Published online Apr 4, 2012. doi: 10.5492/wjccm.v1.i2.50
Revised: October 14, 2011
Accepted: March 10, 2012
Published online: April 4, 2012
To review possible mechanisms and therapeutics for acute lung injury (ALI) and acute respiratory distress syndrome (ARDS). ALI/ARDS causes high mortality. The risk factors include head injury, intracranial disorders, sepsis, infections and others. Investigations have indicated the detrimental role of nitric oxide (NO) through the inducible NO synthase (iNOS). The possible therapeutic regimen includes extracorporeal membrane oxygenation, prone position, fluid and hemodynamic management and permissive hypercapnic acidosis etc. Other pharmacological treatments are anti-inflammatory and/or antimicrobial agents, inhalation of NO, glucocorticoids, surfactant therapy and agents facilitating lung water resolution and ion transports. β-adrenergic agonists are able to accelerate lung fluid and ion removal and to stimulate surfactant secretion. In conscious rats, regular exercise training alleviates the endotoxin-induced ALI. Propofol and N-acetylcysteine exert protective effect on the ALI induced by endotoxin. Insulin possesses anti-inflammatory effect. Pentobarbital is capable of reducing the endotoxin-induced ALI. In addition, nicotinamide or niacinamide abrogates the ALI caused by ischemia/reperfusion or endotoxemia. This review includes historical retrospective of ALI/ARDS, the neurogenic pulmonary edema due to head injury, the detrimental role of NO, the risk factors, and the possible pathogenetic mechanisms as well as therapeutic regimen for ALI/ARDS.
- Citation: Su CF, Kao SJ, Chen HI. Acute respiratory distress syndrome and lung injury: Pathogenetic mechanism and therapeutic implication. World J Crit Care Med 2012; 1(2): 50-60
- URL: https://www.wjgnet.com/2220-3141/full/v1/i2/50.htm
- DOI: https://dx.doi.org/10.5492/wjccm.v1.i2.50
Acute respiratory distress syndrome (ARDS), the most devastating form of acute lung injury (ALI), is a serious clinical disorder with high mortality (30%-60%)[1-3]. The risk factors for ARDS include septicemia, acid aspiration, infection, traumatic injury, fat embolism, ischemia/reperfusion[3-8], and other causes[9-13]. Our cardiopulmonary laboratory has carried out experimental studies and clinical investigations on ALI and ARDS since 1973[3,12,14-18]. The purposes of this review article are: (1) to describe in brief the historical retrospective of ARDS and ALI; (2) to draw attention to an important clinical issue of neurogenic ALI; (3) to present the experimental studies and clinical investigations from our laboratory from 1973 to 2008; (4) to elucidate the functional role of nitric oxide (NO) and other mediators involved in the pathogenesis of ARDS/ALI; (5) To define the risk factors for ARDS and ALI; and (6) to discuss the pathogenetic mechanisms and therapeutic regimen for ARDS/ALI.
In 1967, Ashbaugh and colleagues first described 12 adults with respiratory distress syndrome including cyanosis, reduced lung compliance, and diffuse infiltrates evident on chest radiograph[19]. The initial term of this disorder was “adult respiratory distress syndrome”[20]. This entity is now termed “ARDS”, since it does occur in children[21,22]. Early reports used “pulmonary edema (PE)” because the edematous lesions in the lungs[23-27]. ARDS is now recognized as an important clinical problem[1,3,28-30].
Cushing[31,32] investigated the changes in arterial pressure (AP), heart rate (HR) and respiration in response to an increased intracranial pressure in anesthetized dogs. The Cushing[31,32] responses including systemic hypertension, bradycardia and irregular respiration (dyspnea and apnea) have been used as signs of intracranial hypertension (ICH). Later studies have elucidated and described the hemodynamic consequences and cardiovascular complications of ICH[14,33,34]. In anesthetized dogs, we employed total heart bypass and found that ICH resulted in constriction of the systemic and pulmonary resistance and capacitance vessels[35]. Spectral analysis of the aortic pressure and flow revealed that ICH caused hemodynamic changes of the steady and pulsatile components. ICH not only increased the AP and total peripheral resistance, but also elevated the arterial impedance, pulse wave reflection and ventricular work with a reduction in arterial compliance[36,37]. Evaluation of the HR variability indicated that ICH was associated with augmented sympathetic and attenuated parasympathetic drive[38]. The most serious cardiopulmonary sequelae is fatal PE associated with hypertensive crisis[39,40]. Weissman et al[23] in 1939 presented a comprehensive survey in patients with intracranial hemorrhage. He reported that various degrees of lung edema and congestion were found in 70% of 686 cases with intracranial hemorrhage. Mild lung congestion was found in only 2% of 200 control cases without intracranial lesions. In this report, he also mentioned that Moutier in 1918 was indeed the first to notice severe lung edema in a patient after gunshot injury to the head. In addition, he noted that Hess in 1934 reported several cases of PE in patients with brain tumor, epilepsy and lesions in medulla. Richards et al[25] in 1963 reported fatal PE in 46 cases of 88 patients with brain injury. The most severe cases were found in patients with brain stem distortion, hemorrhage and infarction. Subsequent clinical observations[26,41] confirmed the occurrence of severe PE in patients with intracranial disorders. Jourdan et al[42] revealed that acute PE could dramatically complicate brain injury in four child cases. Today, this serious clinical problem has been overlooked by most clinicians because: (1) Attention on the brain problem always supercedes the lung condition; and (2) The dramatic, fulminating and fatal outcomes often result in sudden death before any emergent intervention[41].
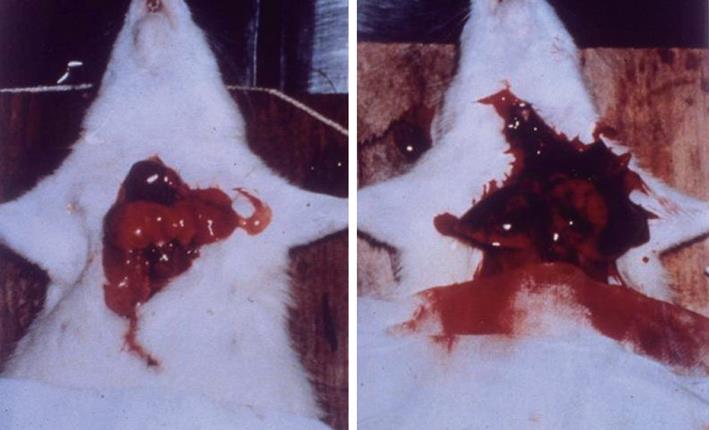
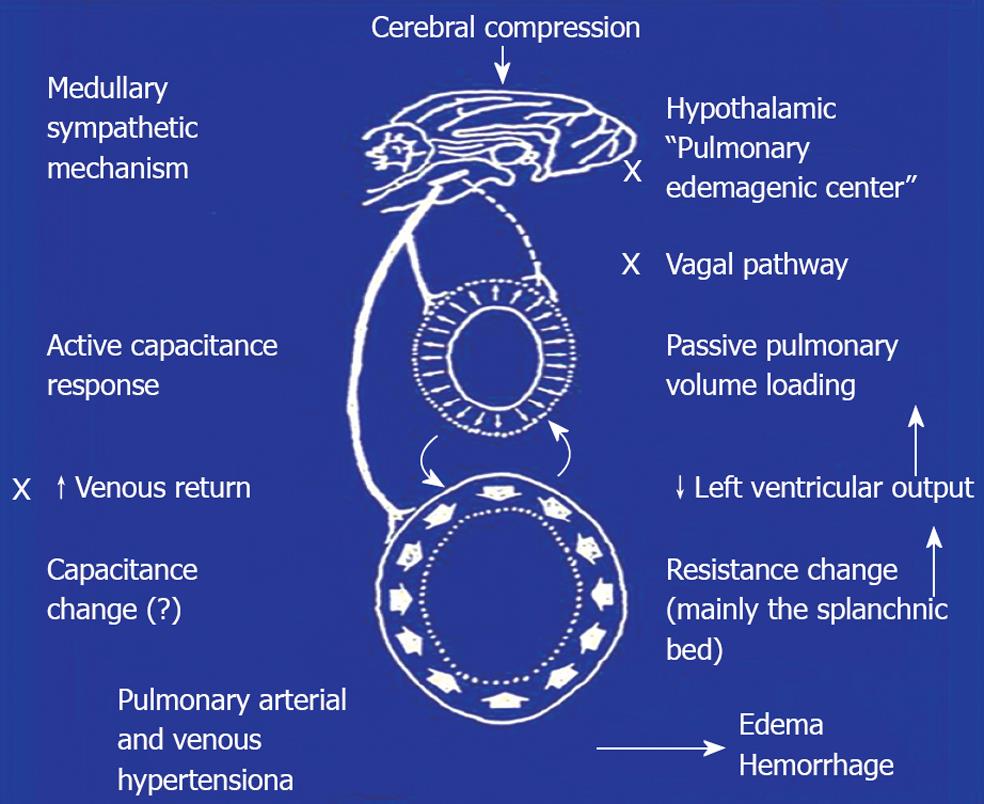
Experimental evidence of pulmonary hypertension followed by edema formation due to ICH was demonstrated in guinea pigs and dogs[24,43]. Similar observations were described in other animal species including rabbits, monkeys and chimpanzees[27,44,45]. These studies mainly confirmed that PE could be induced in animals following ICH. Little information was provided with respect to the pathogenetic mechanisms of the neurogenic PE. In 1973, we began our extensive studies on the neural and hemodynamic mechanisms of neurogenic PE in anesthetized rats. A rapid mass impact into the cranium or distension of a balloon installed intracranially caused systemic hypertension. Subsequently, the insult produced severe pulmonary changes in 3-5 min after cerebral compression (CC). The lungs showed dark-red discoloration, and were swollen and globular in appearance. This was evidence of gross hemorrhage. The lung weight (LW) was increased 3 to 4-fold the normal value (Figure 1). On microscopic examination, the normal configuration of alveolar structure was obscured. There were air spaces filled with red blood cells and exudate. The perivascular space was distended. Electron-microscopic examination revealed marked stretching of the capillary endothelial cells. Disruption of the entire capillary wall with leakage of red cells from the vessel lumen to the interstitium was observed. In comparison with the other animal studies, our findings presented the most drastic lung changes in terms of rapid onset and severity[14,15]. With respect to the central nervous system that is responsible for the pulmonary sequelae following CC, activation of a “hypothalamic PE genetic center” has been implicated to be involved in the centrogenic PE[46]. In our early studies[14,15], we found that a midcollicular decerebration did not affect the centrogenic PE, suggesting that neural structures above the medulla oblongata were not involved in the genesis of CC-induced PE. Later studies demonstrated that sympathetic overactivation leading to systemic vasoconstriction was the major culprit for PE of centrogenic origin. Complicated hemodynamic measurements and heart bypass design were employed to elucidate the hemodynamic events. Relevant studies revealed that ICH caused vasoconstriction of resistance and capacitance vessels in the systemic and pulmonary circulation. Shift of blood from the systemic vascular beds to the lung was the major cause of pressure and volume loading in the pulmonary circulation[35,47] (Figure 2). The hemodynamic alterations have elaborated the classic Cushing[31,32] responses including systemic hypertension, bradycardia and intermittent apnea.
In support of the blood volume shift from the systemic to pulmonary circulation, we used a scintiphotographic method to demonstrate increased lung blood volume[48]. The scintiphotographic study was performed by intravenous injection of a specific isotope, indium-113m. The radioactive isotope forms a large molecular complex with plasma transferrin and is evenly distributed inside the circulatory system. The lungs normally showed little radioactivity. After CC, an increased radioactivity in the lung was evident, indicating blood accumulation in the pulmonary circulation. Regional sympathectomy demonstrated that the splanchnic beds were the major site of vasoconstriction. Direct sympathetic vasoconstriction in the pulmonary circulation only contributed in part to the centrogenic PE[49,50].
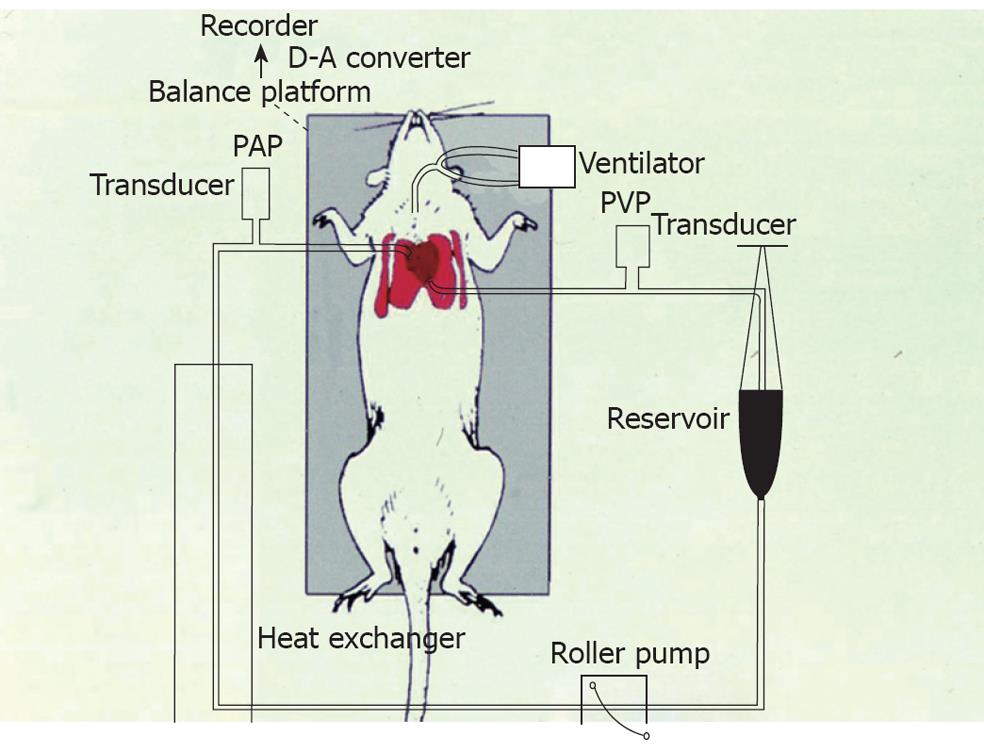
In the early 1990s, our laboratory collaborated with clinicians in the Chest Medicine, Tri-Service General Hospital, Taipei, Taiwan. Many basic and clinical investigators were trained in the laboratory to study on the mechanisms of PE, ALI and ARDS caused by various challenges and disorders, such as phorbol myristate acetate (PMA), platelets, air embolism, ischemia/reperfusion and other challenges. We developed an isolated rat’s lung model that was perfused with constant flow and left in situ (Figure 3). The lungs were not needed to remove from the body. In the preparation, the LW to body weight ratio, LW gain, microvascular permeability, protein concentration in bronchoalveolar lavage, tracer dye leakage, pulmonary arterial and venous pressure and pulmonary vascular resistance could be measured or calculated. Furthermore, biochemical factors and inflammatory cytokines were determined. We have used this preparation in many studies for more than 20 years[3,9,17,51-72].
In these studies, isolated lung preparation was used alone or in combination with the whole rodent model. The main findings were that cyclooxygenase products of arachidonic acid such as thromboxane A2 was involved in the ALI and pulmonary hypertension caused by PMA, air embolism and platelets[51,52]. Furthermore, we found that L-arginine and inhaled NO enhanced the lung injury caused by air embolism, while blockade of NO synthase (NOS) with Nω-nitro-L-arginine methyl ester (L-NAME) attenuated the ALI[52]. The results suggest that NO is detrimental to the lung in air embolism.
Hypoxia-induced pulmonary vasoconstriction has been a well-known physiological phenomenon. The physiological significance is that capillary blood flow to hypoxic alveoli can be reduced, and the blood flow is thus diverted to oxygenated alveoli. It has been speculated that inadequate NO formation is responsible for the increased vascular tone, pulmonary vasoconstriction and hypertension[17,73]. This contention was challenged by a study in our in situ lung preparation[54]. A detector probe for NO was placed in the pulmonary vein and lung tissue in isolated perfused rat’s lungs. Real-time NO monitoring revealed that NO release was increased accompanied by pulmonary arterial hypertension following ventilatory hypoxia. Pretreatment with L-arginine potentiated the NO release and reduced the pulmonary hypertension, while L-NAME produced the opposite effects. These findings suggest that continuous NO release from the lung maintains pulmonary arterial pressure (PAP) and that insufficient NO formation is not the cause of hypoxia-induced pulmonary hypertension. In this connection, studies from our laboratory[74,75] also contest the early consensus that impairment of endothelial function and NO formation is the cause of hypertension due to reduction of the vasodilatory effect of NO[17,73].
Recent experimental studies have investigated the role of NO, free radicals and proinflammatory cytokines in the endotoxin-induced pathophysiological and biochemical changes and the associated ALI. Septicemia or endotoxin shock is one of the major causes of death in the United States and other countries[76-78]. Activation of inducible NO synthase (iNOS) to produce a large amount of NO accounts for the systemic hypotension, hyperreactiveness to vasoconstrictors and finally multiple organ failure in endotoxin shock[77-79]. We found that administration of endotoxin [lipopolysaccharide (LPS)] induced ALI with increases in NO, iNOS, tumor necrosis factor-α (TNF-α) and interleukin-1β (IL-1β). The findings suggest that NO/iNOS and proinflammatory cytokines are involved in the sepsis-induced ALI[56].
Our research team used the isolated perfused lung model to reveal that the lung was the major source of NO production following endotoxemia[58]. NO production mediated by the iNOS system, is toxic to the endothelium in the pulmonary microvasculature in isolated perfused lungs subjected to ischemia/reperfusion[61]. Administration of red blood cells and hemoglobin into the isolated lung as well as static inflation attenuated the ALI following hypoxia or ischemia/reperfusion[9,57].
We have recently advanced our studies on clinical arena[8,10,80]. In patients with Japanese B encephalitis, viral destruction of the depressor area in the medulla oblongata causes central sympathetic activation, whereas rupture of intracranial mycotic aneurysms results in increased intracranial pressure. The hemodynamic mechanism of ALI or ARDS in these two disorders may operate through the similar sequences as proposed by our previous reports, which related to the ALI caused by ICH[14,47]. We also revealed ARDS in cases of lymphangitis in breast carcinoma and fat embolism. Blockade of lymphatics, capillaries, and venules in breast cancer with lymphangitis caused ALI. In cases of ALI associated with fat embolism, we found that ALI could not be solely attributed to fatty embolic blockade of venous and lymphatic drainage. Several mediators, such as cyclic guanosine monophosphate, 5-hydroxytryptamine (serotonin), NO and cytokines might play a contributing role. Kao and coworkers studied 8 patients who died of fat embolism syndrome (FES) with ARDS[10]. Together with the previous report of 6 cases[80], we had the largest group (a total of 14 patients) of autopsy cases with FES and ARDS. These cases were admitted because of long bone fracture (fracture of tibia, femur, combined fracture and fracture of multiple pelvic bones). The chest radiograph revealed clear lung on admission. Subjects developed signs of respiratory distress and progressive loss of consciousness. Chest X-ray showed lung infiltration. Gross inspection revealed diffuse petechial rashes over the skin. Systemic hypotension and bradycardia ensued. Tachypnea, cyanosis, and cardiac arrest developed. Arterial blood pH and PaO2 were decreased, whereas an increase in PaCO2 was observed. Despite intensive care and treatment, the patients expired within 3 h after the crush injury. Before death, measurement of PAP disclosed a high PAP. Biochemical determination further indicates that NO, methyl guanidine (an indicator of hydroxyl radicals), phospholipase A2 are involved in the ARDS due to FES.
During the summers from 2001-2003, we encountered a total of 48 children suffering from hand, foot, and mouth disease[8]. Chest radiography on admission revealed clear lung. However, 21 of these children developed severe dyspnea, hyperglycemia, leukocytosis, and decreased blood oxygen tension. AP and HR fluctuation ensued. Spectral analysis of the AP and HR variabilities showed elevations in sympathetic activity at the onset of respiratory stress. Thereafter, parasympathetic drive increased with declines in AP and HR. These children died within 4 h after the onset of ARDS. Before death, chest radiography revealed severe lung infiltration. Similar to Japanese B encephalitis, destruction of the medullary depressor area caused initial sympathetic activation. Reverse-transcriptase polymerase chain reaction (RT-PCR) found marked iNOS mRNA expression in the lung parenchyma, suggesting iNOS may also be involved in the pathogenesis of ARDS in patients with enterovirus 71 infection.
Furthermore, we have reported ARDS in patients with leptospirosis[13]. In leptospirosis-induced ARDS, histochemical stain demonstrated spirochaetes bacteria in the alveolar space. The pathology included alveolar hemorrhage, myocarditis, portal inflammation and interstitial nephritis. Antigen retrieval immunohistochemical stain disclosed iNOS expression in the alveolar type 1 cells, myocardium, hepatocytes and renal tubules. Spectral analysis of AP and HR variabilities indicated decreased sympathetic drive with increased parasympathetic activity. The changes in autonomic functions led to severe hypotension and bradycardia. Biochemical determinations suggested multiple organ damage. The pathogenesis of lung and organ injury might also involve iNOS and NO production[13,81]. In subjects with scrub typhus, Orientia tsutsugamushi infection caused alveolar injury. Marked iNOS expression was found in the alveolar macrophages with increase in plasma nitrate/nitrite, suggesting that NO production from the alveolar macrophages accounts for the ALI[82]. The victim from rabies was a woman bitten by a wild dog. In addition to sign of hydrophobia, hypoxia, hypercapnia, hyperglycemia and increased plasma nitrate/nitrite were observed. The woman died of alveolar hemorrhage shortly after admission[83]. Recently, we encountered five cases with long-term malignancy. These subjects displayed signs of respiratory distress following an episode of hypercalcemia. Two cases died of ARDS after the plasma calcium was increased above 6 mmol/L (unpublished data). Holmes et al[84], reported a case who died of ARDS following a hypercalcemia crisis caused by a parathyroid adenoma. We conducted animal experiments in whole rodent and isolated perfused rat’s lungs. Our results indicated that hypercalcemia (calcium concentration > 5 mmol/L) caused severe ALI in conscious rats and isolated lungs. Immunohistochemical staining showed iNOS activity in the alveolar macrophages and epithelial cells. RT-PCR found marked increase in iNOS mRNA expression in lung parenchyma. Hypercalcemia also increased plasma nitrate/nitrite, methyl guanidine, proinflammatory cytokines and procalcitonin. Pretreatment with calcitonin or L-N6 (1-iminoethyl)-lysine (L-Nil, an iNOS inhibitor) attenuated the hypercalcemia-induced changes. We proposed that hypercalcemia produced a sepsis-like syndrome. The ALI caused by hypercalcemia may involve NO and iNOS[85].
The aforementioned animal studies and clinical observations indicated that NO production through the iNOS may be involved in the lung injury due to various causes. Our research team demonstrated that endotoxemia produced in anesthetized rats by intravenous administration of LPS (endotoxin) provoked systemic hypotension, endothelial damage and ALI accompanied by increased plasma nitrate/nitrite and expression of iNOS mRNA, TNF-α and IL-1β. The LPS-induced changes were abolished by nonspecific and iNOS-specific (iNOS) inhibitors such as Nω-monomethyl-L-arginine, L-NAME, aminoguanine and dexamethasone[56]. This study suggested that NO/iNOS, TNF-α and IL-1β were involved in the endotoxemia-induced ALI. Generation of NO by the activated neutrophil caused alveolar injury from smoke inhalation[86]. Many laboratories using specific iNOS inhibitors and/or iNOS-knockout animals have supported the contention that NO/iNOS is responsible for the oxidative stress and endothelial damage in the ARDS/ALI caused by endotoxin, ozone exposure, carrageenan treatment, acute hypoxia, bleomycin administration, acid aspiration and other challenges[11,87-96]. Our laboratory further provided evidence to suggest that the NO/iNOS system is involved in the pathogenesis of ALI caused by air embolism[71], fat embolism[10,97,98], ischemia/reperfusion[67], oleic acid[99], and PMA[100]. In these recent studies, various insults caused increase in nitrate/nitrite in plasma or lung perfusate, upregulation of iNOS mRNA in lung parenchyma accompanied with elevation of proinflammatory cytokines such as TNF-α, IL-1β and IL-6. Lin et al[101] have suggested that an increase in iNOS mRNA triggers the release of proinflammatory cytokines in septic and conscious rats. The inflammatory responses results in multiple organ damage including ALI. Inhibition of iNOS with S-methylisothiourea or L-N6-(iminoethyl)-lysine (L-Nil) attenuated the inflammatory changes, release of NO and cytokines, thereby preventing the organ dysfunction and ALI[65].
In animal experimentations and clinical investigations, the risk factors causing ALI/ARDS include head injury, ICH[14,15,23,25-27,39,42,47], sepsis[7,13,56,58,63,64,68,87,89,92,94,102], and infections[4-8,13,30,80-83]. Pulmonary embolic disorders such as fat and air embolism are less common causes[1,10,52,71,80,103,104]. Ischemia/reperfusion lung injury may develop as a consequence of several pulmonary disorders such as pulmonary artery thromboendarterectomy, thrombolysis after pulmonary embolism and lung transplantation[9,61,67,105-107]. Gastric aspiration occurs frequently in surgical patients under anesthesia and other causes such as blunt thoracic trauma, impaired glottis competency, and pregnancy[108-110]. It is one of the major causes of ARDS[111,112]. Intratracheal instillation of hydrochloric acid or gastric particles has been employed as experimental model of ALI[11,113-115]. In addition, amphetamine and oleic acid have been employed for the induction of ALI[59,99,116,117]. PMA (12-O-tetradecanoyl-phorbol-13-acetate), an ester derivative from croton oil has been used to induce ALI[60,69,118,119]. Experiments in vivo and in vitro have demonstrated that PMA is a strong neutrophil activator[119-122]. Activation and recruitment of neutrophils that lead to release of neutrophil elastase and other mediators may play an initial role in the pathogenesis of ALI[123,124].
The oleic acid-induced ALI has several clinical implications. First, the blood level of oleic acid was significantly elevated in patients with ARDS[125,126]. Second, the proportion of oleic acid incorporated into surfactant phospholipids was also increased in patients with ARDS and sepsis[127,128]. These observations suggest that serum level of oleic acid may be a predictor or prognostic factor for ARDS[116,125]. Early study focused on the potential toxic effects of high oxygen fractions on inspired air[129]. Ventilator-induced ALI was attributed to the deleterious effects on capillary stress due to alveolar overdistension. Cyclic opening and closing of atelectatic alveoli during mechanical ventilation might exacerbate lung injury by damaging alveoli. Recent evidence indicated that overdistension coupled with repeated collapse and reopening of alveoli initiated an inflammatory cascade of proinflammatory cytokines release[1,29,130,131].
In spite of the risk factors and causes, the pathophysiology of ARDS/ALI is generally considered to be initiated by formation of alveolar edema (even hemorrhage) that is enriched with protein, inflammatory cells or red blood cells. After damage of alveolar-capillary barrier, impairment of gas exchange occurs, with decrease in lung compliance and increases in dispersion of ventilation and perfusion and intrapulmonary shunt. Hypoxia, reduction in arterial oxygen pressure to fraction of oxygen in inspired air (PaO2/FiO2), and hypercapnia ensued despite ventilation with high oxygen[1,3,28,30,132]. In addition to the potential toxic effects of NO and free radicals, certain chemokines, cytokines, neutrophil elastase, myeloperoxidase and malondialdehyde have been shown to be associated with several types of ARDS/ALI[99,124,133-135]. The balance between proinflammatory and anti-inflammatory mediators is regulated by transcriptional factors mainly nuclear factor-KB[136]. Pulmonary fluid clearance and ion transport are important factors to determine the extent of lung edema. Regulator factors include cystic fibrosis transmembrane conductance regulators, sodium- and potassium-activated adenosine triphophatase, protein kinases, aclenylate cyclase, and cyclic adenosine monophosphate[7,81,137,138].
The treatment of ARDS/ALI is difficult and complex. Several review articles and monographs have addressed the issue of possible therapeutic regimen. The modalities include extracorporeal membrane oxygenation, prone position, mechanical ventilation with appropriate tidal volume and respiratory pressure, fluid and hemodynamic management and permissive hypercapnic acidosis[1,29,139-150].
Other pharmacological treatments are anti-inflammatory and/or antimicrobial agents to control infection and to abrogate sepsis, adequate nutrition, surfactant therapy, inhalation of NO and vasodilators, glucocorticoids and nonsteroid anti-inflammatory drugs, and agents that accelerate lung water resolution and ion transports[1,151-154]. Although most animal studies on these pharmacological options showed favorable results, the effectiveness and outcomes in clinical studies or trials were conflicting[1,12,22].
β-adrenergic agnoists to facilitate water removal and ion transport have been shown to be promising. These agents may also stimulate secretion of surfactant and have no serious side effects. There were several reports on the pharmacological and molecular actions of β agonists, surfactant, vascular endothelial growth factor and related molecules as well as angiotensin-converting enzyme[155,156].
In addition to the experimental studies and clinical investigations on the pathogenesis of ALI/ARDS, our laboratory has carried out several experimentations on the therapeutic regimen for this serious disorder. In conscious rats, regular exercise training attenuates septic responses such as systemic hypotension, increases in plasma nitrate/nitrite, methyl guanidine, blood urea nitrogen, creatinine, amylase, lipase, asparate aminotransferase, alanine aminotransferase, creatine phosphokinase, lactic dehydrogenase, TNF-α, and IL-1β. Exercise training also abrogates the cardiac, hepatic and pulmonary injuries caused by endotoxemia[64]. Insulin exerts anti-inflammatory effects on the ALI and associated biochemical changes following intravenous administration of LPS[62]. Propofol (2, 6-diisopropylphenol) has been commonly used for sedation in critically ill patients[157]. This anesthetic has rapid onset, short duration and rapid elimination[158]. Propofol protects the anesthetized rats from ALI caused by endotoxin[68]. In conscious rats, oleic acid results in sepsis-like responses including ALI, inflammatory reactions and increased in neutrophil-derived factors (neutrophil elastase, myeloperoxidase and malondialdehyde), nitrate/nitrite, methyl guanidine, and inflammatory cytokines. It depresses the sodium-and potassium-activated ATPase, but upregulates the iNOS mRNA expression. Pretreatment and posttreatment with propofol alleviates or reverses the oleic acid-induced lung pathology and associated biochemical changes[99]. Pentobarbital, an anesthetic agent commonly used in experimental studies and a hyponotics for patients. This agent improves the pulmonary and other organ functions following LPS administration. It also increases the survival rate[66]. A later study by Yang et al[159] further revealed that pentobarbital suppressed the expression of TNF-α, which might result from decrease in the activities of nuclear factor-κB and activator protein 1 and reduction in expression of P38 mitogen-activated protein kinase. In vivo examination of cytotoxic effects of LPS disclosed that LPS caused multiple organ dysfunctions. These changes were attenuated by pentobarbital. Pentobarbital also reduced the cell aptosis caused by deforoxamine-induced hypoxia. Nicotinamide or niacinamide (compound of soluble B complex) abrogates the ALI caused by ischemic/reperfusion or endotoxin by mechanism through inhibition on poly (ADP-ribose) synthase and subsequent suppression of iNOS. NO, free radicals and proinflammatory cytokines with restoration of adenosine triphosphate[67,72]. N-acetylcysteine, an antioxidant and cytoprotective agent with scavenging action on reactive oxygen species and inhibitory effects on proinflammatory cytokines ameliorated organ dysfunctions due to sepsis in conscious rats[160,161]. In a similar endotoxin-induced ALI model, we found that N-acetylcysteine improved the LPS-induced systemic hypotension and leukocytopenia. It also reduced the extent of ALI, as evidenced by reductions in LW changes, exhaled NO and lung pathology. In addition, N-acetylcysteine diminished the LPS-induced increases in nitrate/nitrite, TNF-α, and IL-1β[63]. In isolated lungs, N-acetylcysteine attenuated the ALI caused by PMA[69]. In a most recent study, we reported that posttreatment with N-acetylcysteine prevented the ALI caused by fat embolism[98]. Collectively, the results of our studies favored N-acetylcysteine as a therapeutic agent for ALI/ARDS. The conflicting results and practice guidelines from clinical studies in the recommendation of N-acetylcysteine in critically ill patients[162,163] were commented and analyzed by Molnár[164]. The clinical adaptation of results from animal studies requires further investigations.
ARDS or ALI is a serious clinical problem with high mortality. The risk factors leading to ALI/ARDS include head injury, intracranial disorders, sepsis and infections. Pulmonary embolic disorders such as fat and air embolism are less common causes. Ischemia/reperfusion lung injury may develop as a consequence of several pulmonary disorders such as lung transplantation. Gastric aspiration occurs frequently in several conditions such as anesthesia, trauma and pregnancy. The ventilator-induced ALI has been attributed to the deleterious effects on capillary stress due to alveolar overdistension. In experimental studies, PMA and oleic acid have been employed to induce ALI.
The pathogenesis of ALI/ARDS is complex. Experimental studies and clinical investigations from our and other laboratories have indicated the detrimental role of NO through the iNOS. Activation and recruitment of neutrophils that lead to release of neutrophil elastase, myeloperoxidase, malondialdehyde and proinflammatory cytokines may play an initial role in the pathogenesis of ALI/ARDS.
The possible therapeutic regimen for ALI/ARDS include extracorporeal membrane oxygenation, prone position, fluid and hemodynamic management and permissive hypercapnic acidosis etc. Other pharmacological treatments are anti-inflammatory and/or antimicrobial agents, inhalation of NO, glucocorticoids, surfactant therapy and agents that facilitate lung water resolution and ion transports. Adrenergic β agonists are able to accelerate lung fluid and ion removal and to stimulate surfactant secretion. There are reports on the actions of vascular endothelial growth factor and related molecules as well as angiotensin-converting enzyme.
Our laboratory has reported experimental studies on the effectiveness of several regimen for ALI/ARDS. In conscious rats, regular exercise training alleviates the endotoxin-induced ALI. Propofol and N-acetylcysteine exert protective effect on the ALI caused by endotoxin, oleic acid and PMA. We have also provided evidence that insulin possesses anti-inflammatory effect. Pentobarbital is capable of reducing the endotoxin-induced ALI and associated changes. In addition, nicotinamide or niacinamide (soluble B complex) abrogates the ALI caused by ischemia/reperfusion or endotoxemia. These nonpharmacological and pharmacological therapeutic strategies require further investigations for clinical application.
Peer reviewer: Robert T Mallet, Professor, Department of Integrative Physiology, Health Science Center, North Texas University, 3500 Camp Bowie Blvd., Fort Worth, TX 76107-2699, United States
S- Editor Gou SX L- Editor A E- Editor Zheng XM
| 1. | Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000;342:1334-1349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3976] [Cited by in RCA: 3856] [Article Influence: 154.2] [Reference Citation Analysis (0)] |
| 2. | Vincent JL, Sakr Y, Ranieri VM. Epidemiology and outcome of acute respiratory failure in intensive care unit patients. Crit Care Med. 2003;31:S296-S299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 95] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 3. | Chen HI, Kao SJ, Wang D, Lee RP, Su CF. Acute respiratory distress syndrome. J Biomed Sci. 2003;10:588-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 4. | Ksiazek TG, Erdman D, Goldsmith CS, Zaki SR, Peret T, Emery S, Tong S, Urbani C, Comer JA, Lim W. A novel coronavirus associated with severe acute respiratory syndrome. N Engl J Med. 2003;348:1953-1966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3147] [Cited by in RCA: 3072] [Article Influence: 139.6] [Reference Citation Analysis (0)] |
| 5. | Drosten C, Günther S, Preiser W, van der Werf S, Brodt HR, Becker S, Rabenau H, Panning M, Kolesnikova L, Fouchier RA. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N Engl J Med. 2003;348:1967-1976. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3277] [Cited by in RCA: 3314] [Article Influence: 150.6] [Reference Citation Analysis (0)] |
| 6. | Lee N, Hui D, Wu A, Chan P, Cameron P, Joynt GM, Ahuja A, Yung MY, Leung CB, To KF. A major outbreak of severe acute respiratory syndrome in Hong Kong. N Engl J Med. 2003;348:1986-1994. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1563] [Cited by in RCA: 1602] [Article Influence: 72.8] [Reference Citation Analysis (1)] |
| 7. | Eisenhut M, Wallace H, Barton P, Gaillard E, Newland P, Diver M, Southern KW. Pulmonary edema in meningococcal septicemia associated with reduced epithelial chloride transport. Pediatr Crit Care Med. 2006;7:119-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 45] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 8. | Kao SJ, Yang FL, Hsu YH, Chen HI. Mechanism of fulminant pulmonary edema caused by enterovirus 71. Clin Infect Dis. 2004;38:1784-1788. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 72] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 9. | Kao SJ, Wang D, Yeh DY, Hsu K, Hsu YH, Chen HI. Static inflation attenuates ischemia/reperfusion injury in an isolated rat lung in situ. Chest. 2004;126:552-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 10. | Kao SJ, Yeh DY, Chen HI. Clinical and pathological features of fat embolism with acute respiratory distress syndrome. Clin Sci (Lond). 2007;113:279-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 40] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 11. | Jian MY, Koizumi T, Kubo K. Effects of nitric oxide synthase inhibitor on acid aspiration-induced lung injury in rats. Pulm Pharmacol Ther. 2005;18:33-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 12. | Chen HI, Chang HR, Wu CY, Kao SJ, Wang D, Hsieh NK, Hsu YH. Nitric oxide in the cardiovascular and pulmonary circulation--a brief review of literatures and historical landmarks. Chin J Physiol. 2007;50:43-50. [PubMed] |
| 13. | Chen HI, Kao SJ, Hsu YH. Pathophysiological mechanism of lung injury in patients with leptospirosis. Pathology. 2007;39:339-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 14. | Chen HI, Sun SC, Chai CY. Pulmonary edema and hemorrhage resulting from cerebral compression. Am J Physiol. 1973;224:223-229. [PubMed] |
| 15. | Chen HI, Chai CY. Pulmonary adema and hemorrhage as a consequence of systemic vasoconstriction. Am J Physiol. 1974;227:144-151. [PubMed] |
| 16. | Chen HI. Hemodynamic mechanisms of neurogenic pulmonary edema. Biol Signals. 1995;4:186-192. [PubMed] |
| 17. | Chen HI, Hu CT, Wu CY, Wang D. Nitric Oxide in Systemic and Pulmonary Hypertension. J Biomed Sci. 1997;4:244-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 18. | Chen HI, Su CF, Chai CY. [Neural and hemodynamic mechanisms of neurogenic pulmonary edema]. Sheng Li Ke Xue Jin Zhan. 1999;30:203-206. [PubMed] |
| 19. | Ashbaugh DG, Bigelow DB, Petty TL, Levine BE. Acute respiratory distress in adults. Lancet. 1967;2:319-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2410] [Cited by in RCA: 2236] [Article Influence: 38.6] [Reference Citation Analysis (0)] |
| 20. | Petty TL, Ashbaugh DG. The adult respiratory distress syndrome. Clinical features, factors influencing prognosis and principles of management. Chest. 1971;60:233-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 371] [Cited by in RCA: 319] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 21. | Murray JF, Matthay MA, Luce JM, Flick MR. An expanded definition of the adult respiratory distress syndrome. Am Rev Respir Dis. 1988;138:720-723. [PubMed] |
| 22. | Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, Lamy M, Legall JR, Morris A, Spragg R. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994;149:818-824. [PubMed] |
| 23. | Weissman SJ. Edema and congestion of the lungs from intracranial hemorrhage. Surgery. 1939;6:722-729. |
| 24. | Campbell GS, Visscher MB. Pulmonary lesions in guinea pigs with increased intracranial pressure, and the effect of bilateral cervical vagotomy. Am J Physiol. 1949;157:130-134. [PubMed] |
| 25. | Richards P. Pulmonary oedema and intracranial lesions. Br Med J. 1963;2:83-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 30] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 26. | Ducker TB. Increased intracranial pressure and pulmonary edema. 1. Clinical study of 11 patients. J Neurosurg. 1968;28:112-117. [PubMed] |
| 27. | Ducker TB, Simmons RL. Increased intracranial pressure and pulmonary edema. 2. The hemodynamic response of dogs and monkeys to increased intracranial pressure. J Neurosurg. 1968;28:118-123. [PubMed] |
| 28. | Matthay MA, Zimmerman GA. Acute lung injury and the acute respiratory distress syndrome: four decades of inquiry into pathogenesis and rational management. Am J Respir Cell Mol Biol. 2005;33:319-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 439] [Cited by in RCA: 467] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 29. | Bernard GR. Acute respiratory distress syndrome: a historical perspective. Am J Respir Crit Care Med. 2005;172:798-806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 120] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 30. | Ware LB. Clinical Year in Review III: asthma, lung transplantation, cystic fibrosis, acute respiratory distress syndrome. Proc Am Thorac Soc. 2007;4:489-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 31. | Cushing H. Concerning a definite regulatory mechanism of the vasomotor center which controls blood pressure during cerebral compression. Bull Johns Hopkins Hosp. 1901;12:290-292. |
| 32. | Cushing H. Some experimental and clinical observations concerning states of increased intracranial pressure. Am J Med Sci. 1902;124:375-400. |
| 33. | Brashear RE, Ross JC. Hemodynamic effects of elevated cerebrospinal fluid pressure: alterations with adrenergic blockade. J Clin Invest. 1970;49:1324-1333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 44] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 34. | Burch GE, Colcolough H, Giles T. Intracranial lesions and the heart. Am Heart J. 1970;80:574-575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 35. | Chen HI, Wang DJ. Systemic and pulmonary hemodynamic responses to intracranial hypertension. Am J Physiol. 1984;247:H715-H721. [PubMed] |
| 36. | Su CF, Hu CT, Chen HI. Effects of intracranial hypertension on steady and pulsatile haemodynamics in dogs. Clin Exp Pharmacol Physiol. 1999;26:898-902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 37. | Su CF, Yang YL, Lee MC, Chen HI. A severe vicious cycle in uncontrolled subarachnoid hemorrhage: the effects on cerebral blood flow and hemodynamic responses upon intracranial hypertension. Chin J Physiol. 2006;49:56-63. [PubMed] |
| 38. | Su CF, Kuo TB, Kuo JS, Lai HY, Chen HI. Sympathetic and parasympathetic activities evaluated by heart-rate variability in head injury of various severities. Clin Neurophysiol. 2005;116:1273-1279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 63] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 40. | Chen HI, Chai CY. Circulatory and pulmonary segualae in hypertensive crisis-An animal study with intracranial hypertension. In: MRC (RSA)-NSC (ROC) Symposium on Hypertension 1988; 11 (Abstract). |
| 41. | Simon RP. Neurogenic pulmonary edema. Neurol Clin. 1993;11:309-323. [PubMed] |
| 42. | Jourdan C, Convert J, Rousselle C, Wasylkiewicz J, Mircevski V, Mottolese C, Lapras C. [Hemodynamic study of acute neurogenic pulmonary edema in children]. Pediatrie. 1993;48:805-812. [PubMed] |
| 43. | Campbell GS, Haddy FJ. Circulatory changes and pulmonary lesions in dogs following increased intracranial pressure, and the effect of atropine upon such changes. Am J Physiol. 1949;158:96-102. [PubMed] |
| 44. | Simmons RL, Ducker TB, Anderson RW. Pathogenesis of pulmonary edema following head trauma. An experimental study. J Trauma. 1968;8:800-811. [PubMed] |
| 45. | Bean JW, Beckman DL. Centrogenic pulmonary pathology in mechanical head injury. J Appl Physiol. 1969;27:807-812. [PubMed] |
| 46. | Maire FW, Patton HD. Neural structures involved in the genesis of preoptic pulmonary edema, gastric erosions and behavior changes. Am J Physiol. 1956;184:345-350. [PubMed] |
| 47. | Chen HI, Liao JF, Kuo L, Ho ST. Centrogenic pulmonary hemorrhagic edema induced by cerebral compression in rats. Mechanism of volume and pressure loading in the pulmonary circulation. Circ Res. 1980;47:366-373. [PubMed] |
| 48. | Chen HI, Shih WJ, Chen TP. A scintiphotographic study of pulmonary edema and hemorrhage induced by cerebral compression and norepinephrine. Chin J Physiol. 1976;22:65-72. [PubMed] |
| 49. | Chen HI, Lin JD, Liao JF. Participation of regional sympathetic outflows in the centrogenic pulmonary pathology. Am J Physiol. 1981;240:H109-H115. [PubMed] |
| 50. | Chen HI, Wang YC, Chai CY. The Cushing responses in the systemic and pulmonary circulation: the role of adrenal glands, bronchial circulation and pulmonary innervation. Chin J Physiol. 1987;30:29-43. [PubMed] |
| 51. | Wang D, Chou CL, Hsu K, Chen HI. Cyclooxygenase pathway mediates lung injury induced by phorbol and platelets. J Appl Physiol. 1991;70:2417-2421. [PubMed] |
| 52. | Wang D, Li MH, Hsu K, Shen CY, Chen HI, Lin YC. Air embolism-induced lung injury in isolated rat lungs. J Appl Physiol. 1992;72:1235-1242. [PubMed] |
| 53. | Hsu K, Wang D, Wu SY, Shen CY, Chen HI. Ischemia-reperfusion lung injury attenuated by ATP-MgCl2 in rats. J Appl Physiol. 1994;76:545-552. [PubMed] |
| 54. | Wang D, Hsu K, Hwang CP, Chen HI. Measurement of nitric oxide release in the isolated perfused rat lung. Biochem Biophys Res Commun. 1995;208:1016-1020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 55. | Hsu K, Wang D, Chang ML, Wu CP, Chen HI. Pulmonary edema induced by phorbol myristate acetate is attenuated by compounds that increase intracellular cAMP. Res Exp Med (Berl). 1996;196:17-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 56. | Wang D, Wei J, Hsu K, Jau J, Lieu MW, Chao TJ, Chen HI. Effects of nitric oxide synthase inhibitors on systemic hypotension, cytokines and inducible nitric oxide synthase expression and lung injury following endotoxin administration in rats. J Biomed Sci. 1999;6:28-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 57. | Kao SJ, Wei J, Hwang CP, Lieu MW, Jiang JS, Wang D, Chen HI. Modulatory effect of blood cells on hypoxic vasoconstriction response and nitric oxide release in rat lungs. J Formos Med Assoc. 1999;98:39-44. [PubMed] |
| 58. | Lee RP, Wang D, Kao SJ, Chen HI. The lung is the major site that produces nitric oxide to induce acute pulmonary oedema in endotoxin shock. Clin Exp Pharmacol Physiol. 2001;28:315-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 61] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 59. | Huang KL, Shaw KP, Wang D, Hsu K, Huang TS, Chen HI. Free radicals mediate amphetamine-induced acute pulmonary edema in isolated rat lung. Life Sci. 2002;71:1237-1244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 60. | Lin HI, Chu SJ, Wang D, Chen HI, Hsu K. Effects of an endogenous nitric oxide synthase inhibitor on phorbol myristate acetate-induced acute lung injury in rats. Clin Exp Pharmacol Physiol. 2003;30:393-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 61. | Kao SJ, Peng TC, Lee RP, Hsu K, Chen CF, Hung YK, Wang D, Chen HI. Nitric oxide mediates lung injury induced by ischemia-reperfusion in rats. J Biomed Sci. 2003;10:58-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 62. | Chen HI, Yeh DY, Liou HL, Kao SJ. Insulin attenuates endotoxin-induced acute lung injury in conscious rats. Crit Care Med. 2006;34:758-764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 53] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 63. | Kao SJ, Wang D, Lin HI, Chen HI. N-acetylcysteine abrogates acute lung injury induced by endotoxin. Clin Exp Pharmacol Physiol. 2006;33:33-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 48] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 64. | Chen HI, Hsieh SY, Yang FL, Hsu YH, Lin CC. Exercise training attenuates septic responses in conscious rats. Med Sci Sports Exerc. 2007;39:435-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 45] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 65. | Su CF, Yang FL, Chen HI. Inhibition of inducible nitric oxide synthase attenuates acute endotoxin-induced lung injury in rats. Clin Exp Pharmacol Physiol. 2007;34:339-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 51] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 66. | Kao SJ, Su CF, Liu DD, Chen HI. Endotoxin-induced acute lung injury and organ dysfunction are attenuated by pentobarbital anaesthesia. Clin Exp Pharmacol Physiol. 2007;34:480-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 67. | Su CF, Liu DD, Kao SJ, Chen HI. Nicotinamide abrogates acute lung injury caused by ischaemia/reperfusion. Eur Respir J. 2007;30:199-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 32] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 68. | Chu CH, David Liu D, Hsu YH, Lee KC, Chen HI. Propofol exerts protective effects on the acute lung injury induced by endotoxin in rats. Pulm Pharmacol Ther. 2007;20:503-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 38] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 69. | Chuang IC, Liu DD, Kao SJ, Chen HI. N-acetylcysteine attenuates the acute lung injury caused by phorbol myristate acetate in isolated rat lungs. Pulm Pharmacol Ther. 2007;20:726-733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 70. | Liu DD, Hsu YH, Chen HI. Endotoxin-induced acute lung injury is enhanced in rats with spontaneous hypertension. Clin Exp Pharmacol Physiol. 2007;34:61-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 71. | Liu YC, Kao SJ, Chuang IC, Chen HI. Nitric oxide modulates air embolism-induced lung injury in rats with normotension and hypertension. Clin Exp Pharmacol Physiol. 2007;34:1173-1180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 72. | Kao SJ, Liu DD, Su CF, Chen HI. Niacinamide abrogates the organ dysfunction and acute lung injury caused by endotoxin. J Cardiovasc Pharmacol. 2007;50:333-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 73. | Moncada S, Palmer RM, Higgs EA. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991;43:109-142. [PubMed] |
| 74. | Chen HI, Hu CT. Endogenous nitric oxide on arterial hemodynamics: a comparison between normotensive and hypertensive rats. Am J Physiol. 1997;273:H1816-H1823. [PubMed] |
| 75. | Hu CT, Chang KC, Wu CY, Chen HI. Acute effects of nitric oxide blockade with L-NAME on arterial haemodynamics in the rat. Br J Pharmacol. 1997;122:1237-1243. [PubMed] |
| 76. | Bone RC, Grodzin CJ, Balk RA. Sepsis: a new hypothesis for pathogenesis of the disease process. Chest. 1997;112:235-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 620] [Cited by in RCA: 565] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 77. | Parrillo JE. Pathogenetic mechanisms of septic shock. N Engl J Med. 1993;328:1471-1477. [PubMed] |
| 78. | Wheeler AP, Bernard GR. Treating patients with severe sepsis. N Engl J Med. 1999;340:207-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 719] [Cited by in RCA: 658] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 79. | Szabó C, Mitchell JA, Thiemermann C, Vane JR. Nitric oxide-mediated hyporeactivity to noradrenaline precedes the induction of nitric oxide synthase in endotoxin shock. Br J Pharmacol. 1993;108:786-792. [PubMed] |
| 80. | Hsu YH, Kao SJ, Lee RP, Chen HI. Acute pulmonary oedema: rare causes and possible mechanisms. Clin Sci (Lond). 2003;104:259-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 81. | Hsu YH, Chen HI. The involvement of nitric oxide and beta-adrenergic pathway signalling in pulmonary oedema and fluid clearance. Pathology. 2007;39:612-613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 82. | Hsu YH, Chen HI. Pulmonary pathology in patients associated with scrub typhus. Pathology. 2008;40:268-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 83. | Hsu YH, Chen HI. Acute respiratory distress syndrome associated with rabies. Pathology. 2008;40:647-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 84. | Holmes F, Harlan J, Felt S, Ruhlen J, Murphy B. Letter: Pulmonary oedema in hypercalcaemic crisis. Lancet. 1974;1:311-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 85. | Chen HI, Yeh DY, Kao SJ. The detrimental role of inducible nitric oxide synthase in the pulmonary edema caused by hypercalcemia in conscious rats and isolated lungs. J Biomed Sci. 2008;15:227-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 86. | Ischiropoulos H, Mendiguren I, Fisher D, Fisher AB, Thom SR. Role of neutrophils and nitric oxide in lung alveolar injury from smoke inhalation. Am J Respir Crit Care Med. 1994;150:337-341. [PubMed] |
| 87. | Hinder F, Meyer J, Booke M, Ehardt JS, Salsbury JR, Traber LD, Traber DL. Endogenous nitric oxide and the pulmonary microvasculature in healthy sheep and during systemic inflammation. Am J Respir Crit Care Med. 1998;157:1542-1549. [PubMed] |
| 88. | Kristof AS, Goldberg P, Laubach V, Hussain SN. Role of inducible nitric oxide synthase in endotoxin-induced acute lung injury. Am J Respir Crit Care Med. 1998;158:1883-1889. [PubMed] |
| 89. | Evgenov OV, Hevroy O, Bremnes KE, Bjertnaes LJ. Effect of aminoguanidine on lung fluid filtration after endotoxin in awake sheep. Am J Respir Crit Care Med. 2000;162:465-470. [PubMed] |
| 90. | Inoue H, Aizawa H, Nakano H, Matsumoto K, Kuwano K, Nadel JA, Hara N. Nitric oxide synthase inhibitors attenuate ozone-induced airway inflammation in guinea pigs. Possible role of interleukin-8. Am J Respir Crit Care Med. 2000;161:249-256. [PubMed] |
| 91. | Cuzzocrea S, Mazzon E, Calabro G, Dugo L, De Sarro A, van De LOO FA, Caputi AP. Inducible nitric oxide synthase-knockout mice exhibit resistance to pleurisy and lung injury caused by carrageenan. Am J Respir Crit Care Med. 2000;162:1859-1866. [PubMed] |
| 92. | Wang le F, Patel M, Razavi HM, Weicker S, Joseph MG, McCormack DG, Mehta S. Role of inducible nitric oxide synthase in pulmonary microvascular protein leak in murine sepsis. Am J Respir Crit Care Med. 2002;165:1634-1639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 144] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 93. | Agorreta J, Garayoa M, Montuenga LM, Zulueta JJ. Effects of acute hypoxia and lipopolysaccharide on nitric oxide synthase-2 expression in acute lung injury. Am J Respir Crit Care Med. 2003;168:287-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 94. | Razavi HM, Wang le F, Weicker S, Rohan M, Law C, McCormack DG, Mehta S. Pulmonary neutrophil infiltration in murine sepsis: role of inducible nitric oxide synthase. Am J Respir Crit Care Med. 2004;170:227-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 130] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 95. | Hesse AK, Dörger M, Kupatt C, Krombach F. Proinflammatory role of inducible nitric oxide synthase in acute hyperoxic lung injury. Respir Res. 2004;5:11. [PubMed] |
| 96. | Genovese T, Cuzzocrea S, Di Paola R, Failla M, Mazzon E, Sortino MA, Frasca G, Gili E, Crimi N, Caputi AP. Inhibition or knock out of inducible nitric oxide synthase result in resistance to bleomycin-induced lung injury. Respir Res. 2005;6:58. [PubMed] |
| 97. | Kao SJ, Chen HI. Nitric oxide mediates acute lung injury caused by fat embolism in isolated rat's lungs. J Trauma. 2008;64:462-469. [PubMed] |
| 98. | Liu DD, Kao SJ, Chen HI. N-acetylcysteine attenuates acute lung injury induced by fat embolism. Crit Care Med. 2008;36:565-571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 99. | Chen HI, Hsieh NK, Kao SJ, Su CF. Protective effects of propofol on acute lung injury induced by oleic acid in conscious rats. Crit Care Med. 2008;36:1214-1221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 52] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 100. | Yang YL, Huang KL, Liou HL, Chen HI. The involvement of nitric oxide, nitric oxide synthase, neutrophil elastase, myeloperoxidase and proinflammatory cytokines in the acute lung injury caused by phorbol myristate acetate. J Biomed Sci. 2008;15:499-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 101. | Lin NT, Yang FL, Lee RP, Peng TC, Chen HI. Inducible nitric oxide synthase mediates cytokine release: the time course in conscious and septic rats. Life Sci. 2006;78:1038-1043. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 102. | Lee RP, Wang D, Lin NT, Chen HI. Physiological and chemical indicators for early and late stages of sepsis in conscious rats. J Biomed Sci. 2002;9:613-621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 103. | Fabian TC. Unravelling the fat embolism syndrome. N Engl J Med. 1993;329:961-963. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 69] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 104. | Goldhaber SZ. Pulmonary embolism. Lancet. 2004;363:1295-1305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 264] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 105. | Sleiman C, Mal H, Fournier M, Duchatelle JP, Icard P, Groussard O, Jebrak G, Mollo JL, Raffy O, Roue C. Pulmonary reimplantation response in single-lung transplantation. Eur Respir J. 1995;8:5-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 45] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 106. | Levinson RM, Shure D, Moser KM. Reperfusion pulmonary edema after pulmonary artery thromboendarterectomy. Am Rev Respir Dis. 1986;134:1241-1245. [PubMed] |
| 107. | Ward BJ, Pearse DB. Reperfusion pulmonary edema after thrombolytic therapy of massive pulmonary embolism. Am Rev Respir Dis. 1988;138:1308-1311. [PubMed] |
| 108. | Marik PE. Aspiration pneumonitis and aspiration pneumonia. N Engl J Med. 2001;344:665-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1050] [Cited by in RCA: 949] [Article Influence: 39.5] [Reference Citation Analysis (0)] |
| 109. | Olsson GL, Hallen B, Hambraeus-Jonzon K. Aspiration during anaesthesia: a computer-aided study of 185,358 anaesthetics. Acta Anaesthesiol Scand. 1986;30:84-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 382] [Cited by in RCA: 337] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 110. | Warner MA, Warner ME, Weber JG. Clinical significance of pulmonary aspiration during the perioperative period. Anesthesiology. 1993;78:56-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 561] [Cited by in RCA: 486] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 111. | Milberg JA, Davis DR, Steinberg KP, Hudson LD. Improved survival of patients with acute respiratory distress syndrome (ARDS): 1983-1993. JAMA. 1995;273:306-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 86] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 112. | Zilberberg MD, Epstein SK. Acute lung injury in the medical ICU: comorbid conditions, age, etiology, and hospital outcome. Am J Respir Crit Care Med. 1998;157:1159-1164. [PubMed] |
| 113. | Safdar Z, Yiming M, Grunig G, Bhattacharya J. Inhibition of acid-induced lung injury by hyperosmolar sucrose in rats. Am J Respir Crit Care Med. 2005;172:1002-1007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 114. | Davidson BA, Knight PR, Wang Z, Chess PR, Holm BA, Russo TA, Hutson A, Notter RH. Surfactant alterations in acute inflammatory lung injury from aspiration of acid and gastric particulates. Am J Physiol Lung Cell Mol Physiol. 2005;288:L699-L708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 57] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 115. | Brackenbury AM, Puligandla PS, McCaig LA, Nikore V, Yao LJ, Veldhuizen RA, Lewis JF. Evaluation of exogenous surfactant in HCL-induced lung injury. Am J Respir Crit Care Med. 2001;163:1135-1142. [PubMed] |
| 116. | Vadász I, Morty RE, Kohstall MG, Olschewski A, Grimminger F, Seeger W, Ghofrani HA. Oleic acid inhibits alveolar fluid reabsorption: a role in acute respiratory distress syndrome. Am J Respir Crit Care Med. 2005;171:469-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 60] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 117. | de Abreu MG, Quelhas AD, Spieth P, Bräuer G, Knels L, Kasper M, Pino AV, Bleyl JU, Hübler M, Bozza F. Comparative effects of vaporized perfluorohexane and partial liquid ventilation in oleic acid-induced lung injury. Anesthesiology. 2006;104:278-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 118. | Chu SJ, Chang DM, Wang D, Hsu K, Chiang CH. Protective effect of lipophilic antioxidants on phorbol-induced acute lung injury in rats. Crit Care Med. 2001;29:819-824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 119. | Creamer KM, McCloud LL, Fisher LE, Ehrhart IC. Pentoxifylline rescue preserves lung function in isolated canine lungs injured with phorbol myristate acetate. Chest. 2001;119:1893-1900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 120. | Kuraki T, Ishibashi M, Takayama M, Shiraishi M, Yoshida M. A novel oral neutrophil elastase inhibitor (ONO-6818) inhibits human neutrophil elastase-induced emphysema in rats. Am J Respir Crit Care Med. 2002;166:496-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 51] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 121. | Koshika T, Ishizaka A, Nagatomi I, Sudo Y, Hasegawa N, Goto T. Pretreatment with FK506 improves survival rate and gas exchange in canine model of acute lung injury. Am J Respir Crit Care Med. 2001;163:79-84. [PubMed] |
| 122. | Murakami K, Cox RA, Hawkins HK, Schmalstieg FC, McGuire RW, Jodoin JM, Traber LD, Traber DL. Cepharanthin, an alkaloid from Stephania cepharantha, inhibits increased pulmonary vascular permeability in an ovine model of sepsis. Shock. 2003;20:46-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 123. | Kinoshita M, Ono S, Mochizuki H. Neutrophils mediate acute lung injury in rabbits: role of neutrophil elastase. Eur Surg Res. 2000;32:337-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 47] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 124. | Abraham E. Neutrophils and acute lung injury. Crit Care Med. 2003;31:S195-S199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 612] [Cited by in RCA: 676] [Article Influence: 30.7] [Reference Citation Analysis (1)] |
| 125. | Bursten SL, Federighi DA, Parsons P, Harris WE, Abraham E, Moore EE, Moore FA, Bianco JA, Singer JW, Repine JE. An increase in serum C18 unsaturated free fatty acids as a predictor of the development of acute respiratory distress syndrome. Crit Care Med. 1996;24:1129-1136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 69] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 126. | Quinlan GJ, Lamb NJ, Evans TW, Gutteridge JM. Plasma fatty acid changes and increased lipid peroxidation in patients with adult respiratory distress syndrome. Crit Care Med. 1996;24:241-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 90] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 127. | Schmidt R, Meier U, Yabut-Perez M, Walmrath D, Grimminger F, Seeger W, Günther A. Alteration of fatty acid profiles in different pulmonary surfactant phospholipids in acute respiratory distress syndrome and severe pneumonia. Am J Respir Crit Care Med. 2001;163:95-100. [PubMed] |
| 128. | Günther A, Schmidt R, Harodt J, Schmehl T, Walmrath D, Ruppert C, Grimminger F, Seeger W. Bronchoscopic administration of bovine natural surfactant in ARDS and septic shock: impact on biophysical and biochemical surfactant properties. Eur Respir J. 2002;19:797-804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 47] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 129. | Pratt PC, Vollmer RT, Shelburne JD, Crapo JD. Pulmonary morphology in a multihospital collaborative extracorporeal membrane oxygenation project. I. Light microscopy. Am J Pathol. 1979;95:191-214. [PubMed] |
| 130. | Ricard JD, Dreyfuss D, Saumon G. Ventilator-induced lung injury. Eur Respir J Suppl. 2003;42:2s-9s. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 149] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 131. | Vlahakis NE, Hubmayr RD. Cellular stress failure in ventilator-injured lungs. Am J Respir Crit Care Med. 2005;171:1328-1342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 144] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 132. | Piantadosi CA, Schwartz DA. The acute respiratory distress syndrome. Ann Intern Med. 2004;141:460-470. [PubMed] |
| 133. | Lee WL, Downey GP. Leukocyte elastase: physiological functions and role in acute lung injury. Am J Respir Crit Care Med. 2001;164:896-904. [PubMed] |
| 134. | Yoshimura K, Nakagawa S, Koyama S, Kobayashi T, Homma T. Roles of neutrophil elastase and superoxide anion in leukotriene B4-induced lung injury in rabbit. J Appl Physiol. 1994;76:91-96. [PubMed] |
| 135. | Puneet P, Moochhala S, Bhatia M. Chemokines in acute respiratory distress syndrome. Am J Physiol Lung Cell Mol Physiol. 2005;288:L3-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 233] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 136. | Fan J, Ye RD, Malik AB. Transcriptional mechanisms of acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2001;281:L1037-L1050. [PubMed] |
| 137. | Sartori C, Matthay MA. Alveolar epithelial fluid transport in acute lung injury: new insights. Eur Respir J. 2002;20:1299-1313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 86] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 138. | Mutlu GM, Sznajder JI. Mechanisms of pulmonary edema clearance. Am J Physiol Lung Cell Mol Physiol. 2005;289:L685-L695. [PubMed] |
| 139. | Hubmayr RD. Perspective on lung injury and recruitment: a skeptical look at the opening and collapse story. Am J Respir Crit Care Med. 2002;165:1647-1653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 211] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 140. | Angus D, Ishizaka A, Matthay M, Lemaire F, Macnee W, Abraham E. Critical care in AJRCCM 2004. Am J Respir Crit Care Med. 2005;171:537-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 141. | Mols G, Priebe HJ, Guttmann J. Alveolar recruitment in acute lung injury. Br J Anaesth. 2006;96:156-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 71] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 142. | Galiatsou E, Kostanti E, Svarna E, Kitsakos A, Koulouras V, Efremidis SC, Nakos G. Prone position augments recruitment and prevents alveolar overinflation in acute lung injury. Am J Respir Crit Care Med. 2006;174:187-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 158] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 143. | Laffey JG, Honan D, Hopkins N, Hyvelin JM, Boylan JF, McLoughlin P. Hypercapnic acidosis attenuates endotoxin-induced acute lung injury. Am J Respir Crit Care Med. 2004;169:46-56. [PubMed] |
| 144. | Broccard AF, Hotchkiss JR, Vannay C, Markert M, Sauty A, Feihl F, Schaller MD. Protective effects of hypercapnic acidosis on ventilator-induced lung injury. Am J Respir Crit Care Med. 2001;164:802-806. [PubMed] |
| 145. | Ni Chonghaile M, Higgins B, Laffey JG. Permissive hypercapnia: role in protective lung ventilatory strategies. Curr Opin Crit Care. 2005;11:56-62. [PubMed] |
| 146. | Alsaghir AH, Martin CM. Effect of prone positioning in patients with acute respiratory distress syndrome: a meta-analysis. Crit Care Med. 2008;36:603-609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 79] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 147. | Lang JD, Figueroa M, Sanders KD, Aslan M, Liu Y, Chumley P, Freeman BA. Hypercapnia via reduced rate and tidal volume contributes to lipopolysaccharide-induced lung injury. Am J Respir Crit Care Med. 2005;171:147-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 70] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 148. | Feihl F, Eckert P, Brimioulle S, Jacobs O, Schaller MD, Mélot C, Naeije R. Permissive hypercapnia impairs pulmonary gas exchange in the acute respiratory distress syndrome. Am J Respir Crit Care Med. 2000;162:209-215. [PubMed] |
| 149. | Calfee CS, Matthay MA. Nonventilatory treatments for acute lung injury and ARDS. Chest. 2007;131:913-920. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 155] [Cited by in RCA: 132] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 150. | Fan E, Needham DM, Stewart TE. Ventilatory management of acute lung injury and acute respiratory distress syndrome. JAMA. 2005;294:2889-2896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 130] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 151. | Hite RD, Morris PE. Acute respiratory distress syndrome: pharmacological treatment options in development. Drugs. 2001;61:897-907. [PubMed] |
| 152. | Brower RG, Ware LB, Berthiaume Y, Matthay MA. Treatment of ARDS. Chest. 2001;120:1347-1367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 129] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 153. | Moloney ED, Evans TW. Pathophysiology and pharmacological treatment of pulmonary hypertension in acute respiratory distress syndrome. Eur Respir J. 2003;21:720-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 77] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 154. | Griffiths MJ, Evans TW. Inhaled nitric oxide therapy in adults. N Engl J Med. 2005;353:2683-2695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 260] [Cited by in RCA: 243] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 155. | Mura M, dos Santos CC, Stewart D, Liu M. Vascular endothelial growth factor and related molecules in acute lung injury. J Appl Physiol. 2004;97:1605-1617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 140] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 156. | Imai Y, Kuba K, Rao S, Huan Y, Guo F, Guan B, Yang P, Sarao R, Wada T, Leong-Poi H. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature. 2005;436:112-116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1791] [Cited by in RCA: 1998] [Article Influence: 99.9] [Reference Citation Analysis (0)] |
| 157. | Aitkenhead AR, Pepperman ML, Willatts SM, Coates PD, Park GR, Bodenham AR, Collins CH, Smith MB, Ledingham IM, Wallace PG. Comparison of propofol and midazolam for sedation in critically ill patients. Lancet. 1989;2:704-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 148] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 158. | Bryson HM, Fulton BR, Faulds D. Propofol. An update of its use in anaesthesia and conscious sedation. Drugs. 1995;50:513-559. [PubMed] |
| 159. | Yang FL, Li CH, Hsu BG, Tsai NM, Lin SZ, Harn HJ, Chen HI, Liao KW, Lee RP. The reduction of tumor necrosis factor-alpha release and tissue damage by pentobarbital in the experimental endotoxemia model. Shock. 2007;28:309-316. [PubMed] |
| 160. | Hsu BG, Yang FL, Lee RP, Peng TC, Harn HJ, Chen HI. N-acetylcysteine ameliorates lipopolysaccharide-induced organ damage in conscious rats. J Biomed Sci. 2004;11:152-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 161. | Hsu BG, Lee RP, Yang FL, Harn HJ, Chen HI. Post-treatment with N-acetylcysteine ameliorates endotoxin shock-induced organ damage in conscious rats. Life Sci. 2006;79:2010-2016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 78] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 162. | Molnár Z, Shearer E, Lowe D. N-Acetylcysteine treatment to prevent the progression of multisystem organ failure: a prospective, randomized, placebo-controlled study. Crit Care Med. 1999;27:1100-1104. [PubMed] |
| 163. | Berger MM, Chioléro RL. Antioxidant supplementation in sepsis and systemic inflammatory response syndrome. Crit Care Med. 2007;35:S584-S590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 128] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 164. | Molnár Z. N-acetylcysteine as the magic bullet: too good to be true. Crit Care Med. 2008;36:645-646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |