients, but only in experienced centers with the necessary resources. For other less-equipped areas, ensuring availability of more basic equipment such as noninvasive and invasive mechanical ventilation with adequate direction for referral to centers with ECMO expertise is of higher priority, and is projected to save more lives in the current pandemic[18]. However, this approach comes at a cost to the critically ill that may benefit from ECMO in less-equipped areas, and is an ethical dilemma worth mentioning.
Currently, the ELSO requires a set of guidelines to proceed with establishment of ECMO as a viable treatment option. These guidelines mandate ECMO be administered at a tertiary care center or greater with available facilities of a tertiary level Neonatal Intensive Care Unit, Pediatric Intensive Care Unit, and/or Adult Intensive Care Unit[17]. The location of service should also cover a geographic area that can provide a minimum of 6 ECMO patients per year[17]. The center should be actively participating in the ELSO registry[17]. The structure of the center should have a hierarchy including an ECMO program director, multiple associate directors assigned to a specific focus pertinent to ECMO care, an ECMO coordinator, and a multi-disciplinary team responsible for annual internal ECMO evaluation for quality improvement[17]. Every ECMO center should have its set of policies and procedures established with comprehensible indications and contraindications. Moreover, there should be distinct guidelines for clinical management, equipment maintenance, termination of therapy, and follow up of ECMO patients[17].
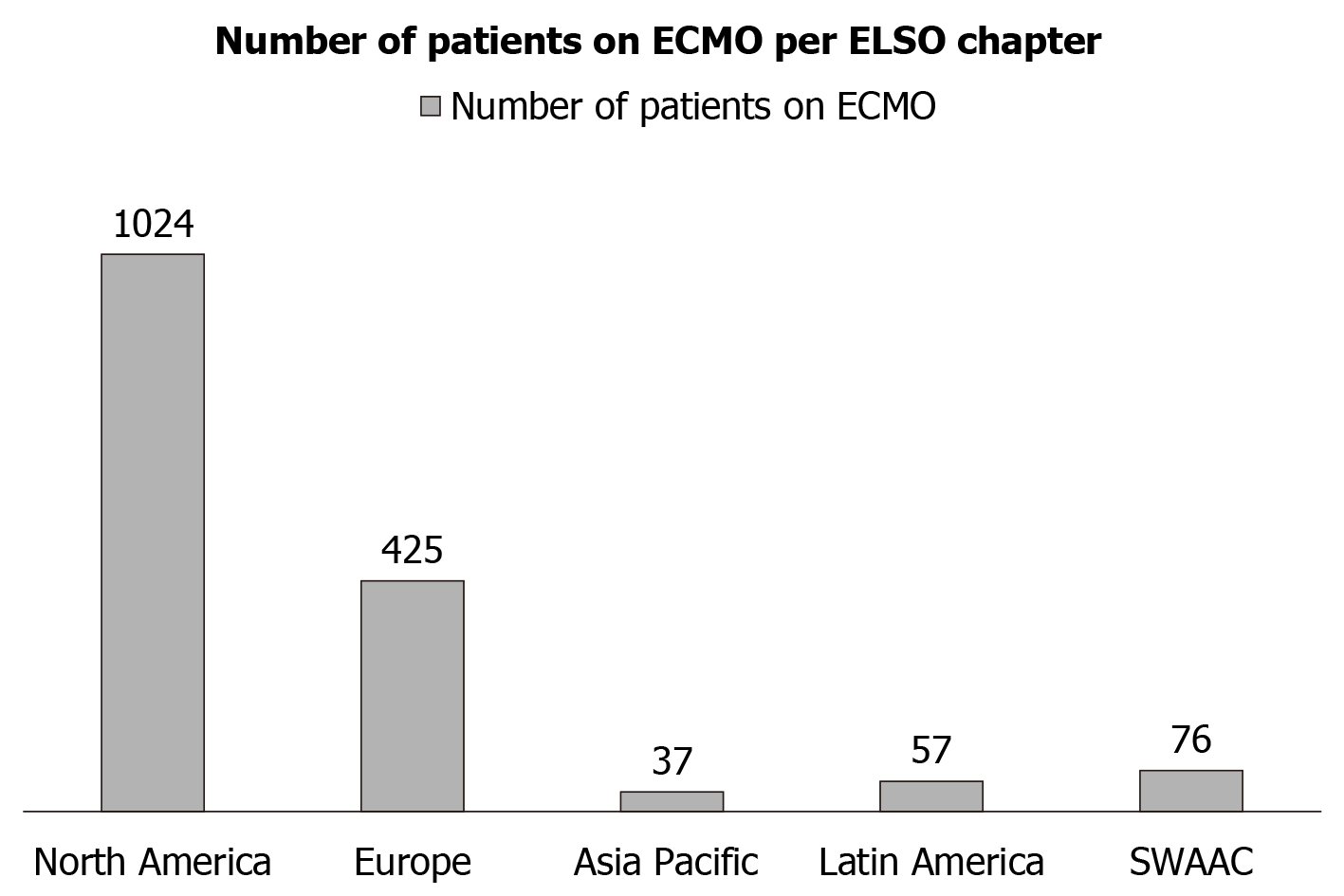
Currently, ECMO is used for respiratory support in 63% of cases, cardiac support in 29% of cases, and both in 8% of cases. The four categories that the ELSO registry considers in its recording of ECMO as it pertains to the pandemic are as follows: COVID-19 confirmed by testing, COVID-19 suspected but no testing confirmation, no clinical suspicion of COVID-19 (and no testing), and COVID-19 confirmed negative[17]. On June 26, 2020, the ELSO registry reported 1619 suspected or confirmed cases of COVID-19 patients on ECMO and specifically listed 1604 confirmed cases of COVID-19 patients on ECMO[17]. The discharged alive rate at 90 d from ECMO was reported at 541/975 patients (55%), and included discharges to rehabilitation facilities and long-term care facilities, indicating a possible lengthier recovery[17]. This rate is not far off from non-COVID-19 ARDS patients on ECMO, where 52% survived to hospital discharge[6]. For reference, in patients who require ECMO for cardiac support due to cardiac arrest or cardiogenic shock, survival rates range from only 20%-30% to hospital discharge[6]. The predominant form of ECMO utilized was VV, which was reported to be used 95% of the time. VA and other configurations were used in 5% cases. The utilization of ECMO as per various ELSO chapters can be seen in Figure 3, where North America demonstrated the highest use of ECMO followed by Europe[17].
The use of ECMO as a rescue therapy in patients with severe ARDS secondary to viral infections has been established in the literature for previous outbreaks of influenza A (H1N1) and SARS-MERS viruses. In a cohort of patients with H1N1-related ARDS, Noah et al[19] demonstrated a hospital mortality of 23.7% for ECMO treated patients vs 52.5% for non-ECMO treated patients. Furthermore, in a retrospective study on MERS-related ARDS, lower mortality was appreciated in the ECMO-treated cohort (65%) compared to the non-ECMO-treated cohort (100%)[7]. There are no definite guidelines established for use of ECMO in COVID-19-related ARDS to-date. However, experience from previous outbreaks can be utilized to determine the guidelines for use of ECMO as a salvage therapy in patients with refractory hypoxemia. Table 1 further elaborates on the indications and contraindications for the use of ECMO in patients with COVID-19 related ARDS. Indications for ECMO use in a mechanically ventilated COVID-19 patient include a PaO2/FiO2 < 60 mmHg for > 6 h, PaO2/FiO2 < 50 mmHg for > 3 h, or a pH < 7.2 + PaCO2 > 80 mmHg for > 6 h[9]. It is important to acknowledge ECMO with consideration of the extent to which the patient will benefit from treatment. Frequent reassessment of the hazard-to-risk ratio is a key factor in evaluation of patients undergoing treatment. In the case of no functional pulmonary or cardiac recovery after 21 d of treatment, an extensive discussion with family members should be made to discuss withdrawing ECMO support[20].
| Indications |
| Refractory hypoxemia despite prone positioning and high PEEP |
| ARDS requiring vasoactive drugs due to COVID-19 (vasopressors) |
| Evidence of one organ failure with minimal co-morbidities |
| Contraindications |
| Multiple comorbidities |
| Immunocompromised status |
| Severe global developmental delay |
| Intracranial hemorrhage |
| Irreversible severe brain damage |
| Severe multiple organ failure |
| Mechanical ventilation for > 14 d before ECMO initiation |
It has been observed that patients with underlying cardiac conditions can also develop cardiogenic and vasogenic shock with COVID-19 infections and can be temporarily managed with ECMO[21]. One 52-year-old male with a known history of congestive heart failure presented with COVID-19-related pneumonia[21]. He was initiated on levosimendan and norepinephrine for combined cardiogenic and vasogenic shock. Subsequently, a peripheral VAD was placed to attempt to mediate the cardiac component of the patient’s shock. A VA ECMO arrangement was then utilized to treat the vasogenic component. The critical care team switched to VV ECMO once the shock resolved[21].
The evidence for long term use of ECMO in COVID-19 patients varies. Zeng et al[22] reported 12 critically ill patients requiring ECMO, where half of them died from septic shock and multi-organ failure. However, Huette et al[23] reported outcomes from 12 patients on ECMO where 10 of 12 patients were weaned from ECMO, 9 patients were weaned from mechanical ventilation, and 8 patients were discharged from the hospital. Patients weaned from ECMO demonstrated an increase in their lymphocyte count and a decrease in their fibrinogen levels[23]. There was also an increase in the PaO2/FiO2 ratio in these patients[23]. A larger systematic review of 331 reported cases of COVID-19 patients receiving ECMO found a mortality rate of 46%[24].
ECMO centers with COVID-19 patients should have special training for members of the ECMO team, regarding personal protective equipment and hospital infection control to contain spread of infection. The ECMO team should practice strict sterile technique along with respiratory droplet precautions including negative airflow isolation at the time of cannulation[25]. To restrict the exposures, the team should consist of a surgeon, an assistant, and a perfusionist[25]. The procedure should be performed in a negative pressure room[25]. The use of ultrasound can decrease the time taken to cannulate, therefore minimizing the risk of exposure[25]. Use of a bi-caval cannula can increase exposure time due to need for TEE and fluoroscopy[25]. To minimize patient contact, the patient can be positioned with the ECMO console facing a window to enable viewing of the control panel without entering the room[25]. Viral particles can disseminate through the gas-port of the membrane lung of the ECMO system. Evacuation of the exhaust port of the oxygenator and vigilance for the plasma leakage signs are measures which can help decrease the risk of spread of aerosols from the membrane lung[26].
There is a need to strengthen the patient referral systems to ECMO centers, including developing strict criteria that considers benefit vs futility of treatment for the patient[27]. This is important in determining the number of candidates that are eligible for ECMO. As patients are transferred to intensive care units (ICUs) for respiratory or other organ failure, there should ideally be guidelines that capture the status of the patient before treatment is futile, but in anticipation of failing traditional invasive ventilation. There should also be strict criteria to decide whether early transfer is appropriate for unpredictable or unclear disease progression[27]. Communication systems should be strong with respect to the availability of resources and personnel for ECMO cannulation[27]. A dedicated ECMO coordinator is instrumental for the success of such a collaboration[27].
An example of a regional framework system encouraging collaboration between remote areas and ECMO centers is discussed by Prekker et al[28]. The framework includes a dedicated ECMO officer overlooking referrals to five established ECMO centers in the state of Minnesota. In countries with expertise and resources, mobile ECMO teams are functional. These teams initiate ECMO on site and transfer the patients to a hospital within the region in less than 45 min[29].
There should also be an effort to increase global participation in data collection registries, such as ELSO, to improve the exchange of expertise and local practices[27]. It has also been suggested that nationwide centralization of ECMO would make the governments more capable of fighting the COVID-19 crisis[30].
As previously discussed, there is a need for additional research related to COVID-19 patients and ECMO. An example of one ongoing global research collaboration is the ECMOCARD trial. It is a prospective/retrospective multi-center short period incidence observational study of COVID-19 patients admitted to the ICU[31]. More than 30 centers in different ELSO member countries are participating and the authors plan to study the clinical characteristics and severity of ARDS in COVID-19 patients on ECMO, including the complications and survival rates[31].
More research is also needed to understand the synergism or lack thereof between ECMO and other COVID-19 therapies. Multiple studies reported the use of IV steroids, IV remdesivir, IV antibiotics, and even hydroxychloroquine in different combinations. However, there is still a lack of consensus as to which combinations are most effective in patients on ECMO with COVID-19 infection. Furthermore, there needs to be more research on the concomitant use of blood filters that remove cytokines from the blood in patients on ECMO[32]. It is unknown if this type of treatment can help with the increase in cytokine production seen in COVID-19 patients[32].
There are ethical dilemmas associated with the use of ECMO in COVID-19 patients. Some of the questions that need extensive discussion with consensus statements are how to define resource conservation during this time. In some practices, extracorporeal CPR is being discontinued for patients with refractory out-of-hospital cardiac arrest. There has also been a recent trend of postponing all procedures that might require post-op ECMO[28]. Another ethical dilemma is the lack of availability of ECMO in many parts of the country, and the harsh reality that some patients may not be able to benefit from this modality of treatment due to the lack of availability[28].
ECMO remains a valid treatment option for patients when other conventional treatment strategies fail. In patients diagnosed with COVID-19, therapy is guided largely from experience with previous coronavirus pandemics such as MERS. North America is the largest geographical region to utilize ECMO in the treatment of COVID-19, and it is without question necessary to have the personnel and infrastructure in place in order to safely treat patients with ECMO. In recent months, new literature continue to demonstrate more clear indications and contraindications for ECMO use, however, much research is still needed to demonstrate clear mortality benefit. Ethical dilemmas also need to be considered, such as ECMO use in the setting of CPR, and modes of expansion need to be examined in order to minimize the treatment availability gap between patients with access ECMO centers and those without access.
Manuscript source: Unsolicited manuscript
Specialty type: Critical care medicine
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gonzalez FM, Wang X S-Editor: Huang P L-Editor: A P-Editor: Li JH
| 1. | Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497-506. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35178] [Cited by in RCA: 30097] [Article Influence: 6019.4] [Reference Citation Analysis (3)] |
| 2. | World Health Organization. COVID-19 situation report [cited 2020 December 15]. Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports. |
| 3. | van Doremalen N, Bushmaker T, Morris DH, Holbrook MG, Gamble A, Williamson BN, Tamin A, Harcourt JL, Thornburg NJ, Gerber SI, Lloyd-Smith JO, de Wit E, Munster VJ. Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1. N Engl J Med. 2020;382:1564-1567. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5894] [Cited by in RCA: 5665] [Article Influence: 1133.0] [Reference Citation Analysis (0)] |
| 4. | Yuki K, Fujiogi M, Koutsogiannaki S. COVID-19 pathophysiology: A review. Clin Immunol. 2020;215:108427. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1218] [Cited by in RCA: 1189] [Article Influence: 237.8] [Reference Citation Analysis (0)] |
| 5. | Lung J, Lin YS, Yang YH, Chou YL, Shu LH, Cheng YC, Liu HT, Wu CY. The potential chemical structure of anti-SARS-CoV-2 RNA-dependent RNA polymerase. J Med Virol. 2020;92:693-697. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 160] [Cited by in RCA: 183] [Article Influence: 36.6] [Reference Citation Analysis (0)] |
| 6. | Makdisi G, Wang IW. Extra Corporeal Membrane Oxygenation (ECMO) review of a lifesaving technology. J Thorac Dis. 2015;7:E166-E176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 175] [Reference Citation Analysis (0)] |
| 7. | Alshahrani MS, Sindi A, Alshamsi F, Al-Omari A, El Tahan M, Alahmadi B, Zein A, Khatani N, Al-Hameed F, Alamri S, Abdelzaher M, Alghamdi A, Alfousan F, Tash A, Tashkandi W, Alraddadi R, Lewis K, Badawee M, Arabi YM, Fan E, Alhazzani W. Extracorporeal membrane oxygenation for severe Middle East respiratory syndrome coronavirus. Ann Intensive Care. 2018;8:3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 115] [Cited by in RCA: 135] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 8. | Napp LC, Kühn C, Hoeper MM, Vogel-Claussen J, Haverich A, Schäfer A, Bauersachs J. Cannulation strategies for percutaneous extracorporeal membrane oxygenation in adults. Clin Res Cardiol. 2016;105:283-296. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 131] [Cited by in RCA: 170] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 9. | Combes A, Hajage D, Capellier G, Demoule A, Lavoué S, Guervilly C, Da Silva D, Zafrani L, Tirot P, Veber B, Maury E, Levy B, Cohen Y, Richard C, Kalfon P, Bouadma L, Mehdaoui H, Beduneau G, Lebreton G, Brochard L, Ferguson ND, Fan E, Slutsky AS, Brodie D, Mercat A; EOLIA Trial Group; REVA; and ECMONet. Extracorporeal Membrane Oxygenation for Severe Acute Respiratory Distress Syndrome. N Engl J Med. 2018;378:1965-1975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1417] [Cited by in RCA: 1479] [Article Influence: 211.3] [Reference Citation Analysis (0)] |
| 10. | Chow J, Alhussaini A, Calvillo-Argüelles O, Billia F, Luk A. Cardiovascular Collapse in COVID-19 Infection: The Role of Venoarterial Extracorporeal Membrane Oxygenation (VA-ECMO). CJC Open. 2020;2:273-277. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 11. | Kowalewski M, Fina D, Słomka A, Raffa GM, Martucci G, Lo Coco V, De Piero ME, Ranucci M, Suwalski P, Lorusso R. COVID-19 and ECMO: the interplay between coagulation and inflammation-a narrative review. Crit Care. 2020;24:205. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 115] [Cited by in RCA: 109] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 12. | Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46:846-848. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2604] [Cited by in RCA: 3192] [Article Influence: 638.4] [Reference Citation Analysis (0)] |
| 13. | Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, Wu Y, Zhang L, Yu Z, Fang M, Yu T, Wang Y, Pan S, Zou X, Yuan S, Shang Y. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475-481. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6231] [Cited by in RCA: 6656] [Article Influence: 1331.2] [Reference Citation Analysis (0)] |
| 14. | Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054-1062. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17476] [Cited by in RCA: 18189] [Article Influence: 3637.8] [Reference Citation Analysis (0)] |
| 15. | Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S, Huang H, Zhang L, Zhou X, Du C, Zhang Y, Song J, Wang S, Chao Y, Yang Z, Xu J, Zhou X, Chen D, Xiong W, Xu L, Zhou F, Jiang J, Bai C, Zheng J, Song Y. Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern Med. 2020;180:934-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4960] [Cited by in RCA: 5515] [Article Influence: 1103.0] [Reference Citation Analysis (1)] |
| 16. | Shen C, Wang Z, Zhao F, Yang Y, Li J, Yuan J, Wang F, Li D, Yang M, Xing L, Wei J, Xiao H, Yang Y, Qu J, Qing L, Chen L, Xu Z, Peng L, Li Y, Zheng H, Chen F, Huang K, Jiang Y, Liu D, Zhang Z, Liu Y, Liu L. Treatment of 5 Critically Ill Patients With COVID-19 With Convalescent Plasma. JAMA. 2020;323:1582-1589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1734] [Cited by in RCA: 1591] [Article Influence: 318.2] [Reference Citation Analysis (0)] |
| 17. | Extracorporeal Life Support Organization (ELSO) [cited June 6 2020]. Available from: https://www.elso.org/. |
| 18. | MacLaren G, Fisher D, Brodie D. Preparing for the Most Critically Ill Patients With COVID-19: The Potential Role of Extracorporeal Membrane Oxygenation. JAMA. 2020;323:1245-1246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 276] [Article Influence: 55.2] [Reference Citation Analysis (0)] |
| 19. | Noah MA, Peek GJ, Finney SJ, Griffiths MJ, Harrison DA, Grieve R, Sadique MZ, Sekhon JS, McAuley DF, Firmin RK, Harvey C, Cordingley JJ, Price S, Vuylsteke A, Jenkins DP, Noble DW, Bloomfield R, Walsh TS, Perkins GD, Menon D, Taylor BL, Rowan KM. Referral to an extracorporeal membrane oxygenation center and mortality among patients with severe 2009 influenza A(H1N1). JAMA. 2011;306:1659-1668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 620] [Cited by in RCA: 613] [Article Influence: 43.8] [Reference Citation Analysis (0)] |
| 20. | Bartlett RH, Ogino MT, Brodie D, McMullan DM, Lorusso R, MacLaren G, Stead CM, Rycus P, Fraser JF, Belohlavek J, Salazar L, Mehta Y, Raman L, Paden ML. Initial ELSO Guidance Document: ECMO for COVID-19 Patients with Severe Cardiopulmonary Failure. ASAIO J. 2020;66:472-474. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 195] [Cited by in RCA: 238] [Article Influence: 47.6] [Reference Citation Analysis (0)] |
| 21. | Bemtgen X, Krüger K, Supady A, Duerschmied D, Schibilsky D, Bamberg F, Bode C, Wengenmayer T, Staudacher DL. First Successful Treatment of Coronavirus Disease 2019 Induced Refractory Cardiogenic Plus Vasoplegic Shock by Combination of Percutaneous Ventricular Assist Device and Extracorporeal Membrane Oxygenation: A Case Report. ASAIO J. 2020;66:607-609. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 22. | Zeng Y, Cai Z, Xianyu Y, Yang BX, Song T, Yan Q. Prognosis when using extracorporeal membrane oxygenation (ECMO) for critically ill COVID-19 patients in China: a retrospective case series. Crit Care. 2020;24:148. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 84] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 23. | Huette P, Beyls C, Guilbart M, Coquet A, Berna P, Haye G, Roger PA, Besserve P, Bernasinski M, Dupont H, Abou-Arab O, Mahjoub Y. Extracorporeal membrane oxygenation for respiratory failure in COVID-19 patients: outcome and time-course of clinical and biological parameters. Can J Anaesth. 2020;67:1486-1488. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 24. | Melhuish TM, Vlok R, Thang C, Askew J, White L. Outcomes of extracorporeal membrane oxygenation support for patients with COVID-19: A pooled analysis of 331 cases. Am J Emerg Med. 2021;39:245-246. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 25. | Jacobs JP, Stammers AH, St Louis J, Hayanga JWA, Firstenberg MS, Mongero LB, Tesdahl EA, Rajagopal K, Cheema FH, Coley T, Badhwar V, Sestokas AK, Slepian MJ. Extracorporeal Membrane Oxygenation in the Treatment of Severe Pulmonary and Cardiac Compromise in Coronavirus Disease 2019: Experience with 32 Patients. ASAIO J. 2020;66:722-730. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 133] [Cited by in RCA: 130] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 26. | Squiccimarro E, Rociola R, Haumann RG, Grasso S, Lorusso R, Paparella D. Extracorporeal Oxygenation and Coronavirus Disease 2019 Epidemic: Is the Membrane Fail-Safe to Cross Contamination? ASAIO J. 2020;66:841-843. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 27. | Ramanathan K, Antognini D, Combes A, Paden M, Zakhary B, Ogino M, MacLaren G, Brodie D, Shekar K. Planning and provision of ECMO services for severe ARDS during the COVID-19 pandemic and other outbreaks of emerging infectious diseases. Lancet Respir Med. 2020;8:518-526. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 337] [Cited by in RCA: 364] [Article Influence: 72.8] [Reference Citation Analysis (0)] |
| 28. | Prekker ME, Brunsvold ME, Bohman JK, Fischer G, Gram KL, Litell JM, Saavedra-Romero R, Hick JL. Regional Planning for Extracorporeal Membrane Oxygenation Allocation During Coronavirus Disease 2019. Chest. 2020;158:603-607. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 29. | Haye G, Fourdrain A, Abou-Arab O, Berna P, Mahjoub Y. COVID-19 Outbreak in France: Setup and Activities of a Mobile Extracorporeal Membrane Oxygenation Team During the First 3 Weeks. J Cardiothorac Vasc Anesth. 2020;34:3493-3495. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 30. | Taniguchi H, Ogawa F, Honzawa H, Yamaguchi K, Niida S, Shinohara M, Takahashi K, Iwashita M, Abe T, Kubo S, Kudo M, Takeuchi I. Veno-venous extracorporeal membrane oxygenation for severe pneumonia: COVID-19 case in Japan. Acute Med Surg. 2020;7:e509. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 31. | Rubin R. Global Effort to Collect Data on Ventilated Patients With COVID-19. JAMA. 2020;323:2233-2234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 32. | Haiduc AA, Alom S, Melamed N, Harky A. Role of extracorporeal membrane oxygenation in COVID-19: A systematic review. J Card Surg. 2020;35:2679-2687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |