Peer-review started: May 10, 2015
First decision: June 18, 2015
Revised: July 9, 2015
Accepted: July 29, 2015
Article in press: August 3, 2015
Published online: November 27, 2015
Processing time: 201 Days and 9.7 Hours
The main task of cancer vaccines is to deliver tumor-specific antigens to antigen-presenting cells for immune recognition that can lead to potent and durable immune response against treated tumor. Using photodynamic therapy (PDT)-generated vaccines as an example of autologous whole-cell cancer vaccines, the importance is discussed of the expression of death-associated molecules on cancer vaccine cells. This aspect appears critical for the optimal capture of vaccine cells by host’s sentinel phagocytes in order that the tumor antigenic material is processed and presented for immune recognition and elimination of targeted malignancy. It is shown that changing death pattern of vaccine cells by agents modulating apoptosis, autophagy or necrosis can significantly alter the therapeutic impact of PDT-generated vaccines. Improved therapeutic effect was observed with inhibitors of necrosis/necroptosis using IM-54, necrostatin-1 or necrostatin-7, as well as with lethal autophagy inducer STF62247. In contrast, reduced vaccine potency was found in case of treating vaccine cells with apoptosis inhibitors or lethal autophagy inhibitor spautin-1. Therefore, PDT-generated cancer vaccine cells undergoing apoptosis or lethal autophagy are much more likely to produce therapeutic benefit than vaccine cells that are necrotic. These findings warrant further detailed examination of the strategy using cell death modulating agents for the enhancement of the efficacy of cancer vaccines.
Core tip: It is discussed how the mode of cell death can be important to the efficacy of cancer vaccine, using the example of photodynamic therapy-generated whole-cell vaccines. With the example of several agents modulating apoptosis, necrosis or autophagy, it is argued that the strategy of using such agents for the enhancement of the efficacy of cancer vaccines deserves serious consideration.
- Citation: Korbelik M. Impact of cell death manipulation on the efficacy of photodynamic therapy-generated cancer vaccines. World J Immunol 2015; 5(3): 95-98
- URL: https://www.wjgnet.com/2219-2824/full/v5/i3/95.htm
- DOI: https://dx.doi.org/10.5411/wji.v5.i3.95
One of the attractive adaptations of photodynamic therapy (PDT), a cancer treatment modality producing cytotoxic lesions by localized generation of reactive oxygen species produced by light-activated drugs (photosensitizers)[1], are PDT-generated autologous cancer vaccines[2]. They are made by exposing surgically removed tumor tissue (or derived tumor cells) to PDT ex vivo and used to vaccinate the same patient against the original malignancy[2,3].
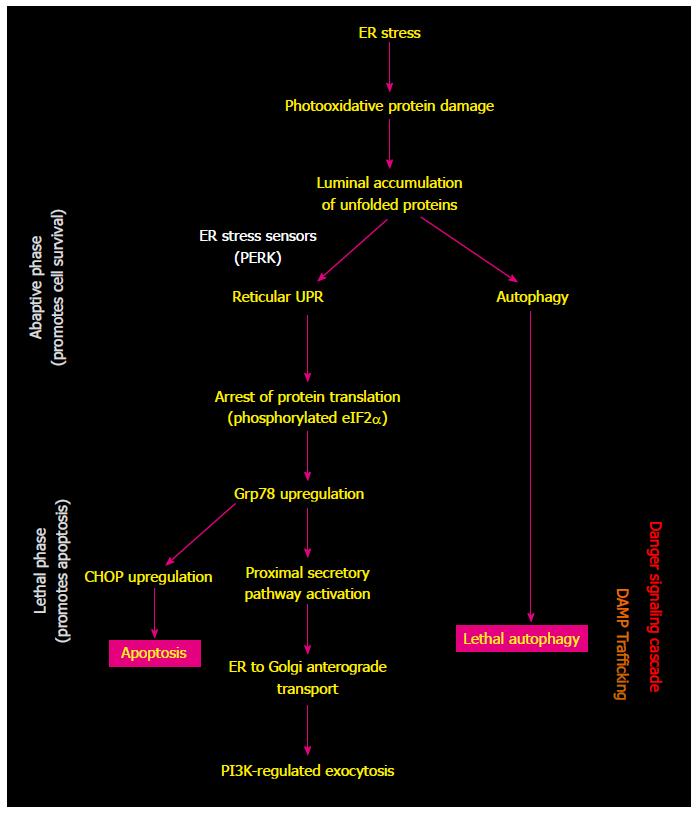
Cell death pathways induced by PDT are dominated by apoptosis, reflecting the activity of produced reactive oxygen species, sensitivity to photodamage of anti-apoptotic protein Bcl-2, engagement of pro-apoptotic mediators such as ceramide and stress kinases, and induction of cytochrome c release from mitochondria[4,5]. Autophagy was also found to be induced in PDT-treated cells and was traced to photodamage induction in endoplasmic reticulum (ER)[4]. The effectiveness of PDT-generated vaccines in orchestrating a strong immune antitumor reaction is considered to result from the nature of PDT-inflicted oxidative stress-based insult expressed, at least in part, in the ER of treated cancer cells[6,7]. Sensors of luminal accumulation of unfolded proteins in ER (appearing as a result of PDT-mediated photooxidative damage) engender unfolded protein response (UPR) as an attempt initially to re-establish homeostasis and this is accompanied with a general arrest of protein translation (Figure 1)[8]. In situation when ER-stress remains unresolved, several signal transduction pathways become activated securing the transition from adaptive to lethal phase of ER stress response. Such induction of reticular UPR promotes the immunogenic cells death (ICD) accompanied with the expression of an abundance of various damage-associated molecular patterns (DAMPs) driving this immune reaction[8-10]. Another suggested contributing element are PDT-induced photooxidative changes in tumor antigenic repertoire that may include the emergence of highly immunogenic tumor neoantigens that could be instrumental in overcoming immune ignorance of the tumor[2].
For optimal efficacy of PDT-generated vaccines, following treatment by PDT in vitro tumor tissue/cells have to be left in culture for 16-24 h before used as a vaccine[3,11]. This post-PDT incubation time interval is considered necessary for the expression of cell death-associated changes on the vaccine cells before they become fully potent vaccine material. Interference with this process, for instance by masking cell surface-exposed phosphatidylserine, inhibiting the induction of heat shock protein 70 (Hsp70), or blocking apoptosis by a caspase-3 inhibitor, will affect negatively the therapeutic impact of PDT-generated vaccines[11,12]. Thus, in order to be captured by host’s sentinel phagocytes and tumor antigenic material contained in them processed and presented in an optimal way for immune recognition and elimination of targeted malignancy, the PDT-generated vaccine cells have to exhibit a particular “eat me” death profile. This revelation inspired the investigation on the prospects of a strategy to enhance the potency of PDT-generated vaccines by modulating the death process in vaccine cells. The findings uncovered thus far by this investigation are summarized in Table 1. In addition to apoptosis, the agents affecting either necrosis or lethal autophagy turned out also to be capable of changing the potency of PDT-generated vaccines.
| Cell death modulating agent | Impact on therapeutic efficacy of PDT-generated cancer vaccine |
| Z-DEVD-FMK (apoptosis inhibitor) | Reduces |
| IM-54 (necrosis inhibitor) | Enhances |
| Necrostatin-1 (necroptosis inhibitor) | Enhances |
| Necrostatin-7 (necroptosis inhibitor) | Enhances |
| STF62247 (lethal autophagy inducer) | Enhances |
| Spautin-1 (lethal autophagy inhibitor) | Reduces |
The importance of apoptotic death of PDT vaccine cells has already been made clear earlier[11]. While physiological apoptosis is immuno-tolerogenic, the type of programmed cell death triggered by oxidative ER stress (induced by PDT and certain other cancer treatments) is associated with the engagement of danger signaling pathways arranging the trafficking of ICD-affiliated DAMPs towards cellular surface and extracellular space[10]. However, not only by blocking the apoptosis but also by impairing lethal autophagy (with agents like spautin-1 in Table 1), PDT-generated tumor cells are rendered less potent source for cancer vaccine. Moreover, the opposite effect was attained by treating these vaccine cells with an autophagy-inducing agent. This reveals that the process of autophagy, which is closely connected with the reticular UPR and is presumably activated along with PDT-induced ER stress response[8,10], also promotes ICD with a consequential boost to immuno-activating potential of PDT vaccine cells. Such action capability is consistent with the paradigm of the contributing role of autophagy in danger signaling activity in dying cancer cells and participation of key autophagy proteins in the endosomal trafficking pathway[10].
In contrast to apoptosis and autophagy, the death of PDT vaccine cells by necrosis and its death receptor-instigated programmed variant necroptosis are detrimental to their therapeutic potential. This is evident from the beneficial effects rendered by vaccine cell treatment with two different necrostatins and IM-54 that selectively blocks oxidative stress-induced necrotic cell death[13] (Table 1). All these three agents, when present during the post-PDT incubation time interval induced an increase in the percentage of apoptotic cells in the PDT vaccine cell samples.
The above examinations on the effects of modulators of cell death by apoptosis, autophagy and necrosis/necroptosis shed novel light on the roles of cell death pathways in the generation of cancer vaccine cells by PDT. It is also becoming increasingly evident that further research is merited for elucidating the potential of agents such as autophagy promoters or necrosis inhibitors for acting as adjuvants to improve therapeutic effectiveness of PDT-generated and similar whole-cell cancer vaccines.
P- Reviewer: Corthay A, Seong SY, Zhang ZX S- Editor: Tian YL L- Editor: A E- Editor: Jiao XK
| 1. | Agostinis P, Berg K, Cengel KA, Foster TH, Girotti AW, Gollnick SO, Hahn SM, Hamblin MR, Juzeniene A, Kessel D. Photodynamic therapy of cancer: an update. CA Cancer J Clin. 2011;61:250-281. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4122] [Cited by in RCA: 3576] [Article Influence: 255.4] [Reference Citation Analysis (0)] |
| 2. | Korbelik M. Cancer vaccines generated by photodynamic therapy. Photochem Photobiol Sci. 2011;10:664-669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 57] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 3. | Korbelik M. Photodynamic therapy-generated cancer vaccines. Methods Mol Biol. 2010;635:147-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 4. | Kessel D. Death pathways associated with photodynamic therapy. Med Laser Appl. 2006;21:219-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 60] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 5. | Mroz P, Yaroslavsky A, Kharkwal GB, Hamblin MR. Cell death pathways in photodynamic therapy of cancer. Cancers (Basel). 2011;3:2516-2539. [PubMed] |
| 6. | Korbelik M, Banáth J, Sun J, Canals D, Hannun YA, Separovic D. Ceramide and sphingosine-1-phosphate act as photodynamic therapy-elicited damage-associated molecular patterns: cell surface exposure. Int Immunopharmacol. 2014;20:359-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 7. | Kepp O, Senovilla L, Vitale I, Vacchelli E, Adjemian S, Agostinis P, Apetoh L, Aranda F, Barnaba V, Bloy N, Bracci L, Breckpot K, Brough D, Buqué A, Castro MG, Cirone M, Colombo MI, Cremer I, Demaria S, Dini L, Eliopoulos A, Faggioni A, Formenti SC, Fučíková J, Gabriele L, Gaipl US, Galon J, Garg A, Ghiringhelli F, Giese NA, Guo ZS, Hemminki A, Herrmann M, Hodge JW, Holdenrieder S, Honeychurch J, Hu HM, Huang X, Illidge TM, Kono K, Korbelik M, Krysko DV, Loi S, Lowenstein PR, Lugli E, Ma Y, Madeo F, Manfredi AA, Martins I, Mavilio D, Menger L, Merendino N, Michaud M, Mignot G, Mossman KL, Multhoff G, Oehler R, Palombo F, Panaretakis T, Pol J, Proietti E, Ricci JE, Riganti C, Rovere-Querini P, Rubartelli A, Sistigu A, Smyth MJ, Sonnemann J, Spisek R, Stagg J, Sukkurwala AQ, Tartour E, Thorburn A, Thorne SH, Vandenabeele P, Velotti F, Workenhe ST, Yang H, Zong WX, Zitvogel L, Kroemer G, Galluzzi L. Consensus guidelines for the detection of immunogenic cell death. Oncoimmunology. 2014;3:e955691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 616] [Cited by in RCA: 651] [Article Influence: 59.2] [Reference Citation Analysis (0)] |
| 8. | Kepp O, Semeraro M, Bravo-San Pedro JM, Bloy N, Buqué A, Huang X, Zhou H, Senovilla L, Kroemer G, Galluzzi L. eIF2α phosphorylation as a biomarker of immunogenic cell death. Semin Cancer Biol. 2015;33:86-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 98] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 9. | Seong SY, Matzinger P. Hydrophobicity: an ancient damage-associated molecular pattern that initiates innate immune responses. Nat Rev Immunol. 2004;4:469-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 961] [Cited by in RCA: 919] [Article Influence: 43.8] [Reference Citation Analysis (0)] |
| 10. | van Vliet AR, Martin S, Garg AD, Agostinis P. The PERKs of damage-associated molecular patterns mediating cancer immunogenicity: From sensor to the plasma membrane and beyond. Semin Cancer Biol. 2015;33:74-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 11. | Korbelik M, Stott B, Sun J. Photodynamic therapy-generated vaccines: relevance of tumour cell death expression. Br J Cancer. 2007;97:1381-1387. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 64] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 12. | Korbelik M, Merchant S. Photodynamic therapy-generated cancer vaccine elicits acute phase and hormonal response in treated mice. Cancer Immunol Immunother. 2012;61:1387-1394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 13. | Dodo K, Katoh M, Shimizu T, Takahashi M, Sodeoka M. Inhibition of hydrogen peroxide-induced necrotic cell death with 3-amino-2-indolylmaleimide derivatives. Bioorg Med Chem Lett. 2005;15:3114-3118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |