Copyright
©The Author(s) 2025.
World J Crit Care Med. Sep 9, 2025; 14(3): 102577
Published online Sep 9, 2025. doi: 10.5492/wjccm.v14.i3.102577
Published online Sep 9, 2025. doi: 10.5492/wjccm.v14.i3.102577
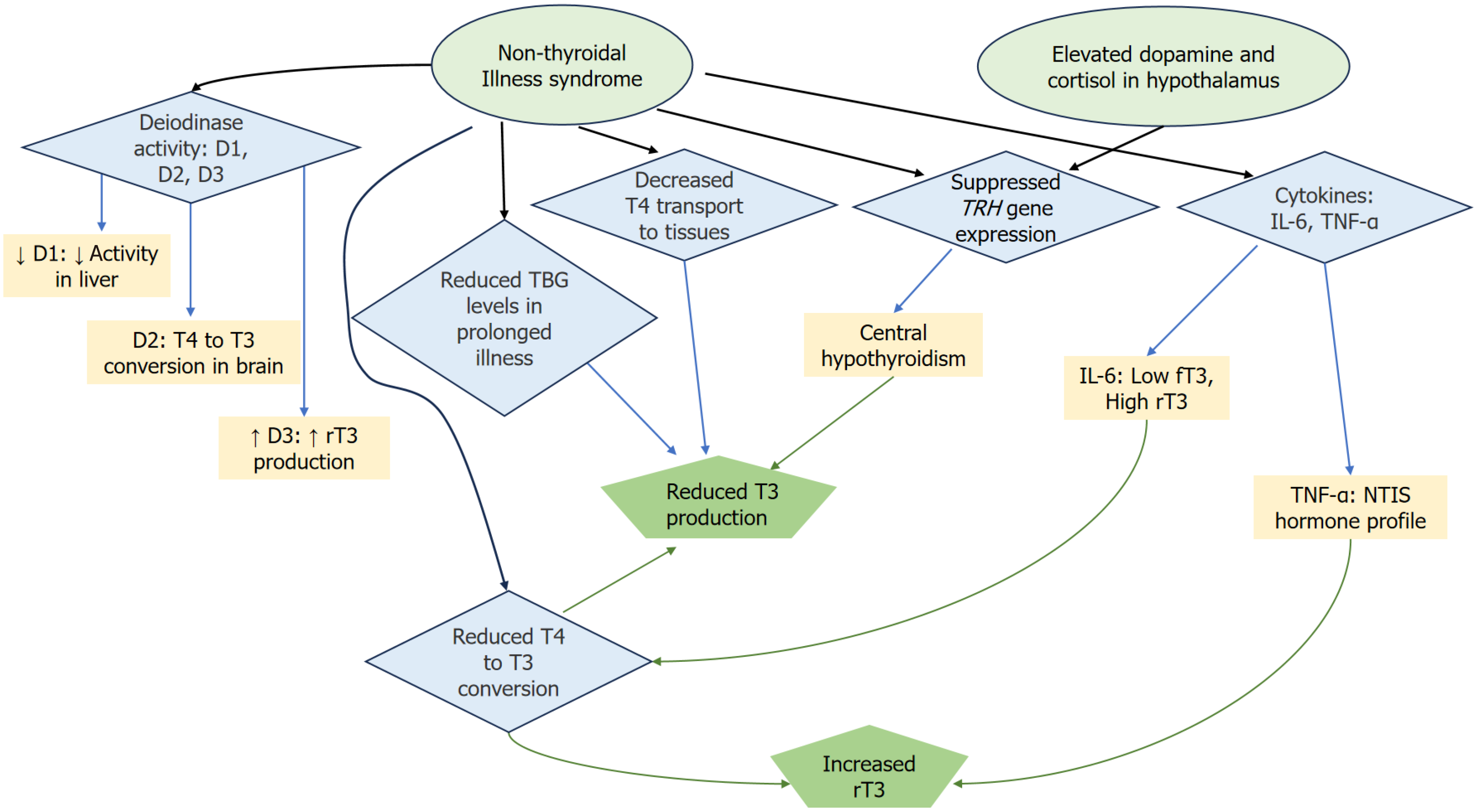
Figure 1 Outline of the pathophysiology of nonthyroidal illness syndrome, focusing on the interactions between deiodinases, cytokines, and hormonal regulation.
Reduced D1 activity in the liver leads to decreased thyroxine (T4) to triiodothyronine (T3) conversion, while D3 increases the production of reverse T3 (rT3). However, D2 in the brain remains active, allowing local T4 to T3 conversion and maintaining brain T3 levels, despite systemic disruptions. Elevated levels of dopamine and cortisol in the hypothalamus lead to the suppression of TRH gene expression, which in turn contributes to central hypothyroidism. The diagram also highlights the role of cytokines, particularly interleukin (IL)-6 and tumor necrosis factor-alpha (TNF-α), in disrupting thyroid hormone metabolism. IL-6 negatively correlates with free T3 and positively with rT3 levels, while TNF-α reproduces the typical hormone profile of NTIS when introduced into healthy individuals. Additionally, a reduction in T4 transport to tissues and lowered thyroxine-binding globulin levels (reduced T4 and T3 transport, impaired hormone availability) further exacerbate the hormonal imbalance during prolonged illness. Together, these mechanisms contribute to the complex hormonal dysregulation seen in nonthyroidal illness syndrome. rT3: Reverse T3; IL: Interleukin; TNF-α: Tumor necrosis factor-alpha; NTIS: Nonthyroidal illness syndrome.
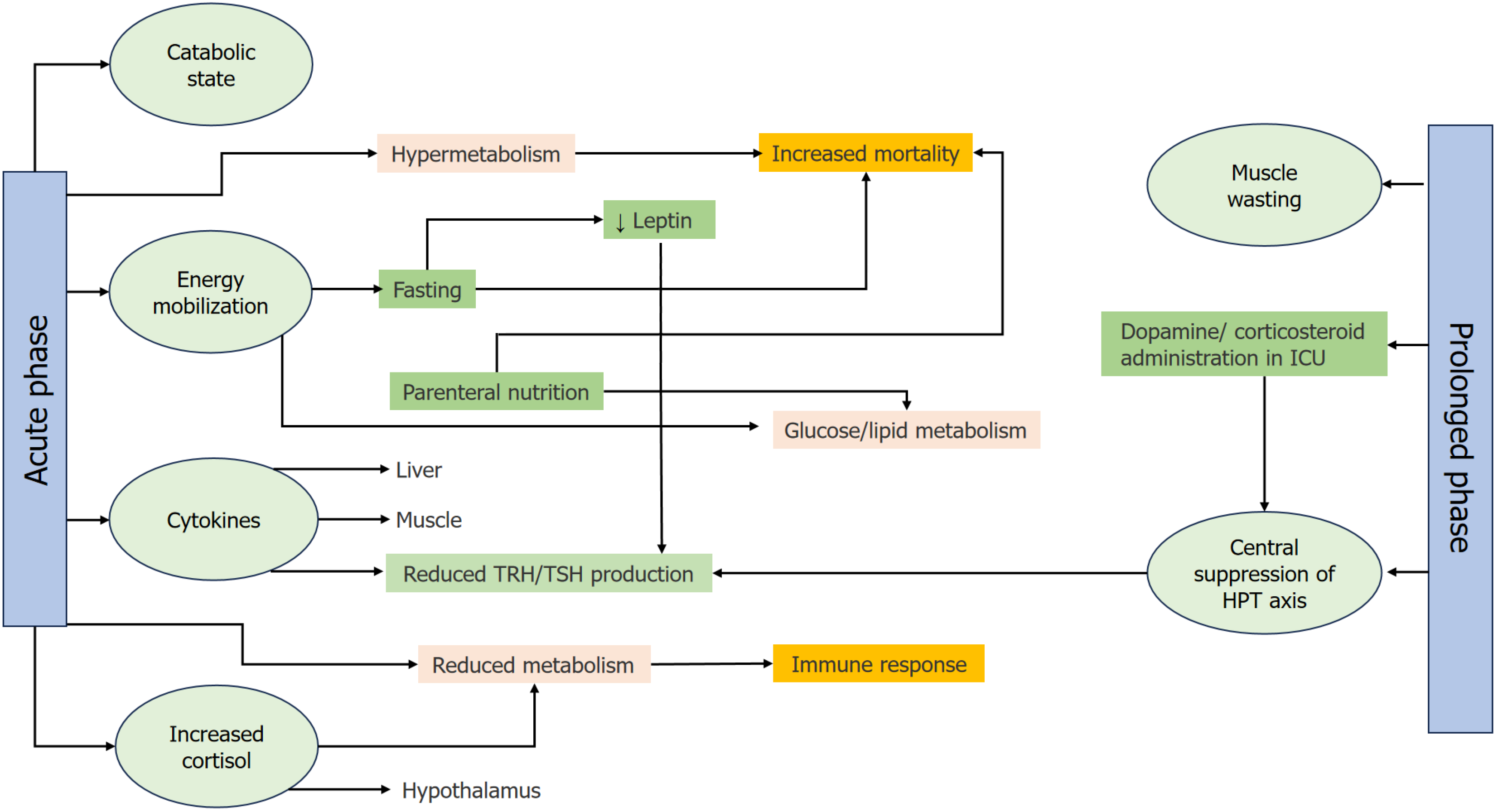
Figure 2 Τhe impact of nonthyroidal illness syndrome on metabolism and clinical outcomes.
The complex interplay of metabolic changes, hormonal suppression, immune function, and clinical interventions during nonthyroidal illness syndrome, which can significantly affect patient outcomes during critical illness. Acute phase: In the early stages of critical illness, the body enters a catabolic state, characterized by the breakdown of muscle protein and mobilization of energy stores (glycogen, muscle, and fat). This phase includes both hypermetabolism (increased energy expenditure) and reduced metabolism, with certain patients experiencing higher mortality if their metabolic response becomes excessive. These changes result in energy mobilization, where the body increases glucose and lipid metabolism to fuel essential processes. Cytokines, such as interleukin-6 and tumor necrosis factor-alpha, and cortisol are elevated during this phase, further influencing the suppression of the hypothalamus-pituitary-thyroid (HPT) axis. Central suppression of the HPT axis: The elevated levels of cytokines and cortisol feedback to the hypothalamus, reducing the secretion of thyrotropin-releasing hormone (TRH), which in turn lowers thyroid-stimulating hormone (TSH) levels [central suppression of the HPT axis, leading to reduced thyroid hormone (T3 and T4) production]. This suppression is critical as it slows down metabolism, possibly as an adaptive mechanism to conserve energy during the acute phase of illness. Fasting and Parenteral Nutrition: During the early acute phase, fasting can exacerbate the downregulation of the HPT axis. Lower leptin levels contribute to this suppression, further reducing TRH and TSH levels. On the other hand, parenteral nutrition influences the body's metabolic responses by potentially increasing mortality if not administered appropriately. Both fasting and parenteral nutrition affect glucose and lipid metabolism, shifting the body's energy balance. Prolonged phase: As the illness persists, patients may enter the prolonged phase, during which prolonged protein breakdown leads to muscle wasting. Central suppression of the HPT axis continues, with further reduction in TRH and TSH levels. This prolonged metabolic dysregulation leads to decreased mitochondrial function, impaired immune responses, and an overall decline in metabolic efficiency. As a result, muscle and liver tissues show increased sensitivity to circulating thyroid hormones due to changes in hormone transport and receptor activity. Clinical outcomes: Throughout both phases, the impact of NTIS on metabolism can lead to different clinical outcomes. Hypermetabolism in the acute phase is associated with increased mortality, especially when the body's energy demands exceed its capacity to mobilize stores. Conversely, reduced metabolism during the acute and prolonged phases may be adaptive in some cases but can also impair immune responses, making recovery more difficult. The interventions, such as fasting and nutritional support, must be carefully managed to optimize clinical outcomes and prevent adverse effects.
- Citation: Savvidis C, Ragia D, Kallistrou E, Kouroglou E, Tsiama V, Proikaki S, Belis K, Ilias I. Critical illness-implications of non-thyroidal illness syndrome and thyroxine therapy. World J Crit Care Med 2025; 14(3): 102577
- URL: https://www.wjgnet.com/2220-3141/full/v14/i3/102577.htm
- DOI: https://dx.doi.org/10.5492/wjccm.v14.i3.102577