Published online Nov 28, 2013. doi: 10.5412/wjsp.v3.i3.29
Revised: August 10, 2013
Accepted: September 14, 2013
Published online: November 28, 2013
Processing time: 190 Days and 20.8 Hours
Platelets contain bio-physiological substances, including insulin-like growth factor-1, vascular endothelial growth factor, platelet-derived growth factor, hepatocyte growth factor, serotonin, transforming growth factor-β, adenosine diphosphate, adenosine tri-phosphate, and epidermal growth factor. Platelets have conventionally been considered to exacerbate the inflammatory response and liver injury. Recently, platelets were discovered to have a positive impact on the liver. In this review, we present experimental and clinical evidence indicating that platelets accelerate liver regeneration and have anti-fibrosis and anti-apoptosis activity, and we detail the mechanisms of action. Platelets accelerate liver regeneration by three different mechanisms: (1) a direct effect on hepatocytes, (2) a cooperative effect with liver sinusoidal endothelial cells, and (3) a collaborative effect with Kupffer cells. Platelets exert anti-fibrotic activity by deactivating hepatic stellate cells via the adenosine-cyclic adenosine 5’-monophosphate signaling pathway. Platelets prevent hepatocyte apoptosis by activating the Akt pathway and up-regulating Bcl-xL, which suppresses caspase-3 activation. Platelet therapy with thrombopoietin, thrombopoietin receptor agonists, and platelet transfusion has the advantages of convenience and cost-efficiency over other treatments. We propose that in the future, platelet therapy will play a promising role in the treatment of the various liver disorders that currently challenge the surgical field, such as liver failure after a massive hepatectomy, hepatectomy of a cirrhotic liver, and small grafts in liver transplantation.
Core tip: Platelets have conventionally been considered to exacerbate the inflammatory response and liver injury. Recently, some studies have demonstrated a role for platelets in promoting liver regeneration, improving liver fibrosis, and attenuating hepatitis. In this review, the experimental and clinical evidence that platelets accelerate liver regeneration and attenuate fibrosis and apoptosis are described, as are the mechanisms of action. Platelet therapies, such as thrombopoietin, thrombopoietin receptor agonists, and platelet transfusion, will play a promising role in the treatment of the various liver disorders that currently challenge the surgical field.
- Citation: Takahashi K, Murata S, Ohkohchi N. Platelet therapy: A novel strategy for liver regeneration, anti-fibrosis, and anti-apoptosis. World J Surg Proced 2013; 3(3): 29-36
- URL: https://www.wjgnet.com/2219-2832/full/v3/i3/29.htm
- DOI: https://dx.doi.org/10.5412/wjsp.v3.i3.29
Platelets contain bio-physiological substances, such as growth factors and cytokines[1,2]. Platelets are activated by various types of stimulation, and they release these physiologically active substances in a context-dependent manner[3]. The predominant function of platelets is in hemostasis and thrombosis, where they play a complex role with other cellular participants[2,4]. Recently, platelets have been determined to have various roles in the body in addition to these primary functions[5-7]. Platelet-rich plasma is a source of platelet growth factors and cytokines[8] and has increased in popularity since the late 1990s[9]. Currently, platelet-rich plasma is widely accepted as the best treatment to promote would healing and tissue regeneration in many fields, including orthopedics[10,11], plastic surgery[12,13], and maxillofacial surgery[14].
Thrombocytopenia is frequently observed in patients with chronic liver disease[15]. This condition results from hypersplenism secondary to portal hypertension and decreased thrombopoietin production by hepatocytes[16]. Liver regeneration after hepatectomy in this patient population is severely impaired, and preventing postoperative liver failure has long been considered a critical issue in the surgical fields[17,18]. Recently, several attempts have been made to overcome this problem, including gene therapy[19], bone marrow cell infusion therapy[20], macrophage therapy[21], and platelet therapy. In platelet therapy, thrombopoietin treatment and platelet transfusions have positive effects on the liver and are innovative treatments for various pathological liver conditions[22,23]. Eltrombopag, an oral thrombopoietin agonist, has recently been developed[24] and is beginning to be utilized to treat various health conditions, including liver disease[25,26].
In this review, we present the experimental and clinical evidence that platelets accelerate liver regeneration and inhibit fibrosis and apoptosis; we also present the mechanisms of action for these functions. We propose that platelet therapy, including thrombopoietin and eltrombopag treatment and platelet transfusion, has a promising role in the treatment of the various liver problems, such as liver failure after a massive hepatectomy[27], hepatectomy of a cirrhotic liver[18], and small grafts in liver transplantation[28], that currently challenge the surgical field.
Liver regeneration after a hepatectomy is accomplished via the proliferation of hepatocytes, biliary epithelial cells, liver sinusoidal endothelial cells, Kupffer cells, and hepatic stellate cells[29-31]. Intercellular interactions between numerous growth factors and cytokines, including hepatocyte growth factor (HGF), tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), transforming growth factor-α, and endothelial growth factor, play important roles during this process[29,30]. Each mediator activates downstream cascades by releasing hepatocytes from a quiescent state and allowing them to enter the cell cycle[29,30]. The TNF-α/nuclear factor-kappa B (NF-KB)[32,33], IL-6/signal transducer and activator of transcription 3 (STAT3)[34], and phosphatidylinositol-3-kinase (PI3K)/Akt[35] pathways are the three major signaling cascades that are engaged during liver regeneration.
In 2004, Murata et al[36] were the first to demonstrate that platelets promoted liver regeneration during the early phases after a partial hepatectomy. Using mouse models, Lesurtel et al[37] reported that platelet-derived serotonin mediated liver regeneration. In this section, the clinical and experimental evidence that platelets promote liver regeneration and the three different mechanisms involved in this process are described.
In a retrospective analysis of 216 consecutive patients who underwent a partial hepatectomy for colorectal metastasis, Alkozai et al[38] reported that an immediate post-operative platelet count below 100000/μL was an independent risk factor for the delayed recovery of postoperative liver function and was associated with an increased risk of postoperative mortality. Kim et al[39] analyzed 87 patients who received adult-to-adult living donor liver transplants and determined that the total number of units of transfused platelet concentrate was significantly associated with graft regeneration, which was assessed by CT scan. Furthermore, the stepwise regression analysis revealed that the total amount of the platelets was independently associated with graft regeneration.
Murata et al[22] determined that a 2- to 3-fold elevation in platelet count induced by thrombopoietin increased the liver/body weight ratio, the hepatocyte Ki-67 labeling index, and the mitotic index after a 70% partial hepatectomy. Myronovych et al[40] reported that the incremental increase in platelet count after thrombopoietin treatment accelerated liver regeneration within 24 h after a 90% hepatectomy and improved the postoperative survival rate. They determined that under thrombocytotic conditions, there was a significant increase in HGF expression in liver tissue and the early phosphorylation of Akt and STAT3. These results implied that the thrombocytotic state induced by thrombopoietin promoted liver regeneration via an early activation of the PI3K/Akt and IL-6/STAT3 pathways, leading to hepatocyte cell cycle entry and mitosis. In both studies, thromboembolic events, organ damage, and other side effects were not observed in response to the increased platelet count.
Matsuo et al[23] examined the effects of platelet transfusion on liver regeneration by transfusing platelet-rich plasma into rats after a 70% partial hepatectomy. After a hepatectomy, platelet transfusion increased the liver/body weight ratio and the hepatocyte Ki-67 labeling index at 24 h without damaging the liver. Furthermore, platelet transfusion accelerated Akt phosphorylation and prolonged the activation of the extracellular signal-regulated kinase 1/2 pathway. These results indicated that platelet transfusion had a positive impact by accelerating liver regeneration after a hepatectomy without damaging the liver.
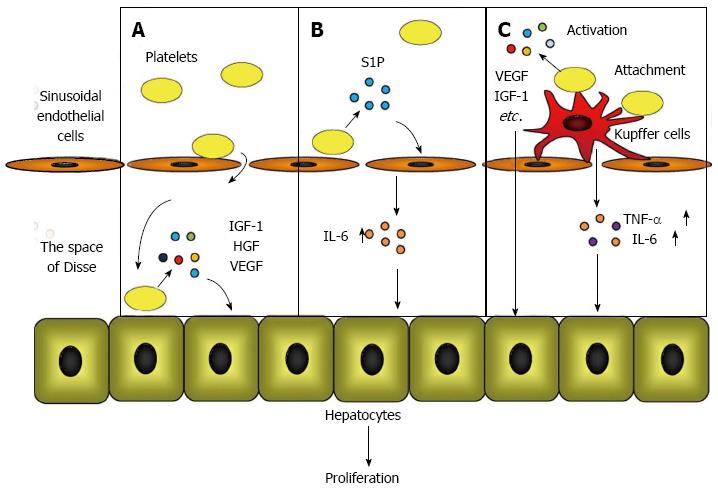
Murata et al[22] observed that platelets accumulated in the liver immediately after a hepatectomy and translocated into the space of Disse to directly contact the hepatocytes. These data implied that platelets in the liver provide signals for hepatocyte proliferation through direct contact with hepatocytes. To prove this hypothesis, Matsuo et al[41] utilized a co-culture chamber system that separates the platelets and hepatocytes with a permeable membrane and clarified that direct contact between platelets and hepatocytes triggered the release of HGF, insulin-like growth factor (IGF)-1, and vascular endothelial growth factor (VEGF) from platelets, resulting in hepatocyte proliferation.
The direct effect of the platelet mechanism occurs when platelets translocate to the space of Disse and directly contact the hepatocytes, which triggers the secretion of HGF, IGF-1, and VEGF from the platelets. These growth factors initiate mitosis in hepatocytes and promote liver regeneration (Figure 1A).
Kawasaki et al[42] studied the role of platelets in liver regeneration in relation to liver sinusoidal endothelial cells using co-culture chamber systems. They demonstrated that the direct contact of platelets with liver sinusoidal endothelial cells increased the release of IL-6 from liver sinusoidal endothelial cells, which accelerated DNA synthesis through the IL-6/STAT3 pathway in hepatocytes. They also proved that platelet-derived sphingosine-1-phosphate (S1P) induced IL-6 secretion from liver sinusoidal endothelial cells.
In the platelet mechanism of action that involves liver sinusoidal endothelial cells, the direct contact between the platelets and the liver sinusoidal endothelial cells induces S1P release from the platelets, which promotes IL-6 secretion from the liver sinusoidal endothelial cells. IL-6 subsequently accelerates hepatocyte mitosis via the IL-6/STAT3 pathway (Figure 1B).
Takahashi et al[43] studied the positive impact of platelets on liver regeneration and focused on the role of Kupffer cells by transfusing platelets into mice. These authors discovered that after a 70% hepatectomy, transfused platelets accumulated and acted locally in the residual liver in the presence of activated Kupffer cells. The hepatic expression of TNF-α and IL-6, which are predominantly produced by Kupffer cells[30,44], increased in response to a platelet transfusion, indicating that the function of the Kupffer cells was enhanced by a platelet transfusion. Furthermore, it was determined by electron microscopy that the transfused platelets were attached to the surface of the Kupffer cells, providing a reason why the platelets accumulated and were activated in the liver after a hepatectomy.
The mechanism involving platelets and Kupffer cells occurs when platelets accumulate and are locally activated in the liver by attaching to the surface of activated Kupffer cells. Liver regeneration is promoted by growth factors that are released from accumulated platelets and by the enhanced release of TNF-α and IL-6 from Kupffer cells (Figure 1C).
Liver fibrosis is a major cause of morbidity and mortality in the world[45]. It results in liver failure, portal hypertension, and an increased risk of carcinogenesis[45,46]; liver transplantation is currently the only cure[47]. Fibrosis is characterized by an excessive deposition of extracellular matrix proteins, which disrupt the liver structure and cause pathophysiological damage to this organ[45,46]. Matrix metallopeptidases (MMPs) are enzymes that are responsible for the degradation of extracellular matrix proteins[48,49], and the production of MMPs is regulated by HGF[50,51]. Activated hepatic stellate cells are the primary cells that are responsible for the excessive synthesis of extracellular matrix proteins[47]. Transforming growth factor-β (TGF-β), which is predominately released from hepatic stellate cells and Kupffer cells[52], is the most potent cytokine that activates hepatic stellate cells. The effects of TGF-β are mediated by intracellular signaling via Smad proteins[53], and TGF-β is suppressed by HGF[19].
Despite improvements in the preoperative assessment of liver function and advances in surgical techniques, liver resection still carries the risk for postoperative hepatic failure, especially in patients with cirrhosis[54]. This risk occurs because a cirrhotic liver has an impaired regenerative ability, and the risk of post-operative hepatic failure correlates with the degree of fibrosis[55]. Accelerating liver regeneration and improving liver fibrosis would avoid liver failure after a hepatectomy. Although previous studies have viewed platelets as promoters of liver fibrosis[56], recent studies have uncovered anti-fibrotic effects of platelets in the liver. This section describes the experimental and clinical evidence that platelets are anti-fibrotic as well as the mechanisms of action.
Maruyama et al[57] conducted a prospective clinical trial of the effect of platelet transfusion on liver fibrosis. Patients with chronic liver disease (Child-Pugh classes A and B) and a platelet count below 100000/μL were registered. Ten patients received ten units of platelet concentrate once 1 wk for 12 wk. Four patients discontinued this treatment because of the appearance of mild hives, anti-human platelet antigen, and anti-human leukocyte antigen. Six patients completed the platelet transfusions and were followed for 9 mo after the last treatment; these patients exhibited increased concentrations of serum albumin and cholinesterase. Furthermore, there was a decrease in the serum hyaluronic acid, one of the serum fibrotic markers. It was determined that platelet transfusion improved liver function and decreased liver fibrosis.
Watanabe et al[58] reported that thrombocytosis induced by thrombopoietin treatment or splenectomy reduced liver fibrosis and the hydroxyproline content of liver tissue. Thrombocytosis suppressed TGF-β mRNA expression and increased MMP-9 expression in the liver. Furthermore, the liver volume, the hepatocyte proliferating cell nuclear antigen (PCNA) labeling index, and the mitotic index in fibrotic liver increased under thrombocytotic conditions. These findings indicated that thrombocytosis reduced liver fibrosis and promoted liver regeneration.
Murata et al[59] examined the effect of a single thrombopoietin treatment on fibrosis and liver regeneration in a cirrhotic liver after a 70% partial hepatectomy. Thrombocytosis improved fibrosis and increased the hepatocyte PCNA labeling index and the mitotic index in the cirrhotic liver. The authors also injected anti-platelet serum after administering thrombopoietin to determine whether the effects were due to the thrombopoietin or to the increased platelet number. The anti-platelet serum injection significantly increased liver fibrosis and decreased liver regeneration. According to these studies, increasing the number of platelets attenuated liver fibrosis and accelerated liver regeneration even in a cirrhotic liver.
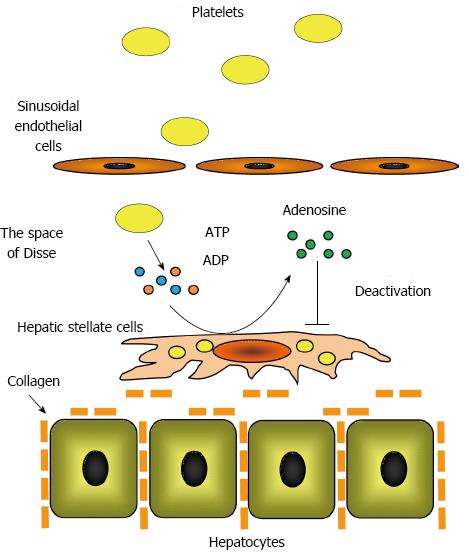
Ikeda et al[60] reported that platelet extracts suppressed hepatic stellate cell activation in vitro. They determined that adenine nucleotides, such adenosine triphosphate and adenosine diphosphate, were enriched in platelets and that ecto-nucleotide triphosphate diphosphodiesterase, ecto-nucleotide pyrophosphatase/phosphodiesterase, and ecto-5’-nucleotidase located on the plasma membrane of hepatic stellate cells degraded these adenine nucleotides to adenosine[61]. The authors demonstrated that adenosine increased the intracellular concentration of cyclic adenosine 5’-monophosphate (cAMP) in hepatic stellate cells, which suppressed hepatic stellate cell activation by phosphorylating cAMP-response element binding protein. These findings indicated that hepatic stellate cell activation is directly suppressed by platelets via the adenosine-cAMP signaling pathway (Figure 2).
Liver failure after a hepatectomy is caused by various events, including a massive hepatectomy, ischemic-reperfusion injury, and a postoperative infection[62]. Hepatocyte apoptosis and diminished liver regeneration are the most important molecular events that occur during liver failure[63]. Apoptosis is an active form of cell death, and two signaling pathways lead to apoptosis: the intrinsic and extrinsic pathways[64]. The intrinsic pathway is characterized by mitochondrial dysfunction. Various stimuli damage the mitochondrial inner membrane, resulting in a permeability transition and the mitochondrial release of cytochrome C[64]. In the cytosol, cytochrome C complexes with Apaf-1 to activate procaspase-9, which in turn activates its downstream effectors, caspases 3, 6, and 7, which are responsible for degrading several cellular substrates that are associated with the morphological changes representative of apoptosis[65]. The Fas/Fas ligand system plays an important role in the extrinsic pathway. Upon activation by the Fas ligand, Fas complexes with procaspase-8. The aggregation of this complex initiates the cleavage of procaspase-8 into its active form, which subsequently activates caspase-3, its downstream effector[66]. Therefore, the Fas/Fas ligand system affects both the intrinsic and extrinsic pathways. Bcl-xL, a member of the Bcl-2 family, prevents mitochondria permeability transition and Fas-mediated apoptosis by inhibiting the signaling cascades[67].
This section describes the effect of thrombocytosis on liver damage and apoptosis. Because the Fas/Fas ligand system and apoptosis are hypothesized to be responsible for hepatitis[68,69], we examined the anti-apoptotic effects using a hepatitis model.
Hisakura et al[70] examined the ability of thrombopoietin-mediated thrombocytosis to protect the liver from damage after an extended hepatectomy using a pig model. The authors discovered that in thrombocytotic conditions, liver cholestasis, ballooning, and necrosis were attenuated and that serum aspartate amino transferase and alkaline phosphatase (ALT) levels were low after an extended hepatectomy. Furthermore, electron transmission microscopy revealed that the structure of the endothelial lining was well preserved in thrombocytotic conditions. These data indicated that thrombocytosis protects the sinusoidal lining and prevents acute liver damage after an extended hepatectomy.
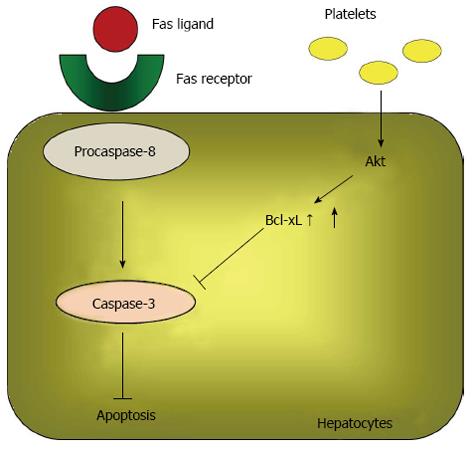
Hisakura et al[71] also investigated the effects of thrombocytosis in acute hepatitis induced by an anti-Fas antibody. The authors demonstrated that serum ALT levels were significantly decreased in thrombocytotic conditions at 6, 24 and 72 h after administering an anti-Fas antibody. They also determined that the percent of TdT-mediated dUTP-biotin nick end labeling-positive hepatocytes and the expression of cleaved caspase-3 in the liver were significantly decreased by thrombocytosis. Furthermore, in vitro Akt phosphorylation, increased Bcl-xL, and decreased cleaved caspase-3 were observed sequentially in hepatocytes co-cultured with platelets. Because Akt is a critical suppressor of apoptosis[72,73], the above data suggested that an increase in the platelet count prevents hepatocyte apoptosis by activating the Akt pathway and up-regulating Bcl-xL, which suppresses caspase-3 activation (Figure 3).
This review describes the published evidence that platelets promote liver regeneration, attenuate liver fibrosis, and prevent liver damage and hepatocyte apoptosis; it also details the mechanisms of action. In the blood, platelets are constituents that contain numerous biologically active growth factors and cytokines, and it was recently determined that platelets have various functions in addition to hemostasis and thrombosis[5-7]. Currently, thrombopoietin[22,40,58,59,70], thrombopoietin receptor agonists[24], artificial platelets[74,75], and freeze-dried platelets[76] are in development and are beginning to be utilized in various clinical settings, and the importance of platelets is becoming more obvious. Despite some side effects[57], platelet therapy has advantages in its convenience and cost-efficiency, and it provides another therapeutic strategy to address the current surgical issues and challenges, such as liver failure after a massive hepatectomy, hepatectomy of a cirrhotic liver, and small grafts in liver transplantation, in the near future.
P- Reviewers: Asahina K, Matsuda Y, Pan GD, Tsuchiya A S- Editor: Zhai HH L- Editor: A E- Editor: Liu XM
| 1. | Polasek J. Platelet secretory granules or secretory lysosomes? Platelets. 2005;16:500-501. [PubMed] |
| 3. | Blair P, Flaumenhaft R. Platelet α-granules: basic biology and clinical correlates. Blood Rev. 2009;23:177-189. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 879] [Cited by in RCA: 857] [Article Influence: 53.6] [Reference Citation Analysis (0)] |
| 4. | Broos K, Feys HB, De Meyer SF, Vanhoorelbeke K, Deckmyn H. Platelets at work in primary hemostasis. Blood Rev. 2011;25:155-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 269] [Cited by in RCA: 321] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 5. | Elzey BD, Sprague DL, Ratliff TL. The emerging role of platelets in adaptive immunity. Cell Immunol. 2005;238:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 119] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 6. | Yamaguchi R, Terashima H, Yoneyama S, Tadano S, Ohkohchi N. Effects of platelet-rich plasma on intestinal anastomotic healing in rats: PRP concentration is a key factor. J Surg Res. 2012;173:258-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 97] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 7. | Mehta P. Potential role of platelets in the pathogenesis of tumor metastasis. Blood. 1984;63:55-63. [PubMed] |
| 8. | Marx RE. Platelet-rich plasma (PRP): what is PRP and what is not PRP? Implant Dent. 2001;10:225-228. [PubMed] |
| 9. | Rozman P, Bolta Z. Use of platelet growth factors in treating wounds and soft-tissue injuries. Acta Dermatovenerol Alp Panonica Adriat. 2007;16:156-165. [PubMed] |
| 10. | Hartmann EK, Heintel T, Morrison RH, Weckbach A. Influence of platelet-rich plasma on the anterior fusion in spinal injuries: a qualitative and quantitative analysis using computer tomography. Arch Orthop Trauma Surg. 2010;130:909-914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 42] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 11. | de Vos RJ, Weir A, van Schie HT, Bierma-Zeinstra SM, Verhaar JA, Weinans H, Tol JL. Platelet-rich plasma injection for chronic Achilles tendinopathy: a randomized controlled trial. JAMA. 2010;303:144-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 591] [Cited by in RCA: 546] [Article Influence: 36.4] [Reference Citation Analysis (0)] |
| 12. | Eppley BL, Pietrzak WS, Blanton M. Platelet-rich plasma: a review of biology and applications in plastic surgery. Plast Reconstr Surg. 2006;118:147e-159e. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 326] [Cited by in RCA: 333] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 13. | Redler LH, Thompson SA, Hsu SH, Ahmad CS, Levine WN. Platelet-rich plasma therapy: a systematic literature review and evidence for clinical use. Phys Sportsmed. 2011;39:42-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 61] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 14. | Dugrillon A, Eichler H, Kern S, Klüter H. Autologous concentrated platelet-rich plasma (cPRP) for local application in bone regeneration. Int J Oral Maxillofac Surg. 2002;31:615-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 133] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 15. | Afdhal N, McHutchison J, Brown R, Jacobson I, Manns M, Poordad F, Weksler B, Esteban R. Thrombocytopenia associated with chronic liver disease. J Hepatol. 2008;48:1000-1007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 380] [Cited by in RCA: 403] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 16. | Rios R, Sangro B, Herrero I, Quiroga J, Prieto J. The role of thrombopoietin in the thrombocytopenia of patients with liver cirrhosis. Am J Gastroenterol. 2005;100:1311-1316. [PubMed] |
| 17. | McCormack L, Capitanich P, Quiñonez E. Liver surgery in the presence of cirrhosis or steatosis: Is morbidity increased? Patient Saf Surg. 2008;2:8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 18. | Llovet JM, Fuster J, Bruix J. Intention-to-treat analysis of surgical treatment for early hepatocellular carcinoma: resection versus transplantation. Hepatology. 1999;30:1434-1440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1331] [Cited by in RCA: 1270] [Article Influence: 48.8] [Reference Citation Analysis (0)] |
| 19. | Ueki T, Kaneda Y, Tsutsui H, Nakanishi K, Sawa Y, Morishita R, Matsumoto K, Nakamura T, Takahashi H, Okamoto E. Hepatocyte growth factor gene therapy of liver cirrhosis in rats. Nat Med. 1999;5:226-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 456] [Cited by in RCA: 471] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 20. | Terai S, Ishikawa T, Omori K, Aoyama K, Marumoto Y, Urata Y, Yokoyama Y, Uchida K, Yamasaki T, Fujii Y. Improved liver function in patients with liver cirrhosis after autologous bone marrow cell infusion therapy. Stem Cells. 2006;24:2292-2298. [PubMed] |
| 21. | Thomas JA, Pope C, Wojtacha D, Robson AJ, Gordon-Walker TT, Hartland S, Ramachandran P, Van Deemter M, Hume DA, Iredale JP. Macrophage therapy for murine liver fibrosis recruits host effector cells improving fibrosis, regeneration, and function. Hepatology. 2011;53:2003-2015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 281] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 22. | Murata S, Ohkohchi N, Matsuo R, Ikeda O, Myronovych A, Hoshi R. Platelets promote liver regeneration in early period after hepatectomy in mice. World J Surg. 2007;31:808-816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 138] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 23. | Matsuo R, Nakano Y, Ohkohchi N. Platelet administration via the portal vein promotes liver regeneration in rats after 70% hepatectomy. Ann Surg. 2011;253:759-763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 97] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 24. | Schipperus M, Fijnheer R. New therapeutic options for immune thrombocytopenia. Neth J Med. 2011;69:480-485. [PubMed] |
| 25. | Boyers D, Jia X, Jenkinson D, Mowatt G. Eltrombopag for the treatment of chronic immune or idiopathic thrombocytopenic purpura: a NICE single technology appraisal. Pharmacoeconomics. 2012;30:483-495. [PubMed] |
| 26. | Giannini EG, Afdhal NH. Eltrombopag in patients with chronic liver disease. Expert Opin Pharmacother. 2013;14:669-678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 27. | Garcea G, Maddern GJ. Liver failure after major hepatic resection. J Hepatobiliary Pancreat Surg. 2009;16:145-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 98] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 28. | Gruttadauria S, Pagano D, Luca A, Gridelli B. Small-for-size syndrome in adult-to-adult living-related liver transplantation. World J Gastroenterol. 2010;16:5011-5015. [PubMed] |
| 31. | Malik R, Selden C, Hodgson H. The role of non-parenchymal cells in liver growth. Semin Cell Dev Biol. 2002;13:425-431. [PubMed] |
| 32. | Cressman DE, Greenbaum LE, Haber BA, Taub R. Rapid activation of post-hepatectomy factor/nuclear factor kappa B in hepatocytes, a primary response in the regenerating liver. J Biol Chem. 1994;269:30429-30435. [PubMed] |
| 33. | FitzGerald MJ, Webber EM, Donovan JR, Fausto N. Rapid DNA binding by nuclear factor kappa B in hepatocytes at the start of liver regeneration. Cell Growth Differ. 1995;6:417-427. [PubMed] |
| 34. | Cressman DE, Diamond RH, Taub R. Rapid activation of the Stat3 transcription complex in liver regeneration. Hepatology. 1995;21:1443-1449. [PubMed] |
| 35. | Jackson LN, Larson SD, Silva SR, Rychahou PG, Chen LA, Qiu S, Rajaraman S, Evers BM. PI3K/Akt activation is critical for early hepatic regeneration after partial hepatectomy. Am J Physiol Gastrointest Liver Physiol. 2008;294:G1401-G1410. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 79] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 36. | Murata S, Ohkohchi N, Abe T, Enomoto Y, Doi H, Satomi S. Platelets promote G1-S progression of liver regeneration after hepatectomy. Bologna: MODIMOND S.r.l 2004; 107-112. |
| 37. | Lesurtel M, Graf R, Aleil B, Walther DJ, Tian Y, Jochum W, Gachet C, Bader M, Clavien PA. Platelet-derived serotonin mediates liver regeneration. Science. 2006;312:104-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 572] [Cited by in RCA: 595] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 38. | Alkozai EM, Nijsten MW, de Jong KP, de Boer MT, Peeters PM, Slooff MJ, Porte RJ, Lisman T. Immediate postoperative low platelet count is associated with delayed liver function recovery after partial liver resection. Ann Surg. 2010;251:300-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 97] [Article Influence: 6.5] [Reference Citation Analysis (3)] |
| 39. | Kim J, Yi NJ, Shin WY, Kim T, Lee KU, Suh KS. Platelet transfusion can be related to liver regeneration after living donor liver transplantation. World J Surg. 2010;34:1052-1058. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 40. | Myronovych A, Murata S, Chiba M, Matsuo R, Ikeda O, Watanabe M, Hisakura K, Nakano Y, Kohno K, Kawasaki T. Role of platelets on liver regeneration after 90% hepatectomy in mice. J Hepatol. 2008;49:363-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 97] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 41. | Matsuo R, Ohkohchi N, Murata S, Ikeda O, Nakano Y, Watanabe M, Hisakura K, Myronovych A, Kubota T, Narimatsu H. Platelets Strongly Induce Hepatocyte Proliferation with IGF-1 and HGF In Vitro. J Surg Res. 2008;145:279-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 99] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 42. | Kawasaki T, Murata S, Takahashi K, Nozaki R, Ohshiro Y, Ikeda N, Pak S, Myronovych A, Hisakura K, Fukunaga K. Activation of human liver sinusoidal endothelial cell by human platelets induces hepatocyte proliferation. J Hepatol. 2010;53:648-654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 81] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 43. | Takahashi K, Kozuma Y, Suzuki H, Tamura T, Maruyama T, Fukunaga K, Murata S, Ohkohchi N. Human platelets promote liver regeneration with Kupffer cells in SCID mice. J Surg Res. 2013;180:62-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 44. | Decker K. The response of liver macrophages to inflammatory stimulation. Keio J Med. 1998;47:1-9. [PubMed] |
| 45. | Bataller R, Brenner DA. Liver fibrosis. J Clin Invest. 2005;115:209-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3381] [Cited by in RCA: 4119] [Article Influence: 206.0] [Reference Citation Analysis (3)] |
| 46. | Friedman SL. Liver fibrosis--from bench to bedside. J Hepatol. 2003;38 Suppl 1:S38-S53. [PubMed] |
| 47. | Parsons CJ, Takashima M, Rippe RA. Molecular mechanisms of hepatic fibrogenesis. J Gastroenterol Hepatol. 2007;22 Suppl 1:S79-S84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 247] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 48. | Hemmann S, Graf J, Roderfeld M, Roeb E. Expression of MMPs and TIMPs in liver fibrosis - a systematic review with special emphasis on anti-fibrotic strategies. J Hepatol. 2007;46:955-975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 349] [Cited by in RCA: 392] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 49. | Roderfeld M, Hemmann S, Roeb E. Mechanisms of fibrinolysis in chronic liver injury (with special emphasis on MMPs and TIMPs). Z Gastroenterol. 2007;45:25-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 36] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 50. | Mizuno S, Matsumoto K, Li MY, Nakamura T. HGF reduces advancing lung fibrosis in mice: a potential role for MMP-dependent myofibroblast apoptosis. FASEB J. 2005;19:580-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 141] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 51. | Higashiyama R, Inagaki Y, Hong YY, Kushida M, Nakao S, Niioka M, Watanabe T, Okano H, Matsuzaki Y, Shiota G. Bone marrow-derived cells express matrix metalloproteinases and contribute to regression of liver fibrosis in mice. Hepatology. 2007;45:213-222. [PubMed] |
| 52. | Knittel T, Mehde M, Kobold D, Saile B, Dinter C, Ramadori G. Expression patterns of matrix metalloproteinases and their inhibitors in parenchymal and non-parenchymal cells of rat liver: regulation by TNF-α and TGF-β1. J Hepatol. 1999;30:48-60. [PubMed] |
| 53. | Flanders KC. Smad3 as a mediator of the fibrotic response. Int J Exp Pathol. 2004;85:47-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 459] [Cited by in RCA: 494] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 54. | Takenaka K, Kanematsu T, Fukuzawa K, Sugimachi K. Can hepatic failure after surgery for hepatocellular carcinoma in cirrhotic patients be prevented? World J Surg. 1990;14:123-127. [PubMed] |
| 55. | Nagasue N, Yukaya H, Ogawa Y, Kohno H, Nakamura T. Human liver regeneration after major hepatic resection. A study of normal liver and livers with chronic hepatitis and cirrhosis. Ann Surg. 1987;206:30-39. [PubMed] |
| 56. | Zaldivar MM, Pauels K, von Hundelshausen P, Berres ML, Schmitz P, Bornemann J, Kowalska MA, Gassler N, Streetz KL, Weiskirchen R. CXC chemokine ligand 4 (Cxcl4) is a platelet-derived mediator of experimental liver fibrosis. Hepatology. 2010;51:1345-1353. [PubMed] |
| 57. | Maruyama T, Murata S, Takahashi K, Tamura T, Nozaki R, Ikeda N, Fukunaga K, Oda T, Sasaki R, Ohkohchi N. Platelet transfusion improves liver function in patients with chronic liver disease and cirrhosis. Tohoku J Exp Med. 2013;229:213-220. [PubMed] |
| 58. | Watanabe M, Murata S, Hashimoto I, Nakano Y, Ikeda O, Aoyagi Y, Matsuo R, Fukunaga K, Yasue H, Ohkohchi N. Platelets contribute to the reduction of liver fibrosis in mice. J Gastroenterol Hepatol. 2009;24:78-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 89] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 59. | Murata S, Hashimoto I, Nakano Y, Myronovych A, Watanabe M, Ohkohchi N. Single administration of thrombopoietin prevents progression of liver fibrosis and promotes liver regeneration after partial hepatectomy in cirrhotic rats. Ann Surg. 2008;248:821-828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 68] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 60. | Ikeda N, Murata S, Maruyama T, Tamura T, Nozaki R, Kawasaki T, Fukunaga K, Oda T, Sasaki R, Homma M. Platelet-derived adenosine 5’-triphosphate suppresses activation of human hepatic stellate cell: In vitro study. Hepatol Res. 2012;42:91-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 61. | Dranoff JA, Ogawa M, Kruglov EA, Gaça MD, Sévigny J, Robson SC, Wells RG. Expression of P2Y nucleotide receptors and ectonucleotidases in quiescent and activated rat hepatic stellate cells. Am J Physiol Gastrointest Liver Physiol. 2004;287:G417-G424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 75] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 62. | Schreckenbach T, Liese J, Bechstein WO, Moench C. Posthepatectomy liver failure. Dig Surg. 2012;29:79-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 103] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 63. | Yoshida N, Iwata H, Yamada T, Sekino T, Matsuo H, Shirahashi K, Miyahara T, Kiyama S, Takemura H. Improvement of the survival rate after rat massive hepatectomy due to the reduction of apoptosis by caspase inhibitor. J Gastroenterol Hepatol. 2007;22:2015-2021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 64. | Malhi H, Gores GJ, Lemasters JJ. Apoptosis and necrosis in the liver: a tale of two deaths? Hepatology. 2006;43:S31-S44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 514] [Cited by in RCA: 499] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 65. | Guicciardi ME, Gores GJ. Apoptosis: a mechanism of acute and chronic liver injury. Gut. 2005;54:1024-1033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 381] [Cited by in RCA: 390] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 66. | Bai J, Odin JA. Apoptosis and the liver: relation to autoimmunity and related conditions. Autoimmun Rev. 2003;2:36-42. [PubMed] |
| 67. | Medema JP, Scaffidi C, Krammer PH, Peter ME. Bcl-xL acts downstream of caspase-8 activation by the CD95 death-inducing signaling complex. J Biol Chem. 1998;273:3388-3393. [PubMed] |
| 68. | Leifeld L, Nattermann J, Fielenbach M, Schmitz V, Sauerbruch T, Spengler U. Intrahepatic activation of caspases in human fulminant hepatic failure. Liver Int. 2006;26:872-879. [PubMed] |
| 69. | Mita A, Hashikura Y, Tagawa Y, Nakayama J, Kawakubo M, Miyagawa S. Expression of Fas ligand by hepatic macrophages in patients with fulminant hepatic failure. Am J Gastroenterol. 2005;100:2551-2559. [PubMed] |
| 70. | Hisakura K, Murata S, Fukunaga K, Myronovych A, Tadano S, Kawasaki T, Kohno K, Ikeda O, Pak S, Ikeda N. Platelets prevent acute liver damage after extended hepatectomy in pigs. J Hepatobiliary Pancreat Sci. 2010;17:855-864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 71. | Hisakura K, Murata S, Takahashi K, Matsuo R, Pak S, Ikeda N, Kawasaki T, Kohno K, Myronovych A, Nakano Y. Platelets prevent acute hepatitis induced by anti-fas antibody. J Gastroenterol Hepatol. 2011;26:348-355. [PubMed] |
| 72. | Datta SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, Greenberg ME. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997;91:231-241. [PubMed] |
| 73. | Stiles BL. PI-3-K and AKT: Onto the mitochondria. Adv Drug Deliv Rev. 2009;61:1276-1282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 118] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 74. | Bode AP, Fischer TH. Lyophilized platelets: fifty years in the making. Artif Cells Blood Substit Immobil Biotechnol. 2007;35:125-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 54] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 75. | Okamura Y, Takeoka S, Eto K, Maekawa I, Fujie T, Maruyama H, Ikeda Y, Handa M. Development of fibrinogen gamma-chain peptide-coated, adenosine diphosphate-encapsulated liposomes as a synthetic platelet substitute. J Thromb Haemost. 2009;7:470-477. [PubMed] |
| 76. | Hoshi R, Murata S, Matsuo R, Myronovych A, Hashimoto I, Ikeda H, Ohkohchi N. Freeze-dried platelets promote hepatocyte proliferation in mice. Cryobiology. 2007;55:255-260. [PubMed] |