Published online Jul 28, 2015. doi: 10.5412/wjsp.v5.i2.208
Peer-review started: November 8, 2014
First decision: January 20, 2015
Revised: February 23, 2015
Accepted: March 30, 2015
Article in press: April 2, 2015
Published online: July 28, 2015
Processing time: 242 Days and 1.1 Hours
Acute type B aortic dissection (TBAD) occurs as a result of an intimal tear within the proximal thoracic aorta. Patients are typically managed acutely with aggressive antihypertensive therapy. Surgical repair is reserved for those who develop complications such as rupture or malperfusion. The surgical management of acute TBAD has changed considerably in the last decade secondary to the advent of thoracic stent grafting. Thoracic endovascular aortic repair (TEVAR) has improved early mortality and morbidity rates for patients presenting with complicated TBAD. The role of TEVAR in patients presenting with acute and subacute uncomplicated TBAD is less clear. TEVAR has been associated with increased late survival and better aortic remodeling, with low perioperative morbidity in selected patients. Recent literature suggests certain radiographic criteria may be used to predict patients developing late aortic events who would benefit from early TEVAR. The purpose of this article is to review the contemporary management of acute TBAD, discuss controversies in management and evaluate the latest research findings.
Core tip: Current recommendations and controversies within the surgical management of acute type B aortic dissection are discussed. The increased use of thoracic endovascular aortic repair has been associated with improved patient outcomes, though data on patients presenting with acute and subacute dissection is less clear. Certain radiographic findings may predict those at higher risk of developing late aortic-related complication.
- Citation: Iranmanesh S, Ricotta JJ. Current management of acute type B aortic dissection. World J Surg Proced 2015; 5(2): 208-216
- URL: https://www.wjgnet.com/2219-2832/full/v5/i2/208.htm
- DOI: https://dx.doi.org/10.5412/wjsp.v5.i2.208
Acute type B aortic dissection (TBAD) remains a complex clinical entity associated with a high rate of morbidity and mortality[1]. The majority of patients are able to be managed medically in the acute setting, though a subset of patients require acute surgical intervention. Open surgical therapy has traditionally been associated with high rates of in hospital death and morbidity. Surgical complications have been reduced by endovascular technology, specifically thoracic endovascular aortic repair (TEVAR). Unfortunately strong evidence is lacking regarding the optimal management of patients with acute TBAD. One of the difficulties in interpreting the literature on this topic involves the retrospective, single-institution nature of most studies. Few prospective, randomized trials exist to help guide vascular surgeons in selecting optimal management strategies. This paper will focus on reviewing the contemporary management of acute TBAD, controversies and future directions.
The primary etiology of TBAD is the separation of the layers of the aortic wall from each other, originating at a site known as the entry tear. This injury occurs within the intima at the proximal descending aorta, most often just distal to the origin of the left subclavian artery. A study of hemodynamic forces within the aortic arch by Nathan et al[2] demonstrates this area to be particularly susceptible to shear forces. This, in part, explains the frequency with which this location is involved. Microscopic analysis reveals that the dissection occurs into the media, functionally separating the intima from the adventitia. The “false lumen” (between the intima and adventitia) becomes pressurized, and, since the adventitia is stronger than the intima, the true lumen may become compressed. Compression of the true lumen may result in propagation of the dissection in a caudal (or occasionally cranial) direction and compromise of the distal branch arteries to the viscera, spinal cord or extremities. A novel ex vivo model for aortic dissection by Faure et al[3] highlights the spiral dissection plane that descends caudally. Often the celiac, superior mesenteric and right renal arteries originate from the true lumen while the left renal originates from the false lumen.
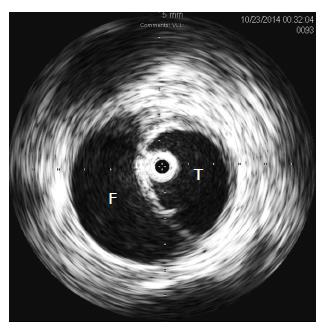
Symptoms from malperfusion may result from either static or dynamic obstruction. Static obstruction occurs when a highly pressurized false lumen dissects around, and circumferentially occludes, the orifice of a branch vessel. In contrast, dynamic obstruction occurs when a branch vessel orifice is occluded intermittently by extrinsic compression of the true lumen by pulsatile flow within the false lumen. This phenomenon is best observed using intravascular ultrasound (IVUS) to evaluate a patient with severe true lumen compression (Figure 1).
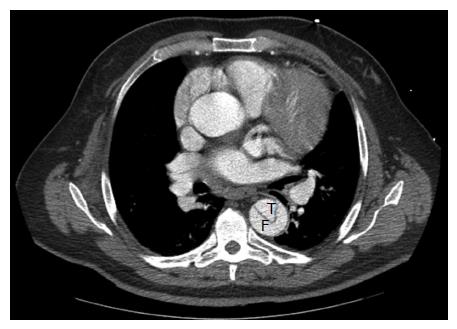
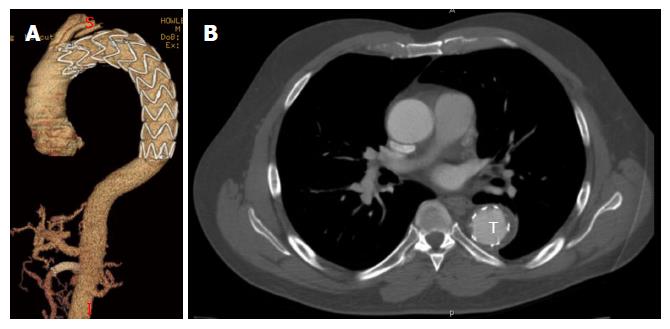
The initial presentation of dissection is that of tearing chest pain radiating to the back. This may be accompanied by symptoms of end-organ ischemia such as abdominal pain, oligo-anuria, lower extremity ischemia, paresis or paraplegia depending on the end organs involved. When malperfusion occurs, often several vascular territories are involved[4]. In the setting of rupture, patients may develop hypotension, abdominal distention or a left pleural effusion. Diagnosis is most commonly made by computed tomography angiography (CTA) or transesophageal echocardiography (TEE). TEE, when readily available, can identify the proximal entry tear and its origin. It is also effective in differentiating type A and type B dissections, and can assess cardiac function without the use of contrast or ionizing radiation. CTA, however, has the advantage of being readily available in most emergency rooms and is less operator dependent. It can also identify rupture, end organ ischemia, the extent of distal dissection and the relative size of the true and false lumens. For this reason CTA has emerged as the study of choice in acute TBAD[5] (Figures 2 and 3).
Medical management is critical for all patients with acute TBAD, whether or not surgery is performed. Initial management is focused on strict blood pressure and heart rate control. At our institution we favor initiation of anti-impulse therapy with a beta blocker followed by a vasodilator to prevent further propagation of the dissection and to manage the patient’s symptoms. These medications are best administered in a closely monitored unit aided by an arterial line and urinary catheter. Target systolic blood pressure goals include 110-120 mmHg, with heart rate goals between 60-80 BPM[5]. These targets may be lowered if the patient’s symptoms persist, as long as adequate perfusion as judged by urine output and mentation persists. Patients who respond to this regimen are transitioned to oral antihypertensive medications once their hypertension is controlled. Repeat imaging is typically performed prior to discharge and at regular outpatient intervals, evaluating for patency of the false lumen and aneurysmal degeneration. Established indications to proceed with operative intervention in the acute setting include: rupture, malperfusion, and persistent/refractory pain in the face of maximal medical therapy. The existence of one of these criteria is defined as complicated aortic dissection.
Estrera et al[6] evaluated 159 patients presenting with acute TBAD in a single center. In-hospital mortality for patients requiring only medical therapy (i.e., uncomplicated) was 7.3%. Complication rates in medically managed patients included rupture in 5%, stroke in 5%, spinal cord ischemia in 8.2%, mesenteric ischemia in 5.7%, dialysis dependence in 13.8%, and lower extremity ischemia in 3.8%. Survival at 1 year and 5 years was 83% and 75%, respectively. Approximately 14.5% of patients progressed to complicated aortic dissection requiring intervention; the in-hospital mortality for this cohort rose to 17%. Tsai et al[7] reviewed data from the multi-institution International Registry of Acute Aortic Dissection(IRAD). They identified a 10% in hospital mortality rate for patients receiving medical therapy alone. They reported a similar incidence of overall morbidities as Estrera et al[6] Approximately 11% of patients in that cohort required surgical intervention. In addition, they reported 1 year and 3 years survival rates for patients treated initially with medical therapy at 90.3% and 77.6%. These data show that the overwhelming majority of patients present with uncomplicated aortic dissection, and they can safely be managed medically. There is, however, a notable incidence of late aortic events and decline in survival in the medically managed patients after several years.
The goals of surgical management are to prevent or treat rupture and/or ischemia from vessel malperfusion. This can be accomplished in one of two ways: (1) sealing the entry tear to promote false lumen thrombosis; or (2) equalizing the pressure between the true and false lumen by fenestration of the dissection septum to prevent progression of the dissection and reestablish perfusion to compromised end organs. The choice of therapy depends on the clinical and anatomic presentation of the patient. Efforts at sealing the entry tear are most likely to cause false lumen thrombosis and restore distal perfusion through the true lumen when there is a relatively discrete entry tear with a highly pressurized false lumen. However, when a major branch vessel is perfused exclusively through the false lumen, successfully sealing the entry tear may induce ischemia in the territory that vessel supplies. This can result in renal, intestinal, extremity or spinal cord compromise. Furthermore, when multiple entry and re-entry tears are present, sealing the proximal entry tear alone often will not be sufficient to depressurize the false lumen. Our current diagnostic capabilities make it difficult to definitively predict when such conditions may occur and this uncertainty has tempered enthusiasm for surgery as a first approach.
The principle of fenestration is the opposite of that underlying entry tear coverage. The aim of this technique is to increase communication between the true and false lumen, equalizing pressures within them and stabilizing the dissection process. The technique seeks to create the situation that occurs in many TBADs that respond to medical management alone, i.e., equilibrium between true and false lumens. This technique is most often performed percutaneously and will be described under “endovascular approaches.” It is important to recognize that this technique does not “treat” dissection, only malperfusion, and cannot prevent rupture or late aneurysmal dilation of the dissected arterial segment.
Correction of malperfusion may require more than one approach. When the entry tear is sealed and the false lumen depressurized, dynamic malperfusion will be reversed. Equilibration of the pressure in the true and false lumens may also reverse dynamic obstruction. Therefore sealing the entry tear, or fenestration of the aorta may be all that is necessary in some cases. However when a static obstruction exists, flow must be restored by another means. When ischemia is restricted to the lower extremities this may be accomplished by extra-anatomic bypass without addressing the aortic dissection itself. However when ischemia persists after initial treatment of malperfusion, vascular reconstruction directed at the ischemic territory is required. When the viscera are involved this is most often done from and endovascular approach using self-expanding stents or covered stents, since aortovisceral bypass in these circumstances is hazardous. These will be discussed in more detail in the “endovascular management” section. When lower extremity ischemia is present either endovascular stents or extra-anatomic bypass may be performed.
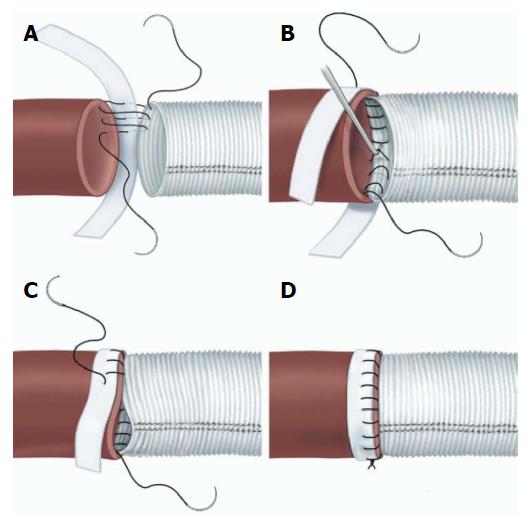
Open surgical management is generally directed at sealing the entry tear and treating any acute complication (rupture or malperfusion) rather than definitive treatment of the aortic pathology. The urgent nature of the operation and unstable character of the aorta dictates a focal approach directed at saving life and limb. Classically, open surgical management of ruptured TBAD involves direct aortic replacement of the ruptured area. When malperfusion is present rather than rupture, management options include a short interposition graft to covering the proximal entry tear, aortic fenestration, or extra-anatomic bypass. Coverage of the entry tear requires a proximal suture line in an area of aorta free of dissection. The graft itself may be relatively short since the goals are simply to seal the entry tear and direct blood into the true lumen. This technique relieves malperfusion secondary to dynamic obstruction. Fenestration involves a transverse aortotomy at or below the location of the branch vessels at risk, with partial resection of the septum to equalize pressure in the true and false lumens[8]. Distal flow is directed exclusively into the true lumen. In both approaches, accurate identification of the distal true lumen and obliteration of the false lumen is critical and this may sometimes be difficult. The suture lines require reinforcement with pledget strips, placed circumferentially (Figure 4), both between the intima and adventitia in the false lumen of the dissected aorta and outside the adventitia at both proximal and distal suture lines, to maintain anastomotic integrity[9]. Aorto-visceral bypass, if required, should originate from the graft itself since the aorta is diseased. Definitive aortic repair is not the goal of open treatment in the acute setting. Spinal cord ischemia, when it occurs, is not reversible.
In patients who manifest only lower extremity ischemia, extra-anatomic bypass grafting, directed at restoring perfusion to the ischemic extremity, may be undertaken without addressing the aortic dissection itself, which is managed medically. In patients with unilateral ischemia a femoral-femoral bypass may be sufficient while in patients with bilateral ischemia axillo-bifemoral grafting is appropriate. As in the thoracic aorta, accurate identification of the distal true lumen is critical to avoid perpetuating the dissection distally. External reinforcement with pledgets may be required.
In a high volume single institution, Bozinovski et al[10] retrospectively reviewed 76 patients who underwent aortic replacement. Operative mortality was reported to be 22.4%. The relevant morbidity rates included: stroke (6.6%), paraplegia (6.6%), dialysis dependence (10.5%), left vocal cord paralysis (39.5%) and cardiac complications (43.4%). In their examination of the multi-institution IRAD dataset, Trimarchi et al[11] found a 29.3% mortality rate for 82 patients undergoing any open intervention for complicated TBAD. The majority (69.3%) of these patients underwent aortic replacement. Stroke and paralysis occurred in 9.0% and 4.5%, respectively. Sachs et al[12] analyzed data from the Nationwide Inpatient Sample (NIS), identifying a 20% in-hospital mortality rate for patients undergoing emergent open aortic replacement, despite being utilized in a younger, less comorbid patient population. Taken as a whole, open surgical intervention is associated with significant mortality and morbidity rates. For this reason it is not recommended in patients without life threatening complications.
The principles of therapy using endovascular techniques remain the same as those with open surgery: either covering the entry tear to induce false lumen thrombosis or equalizing the pressure in the true and false lumen by fenestration. As with open fenestration, percutaneous fenestration treats malperfusion secondary to dynamic obstruction. Its advantages over open fenestration include avoidance of aortic cross clamping and general anesthesia. It can be performed rapidly in an interventional suite and document the perfusion of branch vessels. Furthermore in patients where visceral vessels are perfused through both the true and false lumens the risk of inducing ischemia by false lumen thrombosis is eliminated. Though the technique is not standardized, common methods include the use of IVUS to determine the locations of the true and false lumens. With a wire passed from one lumen into the other, a fenestration is created then enlarged via large balloon angioplasty or balloon-expandable stent placement. When visceral/extremity malperfusion occurs secondary to static obstruction, percutaneous branch vessel stent placement (via bare-metal or covered stents) may be utilized alone or in conjunction with other endovascular techniques described in this article.
There has been a robust experience with this technique to treat malperfusion in selected centers of excellence. Patel et al[4] published their results in treating 69 patients presenting with acute TBAD with visceral malperfusion. Treatment options included true lumen stenting, branch vessel stenting, fenestration, and a combination of all three modalities. When all ischemic territories were examined, angiographic reperfusion was obtained in 95.7% of cases. Early mortality was reported at 17.4%, with a 4.3% incidence of stroke, 2.9% incidence of spinal cord ischemia, and 14.5% of dialysis dependent renal failure. During the follow-up period, the authors noted 1 year and 3 years survival rates of 76.2% and 63.5%, respectively. Despite the immediate success with endovascular fenestration, the authors documented the technique’s shortcomings – the inability to reduce long term aortic-related events. After successful fenestration the dissection will persist, the false lumen will not thrombose and the risk of late aneurysmal dilation persists. At 5 years, the rate of freedom from aortic rupture or repair was 67.7%. With the advent of stent graft coverage of the entry tear, the use of fenestration has diminished.
The biggest change in surgical management of TBAD is the evolution of TEVAR to substitute for open surgical sealing of the entry tear. Like percutaneous fenestration, TEVAR has the potential benefit of an “indirect” intraluminal approach to the dissected aorta as well as the ability to avoid aortic cross clamping and minimize additional end organ ischemia. Through this minimally invasive approach, TEVAR has significantly altered treatment algorithms in patients presenting acutely. The goals of TEVAR use in the acute setting are to seal the entry tear, decompress the false lumen, expand the true lumen, and prevent rupture. Until recently, thoracic endografts were being utilized in an off-label fashion in the United States. In 2014, two endografts, the TAG device (WL GORE) and the Valiant device (Medtronic), received United States Food and Drug Administration approval for use specifically in aortic dissection[13,14]. Several other devices remain under investigation.
Qin et al[15] recently reviewed their single center experience performing TEVAR in 152 patients presenting with complicated TBAD. They achieved technical success in 94.7% of cases, with an in-hospital mortality rate of 2%, stroke rate of 1.3%, and paralysis rate of 1.3%. They also reported a 2.6% incidence of type I endoleak formation and a 1.3% rate of retrograde dissection. Fattori et al[16] reported a slightly higher mortality rate of 10.9% in their review of 290 patients from the IRAD dataset. Rates of stroke (2.3%) and paralysis (1.3%) remained low. In the long term follow up, the group did note that 30.6% of patients required a repeat intervention, and 13.4% developed any endoleak. The 5 year mortality rate was reported at 15.5%. Data from the NIS dataset revealed similar rates of in-hospital mortality (13.1%) and related morbidities[12]. Sachs et al[12] also documented a continual increase in the utilization of TEVAR throughout the study period. Hanna et al[17] reviewed their experience performing endovascular repair in 50 patients presenting with complicated TBAD. They reported no in-hospital deaths, with low (2%) rates of stroke and spinal cord ischemia. They noted a 20% utilization of adjunct procedures (branch vessel stenting and extra anatomic bypass). Though studied only retrospectively, TEVAR utilized in the acute complicated setting is associated with overall lower rates of mortality and morbidity compared with open repair.
TEVAR and percutaneous fenestration may not completely resolve end organ ischemia and supplemental endovascular techniques may be required[18]. Persistence of visceral malperfusion after true lumen expansion with TEVAR, or in the setting of static obstruction, typically warrants treatment with visceral branch vessel stenting. The choice of using bare-metal, covered, self-expanding or balloon expandable stents is left to the discretion of the surgeon, as all devices have been used to manage branch vessel malperfusion[19,20].
The reduced morbidity and mortality of TEVAR compared to open repair raises the question of prophylactic TEVAR in asymptomatic patients. The rationale of such an approach would be to seal the entry tear at an early point in the process, depressurizing the false lumen and thereby reducing risk of rupture and progression to malperfusion in the acute setting or aneurysmal dilation in the long term. It is well known that in chronic dissection the septum between the true and false lumen becomes stiff and repair by endovascular means is more complex and often impossible. The goal of early prophylactic intervention would be to promote false lumen thrombosis, thereby increasing aortic remodeling and reducing the incidence of late aneurysmal degeneration and the frequency of late open repair.
In an attempt to evaluate the role of TEVAR in uncomplicated TBAD, the Investigation of Stent Grafts in Aortic Dissection (INSTEAD) trial randomized approximately 140 patients presenting with subacute (> 14 d) uncomplicated TBAD to best medical therapy with TEVAR or best medical therapy alone[21]. Perioperative mortality rates in the TEVAR group were reported at 2.8%, with a 2.9% incidence of spinal cord ischemia and a 1.5% incidence in major stroke. At 2 years of follow up, the investigators were unable to demonstrate any mortality benefit from TEVAR compared with medical management, with an 88.9% survival in the TEVAR arm and a 95.6% survival in the medical therapy arm. There was no statistical difference seen in the rates of aortic-related deaths (2.9% medical vs 5.6% TEVAR), secondary interventions (22.1% medical vs 18.1% TEVAR) or spinal cord ischemia (1.4% medical vs 2.8% TEVAR) at the end of the 2 years study period. The authors concluded that there was no short or midterm benefit for TEVAR in patients with uncomplicated TBAD and the technique should be reserved for use in those presenting with complications.
There are several shortcomings of the INSTEAD Trial. The major criticisms were that the endpoints of death and complications at two years may not reflect the potential late benefits of TEVAR on false lumen thrombosis, aortic remodeling and late aortic related events and that the trial did not address the role of TEVAR in acute (< 14 d) aortic dissection.
The INSTEAD investigators acknowledged that two years may have been inadequate to capture enough aortic-related deaths within the medical therapy group. To that end, they published outcomes on the same cohort patients followed from 2-5 years from the initial randomization. At 5 years, all-cause mortality statistically differed between the medical (19.3%) and the TEVAR (11.1%) arms[22]. When examining aortic specific mortality, the difference between the medical (19.3%) and TEVAR (6.9%) groups is even more pronounced, with the majority of aortic-related deaths in the medical arm occurring between 2 and 5 years. The authors demonstrated a late survival benefit occurring between 2 and 5 years in patients undergoing TEVAR. It was concluded the survival benefit with TEVAR occurs at a cost of initially increased perioperative morbidity and mortality.
The INSTEAD investigators were also able to demonstrate an improvement in false lumen thrombosis and aortic remodeling in the TEVAR patients. Aortic remodeling is defined as an increase in the true lumen diameter with a subsequent reduction in the false lumen diameter over time, reflecting resolution of the dissection process (Figure 5). No specific criteria exist for objectively quantifying this phenomenon, though several techniques include measuring the true and false lumen diameters at different sites along the thoracic aorta, measuring luminal cross-sectional area, and by volumetric analysis[23]. At 2 years in the INSTEAD trial, only 19.4% of patients undergoing medical therapy were noted to have complete false lumen thrombosis, in contrast to 91.3% of patients undergoing TEVAR[21]. When carried out to 5 years, 22% of patients treated medically showed complete false lumen thrombosis compared with 90.6% of patients undergoing TEVAR[22]. Patterson et al[24] attempted to review the available literature on aortic remodeling. Despite being limited by multiple small-sized retrospective series, series with both acute and chronic dissection, and the heterogeneity in which aortic remodeling was quantified, the authors were able to confirm a high (80% to 90%) rate of complete false lumen thrombosis within the proximal thoracic aorta in patients with TBAD undergoing TEVAR. There is evidence to support the connection between aortic remodeling and improvement in long term survival, albeit limited. In a series of patients treated with TEVAR for chronic TBAD, Mani et al[25] demonstrated an 89% 3-year survival in patients with evidence of aortic remodeling, in contrast to a 54% 3-year survival in patients who did not show this feature.
It is important to note that the INSTEAD trial did not address the optimal management of acute TBAD; i.e., all patients survived at least two weeks without developing complications related to their dissection. In patients randomized to TEVAR, the time from diagnosis to treatment averaged 51 d. This may reflect a group of patients in whom the dissection process has already stabilized and who are less likely to develop early or mid-term complications with persistent medical management. Indeed the medical arm had a 95.6% survival and 2.9% aorta related mortality, lower than the 10% mortality reported form the medically managed patients in the IRAD registry[7]. Thus the proper endpoints might have been late rather than early mortality. In fact the 5 years results suggest that the impact of TEVAR is significant in patients who have a longer life expectancy.
INSTEAD did not address the question of how best to deal with patients with acute TBAD who remain asymptomatic but may be at risk for developing complications. While it is clear that this will not occur in the majority of patients, it is equally intuitive that intervention before rupture or malperfusion occurs would be the optimal way to reduce overall morbidity and mortality. The Acute Dissection: Stent Graft or Best Medical Therapy (ADSORB) trial is underway to clarify this issue. A prospectively randomized control study, the ADSORB trial randomized approximately 60 patients presenting with TBAD of less than 14 d duration to either best medical therapy or TEVAR utilizing a Gore TAG device. In contrast to the INSTEAD trial, the ADSORB trial’s primary composite endpoint was freedom from either false lumen patency, aortic dilation, or aortic rupture. Mean time to randomization was 4.77 d, with 0.88 d to treatment. Although the study is ongoing, preliminary one year data has been presented. There were no in hospital occurrences of death, stroke or spinal cord ischemia. False lumen thrombosis and freedom from the composite endpoint was reported to be markedly higher in the TEVAR group (57%) compared to the medical only group (3%)[26].
It would be ideal to identify patients at high risk for developing complicated TBAD so that selective use of TEVAR in an asymptomatic setting could occur in at-risk patients, while patients likely to develop false lumen thrombosis with medical management alone could be spared surgical intervention. Several reports have been published that highlight specific cohorts of patients (identified via specific radiographic findings) that would potentially benefit the most from early TEVAR. In a recent retrospective review of 228 patients presenting with acute TBAD, Ueki et al[27] identified the descending aortic diameter and location of the entry tear as predictors of aortic-related events (dissection-related death, surgical intervention, aneurysmal degeneration or retrograde dissection). In patients treated medically, those with an aortic diameter less than 40 mm and an entry tear located greater than 50 mm from the left subclavian artery experienced an 82.5% rate of freedom from aortic events by 5 years. In contrast, those with aortic diameters greater than 40 mm and a proximal (less than 50 mm from the left subclavian) entry tear experienced a 53.5% freedom from aortic event rate over a similar time period. Marui et al[28] also retrospectively examined a group of patients with TBAD treated medically. They identified an aortic diameter greater than 40 mm, persistent false lumen patency and a fusiform dilation index as significantly associated with late aortic events. In a retrospective review of 110 patients presenting with TBAD, Akutsu et al[29] identified an aortic diameter of 45 mm on presentation and false lumen patency as independent risk factors for future dissection-related mortality. When examining a series of patients presenting with acute type A and TBADs, Song et al[30] identified a false lumen diameter of 22 mm or greater as an independent predictor of late aneurysmal degeneration and aneurysm related death.
Management of TBAD has undergone dramatic alterations within the past decade and the management of this problem continues to evolve. The high mortality associated with open repair of patients with complicated TBAD has been reduced by the increasing use of thoracic stent grafts to seal the entry tear and restore perfusion. Initial enthusiasm for percutaneous fenestration is being replaced for the most part by TEVAR, which affords entry tear sealing (and subsequent aortic remodeling) in a minimally invasive fashion. Moreover, the success of TEVAR in managing malperfusion has led investigators to study its use in uncomplicated TBAD. Data supporting this indication is not definitive, but what exists suggests that elective TEVAR in the subacute phase is associated with an improvement in 5 years aortic-related survival, at the cost of some increase in perioperative morbidity. The use of TEVAR also appears to improve aortic morphology over time, potentially explaining its long term survival benefit. This causal relationship, however, has not been definitely proven. Current trials are underway to determine feasibility in applying TEVAR in cases of early (< 14 d) uncomplicated TBAD, although the optimal timing of intervention and criterion for patient selection remain unclear. Observational data has aided in identifying specific radiographic criteria that may select out potential subgroups that may be more likely to benefit from TEVAR than medical therapy alone. Fruitful areas for further investigation include: the development of new devices with lower profile and better conformability to reduce perioperative complications; new techniques to increase incidence of false lumen thrombosis and identifying clinical and radiographic characteristics which can predict patients at high and low risk of developing complications with medical management.
P- Reviewer: Kin T, Li XL, Paraskevas KI S- Editor: Tian YL L- Editor: A E- Editor: Jiao XK
| 1. | Jimenez JC. Acute and Chronic Dissection: Medical Management, Surgical Management, Endovascular Management, and Results. Vasc Endovasc Surg A Compr Rev. 8th ed. Philadelphia: Saunders; 2013; 638-649. |
| 2. | Nathan DP, Xu C, Gorman JH, Fairman RM, Bavaria JE, Gorman RC, Chandran KB, Jackson BM. Pathogenesis of acute aortic dissection: a finite element stress analysis. Ann Thorac Surg. 2011;91:458-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 97] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 3. | Faure EM, Canaud L, Cathala P, Serres I, Marty-Ané C, Alric P. Human ex-vivo model of Stanford type B aortic dissection. J Vasc Surg. 2014;60:767-775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 4. | Patel HJ, Williams DM, Meerkov M, Dasika NL, Upchurch GR, Deeb GM. Long-term results of percutaneous management of malperfusion in acute type B aortic dissection: implications for thoracic aortic endovascular repair. J Thorac Cardiovasc Surg. 2009;138:300-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 61] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 5. | Hiratzka LF, Bakris GL, Beckman JA, Bersin RM, Carr VF, Casey DE, Eagle KA, Hermann LK, Isselbacher EM, Kazerooni EA. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with Thoracic Aortic Disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. Circulation. 2010;121:e266-e369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 952] [Cited by in RCA: 1210] [Article Influence: 80.7] [Reference Citation Analysis (1)] |
| 6. | Estrera AL, Miller CC, Goodrick J, Porat EE, Achouh PE, Dhareshwar J, Meada R, Azizzadeh A, Safi HJ. Update on outcomes of acute type B aortic dissection. Ann Thorac Surg. 2007;83:S842-S485; discussion S846-S850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 97] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 7. | Tsai TT, Fattori R, Trimarchi S, Isselbacher E, Myrmel T, Evangelista A, Hutchison S, Sechtem U, Cooper JV, Smith DE. Long-term survival in patients presenting with type B acute aortic dissection: insights from the International Registry of Acute Aortic Dissection. Circulation. 2006;114:2226-2231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 430] [Cited by in RCA: 438] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 8. | Trimarchi S, Segreti S, Grassi V, Lomazzi C, Cova M, Piffaretti G, Rampoldi V. Open fenestration for complicated acute aortic B dissection. Ann Cardiothorac Surg. 2014;3:418-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 9. | Strauch JT, Spielvogel D, Lansman SL, Lauten AL, Bodian C, Griepp RB. Long-term integrity of teflon felt-supported suture lines in aortic surgery. Ann Thorac Surg. 2005;79:796-800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 10. | Bozinovski J, Coselli JS. Outcomes and survival in surgical treatment of descending thoracic aorta with acute dissection. Ann Thorac Surg. 2008;85:965-970; discussion 970-971. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 73] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 11. | Trimarchi S, Nienaber CA, Rampoldi V, Myrmel T, Suzuki T, Bossone E, Tolva V, Deeb MG, Upchurch GR, Cooper JV. Role and results of surgery in acute type B aortic dissection: insights from the International Registry of Acute Aortic Dissection (IRAD). Circulation. 2006;114:I357-I364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 159] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 12. | Sachs T, Pomposelli F, Hagberg R, Hamdan A, Wyers M, Giles K, Schermerhorn M. Open and endovascular repair of type B aortic dissection in the Nationwide Inpatient Sample. J Vasc Surg. 2010;52:860-866; discussion 866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 105] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 13. | GORE TAG Thoracic Endoprosthesis - P040043/S051. [approval 2013 Sept 10]. Available from: http://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/DeviceApprovalsandClearances/Recently-ApprovedDevices/ucm372107.htm. |
| 14. | Medtronic Valiant Thoracic Stent Graft with Captivia Delivery System - P100040/S012. [approval 2014 Jan 22]. Available from: http: //www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/DeviceApprovalsandClearances/Recently-ApprovedDevices/ucm384549.htm. |
| 15. | Qin YL, Deng G, Li TX, Wang W, Teng GJ. Treatment of acute type-B aortic dissection: thoracic endovascular aortic repair or medical management alone? JACC Cardiovasc Interv. 2013;6:185-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 68] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 16. | Fattori R, Montgomery D, Lovato L, Kische S, Di Eusanio M, Ince H, Eagle KA, Isselbacher EM, Nienaber CA. Survival after endovascular therapy in patients with type B aortic dissection: a report from the International Registry of Acute Aortic Dissection (IRAD). JACC Cardiovasc Interv. 2013;6:876-882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 335] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 17. | Hanna JM, Andersen ND, Ganapathi AM, McCann RL, Hughes GC. Five-year results for endovascular repair of acute complicated type B aortic dissection. J Vasc Surg. 2014;59:96-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 121] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 18. | van Bogerijen GH, Williams DM, Patel HJ. TEVAR for complicated acute type B dissection with malperfusion. Ann Cardiothorac Surg. 2014;3:423-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 19. | Uchida N, Shibamura H, Katayama A, Aishin K, Sutoh M, Kuraoka M. Surgical strategies for organ malperfusions in acute type B aortic dissection. Interact Cardiovasc Thorac Surg. 2009;8:75-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 20. | Feezor RJ, Martin TD, Hess PJ, Beaver TM, Klodell CT, Lee WA. Early outcomes after endovascular management of acute, complicated type B aortic dissection. J Vasc Surg. 2009;49:561-566; discussion 566-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 51] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 21. | Nienaber CA, Rousseau H, Eggebrecht H, Kische S, Fattori R, Rehders TC, Kundt G, Scheinert D, Czerny M, Kleinfeldt T. Randomized comparison of strategies for type B aortic dissection: the INvestigation of STEnt Grafts in Aortic Dissection (INSTEAD) trial. Circulation. 2009;120:2519-2528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 530] [Cited by in RCA: 545] [Article Influence: 34.1] [Reference Citation Analysis (0)] |
| 22. | Nienaber CA, Kische S, Rousseau H, Eggebrecht H, Rehders TC, Kundt G, Glass A, Scheinert D, Czerny M, Kleinfeldt T. Endovascular repair of type B aortic dissection: long-term results of the randomized investigation of stent grafts in aortic dissection trial. Circ Cardiovasc Interv. 2013;6:407-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 644] [Cited by in RCA: 796] [Article Influence: 66.3] [Reference Citation Analysis (0)] |
| 23. | Stanley GA, Murphy EH, Knowles M, Ilves M, Jessen ME, Dimaio JM, Modrall JG, Arko FR. Volumetric analysis of type B aortic dissections treated with thoracic endovascular aortic repair. J Vasc Surg. 2011;54:985-992; discussion 992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 56] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 24. | Patterson BO, Cobb RJ, Karthikesalingam A, Holt PJ, Hinchliffe RJ, Loftus IM, Thompson MM. A systematic review of aortic remodeling after endovascular repair of type B aortic dissection: methods and outcomes. Ann Thorac Surg. 2014;97:588-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 73] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 25. | Mani K, Clough RE, Lyons OT, Bell RE, Carrell TW, Zayed HA, Waltham M, Taylor PR. Predictors of outcome after endovascular repair for chronic type B dissection. Eur J Vasc Endovasc Surg. 2012;43:386-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 111] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 26. | Hughes GC. Management of acute type B aortic dissection; ADSORB trial. J Thorac Cardiovasc Surg. 2015;149:S158-S162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 58] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 27. | Ueki C, Sakaguchi G, Shimamoto T, Komiya T. Prognostic factors in patients with uncomplicated acute type B aortic dissection. Ann Thorac Surg. 2014;97:767-773; discussion 773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 28. | Marui A, Mochizuki T, Koyama T, Mitsui N. Degree of fusiform dilatation of the proximal descending aorta in type B acute aortic dissection can predict late aortic events. J Thorac Cardiovasc Surg. 2007;134:1163-1170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 83] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 29. | Akutsu K, Nejima J, Kiuchi K, Sasaki K, Ochi M, Tanaka K, Takano T. Effects of the patent false lumen on the long-term outcome of type B acute aortic dissection. Eur J Cardiothorac Surg. 2004;26:359-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 162] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 30. | Song JM, Kim SD, Kim JH, Kim MJ, Kang DH, Seo JB, Lim TH, Lee JW, Song MG, Song JK. Long-term predictors of descending aorta aneurysmal change in patients with aortic dissection. J Am Coll Cardiol. 2007;50:799-804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 270] [Article Influence: 15.0] [Reference Citation Analysis (0)] |