Published online Mar 1, 2024. doi: 10.5412/wjsp.v14.i2.8
Peer-review started: November 21, 2023
First decision: December 29, 2023
Revised: January 6, 2024
Accepted: February 1, 2024
Article in press: February 1, 2024
Published online: March 1, 2024
Processing time: 98 Days and 2 Hours
The management of tongue carcinoma is excision and radical neck dissection followed with reconstruction. This is a case report of a patient with tongue squamous cell carcinoma (SCC) who underwent the procedure with sternocleidomastoid (SCM) flap reconstruction.
A 52-year-old woman without smoking history complained tongue ulcer since 3 years ago. Based on the histopathological examination, the patient was diagnosed with T2N2M0 right tongue SCC and underwent wide excision of tumor; right mandibular; neck dissection and were reconstructed with SCM flap.
SCC of the tongue requires wide excision and dissection of the neck and mandible if infiltration into the surrounding lymph nodes has been found. The SCM flap reconstruction could be used post-surgery.
Core Tip: The sternocleidomastoid flap can be used for closing defects after resection of the tongue and floor of the mouth. We can choose this flap if there is no lymph node enlargement or metastasis at level 2, level 3, and level 4.
- Citation: Irawan H, Bharata MBS. Sternocleidomastoid flap for reconstruction of tongue small cell carcinoma: A case report. World J Surg Proced 2024; 14(2): 8-14
- URL: https://www.wjgnet.com/2219-2832/full/v14/i2/8.htm
- DOI: https://dx.doi.org/10.5412/wjsp.v14.i2.8
Carcinoma originating from the tongue tissue is an uncommon disease. The incidence of tongue carcinoma in Africa is 2.6 over 100000 populations, while in the United States the incidence number reaches 1.6-2.9 over 100000 populations. On the other hand, the incidence of tongue carcinoma in the Europe is 1.4 over 100000 populations, yet the information of tongue carcinoma prevalence in Indonesia has not been established[1]. About ninety percent of this neoplasm is squamous cell carcinoma (SCC)[2]. Histologically, carcinoma of the tongue has similar figures with carcinoma of the oral cavity. However, carcinoma from the base of the tongue has an important implication which makes this malignancy a disease to consider, with different managements, prognosis, and follow-ups[3]. Carcinoma of the tongue is usually found in elderly men with history of smoking and alcohol consumption. Nevertheless, in the last decades carcinoma of the tongue incidences in women and young patients with no history of smoking or alcohol consumption have increased. This may be associated with HPV infection or changes of genetic factors[4].
The primary management of tongue carcinoma is through surgery. The surgery is conducted in order to remove the primary tumor with wide margins from the normal surrounding tissues. The current standard is to eliminate the primary lesion with a macroscopic margin of 1.5-2 cm. With this standard, the parts of the tongue musculature that is needed to be removed could not be clearly determined by the pre-operative process. Other considerations that might complicate the surgery are the anatomy of the tongue, the size of the tumor, the extrinsic muscle invasion depth, and the presence of non-functional muscles for reconstructive purposes. In the last ten years, these considerations have been the main thoughts in developing surgical techniques for carcinoma of the tongue. Neck and mandibular dissection for the management of tongue carcinoma will leave defects that could not be closed directly, hence reconstruction by using flaps is required. One kind of flap which may be used to reconstruct 68 the neck and mandible is sternocleidomastoid (SCM) flap[5]. This case report aims to report a case of patient with SCC of the tongue who had undergone a wide excision along with neck and mandibular dissection, continued by reconstruction with SCM flap.
A 52-year-old woman came with mouth ulcer on the tongue in the last three years ago.
This complaint was also accompanied by pain. The lesion size and the pain were said to be increasing over time. These complaints were aggravated especially while eating. The patient said that the mouth ulcer appeared suddenly without any previous history of trauma. The patient brushed her teeth twice a day. History of weight lost, shortness of breath, and active bleeding were denied. History of smoking and consuming alcohol were denied.
The patient claimed that she had not experienced any similar complaints before. There was no history of hypertension, diabetes mellitus, heart disease, or allergies.
From the physical examinations, it was found that the vital signs and general status were in normal limits. The general condition of the patient was sufficient. From the local status of the tongue region, a mass was found, sized 6 cm × 6 cm, with a solid and bumpy consistency, and unclearly-defined irregular margin.
Laboratory findings such as complete blood count, electrolyte, kidney and liver function, blood sugar, as well as prothrombin time and activated partial thromboplastin time examination were within normal limits.
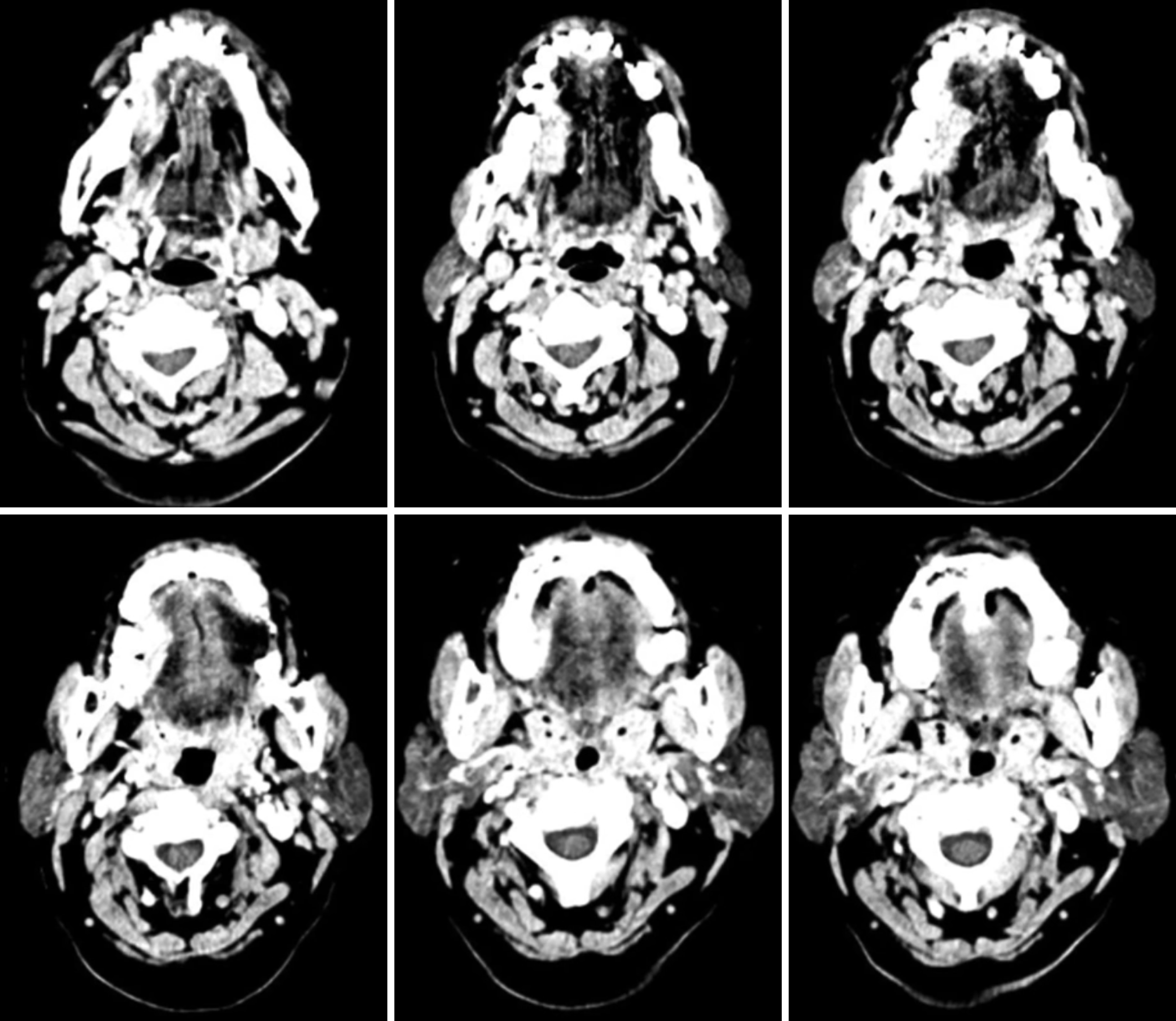
Chest X-ray results did not show any sign of lung metastasis. On the other hand, multi slice computed tomography (MSCT) with contrast examination showed partial compaction on the edge of the right tongue which on contrast administration appears to be heterogeneous contrast enhancement as shown on Figure 1.
The infiltration was suspected to be found on the right mylohyoid and hyoglossus muscles. Other findings were atypical right and left lymphadenopathy with the grade of IA/B, IIA/B, III, and IV, right sphenoid sinusitis, bilateral ethmoid and maxillary sinusitis, bilateral inferior nasal conchae hypertrophy, conchae bullosa of the right middle conchae, and cervical spondylosis as shown on Figure 1. Thyroid ultrasonography (USG) examination showed normal figures of the right and left thyroid glands and isthmus. In addition, multiple atypical lymphadenopathies were found on the right submandibular, right neck, and right post auricular region.
The patients tongue histopathological examination result showed there were moderately differentiated SCC.
The patient was diagnosed with right tongue SCC with moderately differentiated, T2N2M0.
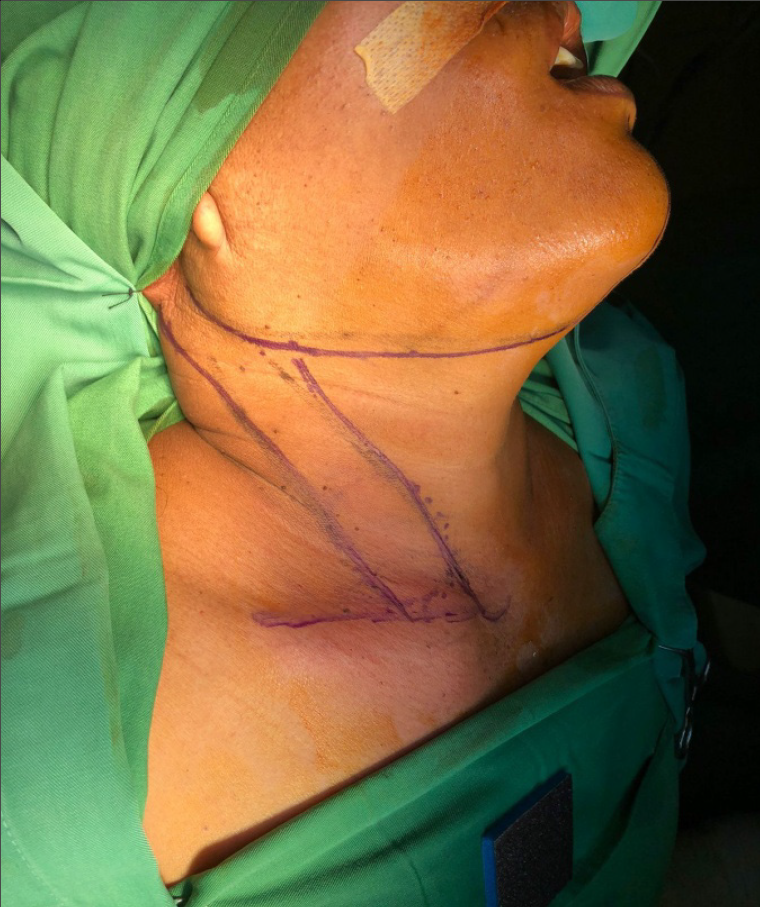
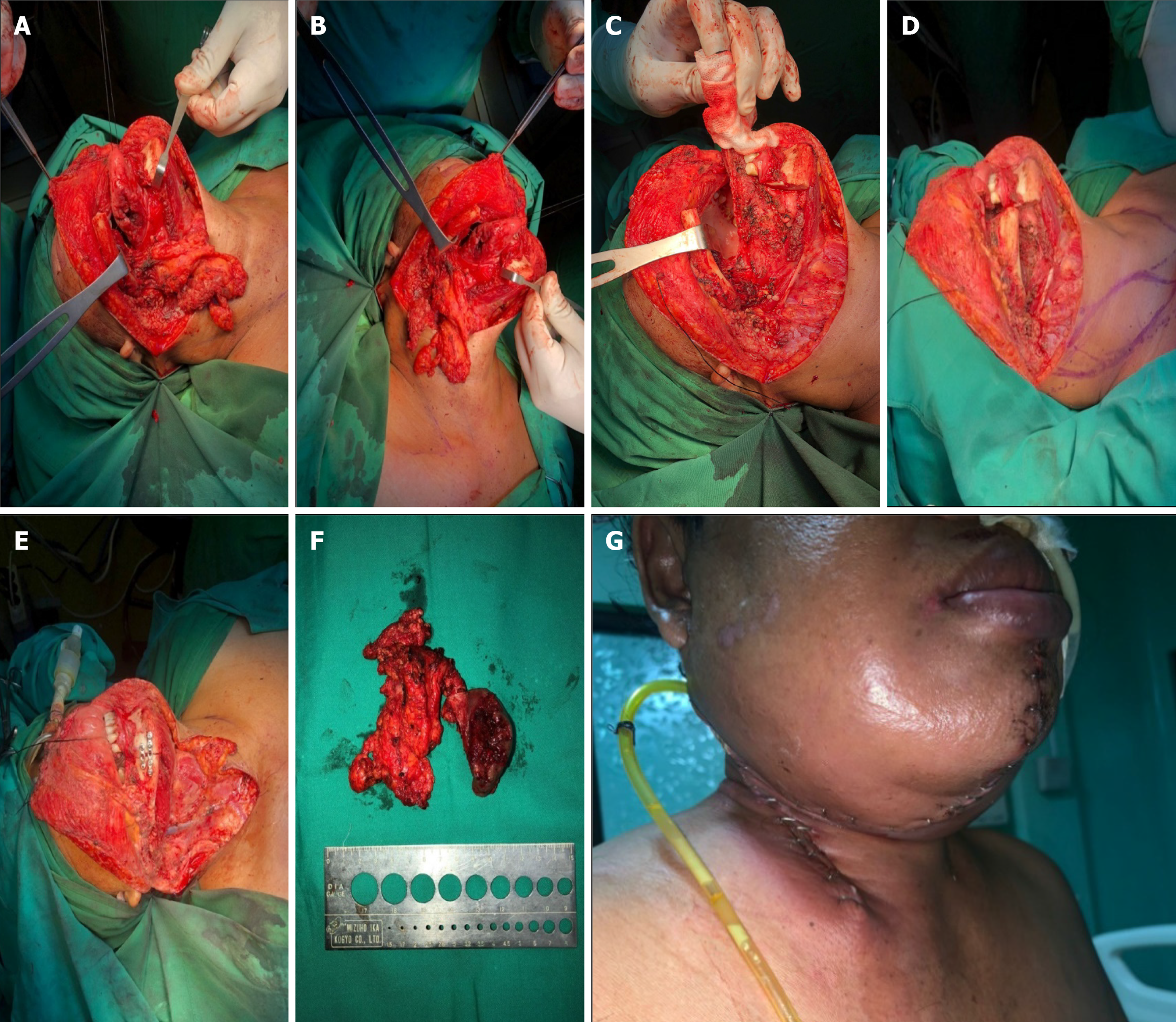
The patient was diagnosed with right tongue SCC with moderately differentiated, T2N2M0. The surgical site marking was done on the patient’s right side of the neck as shown on Figure 2. It was planned to undergo a wide excision of tumor, right mandibular and neck dissection as it is shown on Figure 3. The surgery was performed perfectly without any complication. Pathological result of the surgery was moderately differentiated SCC (pT2), with one metastatic lymph node of 4 at level IB, no metastatic of three lymph nodes at level IA, no metastatic of seven lymph nodes at level II, and the free margin was very close.
The therapy plan continued with chemotherapy but the patient died more than two months after surgery due to treatment for coronavirus disease 2019 infection.
SCC of the tongue is one of the malignancies that is found in the oral cavity. In this case, the patient was an elderly woman. Tongue SCC are usually found in elderly men populations with a history of smoking however there are numerous studies from the last decades showed that the incidence of this carcinoma has been increasing in women and younger patients without any history of smoking nor alcohol consumption. This condition could be associated with the incidence of HPV infection or genetic alterations[4].
The clinical manifestation that is found in this patient was typical for SCC of the tongue, which was a lesion on the tongue that had not been resolved for three years. Besides, the patient also complained local pain on the surrounding tissues. Physical examinations had been comprehensively done on this patient, along with a specific examination of the tongue as well as an investigation for metastatic signs on the lymph nodes[6]. The gold standard in diagnosing carcinoma of the tongue is through histopathological examination from tongue tissue biopsy. Histopathological examination of the lymph nodes may also be conducted to evaluate the signs of ongoing metastatic process. The pathological anatomy findings of this patient supported the theory that about ninety percent of carcinoma of the tongue are SCC[2]. Radiological examinations that are usually conducted for the suspicion of carcinoma of the head and neck include head and neck CT scan, and USG to observe any possible spreading to the thyroid glands and their surroundings. Moreover, radiological findings might also be useful as an assessment tool for any anatomical defect and planning the following reconstruction needed to optimize patient’s function and life quality[3].
The management that is chosen for this patient had been consistent according to the theory, in which the patient with the stadium of T2 should be treated through surgical approaches. Patients in the early stadium, T1 and T2, could be managed with single modality like surgery or radiation, with surgical approaches being the most commonly picked modality[7]. Another treatment such as drainage insertion was conducted in order to anticipate possible hematoma formation. In addition, wound care and antibiotic administration were executed to prevent furthermore infection of the wound and oro-cutaneous fistula. These are the complications that are commonly found subsequently the surgical managements in patients with SCC of the tongue[8].
There are several surgical techniques for carcinoma of the tongue, such as: (1) Type I mucosectomy; (2) Type II partial glossectomy; (3) Type III glossectomy: IIIa hemiglossectomy and IIIb compartmental hemiglossectomy; (4) Type IV glossectomy: IVa subtotal glossectomy and IVb near total glossectomy; and (5) Type V total glossectomy. The technique that were chosen for this patient was compartmental hemiglossectomy, in which the specimens that were removed includes the mucosal membrane, submucosal membrane, intrinsic and extrinsic muscles on the ipsilateral side of the lesion, genioglossus muscle, hyoglossus muscle, styloglossus muscle, and the inferior part of palatoglossal muscle. The lingual nerve was resected as far as possible towards the cranium and the hypoglossal nerve were also eliminated. The lingual artery and vein will also be ligated at the proximal edge of the hyoid bone. All the specimens and nodes in the neck will be removed en bloc[9]. This was the appropriate technique for this patient, considering the MSCT results that showed the figures of atypical lymphadenopathy which required resection of the nodes.
Compartmental tongue surgery (CTS) technique is the most commonly picked modality of the surgery due to its ability to maintain the function of every tongue compartment and explore tumor better. This technique allows the muscles in the compartment to be removed totally, which is essential to prevent any potential of micro metastatic process in partial tumors. This approach is a game-changer that creates a new paradigm for the resection methods, which were resection around the lesion into longitudinal resection. By this method, the surgeon will be able to do resection radically as well as non-selective demolition elimination with wide margin resection principle, especially on the early stages. However, this surgical approach by using CTS technique requires a wide resection area, therefore some anatomical and functional defects would be left. These functional defects are only associated with the muscles that were removed, which eventually considered as a similar outcome as the other surgical technique that only removes the muscles partially. The technique itself could be used to reconstruct and maintain the remaining function of the tongue aside from preventing functional[10]. A study by Piazza et al[11] stated that CTS showed remarkable oncological outcomes. The overall survival of 2 years was 80%, the disease-free survival was 91%, the local control was 100%, and the loco-regional control was 94%. Another study by Grammatica et al[12] with 1 year of follow-up found that CTS did not alter the speech function significantly.
The mandible and neck dissection will leave defects that require reconstruction. One of the considerable reconstruction techniques for head and neck surgery is by using SCM flap. This technique is usually conducted for intraoral and pharyngeal reconstruction. The principle of the reconstruction by using SCM flap is the utilization of the blood supplies from occipital artery, superior thyroid artery, and supracapsullar artery. The SCM flap can be a useful alternative in patients who are unable to have prolonged free flap surgery, or in the absence of a microvascular surgeon. Some advantages that make the SCM flap is preferable include shorter operating time compared to the free flap, reduction of muscle mass, lower morbidity, easily-shaped characteristic, and better comfort and hairless[13]. From a study of 20 patients with oral carcinoma, it was found that the application of the SCM flap for reconstruction purposes had great and favorable results, with only one case of partial flap loss[5].
SCC of the tongue should be managed through wide excision along with neck and mandibular dissection if infiltration to the surrounding lymph nodes has already been found. The SCM flap could be utilized to reconstruct the defect remained after the neck and mandibular dissection.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Dentistry, oral surgery and medicine
Country/Territory of origin: Indonesia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Boffano P, Italy S-Editor: Zhang H L-Editor: A P-Editor: Zhao YQ
| 1. | Hamdy R, Halim A. Squamous Cell Carcinoma of the Oral Tongue: A Single Institution Retrospective Cohort Study from Mansoura University Hospital. Indones J Cancer. 2019;12:102-108. [DOI] [Full Text] |
| 2. | Ettinger KS, Ganry L, Fernandes RP. Oral Cavity Cancer. Oral Maxillofac Surg Clin North Am. 2019;31:13-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 89] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 3. | Rivera C. Essentials of oral cancer. Int J Clin Exp Pathol. 2015;8:11884-11894. [PubMed] |
| 4. | Leoncini E, Ricciardi W, Cadoni G, Arzani D, Petrelli L, Paludetti G, Brennan P, Luce D, Stucker I, Matsuo K, Talamini R, La Vecchia C, Olshan AF, Winn DM, Herrero R, Franceschi S, Castellsague X, Muscat J, Morgenstern H, Zhang ZF, Levi F, Dal Maso L, Kelsey K, McClean M, Vaughan TL, Lazarus P, Purdue MP, Hayes RB, Chen C, Schwartz SM, Shangina O, Koifman S, Ahrens W, Matos E, Lagiou P, Lissowska J, Szeszenia-Dabrowska N, Fernandez L, Menezes A, Agudo A, Daudt AW, Richiardi L, Kjaerheim K, Mates D, Betka J, Yu GP, Schantz S, Simonato L, Brenner H, Conway DI, Macfarlane TV, Thomson P, Fabianova E, Znaor A, Rudnai P, Healy C, Boffetta P, Chuang SC, Lee YC, Hashibe M, Boccia S. Adult height and head and neck cancer: a pooled analysis within the INHANCE Consortium. Eur J Epidemiol. 2014;29:35-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 66] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 5. | Chen HC, Chang HS. The Sternocleidomastoid Flap for Oral Cavity Reconstruction: Extended Indications and Technical Modifications. J Oral Maxillofac Surg. 2015;73:2429-2439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 6. | Smith EM, Wang D, Kim Y, Rubenstein LM, Lee JH, Haugen TH, Turek LP. P16INK4a expression, human papillomavirus, and survival in head and neck cancer. Oral Oncol. 2008;44:133-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 75] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 7. | Abu-Ghanem S, Yehuda M, Carmel NN, Leshno M, Abergel A, Gutfeld O, Fliss DM. Elective Neck Dissection vs Observation in Early-Stage Squamous Cell Carcinoma of the Oral Tongue With No Clinically Apparent Lymph Node Metastasis in the Neck: A Systematic Review and Meta-analysis. JAMA Otolaryngol Head Neck Surg. 2016;142:857-865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 132] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 8. | Kim YJ, Kim JH. Increasing incidence and improving survival of oral tongue squamous cell carcinoma. Sci Rep. 2020;10:7877. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 53] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 9. | Ansarin M, Bruschini R, Navach V, Giugliano G, Calabrese L, Chiesa F, Medina JE, Kowalski LP, Shah JP. Classification of GLOSSECTOMIES: Proposal for tongue cancer resections. Head Neck. 2019;41:821-827. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 77] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 10. | Paderno A, Morello R, Piazza C. Tongue carcinoma in young adults: a review of the literature. Acta Otorhinolaryngol Ital. 2018;38:175-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 44] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 11. | Piazza C, Grammatica A, Montalto N, Paderno A, Del Bon F, Nicolai P. Compartmental surgery for oral tongue and floor of the mouth cancer: Oncologic outcomes. Head Neck. 2019;41:110-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 12. | Grammatica A, Piazza C, Montalto N, Del Bon F, Frittoli B, Mazza M, Paderno A, Lancini D, Fior M, Deganello A, Lombardi D, Nicolai P. Compartmental Surgery for Oral Tongue Cancer: Objective and Subjective Functional Evaluation. Laryngoscope. 2021;131:E176-E183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 13. | Jones LF, Farrar EM, Roberts DJH, Moor JW. Revisiting the sternocleidomastoid flap as a reconstructive option in head and neck surgery. J Laryngol Otol. 2019;133:742-746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |