Published online Dec 30, 2021. doi: 10.5412/wjsp.v11.i1.1
Peer-review started: June 26, 2021
First decision: September 2, 2021
Revised: September 6, 2021
Accepted: December 7, 2021
Article in press: December 7, 2021
Published online: December 30, 2021
Processing time: 186 Days and 22.7 Hours
Post-arachnoiditis syringomyelia is a condition in which there is an intraspinal cerebrospinal fluid (CSF) blockade due to arachnoidal adhesions and bands. Although many of the techniques currently in use, namely, the theco-peritoneal, syringo-pleural, syringo-peritoneal, and syringo-subarachnoid shunts, are effective, the results are often variable.
A 36-year-old man with a past history of pulmonary tuberculosis, presented with progressive paraesthesia in the feet and progressive paraparesis along with constipation, difficulty in micturition, and decreased libido. He was bedridden a month before presentation. Magnetic resonance imaging revealed a dorsal multiloculated syrinx from D3-D10 vertebral levels. He underwent a D1-2 to D11 theco-thecal shunt bilaterally to abolish the CSF gradient across the level of the syrinx. There was no direct surgical handling of the spinal cord involved. At the 15-mo follow up, the patient had significant improvement in his symptoms and function.
We present a novel technique aimed at correcting the primary cause of a post-arachnoiditis syrinx, the subarachnoid cerebrospinal flow obstruction or block, which we believe is simple and effective, involves minimal handling of the normal neural structures, and attempts to restore the physiology of CSF flow across the obstruction, with favorable clinical results.
Core Tip: Theco-thecal bypass technique was envisaged to achieve a long lasting zero net pressure difference via free flow of cerebrospinal fluid above and below the level of spinal subarachnoid adhesions with minimal handling of the spinal cord and avoidance of extensive adhesiolysis that is associated with a high rate of re-adhesions and failure. This surgical technique involves a valve-less shunt conduit connecting the subarachnoid space above and below the level of adhesions and thereby abolishing the trans-mural pressure gradient causing the syringomyelia, leading to a drastic clinical improvement, as elaborated in this case report.
- Citation: Bhatjiwale M, Bhatjiwale M. Theco-thecal bypass technique elucidating a novel procedure and perspective on treatment of post-arachnoiditis syringomyelia: A case report. World J Surg Proced 2021; 11(1): 1-9
- URL: https://www.wjgnet.com/2219-2832/full/v11/i1/1.htm
- DOI: https://dx.doi.org/10.5412/wjsp.v11.i1.1
A syrinx is a fluid-filled cavity that anatomically lies within the spinal cord paren
Despite the several proposed etiopathogenetic mechanisms and therefore a number of different treatment strategies[5], the management of post-arachnoiditis syrin
We present the case of a patient with post-arachnoiditis syringomyelia who was treated surgically by reconstituting CSF flow without passing through the adhesion zone, using a theco-thecal bypass. This new technique, its theoretical safety, and clinical efficacy are described in detail.
A 36-year-old Indian man presented with progressive paraesthesia and dysesthesia in the plantar aspect of both feet for 3 years, along with progressive paraparesis and low backache.
The patient was bedridden a month before presentation and was brought in on a wheelchair. He also complained of constipation, difficulty and straining while micturating, and decreased libido.
The patient was diagnosed with pulmonary tuberculosis 20 years ago, had received anti-tubercular treatment for 6 mo as per national protocols, and was declared cured. Treatment was sought after 3 years of onset symptoms in view of recent aggravation.
The family history was unremarkable.
A neurological examination revealed normal upper extremity power, reflexes, and sensations bilaterally. There was mild dissociated sensory loss below the D4 dermatome along with spastic paraparesis with a modified Medical Research Council (mMRC) grade 2 power in the right and grade 3 in the left lower limb muscle groups. Grade 4 spasticity as per the modified Ashworth Spasticity Score (mASS) was noted in bilateral lower limbs along with exaggerated deep tendon reflexes and extensor plantar responses.
The routine hematological workup, including complete blood counts, and renal and hepatic function tests were normal.
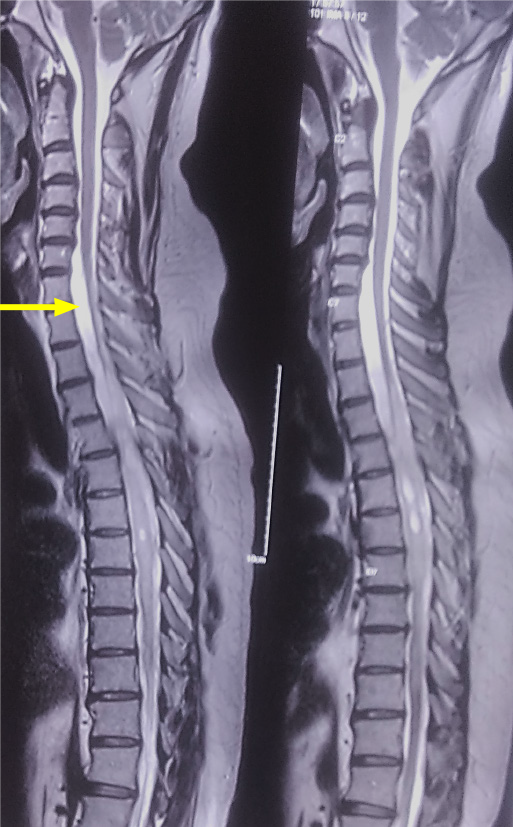
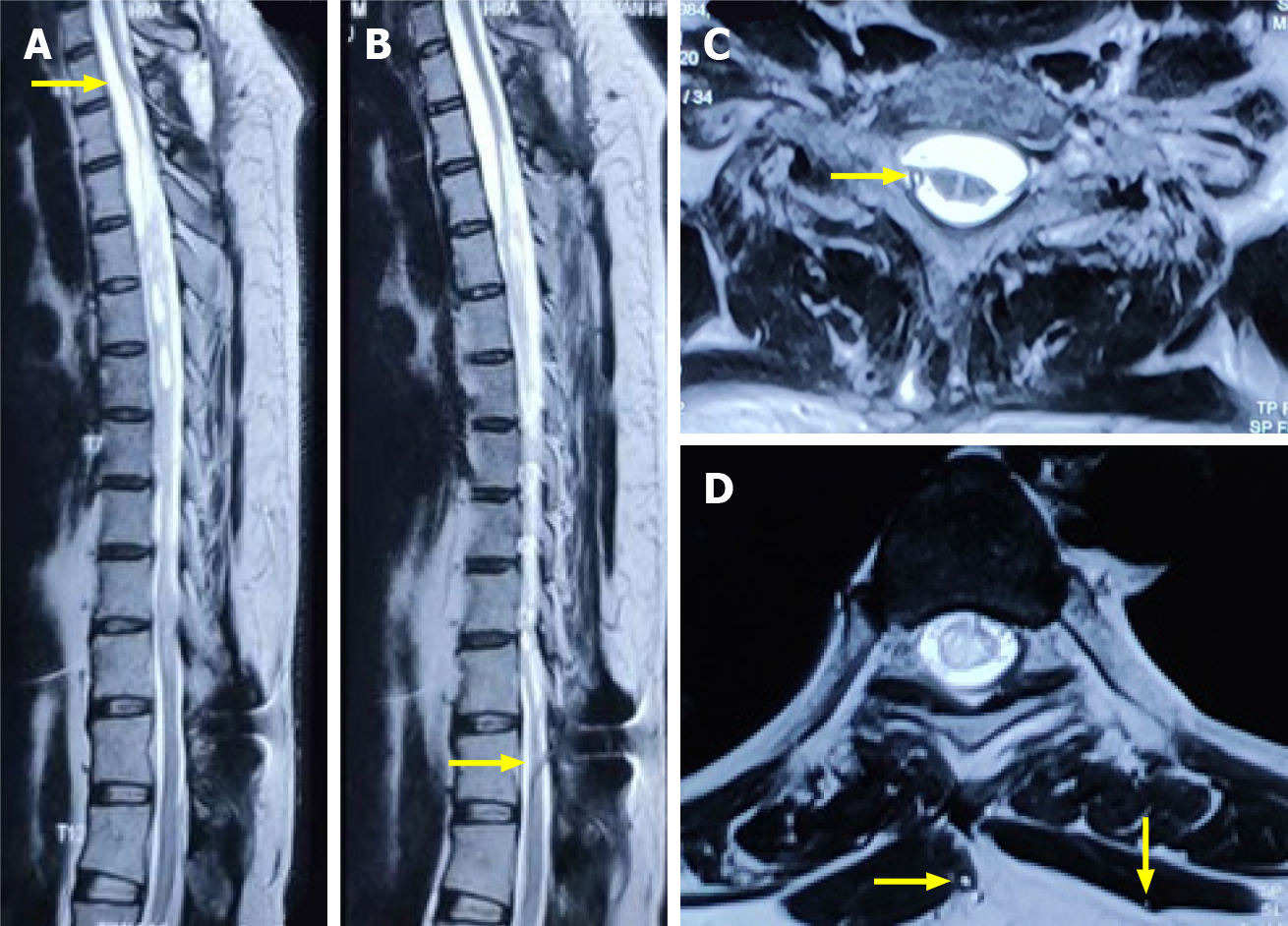
Magnetic resonance imaging (MRI) of the dorsal spine was performed with whole spine screening, which revealed a multiloculated syrinx from D3-D10 vertebral levels with a CSF loculation anterior to the lower cervical and upper dorsal cord (Figure 1).
There were features of extensive subarachnoid adhesions in the form of greatly varying peri-myelic CSF along with areas of absent CSF intensity. To minimize cord handling and avoid extensive adhesiolysis with its associated risks, a first-in-human theco-thecal bypass procedure (TTBP) was performed after obtaining informed consent from the patient and due permission from the local institutional body. The patient's consent for publication was also obtained at this stage.
A final diagnosis of post-arachnoiditis syringomyelia was made.
To minimize cord handling and avoid extensive adhesiolysis with its associated risks, a first-in-human TTBP was performed after obtaining informed consent from the patient and due permission from the local institutional body. The patient's consent for publication was also obtained at this stage.
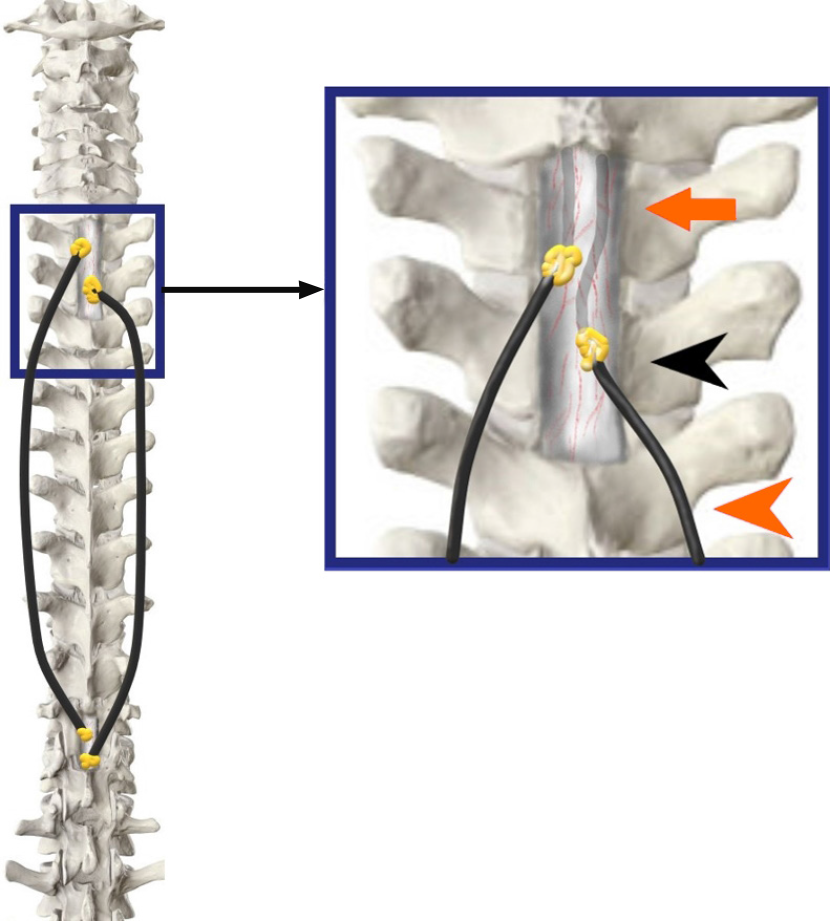
The surgery was performed by a senior neurosurgeon and a spine surgeon at a tertiary care community center. The laminectomy level above and below the extent of syringomyelia was marked on the previous day under CT guidance for precision and to reduce operative time. The entry points both above at D1-2, and below at D11-12 were selected based on sagittal MRI films, away from areas of possible adhesions in order to ensure a free passage of the shunt tube within the subarachnoid space. The patient was positioned prone on bolsters in a standard fashion as for a dorsal laminectomy. Preoperative preparation, prepping, and draping were performed. In addition to laminectomy instruments, a regular adult ventriculoperitoneal shunt ‘tunneller’ and basic micro-neurosurgical and spinal instruments were kept ready. Silicone tubes of the distal catheters of a shunt system with valves subtracted were obtained from two ventriculoperitoneal shunt sets (Chhabra, Hydrocephalus Shunt System, Surgiwear, India). Each conduit was fashioned on the table considering three principal measurements: (1) Length inside the theca above and below of arbitrarily 4-5 cm on each side; (2) Distance from the durotomy to subcutaneous tissue at the proximal and distal end; and (3) Intermediate-length from one laminectomy incision site to another. A connector and further tubing were used until the desired length was obtained.
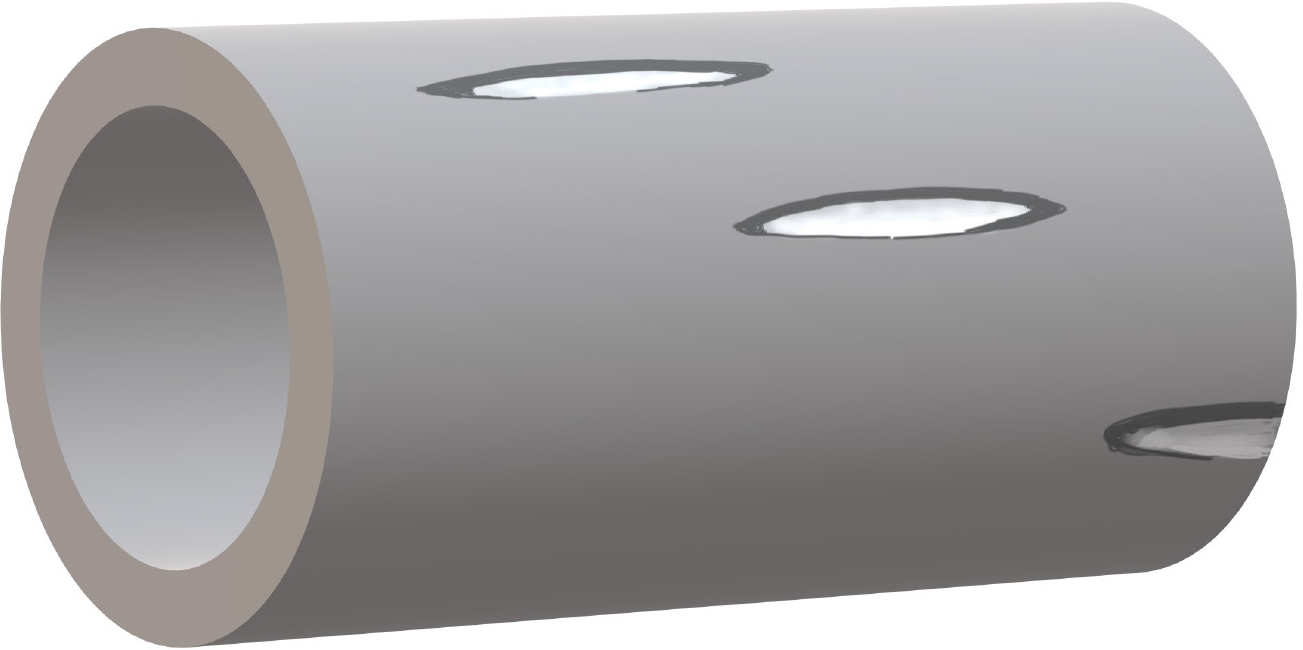
Five 1-mm fenestrations were made with fine micro-scissors over a length of 3-5 cm from either end of the conduit in an equidistant helical fashion. No two fenestrations were in close approximation or in one horizontal or vertical plane. Such an arrangement ensured the structural stability of the tube and minimized the risk of tube collapse or kinks (Figure 2).
The goal of surgery was to achieve a long-lasting zero net pressure difference via a free flow of CSF above and below the level of spinal subarachnoid adhesions, mimicking a physiological subarachnoid environment and reversing pathological forces leading to syringomyelia. Therefore, it is imperative that no valve be used while designing the conduit.
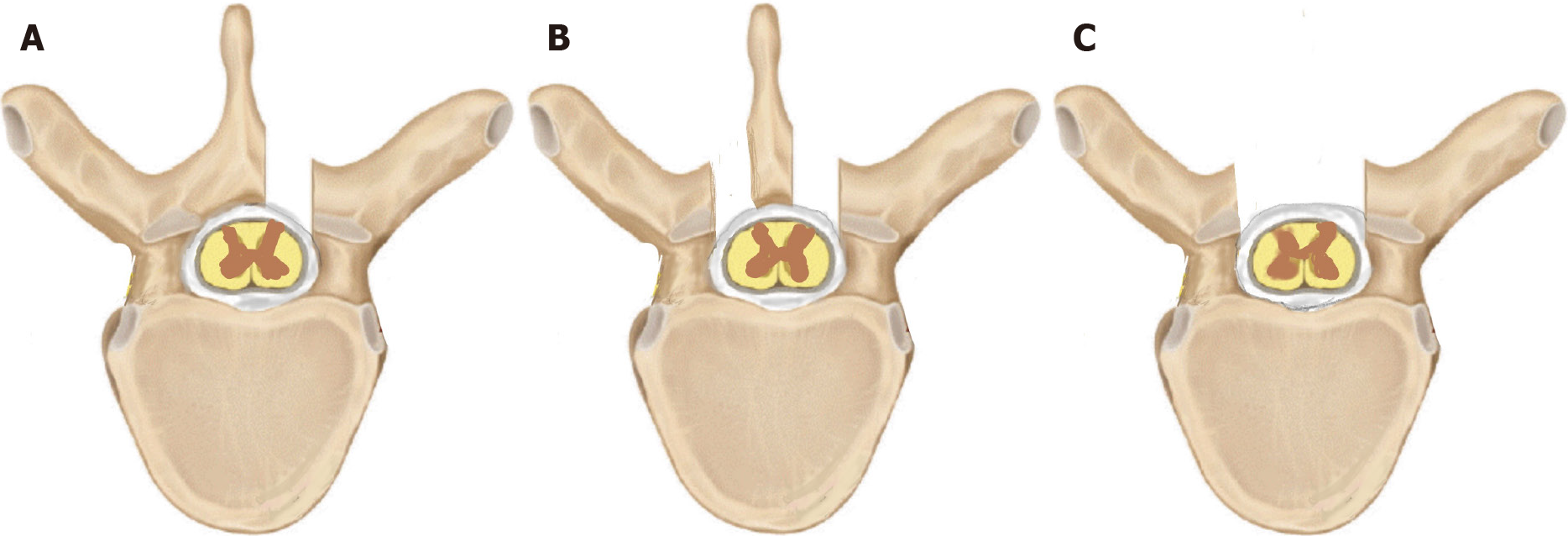
Using two midline incisions approximately 4 cm in length, the D1, superior aspect of D2, and the D11 laminae were exposed subperiosteally. Depending on the laterality and ease of access, bony work may range from a limited unilateral fenestration to a complete laminectomy (Figure 3).
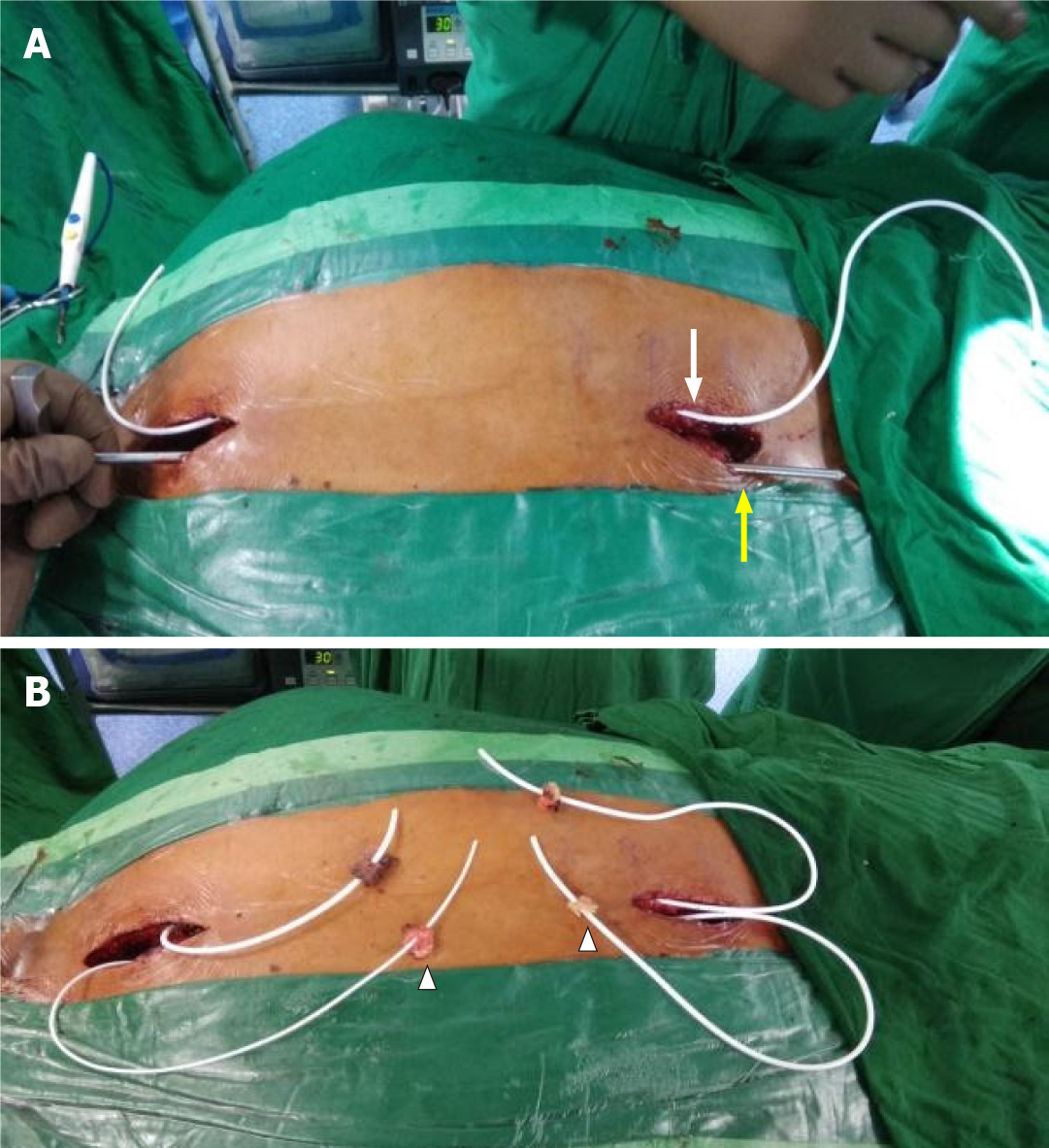
Limited midline D1, partial D2, and D11 vertebral laminectomies were performed until adequate thecal tube was visualized at both levels. Connecting passages were created by subcutaneous tunneling with the help of an adult ventriculoperitoneal shunt tunneller to emerge at either side of the incision and positioned at the mouth of each incision (Figure 4).
Fat cuffs harvested from the surrounding subcutaneous tissues were placed to rest a few centimeters proximal to the catheter ends. The conduits were pre-flushed with sterile Amikacin-saline and kept fluid-filled to eliminate any air pockets. Two 2-3 mm nicks were made in the dura on either side of the midline at the upper dorsal and lower dorsal exposed levels. The incisions were just adequate to permit free entry and further passage of the catheter tubes to the predetermined extent. Care was taken to ensure that the catheter fenestrations were well within the theca to preclude CSF egress into the surgical wound cavity. The four ends of tubing were inserted through four small durotomies which were sealed with the help of Tisseel fibrin glue (Baxter, Illinois, United States) and fat grafts (Figure 5).
The tubes were secured with silicone collars to the fascia. Incisions were closed after ensuring hemostasis and antibiotic-saline lavages in a routine layered fashion.
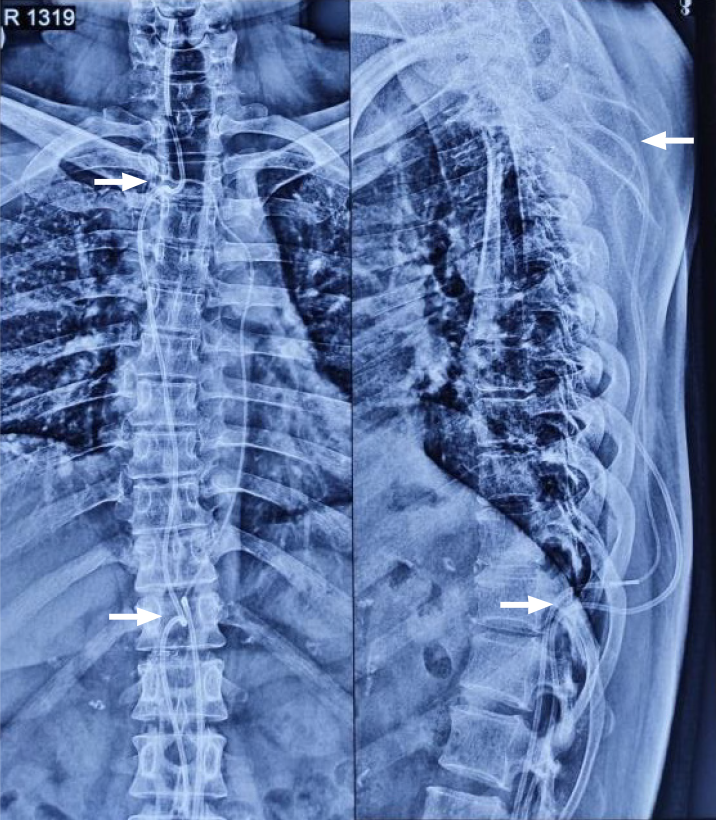
The patient was discharged on the second postoperative day and had an uneventful surgical recovery. Plain radiographs confirmed the levels and structural continuity of the conduits (Figure 6).
At the 4-mo follow-up, the patient had a 50% reduction in the burning and tingling sensations in his feet, improved sensations of temperature and pain, and a subjective reduction in stiffness by 30%-40% in his legs. Objectively, spasticity had decreased to an mASS grade 3 in the right lower limb and to grade 2 in the left lower limb. Power had improved to mMRC grade 3 in the right lower limb and grade 4 in the left lower limb muscle groups and he could stand with support. There was a significant improvement in the bowel-bladder functions and improved libido. Deep tendon reflexes remained exaggerated and bilateral plantars showed extensor responses.
An MRI scan obtained at this stage showed subtle changes in the morphology of loculated CSF anterior to the lower cervical and upper dorsal cord with no significant change in the size or shape of the syrinx (Figure 7).
The patient has since returned to his remote village and on telephonic follow-up at 15 mo, the time of writing this article, the patient had a near-total reduction in the sensory discomfort in his feet, significantly improved temperature and pain sensations in lower limbs, and reduced stiffness in lower limbs by 70%-80%. Bowel and bladder functions, as well as libido, had returned to normal. He was ambulant with minimal support and had near-complete recovery of his lower limb power with a drastic functional improvement. There was no evidence of conduit infection, malfunction, or any other procedure-related complication.
The challenges involved in treating syringomyelia due to tubercular arachnoiditis are well known and several procedures are currently employed to address themwith no conclusively efficacious treatment[7,9,11]. Current shunting techniques including syringo-pleural, syringo-peritoneal, and syringo-subarachnoid shunts decompress the syrinx by draining it into an ‘external’ non-neurological space like the pleura or peritoneum or into an ‘internal’ neurological adjoining subarachnoid compartment. The theco-peritoneal or lumbo-peritoneal shunt is an external diversion of CSF from spinal subarachnoid space to the peritoneum. It provides an alternative path of low resistance to CSF flow and thus, in theory, decreases the spinal cord trans-mural pressure and halts syrinx progression.
In patients with post-inflammatory arachnoid scarring and post-traumatic syringomyelia, the operative procedure is directed toward reconstituting spinal subarachnoid CSF flow by arachnoid scar membrane resection, microsurgical lysis of arachnoid adhesions, and dural reconstruction[12,13]. As extensive spinal adhesiolysis can lead to postoperative scarring within the subarachnoid space, any surgery for this condition should be as minimally invasive as possible[8].
The TTBP described herewith addresses the question of this intraspinal CSF block in a simple minimally invasive fashion. The procedure was developed on the principle that the pathological mechanism directly causing the syrinx is CSF flow obstruction and pressure imbalance within the subarachnoid compartment. Treatments aimed at adhesiolysis carry a high rate of re-scarring and failure. This method reconstitutes the CSF flow from the normal-above to the normal-below subarachnoid space, bypassing the pathological area of obstruction, and proposes to normalize CSF pressure hydrodynamics across the area of the blockade. Exposure or handling of the syrinx affected and compromised spinal cord is entirely avoided. The procedure is simple, effective, and in theory, less hazardous than physical manipulation involved in a microsurgical procedure, of an already disturbed spinal cord region. It employs existing low-cost silicone implants that are in common use and combines techniques of laminectomy, shunt tunneling, etc., familiar to most neurosurgeons worldwide.
The use of two conduits in our case was to maintain the functionality and physiology of uninterrupted CSF flow in the scenario of one conduit being inadvertently blocked. As in foramen magnum decompression procedures for symptomatic C-I-M with syringomyelia[14], collapse of the syrinx and visible resolution on imaging are expected to be delayed and might not always occur, as the pathological cavity is not directly deroofed or decompressed.
The clinical benefit in our patient is possibly the result of lowered pressure in the syrinx cavity and an arrest of its progression leading to the gradual restoration of spinal cord function. Altered CSF dynamics is known to be a causative factor in syringomyelia[15] and it is speculated that the rostrocaudal CSF pulse wave is misdirected into the spinal cord parenchyma. The resulting high trans-mural pressure and decreased compliance of the subarachnoid space favor the flow of fluid into the spinal cord, possibly through perivascular spaces, resulting in a destructive cavitation process and eventually the formation of a syrinx cavity[16-19]. We posit that the insertion of a fluid diverting shunt in our surgical procedure could attenuate and possibly redirect this misdirected rostrocaudal CSF pulse wave and consequently have a bearing on pressure hydrodynamics in the syrinx cavity. In surgical procedures directed towards optimization of CSF dynamics and no direct drainage of the syrinx cavity, patients may experience significant improvement in clinical symptoms in the absence of complete syrinx resolution on imaging[20]. Hence, with regard to different surgical procedures for syringomyelia, clinical outcome, rather than radiological improvement, is the more appropriate indicator of the surgical result[10,21,22].
We acknowledge the need for larger randomized control studies with a longer follow-up period before TTBP can be established as a regular treatment for post-arachnoiditis syringomyelia. With refinements and further controlled trials, one conduit may possibly suffice for the optimization of CSF flow. Evolutions in the method by utilizing minimally invasive techniques in spine surgery, using preformed factory generated perforated tube endings and innovative percutaneous manipulations in the lumbar region for intrathecal tube insertion, are promising.
The TTBP is a novel simple technique that may be used to treat certain cases of syringomyelia secondary to arachnoiditis. The procedure aims at reversing the direct pathology of altered CSF dynamics and increased transmural pressure leading to a syrinx. In selected patients, advantages over currently employed methods include minimal handling of the spinal cord, avoidance of extensive adhesiolysis and its associated complications, and a small incision minimally invasive approach with a shorter operative time.
We acknowledge assistance from Dr. Amit Jain, MS (Ortho) during the procedure and help from Dr. Priyam B, MD and Dr. Salila MB, MBBS for their guidance and assistance in the preparation of this manuscript. We also acknowledge the contribution of the late Dr. US Vengsarkar, our teacher and one of the first neurosurgeons in the world to pioneer the use of the theco-peritoneal shunt for syringomyelia.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Neurosciences
Country/Territory of origin: India
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Navarrete Arellano M S-Editor: Liu JH L-Editor: Wang TQ P-Editor: Liu JH
| 1. | Greitz D. Unraveling the riddle of syringomyelia. Neurosurg Rev. 2006;29:251-63; discussion 264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 196] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 2. | Milhorat TH, Chou MW, Trinidad EM, Kula RW, Mandell M, Wolpert C, Speer MC. Chiari I malformation redefined: clinical and radiographic findings for 364 symptomatic patients. Neurosurgery. 1999;44:1005-1017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 880] [Cited by in RCA: 840] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 3. | Roy AK, Slimack NP, Ganju A. Idiopathic syringomyelia: retrospective case series, comprehensive review, and update on management. Neurosurg Focus. 2011;31:E15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 4. | Klekamp J, Batzdorf U, Samii M, Bothe HW. Treatment of syringomyelia associated with arachnoid scarring caused by arachnoiditis or trauma. J Neurosurg. 1997;86:233-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 143] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 5. | Milhorat TH, Johnson WD, Miller JI, Bergland RM, Hollenberg-Sher J. Surgical treatment of syringomyelia based on magnetic resonance imaging criteria. Neurosurgery. 1992;31:231-44; discussion 244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 98] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 6. | Kaynar MY, Koçer N, Gençosmanoğlu BE, Hanci M. Syringomyelia--as a late complication of tuberculous meningitis. Acta Neurochir (Wien). 2000;142:935-8; discussion 938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 28] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 7. | Shenoy VS, Sampath R. Syringomyelia. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021 Jun 29. [PubMed] |
| 8. | Gottschalk A, Schmitz B, Mauer UM, Bornstedt A, Steinhoff S, Danz B, Schlötzer W, Rasche V. Dynamic visualization of arachnoid adhesions in a patient with idiopathic syringomyelia using high-resolution cine magnetic resonance imaging at 3T. J Magn Reson Imaging. 2010;32:218-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 9. | Canova G, Boaro A, Giordan E, Longatti P. Treatment of Posttubercular Syringomyelia Not Responsive to Antitubercular Therapy: Case Report and Review of Literature. J Neurol Surg Rep. 2017;78:e59-e67. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | Oluigbo CO, Thacker K, Flint G. The role of lumboperitoneal shunts in the treatment of syringomyelia. J Neurosurg Spine. 2010;13:133-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Lee JS, Song GS, Son DW. Surgical Management of Syringomyelia Associated with Spinal Adhesive Arachnoiditis, a Late Complication of Tuberculous Meningitis: A Case Report. Korean J Neurotrauma. 2017;13:34-38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 12. | Attal N, Parker F, Tadié M, Aghakani N, Bouhassira D. Effects of surgery on the sensory deficits of syringomyelia and predictors of outcome: a long term prospective study. J Neurol Neurosurg Psychiatry. 2004;75:1025-1030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 13. | Lee TT, Alameda GJ, Camilo E, Green BA. Surgical treatment of post-traumatic myelopathy associated with syringomyelia. Spine (Phila Pa 1976). 2001;26:S119-S127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 54] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 14. | Kennedy BC, Nelp TB, Kelly KM, Phan MQ, Bruce SS, McDowell MM, Feldstein NA, Anderson RCE. Delayed resolution of syrinx after posterior fossa decompression without dural opening in children with Chiari malformation Type I. J Neurosurg Pediatr. 2015;16:599-606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 15. | Morisako H, Takami T, Yamagata T, Chokyu I, Tsuyuguchi N, Ohata K. Focal adhesive arachnoiditis of the spinal cord: Imaging diagnosis and surgical resolution. J Craniovertebr Junction Spine. 2010;1:100-106. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 16. | Batzdorf U. Primary spinal syringomyelia: a personal perspective. Neurosurg Focus. 2000;8:E7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 17. | Brodbelt AR, Stoodley MA. Syringomyelia and the arachnoid web. Acta Neurochir (Wien). 2003;145:707-11; discussion 711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 51] [Article Influence: 2.3] [Reference Citation Analysis (1)] |
| 18. | Brodbelt AR, Stoodley MA, Watling AM, Tu J, Burke S, Jones NR. Altered subarachnoid space compliance and fluid flow in an animal model of posttraumatic syringomyelia. Spine (Phila Pa 1976). 2003;28:E413-E419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 19. | Milhorat TH, Capocelli AL Jr, Anzil AP, Kotzen RM, Milhorat RH. Pathological basis of spinal cord cavitation in syringomyelia: analysis of 105 autopsy cases. J Neurosurg. 1995;82:802-812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 233] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 20. | Porensky P, Muro K, Ganju A. Nontraumatic cervicothoracic syrinx as a cause of progressive neurologic dysfunction. J Spinal Cord Med. 2007;30:276-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 21. | Goel A, Desai K. Surgery for syringomyelia: an analysis based on 163 surgical cases. Acta Neurochir (Wien). 2000;142:293-301; discussion 301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 76] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 22. | Pillay PK. Thecoperitoneal shunting for syringomyelia. J Neurosurg. 1991;75:835-836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |