Published online Dec 28, 2023. doi: 10.5412/wjsp.v13.i3.22
Peer-review started: October 14, 2023
First decision: October 24, 2023
Revised: November 8, 2023
Accepted: November 21, 2023
Article in press: November 21, 2023
Published online: December 28, 2023
Processing time: 74 Days and 5.8 Hours
Endoscopic submucosal dissection is considered curative for patients with early rectal cancer when level of submucosal invasion is < 1000 microns with fa
A 66 year old New Zealand European male presented with 3 mo history of per rectal bleeding. He was referred for a colonoscopy test to investigate this further. This revealed a malignant appearing lesion in the rectum. Biopsies however showed high grade dysplasia only. Given endoscopic appearances suspicious for deep submucosal invasion, patient was consented for endoscopic intermuscular dissection (EID). The case was successfully performed, and the presence of mu
EID is a safe and feasible option for management of rectal cancer in highly selected patients.
Core Tip: Endoscopic intermuscular dissection is a novel technique for management of locally advanced rectal cancer especially for patients who are not fit for oncological surgery or chemoradiotherapy. This technique ensures local resection of the tumour which is safe and feasible with minimal recovery times.
- Citation: Sekra A, Tan T. Endoscopic intermuscular dissection for locally advanced rectal cancer: A case report. World J Surg Proced 2023; 13(3): 22-28
- URL: https://www.wjgnet.com/2219-2832/full/v13/i3/22.htm
- DOI: https://dx.doi.org/10.5412/wjsp.v13.i3.22
Endoscopic submucosal dissection (ESD), for early rectal cancer, is considered a curative option for patients. It is an organ preserving surgery with higher rates of en bloc resection and lower risk of local recurrence compared with endoscopic mucosal resection[1]. It is significantly cheaper than radical surgery, with significantly fewer days in hospital and has lower morbidity and mortality[2-4].
Early rectal cancer is defined as T1 cancer with invasion < 1000 microns into the submucosa (SM) or SM1 Kikuchi levels, as the risk of nodal metastasis is close to 0%. ESD is considered curative for these lesions[5].
Recent European Society of Gastrointestinal Endoscopy guidelines also recommend that an en bloc R0 resection of a superficial lesion with histology no more advanced than well-differentiated adenocarcinoma (G1/G2), sm1 (< 1 mm SM invasion) with no lymphovascular invasion (LVI), would be considered curative. Surgery, on the other hand, is recommended when LVI (deeper infiltration than SM1), positive/ non evaluable vertical margins, or poorly differentiated tumour with SM invasion is diagnosed[6]. However, more recent data suggests that the depth of SM invasion is not an independent risk factor for lymph node metastasis (LNM) in T1 colorectal cancer (CRC)[7]. Several studies have shown that in the absence of adverse histopathological risk factors, such as; poor differentiation, high tumour budding, LVI, and perineural invasion, the risk of LNM (regardless of depth of SM invasion) is extremely low[8-11].
With ESD the dissection plane is limited to SM. As a result, the vertical margins are more likely to be positive if the lesions invade deeper into the SM[12]. More recently, endoscopic intermuscular dissection (EID) technique has been described to achieve R0 resection for the management of lesions with deeper submucosal invasion[13,14].
EID technique has evolved from ESD. The principle of ESD is based on the dissection of the submucosal plane. EID, on the other hand, focuses on the dissection of the superficial circular muscle layer of the rectum, allowing the longitudinal layer to remain intact. This essentially means that the EID can achieve a deeper dissection compared to ESD.
A 66 year old New Zealand European male presented with per rectal bleeding.
Patient presented with 3 mo history of intermittent per rectal bleeding. He reported that the blood was often mixed in the stools. He did not report any history of of changes in bowel habits or weight loss.
Patient has known history of ischaemic heart disease and he underwent a previous angioplasty. He has known congestive heart failure from ischaemic cardiomyopathy. He also had poorly controlled type 2 diabetes mellitus and is on insulin.
There was no known personal or family history of bowel cancer.
A physical exam including a digital rectal exam was completely normal.
Patient's full blood count, iron studies, and carcinoembryonic antigen were all normal.
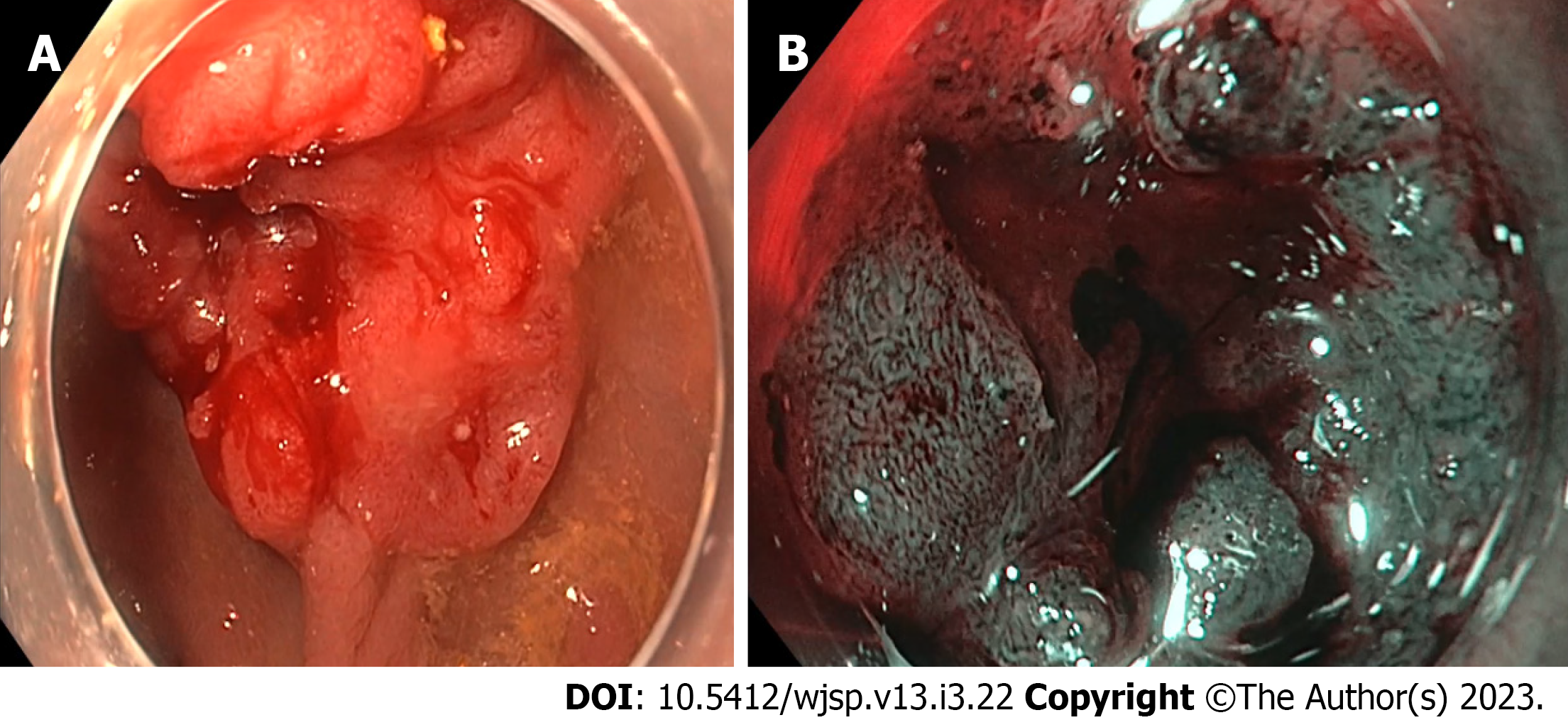
The colonoscopy revealed a 30 mm rectal tumour, located 10 cm from anal verge, at the posterior wall of the rectum. The lesion was IIc/IIa lesion as per Paris classification (Figure 1A). There was a type Vn Kudo pit pattern in the area of the depression. Magnification virtual chromoendoscopy revealed a Narrow Band Imaging International Colorectal Endoscopic classification 3, Japan Narrow Band Imaging Expert Team classification 3 lesion (Figure 1B). Biopsies taken from the lesion showed high grade dysplasia only. The patient underwent a staging computed tomography scan which did not shown any evidence of metastatic disease. Magnetic resonance imaging showed a T2N0M0 rectal lesion at the posterior wall.
An invasive cancer was suspected based on the endoscopic features. Consequently, the patient was referred to the colorectal surgeons for further management. Since initial biopsies had shown high grade dysplasia only, the patient underwent a second biopsy, which again showed high grade dysplasia. Due to the clinical suspicion of invasive cancer, the patient was then considered for radical surgery. Unfortunately, he had significant comorbidities and was therefore deemed an unsuitable candidate for radical surgery. He was further evaluated for a transanal minimally invasive surgery for accurate staging and diagnosis, but was deemed extremely high risk to even receive a general anaesthesia.
The case was discussed in the multidisciplinary team meeting and the consensus was to perform an endoscopic resection with a view to; confirm the diagnosis, accurately stage the disease, and to attempt a curative resection. Because a deep invasion was suspected, the patient was consented for an EID under conscious sedation.
Invasive rectal cancer.
Patient underwent EID procedure. The procedure was performed under conscious sedation with midazolam and fentanyl. A standard gastroscope (Olympus GIF-HQ190, Olympus, Tokyo, Japan) with a transparent hood (Olympus, Tokyo, Japan) was used. Submucosal lifting was performed with a mixture of adrenaline, methylene blue, and hydroethyl starch (Voluven® 6%). A 1.5 mm dual knife J (Olympus, America) was used, with an endocut mode of the ERBE VIO 300D (ERBE Elektromedizin, Tübingen, Germany).
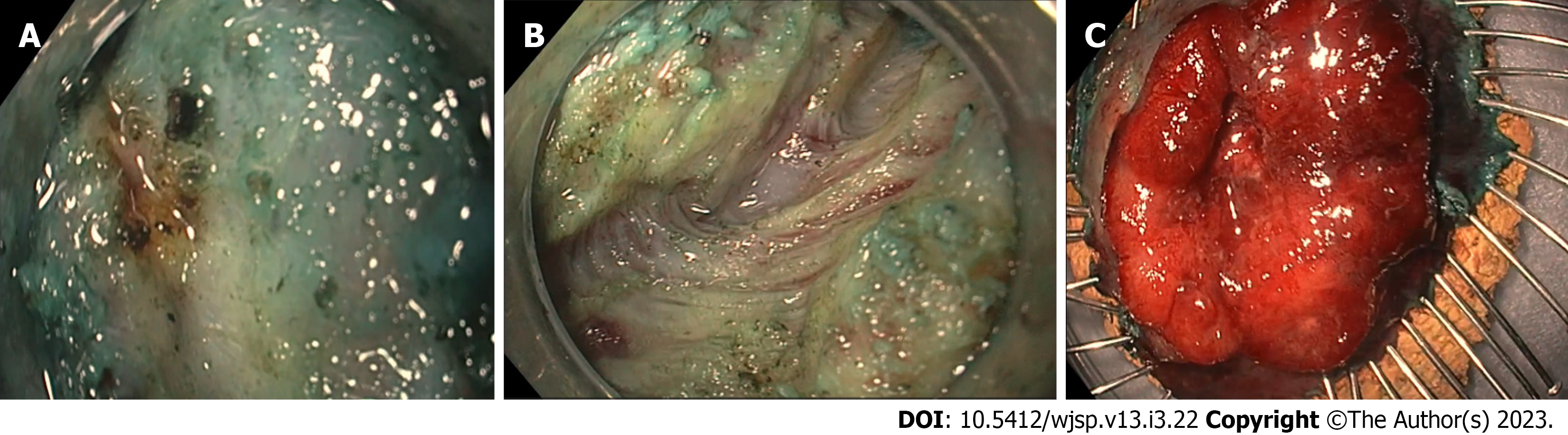
Firstly, a mucosal incision was created at the anal side of the lesion. Submucosal dissection was then performed in the same area. This was, however, stopped due to poor access and visualisation of the submucosal space. An incision was then made at the oral side and the circumferential incision was completed. The scope was entered into the submucosal space using the transparent hood. Significant fibrosis was encountered at this point. The fibrotic area was dissected (Figure 2A). The inner circular layer was visible at this point. An attempt was made to inject just above the muscle layer; however, this could not be achieved due to fibrosis. The inner circular layer was then dissected from the oral side to the anal side of the lesion, managing to keep the outer longitudinal layer intact (Figure 2B). The tumour was finally released from the muscle layer and was resected en bloc. The lesion was pinned on the cork and then submitted for histology (Figure 2C). The total procedure time was 124 min. The patient was given IV antibiotics intraoperatively and was discharged home after 2 h of observation. He received a one-week course of oral antibiotics. There were no immediate or delayed complications noted.
Figure 2A shows the significance of the fibrosis encountered during Endoscopic Intermuscular Dissection. After dissecting the fibrotic area, the inner muscle layer was dissected, keeping the outer longitudinal layer intact, as shown in Figure 2B. The tumour was released from the muscle layer and pinned on the cork board and submitted for histological analysis (Figure 2C).
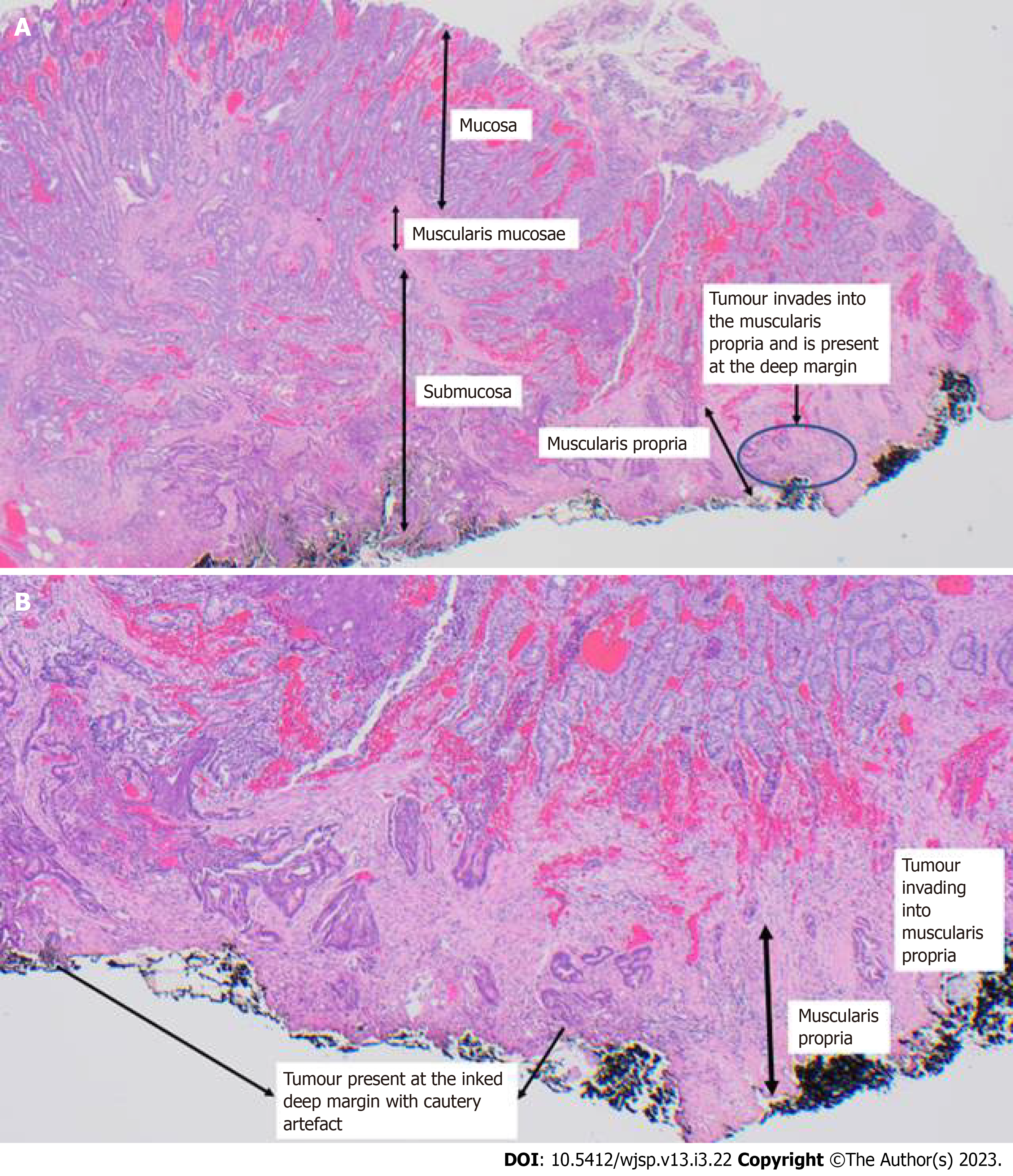
The histology confirmed the presence of muscularis propria in the specimen. This is a low-grade adenocarcinoma, with absent LVI, absent perineural invasion and low tumour budding. The lesion was clear of peripheral margins by 7 mm. Vertical margins, however, confirmed infiltration of the tumour into the muscularis propria and were positive. This confirmed it as at least a pT2 lesion (Figure 3).
This histology was again discussed in a multidisciplinary meeting, and it was concluded that the patient will require additional treatment. He was referred for long course chemoradiotherapy, which he successfully completed without any side effects. A follow up colonoscopy, 6 mo after the resection, showed no residual disease. An MRI scan of the rectum was performed 9 mo after the resection and showed no recurrence of disease. After 9 mo of follow up, the patient had remained well, with no symptoms and no delayed complications from the EID procedure.
EID was first described by Rahni et al[13] for resection of rectal lesions with significant fibrosis. It is a new technique where a dissection is carried out of the inner circular muscle layer in the intermuscular plane, while keeping the outer longitudinal layer in the rectum intact. A recent case series of 67 patients described this technique for resection of rectal cancers with suspected invasion beyond the SM1 layer. This series concluded that EID is a feasible technique, with a technical success of 96% and a safe procedure requiring no surgery in EID related complications. 12% of patients had minor adverse events[14].
The case discussed above, was the first such procedure to be performed in New Zealand. Although the patient did not have R0 resection and ideally should have had radical surgery, his comorbidities meant that such surgery was prohibitive. Radical surgery can still be safely performed in low risk patients who have non curative ESD[15]. Our patient underwent long course chemoradiotherapy instead, with no disease recurrence after 9 mo of follow up.
There is preliminary data to suggest that patients who undergo local resection, followed by adjuvant chemoradiotherapy, can preserve their rectum and have better QoL[16]. A large, randomized trial (TESAR trial) is underway and results are awaited with interest[17]. This trial will consider this issue and, if positive, radical surgery might be avoidable for high risk patients in future.
A recent study evaluated the clinical outcome of non-curative ESD for early CRC. This study included 207 non-curative ESD cases and showed the tumour recurrence and disease specific survival rates were similar in patients who had radical surgery vs those who were followed up by endoscopy (after a median follow-up of 30 mo)[18]. Additional treatment decisions are often based on patient comorbidities and risks of additional treatment should be carefully weighed against the benefits. The patient discussed above was considered fit enough for long course chemoradiotherapy, with the aim to treat both the local residual disease and LNM if present.
This groundbreaking EID case, the first to be performed in New Zealand, highlights that this technique is an option worth considering for some patients. It is feasible and can be performed successfully, without any major complications, in highly selected lesions and patient groups. It can potentially offer curative local resection of rectal cancer despite deep submucosal invasion, showing favourable histopathological features in carefully selected patients.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Surgery
Country/Territory of origin: New Zealand
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Li JJ, China S-Editor: Qu XL L-Editor: A P-Editor: Qu XL
| 1. | Wang J, Zhang XH, Ge J, Yang CM, Liu JY, Zhao SL. Endoscopic submucosal dissection vs endoscopic mucosal resection for colorectal tumors: a meta-analysis. World J Gastroenterol. 2014;20:8282-8287. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 87] [Cited by in RCA: 100] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 2. | Arthursson V, Rosén R, Norlin JM, Gralén K, Toth E, Syk I, Thorlacius H, Rönnow CF. Cost comparisons of endoscopic and surgical resection of stage T1 rectal cancer. Endosc Int Open. 2021;9:E1512-E1519. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 3. | Steele SR, Bleier J, Champagne B, Hassan I, Russ A, Senagore AJ, Sylla P, Pigazzi A. Improving outcomes and cost-effectiveness of colorectal surgery. J Gastrointest Surg. 2014;18:1944-1956. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 4. | Lee CM, Huh JW, Yun SH, Kim HC, Lee WY, Park YA, Cho YB, Chun HK. Laparoscopic versus open reintervention for anastomotic leakage following minimally invasive colorectal surgery. Surg Endosc. 2015;29:931-936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 5. | Eleftheriadis N, Inoue H, Ikeda H, Onimaru M, Yoshida A, Maselli R, Santi G, Kudo S. Definition and staging of early esophageal, gastric, and Colorectal Cancer. Journal of tumour. 2014;18:161-178. |
| 6. | Pimentel-Nunes P, Dinis-Ribeiro M, Ponchon T, Repici A, Vieth M, De Ceglie A, Amato A, Berr F, Bhandari P, Bialek A, Conio M, Haringsma J, Langner C, Meisner S, Messmann H, Morino M, Neuhaus H, Piessevaux H, Rugge M, Saunders BP, Robaszkiewicz M, Seewald S, Kashin S, Dumonceau JM, Hassan C, Deprez PH. Endoscopic submucosal dissection: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2015;47:829-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 817] [Cited by in RCA: 928] [Article Influence: 92.8] [Reference Citation Analysis (0)] |
| 7. | Rönnow CF, Arthursson V, Toth E, Krarup PM, Syk I, Thorlacius H. Lymphovascular Infiltration, Not Depth of Invasion, is the Critical Risk Factor of Metastases in Early Colorectal Cancer: Retrospective Population-based Cohort Study on Prospectively Collected Data, Including Validation. Ann Surg. 2022;275:e148-e154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 72] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 8. | Kitajima K, Fujimori T, Fujii S, Takeda J, Ohkura Y, Kawamata H, Kumamoto T, Ishiguro S, Kato Y, Shimoda T, Iwashita A, Ajioka Y, Watanabe H, Watanabe T, Muto T, Nagasako K. Correlations between lymph node metastasis and depth of submucosal invasion in submucosal invasive colorectal carcinoma: a Japanese collaborative study. J Gastroenterol. 2004;39:534-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 468] [Cited by in RCA: 487] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 9. | Nakadoi K, Tanaka S, Kanao H, Terasaki M, Takata S, Oka S, Yoshida S, Arihiro K, Chayama K. Management of T1 colorectal carcinoma with special reference to criteria for curative endoscopic resection. J Gastroenterol Hepatol. 2012;27:1057-1062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 152] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 10. | Sohn DK, Chang HJ, Park JW, Choi DH, Han KS, Hong CW, Jung KH, Kim DY, Lim SB, Choi HS, Jeong SY. Histopathological risk factors for lymph node metastasis in submucosal invasive colorectal carcinoma of pedunculated or semipedunculated type. J Clin Pathol. 2007;60:912-915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 76] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 11. | Oka S, Tanaka S, Nakadoi K, Kanao H, Chayama K. Risk analysis of submucosal invasive rectal carcinomas for lymph node metastasis to expand indication criteria for endoscopic resection. Dig Endosc. 2013;25 Suppl 2:21-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 12. | Watanabe D, Toyonaga T, Ooi M, Yoshizaki T, Ohara Y, Tanaka S, Kawara F, Ishida T, Morita Y, Umegaki E, Matsuda T, Sumi Y, Nishio M, Yokozaki H, Azuma T. Clinical outcomes of deep invasive submucosal colorectal cancer after ESD. Surg Endosc. 2018;32:2123-2130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 13. | Rahni DO, Toyonaga T, Ohara Y, Lombardo F, Baba S, Takihara H, Tanaka S, Kawara F, Azuma T. First reported case of per anal endoscopic myectomy (PAEM): A novel endoscopic technique for resection of lesions with severe fibrosis in the rectum. Endosc Int Open. 2017;5:E146-E150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 14. | Moons LMG, Bastiaansen BAJ, Richir MC, Hazen WL, Tuynman J, Elias SG, Schrauwen RWM, Vleggaar FP, Dekker E, Bos P, Fariña Sarasqueta A, Lacle M, Hompes R, Didden P. Endoscopic intermuscular dissection for deep submucosal invasive cancer in the rectum: a new endoscopic approach. Endoscopy. 2022;54:993-998. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 62] [Article Influence: 20.7] [Reference Citation Analysis (1)] |
| 15. | Chen T, Zhang YQ, Chen WF, Hou YY, Yao LQ, Zhong YS, Xu MD, Zhou PH. Efficacy and safety of additional surgery after non-curative endoscopic submucosal dissection for early colorectal cancer. BMC Gastroenterol. 2017;17:134. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 16. | Coco C, Magistrelli P, Netri G, Cogliandolo S, Carbone L, Morganti AG, Ziccarelli L, Valentini V. Combined modality therapy in low risk (T2N0) rectal cancer. Rays. 1995;20:156-164. [PubMed] |
| 17. | Borstlap WA, Tanis PJ, Koedam TW, Marijnen CA, Cunningham C, Dekker E, van Leerdam ME, Meijer G, van Grieken N, Nagtegaal ID, Punt CJ, Dijkgraaf MG, De Wilt JH, Beets G, de Graaf EJ, van Geloven AA, Gerhards MF, van Westreenen HL, van de Ven AW, van Duijvendijk P, de Hingh IH, Leijtens JW, Sietses C, Spillenaar-Bilgen EJ, Vuylsteke RJ, Hoff C, Burger JW, van Grevenstein WM, Pronk A, Bosker RJ, Prins H, Smits AB, Bruin S, Zimmerman DD, Stassen LP, Dunker MS, Westerterp M, Coene PP, Stoot J, Bemelman WA, Tuynman JB. A multi-centred randomised trial of radical surgery versus adjuvant chemoradiotherapy after local excision for early rectal cancer. BMC Cancer. 2016;16:513. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 76] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 18. | Spadaccini M, Bourke MJ, Maselli R, Pioche M, Bhandari P, Jacques J, Haji A, Yang D, Albéniz E, Kaminski MF, Messmann H, Herreros de Tejada A, Sferrazza S, Pekarek B, Rivory J, Geyl S, Gulati S, Draganov P, Shahidi N, Hossain E, Fleischmann C, Vespa E, Iannone A, Alkandari A, Hassan C, Repici A; ESD Western Alliance (EWA). Clinical outcome of non-curative endoscopic submucosal dissection for early colorectal cancer. Gut. 2022;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 11.7] [Reference Citation Analysis (0)] |