Copyright
©The Author(s) 2021.
World J Crit Care Med. Sep 9, 2021; 10(5): 260-277
Published online Sep 9, 2021. doi: 10.5492/wjccm.v10.i5.260
Published online Sep 9, 2021. doi: 10.5492/wjccm.v10.i5.260
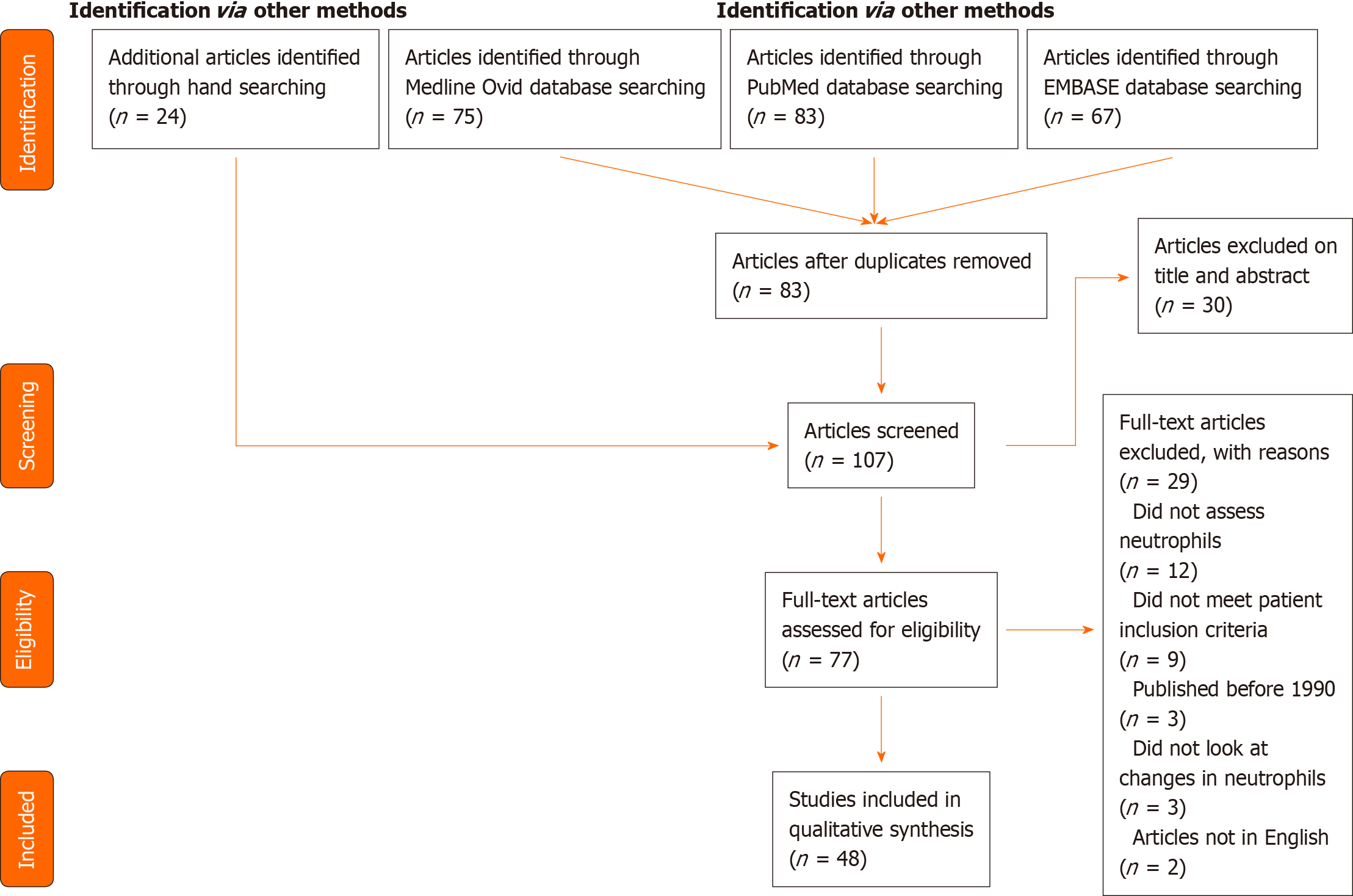
Figure 1 PRISMA diagram summarising included studies.
Further characteristics of each study are available in Table 1.
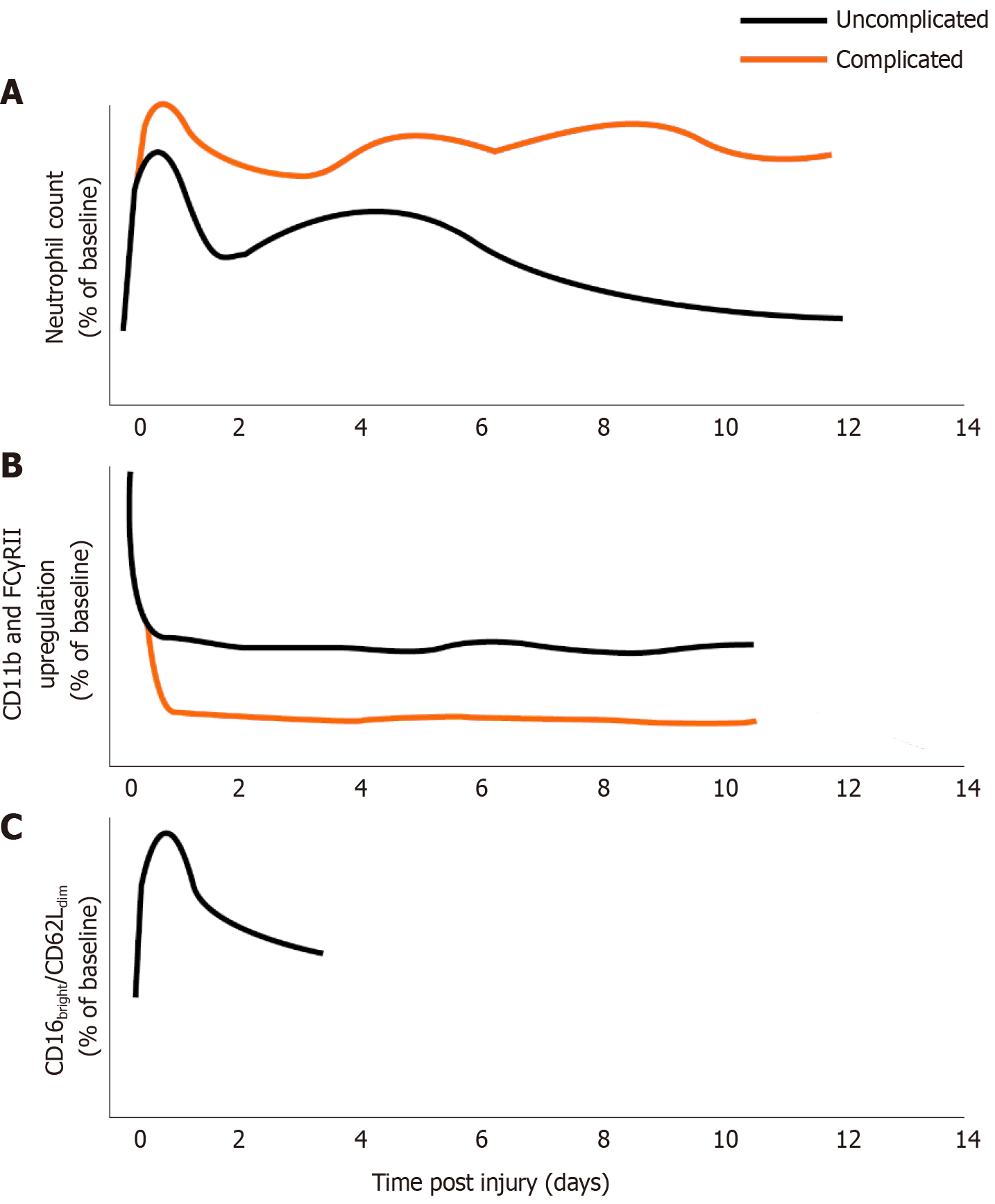
Figure 2 Schematical representation of neutrophil changes over time following major trauma, differentiated by complicated or uncomplicated clinical course.
Complicated course indicates subsequent sepsis or multiple organ dysfunction, where evidence exists in the literature. A: Neutrophil number post injury relative to pre-injury/control levels; B: Neutrophil responsiveness (CD11b or FC Gamma receptor II upregulation in response to fMLP) following injury relative to control; C: Immunosuppressive CD16bright/CD62Ldim neutrophils as percentage of total neutrophils relative to control. No data exist for the presence of these cells more than 4 d after trauma or in complicated/uncomplicated courses.
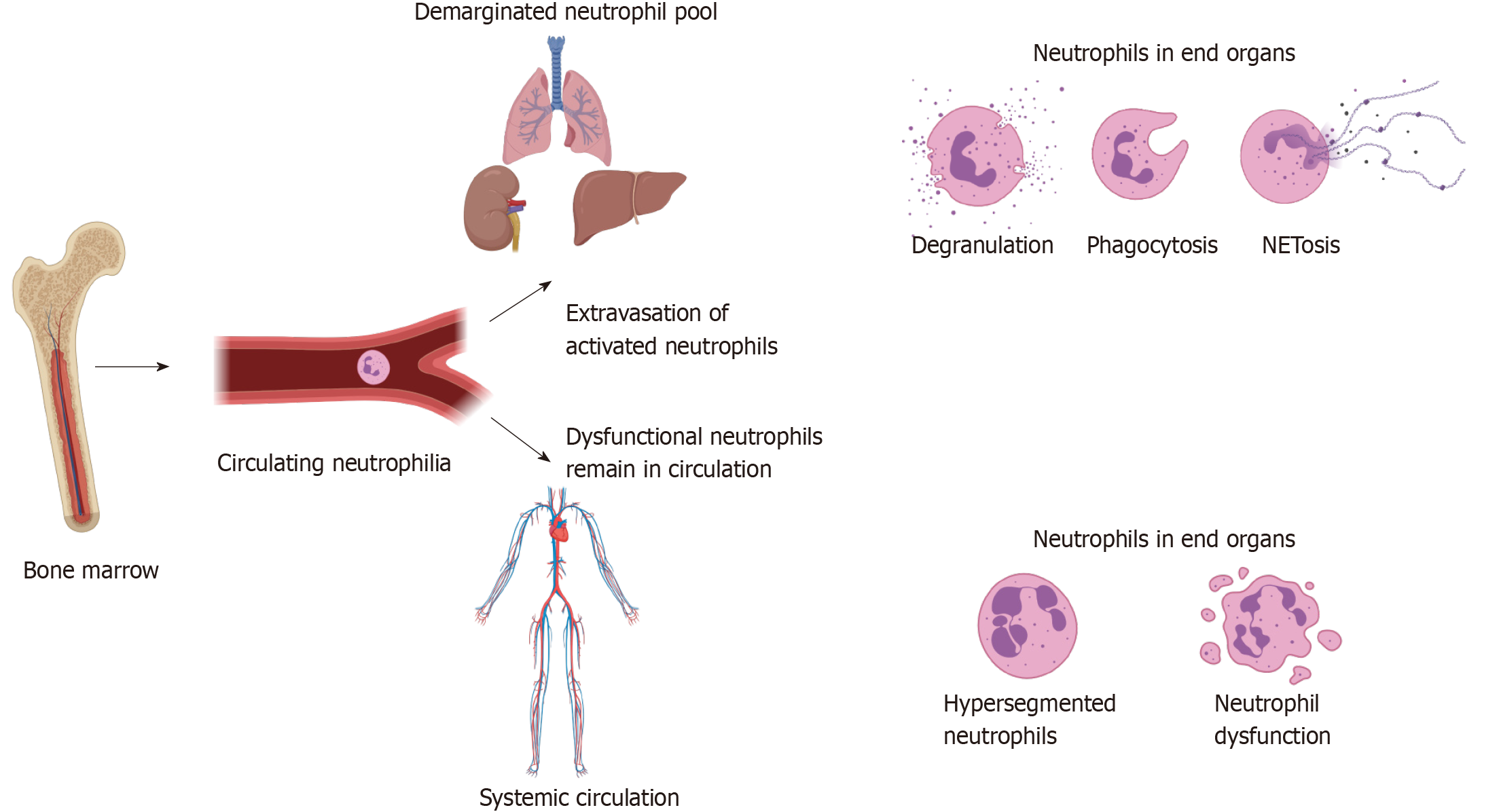
Figure 3 Neutrophil extravasation and resultant immune dysfunction.
Tissue damage caused by injury leads to danger associated molecular pattern, catecholamine and corticosteroid release, which result in neutrophil egress from the bone marrow, as well as increased production through emergency granulopoiesis. This leads to a circulating neutrophilia and altered phenotypes of circulating neutrophils as discussed in text. It is hypothesised that hyperinflammatory cells expressing high levels of adhesion markers transmigrate into the demarginated pool in the lungs, spleen, liver and other end organs, where they may cause further inflammation through NETosis, degranulation and phagocytosis, leading to organ dysfunction. The loss of these highly inflammatory cells from the circulation leads to remaining neutrophils being dysfunctional, predisposing the individual to immune failure and secondary infection. Figure produced using Biorender.
- Citation: Finlay LD, Conway Morris A, Deane AM, Wood AJ. Neutrophil kinetics and function after major trauma: A systematic review. World J Crit Care Med 2021; 10(5): 260-277
- URL: https://www.wjgnet.com/2220-3141/full/v10/i5/260.htm
- DOI: https://dx.doi.org/10.5492/wjccm.v10.i5.260