Published online Feb 28, 2012. doi: 10.5412/wjsp.v2.i1.1
Revised: December 5, 2011
Accepted: December 20, 2011
Published online: February 28, 2012
A new procedure known as mid-colon esophagocoloplasty for corrosive esophageal strictures is described. This has been used in 112 consecutive patients over thirty years from 1977 to 2010 with excellent results. The conduit is a modification of the left colon conduit. It is based primarily on the left colic pedicle and includes the entire transverse colon with both flexures. It has several advantages over conventional methods of esophageal bypass for corrosive strictures. The major advantages of this procedure are excellent vascularity of the conduit, adequate length giving scope for operative decision making on the length required for the conduit, the possibility of a wide cervical esophago-colic anastomosis, negligible necrosisand stricture rates and excellent long-term functional results. The procedure, its differences and merits compared to conventional colonic conduits for esophageal replacement are described in detail.
- Citation: Ananthakrishnan N. Esophageal bypass using the midcolon: A modified technique for corrosive esophageal strictures. World J Surg Proced 2012; 2(1): 1-4
- URL: https://www.wjgnet.com/2219-2832/full/v2/i1/1.htm
- DOI: https://dx.doi.org/10.5412/wjsp.v2.i1.1
The chronic sequelae of corrosive ingestion such as esophageal strictures continue to remain a significant public health problem in many developing countries due to poor regulatory control and unrestricted availability of caustic substances which are sold across the counter for domestic use. The very nature of these injuries often dictates surgery for their amelioration since they are not amenable for dilatation. Over a thirty year period, a modification of the left colon coloplasty was developed in the unit. This procedure differs in several respects from the conventional left colon conduit, in so far as the segment of colon used, the procedure for mobilization of the conduit, the vascular supply and the nature of cervical esophago-colic anastomosis. The procedure was described briefly in an earlier publication from the unit[1]. The technique is described in detail and its advantages over conventional procedures are highlighted so that it may be tried by others managing this condition.
Midcolon esophagocoloplasty is offered to all patients with corrosive esophageal strictures including long narrow strictures, multiple strictures, non-dilatable strictures, recurrent strictures after repeated dilatation or previous esophageal perforation. All other patients with corrosive strictures are initially offered treatment by dilatation. Over the last thirty years, midcolon esophagocoloplastyhas been performed in 112 consecutive patients.
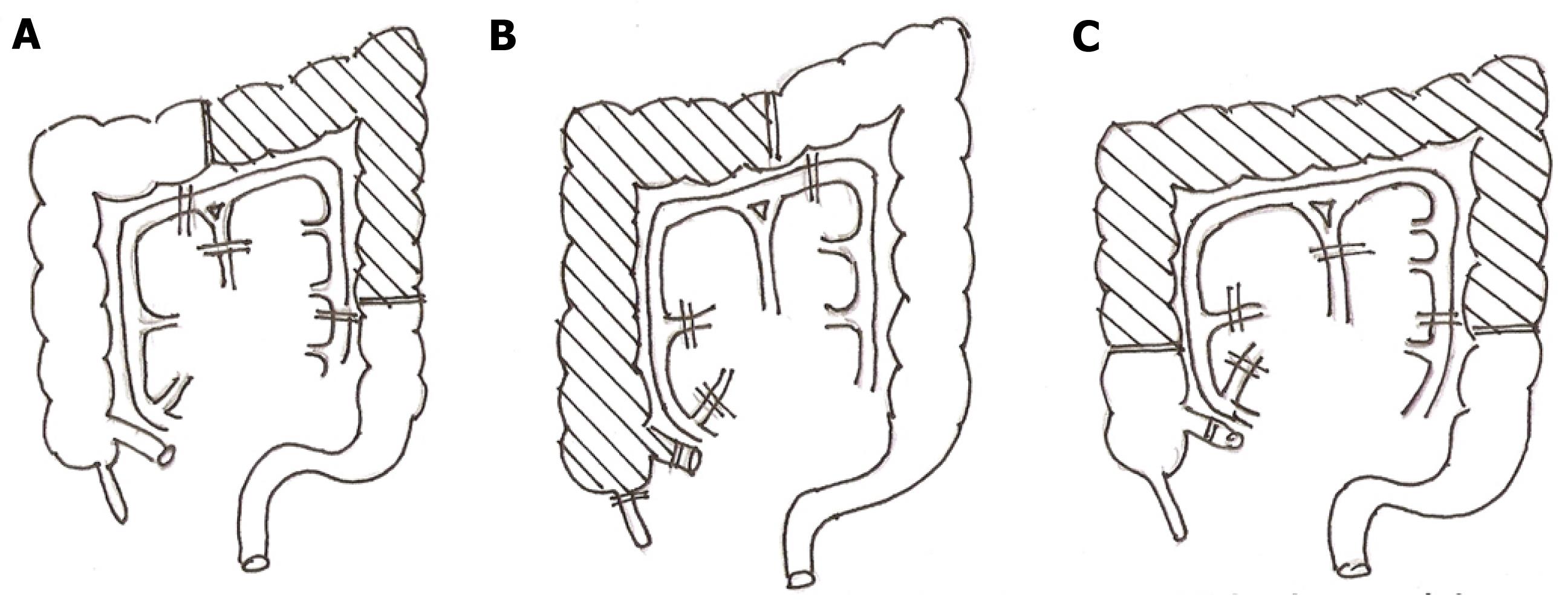
The segment of colon used for bypass extends from the mid ascending colon to the mid descending colon and is based on the left colic pedicle. It, therefore, differs from the conventional right colon bypass based on the middle colic vessel or the left colon bypass which extends from the mid transverse colon to the descending colon based on the left colic vessels. The segment of colon used and its pedicle in midcolon esophagocoloplastyand its differences compared with the conventional methods of coloplastyare shown in Figure 1.
In order to save time, the procedure is carried out by two teams operating simultaneously from the neck and abdomen. Access to the abdomen is through a long midline laparotomy and to the cervical esophagus through an incision along the anterior border of the left sternomastoid which curves at its lower end across the supra-sternal fossa. After ruling out corrosive gastric injury at laparotomy, the omentum is completely detached from the transverse colon and the pattern of colonic vasculature and the integrity of the marginal arcade is confirmed by transillumination of the mesocolon. Once the arcade is found to be complete with no interruption and the left colic vessels are found to be of sufficient caliber, the colon is mobilized from the terminal ileum to the mid descending colon. The ileo-colic and the right colic vessels are divided ensuring that the integrity of the marginal arcade is maintained. If the left colic pedicle consists of two or more vessels instead of a single dominant vessel, all of them are used for conduit vascularization. In most cases, if the left colic vessels are robust, the middle colic arterial pedicle is divided after prior temporary vascular clamping to ensure viability of the colon. The middle colic vessels are retained only under the following circumstances: (1) clamping of the middle colic leads to ischemia of the end of the colon most distal from the vascular pedicle; (2) a narrow marginal arcade between the left colic vessels and the middle colic vessels; (3) a thin and unsatisfactory left colic pedicle; and (4) a long middle colic vessel retention of which would not compromise the conduit length and its ability to reach theneck without tension. All these are uncommon and in the whole series of 112 patients, the middle colic vessel was retained in only thirteen instances (12%). Once colonic vascularity is ensured the terminal ileum is divided 5 cm, proximal to the ileo-cecal valve and the mid descending colon distal to the left colic vessels. Bowel continuity is restored by an end to end ileo-colic anastomosis in two layers using interrupted 2 “0” silk sutures.
A retrosternal tunnel of sufficient width to accommodate the conduit is created simultaneously from the neck and the abdomen after excising the xiphisternum. This is ensured by sequentially drawing thicker and thicker abdominal pads through the tunnel using a long Robert’s forceps after initial finger dissection from the neck and the abdomen until the fingers meet. Routine division of the clavicular head of the sternomastoid and separation of all adhesions at the thoracic inlet behind the manubrium and clavicles ensures that the space at the thoracic inlet is sufficient for the conduit. In no case was it necessary to resect the manubrium, the left sternoclavicular joint or the sternal ends of the clavicle to create this space.The colon is drawn into the neck through the mediastinal tunnel with the help of an umbilical tape tied around the terminal ileum guiding it from below with minimal traction from the neck until about four to six inches of colon is available in the neck above the manubrial notch. Tying the tape around the ileum instead of the colon ensures that the full length of the colon is available as required for the bypass. The pedicle of the conduit is carried anterior to the stomach taking care to ensure that there is no undue traction on it.
In patients in whom the mediastinal space is inadequate, the conduit is placed subcutaneously after creating sufficient space for it to lie without compression. The subcutaneous route is also used in patients with dense mediastinal adhesions following previous esophageal perforation or occasionally in children where there is insufficient room in the anterior mediastinum. The subcutaneous route was used in 16 patients (14%).
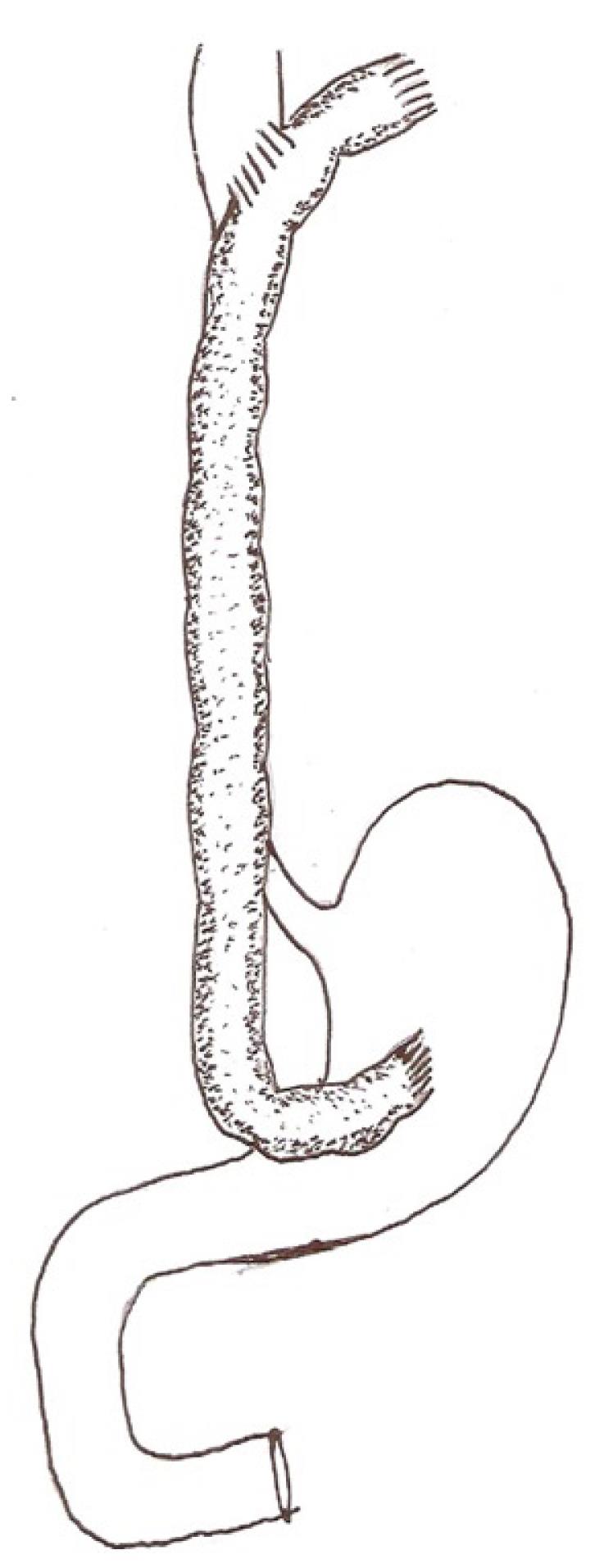
Cologastric continuity is established, end to side, using interrupted 2 “0” silk sutures. Before completing this anastomosis, a finger is passed through the gastrotomy created for the anastomosis beyond the pylorus to ensure that there is no unrecognized gastric outlet obstruction due to corrosive injury. In instances where the stomach has been previously removed or is densely contracted, the abdominal end of the conduit is anastomosed end to side to the proximal jejunum. The proximal end of the conduit is anastomosed side to side to the esophagus above the stricture, creating a wide 5 cm stoma, using interrupted 2 “0” silk sutures in two layers. Care is taken to ensure that the segment of colon selected for the esophago-colic anastomosis has good vascularity and can lie adjacent to the esophagus without tension. Interrupted sutures are used for all anastomoses to ensure that there is no ischemia at the site. Once the esophago-colic anastomosis is completed, the conduit is divided about 5 cm cephalad of the anastomosis and the blind end closed in two layers of interrupted sutures. The segment of excess conduit is excised only after ensuring that there is no tension at the cervical anastomosis, the conduit is viable and that there is sufficient conduit available proximal to the cervical anastomosis to correct stenosis at this site if it becomes necessary later on. The segment of excised bowel after completing the cervical anastomosis usually comprises about 5 cm of colon and the last 5 cm of terminal ileum. A feeding jejunostomy is always created. The details of this technique are shown in Figure 2.
This procedure has now been performed in 112 consecutive patients with only one instance of conduit necrosis. Contrary to usual perception, vascularity of this long segment of colon can be sustained by the left colic pedicle as seen by the fact that there was brisk bleeding from the cut end of the colon after completion of the cervical anastomosis in all patients. This vascularity is dependent on the integrity of the marginal arcade between the left colic system and the middle colic system which is normal. In those where this is deficient, retention of the middle colic vessel also ensures conduit vascularity. Overall cervical fistula rate was 13%. Cervical anastomotic stenosis was observed which was easily corrected by widening the anastomosis by extending into the conduit cephalad of the previous esophago-colic anastomosis with excellent functional results.
Midcolon esophagocoloplasty has several advantages: (1) not being based on preconceived vascular pedicles, it is adaptable on the table and can accommodate variations in vascular anatomy; (2) the conduit has excellent vascular supply; there was only one instance of conduit necrosis in 112 cases and this was due to accidental injury to the marginal arcade while the conduit was being placed in the mediastinal tunnel; (3) there is no problem of insufficient conduit length. In fact there is excess length which is excised only after the cervical anastomosis is completed without tension leaving behind an additional 5 cm. Shortage of conduit length and tension at the anastomosis are the main causes of cervical salivary fistulae, cervical anastomosis stenosis and conduit necrosis which are all avoided by this technique. Conventional methods of coloplasty involve preformed ideas of the length of the colon required for bypass and the pedicle to be used and separation of this conduit segment from the rest of the colon. This is responsible for subsequent realization that the length is inadequate and there is tension at the anastomotic site. These errors are avoided using the current technique; (4) by using the ileum to pull the colon into the neck, injury to the vascular pedicle and the marginal arcade of the colon is avoided; (5) the wide side to side esophago-colic anastomosis in the neck reduces the incidence of stricture at this site which is a common problem with gastric pull ups and conventional right colon coloplasty; (6) avoidance of an end to side esophago-colic anastomosis ensures that the terminal end of the conduit, which is furthest from the pedicle and is potentially ischemic, does not form part of the anastomosis. Ischemia of the anastomosis is by far the most common cause of cervical salivary fistulae. Use of a side to side anastomosis and interrupted sutures markedly decreased the incidence of cervical fistulae in our hands to less than 3% in the last 80 cases; (7) the extra length of the colon available cephalad of the anastomosis provides scope for future enlargement of the stoma should a stricture develop which is resistant to dilatation. This was required in one instance in our series; (8) a side to side colo-esophageal anastomosis avoids complications such asmucoceles or abscess in the residual esophagus which remains in continuity with the anastomotic site; (9) a mid colon conduit is possible irrespective of gastric involvement; and (10) previous gastroenterostomy for corrosive gastric outlet obstruction which would rule out a gastric conduit does not preclude its use.
Peer reviewer: Steven N Hochwald, MD, Chief, Associate Professor of Surgery, Edward M Copeland, III Professor of Surgical Oncology, Molecular Genetics and Microbiology, Division of Surgical Oncology, University of Florida College of Medicine, 1600 SW Archer Road, Room 6165, PO Box 100109, Gainesville, FL 32610, United States
S- Editor Yang XC L- Editor Webster JR E- Editor Yang XC
| 1. | Ananthakrishnan N, SubbaRao KSVK, Radjendirin P. Midcolon esophagocoloplasty for corrosive esophageal strictures. Aust N Z J Surg. 1993;63:389-395. [PubMed] [DOI] [Full Text] |