Published online Nov 27, 2015. doi: 10.5411/wji.v5.i3.152
Peer-review started: July 8, 2015
First decision: September 18, 2015
Revised: September 22, 2015
Accepted: November 13, 2015
Article in press: November 17, 2015
Published online: November 27, 2015
Processing time: 148 Days and 14.1 Hours
Sjögren’s syndrome (SS) is a systemic autoimmune disease that affects primarily the lacrimal and salivary glands. In addition to a systemic autoimmune response directed against ubiquitous antigens (such as Ro and La antigens), patients with SS mount a localized response that affects the epithelial component of exocrine glands leading to the establishment of a destructive inflammatory infiltrate comprised of activated T and B cells. Local chemokine and cytokine production drive the recruitment and local activation of immune cells that cause injury to acinar cells. CD4 T cells with different functional differentiation programs including Th1 (IFN-γ), Th2 (IL-13, IL-4) and Th17 (IL-17, IL-21, IL-22) as well as diverse cytokine signaling pathways, are involved at the initiation, perpetuation, and progression of the disease. Which factors initiate this response and allow it to become chronic are unknown. Proposed mechanisms include viral infections and acinar cell apoptosis. Moreover risk-conferring genetic variants, probably through the facilitation of innate and adaptive immune activation, most certainly contribute to the creation of an underlying environment that fosters tolerance loss and facilitates perpetuation of the autoimmune response. In this review, we describe the mechanisms through which the immune response causes SS and emphasize the pathways that are amenable of being targeted with therapeutic purposes.
Core tip: Sjögren’s syndrome (SS) is a complex entity caused by an autoimmune process that encompasses both an anti-acinar and a systemic response. Exocrine gland infiltration is probably primarily responsible for the destruction of the acinar cells and consequently for the development of sicca symptoms. The participation of diverse chemokines, activated T cells and B cells, cytokines and cytokine signaling pathways has been recognized. The aim of this review is to discuss some aspects of SS pathogenesis and emphasize the potential opportunities where therapeutic interventions might be useful.
- Citation: Crispín JC, Rosetti F, Hernández-Molina G. Lessons from Sjögren’s syndrome etiopathogenesis: Novel cellular and molecular targets. World J Immunol 2015; 5(3): 152-159
- URL: https://www.wjgnet.com/2219-2824/full/v5/i3/152.htm
- DOI: https://dx.doi.org/10.5411/wji.v5.i3.152
Primary Sjögren’s syndrome (PSS) is a chronic systemic autoimmune disease. Clinical and pathological manifestations in patients with PSS are most evident in exocrine glands, in particular in salivary and lacrimal glands. However, patients frequently also exhibit extraglandular manifestations[1].
The origin of the disease is unknown. An autoimmune response primarily directed towards exocrine glands develops in the midst of a less well defined systemic loss of tolerance. Why some patients with systemic autoimmunity develop a response against lacrimal and salivary glands is still poorly understood, but local factors that induce infectious and non-infectious cellular injury are probably involved. Risk-conferring genetic variants, probably through the facilitation of innate and adaptive immune activation, most certainly contribute to the creation of an underlying environment that fosters tolerance loss and facilitates perpetuation of the autoimmune response upon environmental triggering elements[2].
Sicca symptoms, the hallmark manifestation of Sjögren’s syndrome (SS) are the result of acinar cell dysfunction and destruction (Figure 1). Because both phenomena are primarily immune-mediated, the interruption of the anti-acinar immune response has been the focus of most therapeutic proposals. T cells comprise a large fraction of the inflammatory infiltrate of affected salivary glands. Therefore, they are considered important players in the pathogenesis of SS[3]. Other cells, including B cells which undergo local activation and proliferation, also play a central roles in the development of the disease[4].
The aim of this review is to discuss some aspects of SS pathogenesis and emphasize potential opportunities where therapeutic interventions might be useful. As targeting B cells has been recently discussed in the literature[5,6], we have decided to focus in other potential approaches.
SS is considered a systemic autoimmune disease, because in contrast to patients with organ-specific autoimmune conditions such as type 1 diabetes, patients with SS mount a robust immune response mostly directed against ubiquitously expressed molecules (e.g., Ro antigen). Amid this systemic autoimmune response, patients with SS develop a response that preferentially affects exocrine glandular tissue, most notably acinar cells from lacrimal and salivary glands. This anti-acinar response is a unifying characteristic, a sine qua non that defines SS present either as a primary disease or as a condition associated to other systemic autoimmune diseases (secondary SS).
The systemic autoimmune response probably precedes and underlies the anti-acinar response. Further, it contributes to the pathogenesis of extraglandular manifestations present in up to 60% of patients with SS (Figure 1). Some autoantibodies commonly found in patients with SS, in particular anti-Ro and anti-La, are directed against ribonucleoproteins that are ubiquitously expressed. Interestingly, these autoantibodies may exert effects locally, at the exocrine gland level, where they affect cellular function and may contribute to acinar cell apoptosis.
The most distinctive feature of SS is the development of a well-organized immune response in exocrine glands. Which factors initiate this response and allow it to become chronic are unknown. Proposed mechanisms include viral infections and acinar cell apoptosis. Alternatively, products of an underlying systemic autoimmune response, for example anti-Ro antibodies, may initiate a local inflammatory response by inciting acinar cell damage that is amplified and perpetuated by the infiltrating immune cells.
Chemokines are small molecules that exert powerful chemoattractant effects on a variety of cells. The local secretion of chemokines guides the amount and type of cells that infiltrate and therefore cause inflammation in a tissue. Therefore, the type and abundance of chemokines present in a tissue determine the intensity of inflammation and its characteristics. High concentrations of several chemokines including CCL5, CCL3, CCL17, CCL18, CCL19, CCL21, CXCL9, CXCL10, CXCL11, and CXCL13, have been reported in affected glands of patients with SS[7]. CXCL12 and CXCL13 are strongly associated with the development of ectopic germinal center (GC) like structures[8] and CXCL10 has been proposed as a biomarker for early salivary gland inflammation[9].
The therapeutic usefulness of targeting chemokines has been tested in animal models. In MRL/lpr mice, the use of an N-terminal-truncated IP-10 analogue that possessed IP-10 receptor antagonist capacities ameliorated the progression of autoimmune sialadenitis[10]. Likewise, CXCL13 blockade reduced the number of lymphocytic foci in salivary gland in the non-obese diabetic mouse model when administered before disease onset[11]. Conversely, CCL28 a mucosal chemokine that attracts CD4 and CD8 T cells was diminished in saliva of patients with SS[12]. These preliminary data support the hypothesis that blocking chemokines may represent a therapeutic strategy in SS.
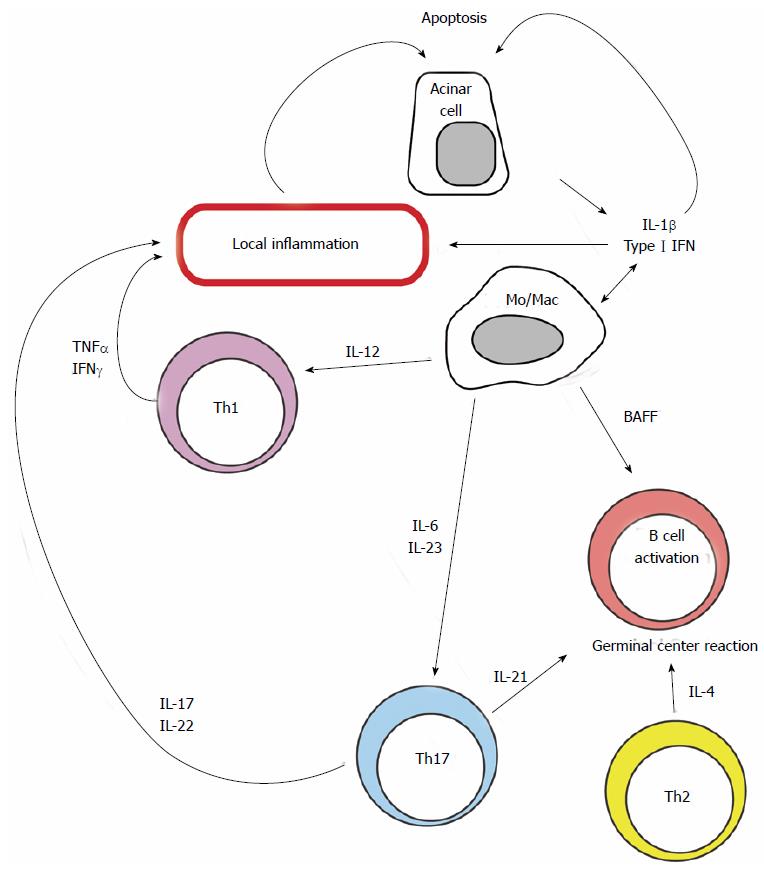
Cytokine gene knock-out mouse models as well as clinical evidence have shown that effector CD4 T cells with different functional differentiation programs (including Th1, Th2 and Th17) are involved at the initiation, perpetuation, and progression of the disease[13,14]. However data concerning the time when each of these responses is most relevant is conflicting. Some authors propose that a Th2 response dominates in early lesions[15] while others consider that Th1 and Th17 cytokines are essential for the induction and/or maintenance[7]. Th1 cells produce IFN-γ and IL-2 whereas Th2 cells secrete IL-4, IL-5, IL-6, IL-10, and IL-13[16,17]. On the other hand, Th17 cells represent a major source of IL-17A, IL-17F, and IL-22. Thus blocking one or a cluster of cytokines may be potentially useful therapeutic strategies (Figure 2).
Increased levels of IL-1 in saliva and peripheral blood of patients with PSS have been reported[18]. In the salivary and lacrimal glands, IL-1β production from infiltrating immune cells and also from local epithelial cells has been described[19]. This illustrates how damaged acinar cells may promote the initiation and perpetuation of the response that will further injure them by facilitating their apoptosis and contributing to the recruitment and local activation of T cells[19]. The IL-1R antagonist, anakinra, demonstrated therapeutic benefits as a topical treatment for dry eye in a spontaneous mouse model of autoimmune keratoconjunctivitis sicca that mimics SS[20]. On the other hand, a randomized, double-blind, placebo-controlled trial of IL-1 blockade did not find a significant reduction in fatigue in PSS[21].
Type I interferons are produced by virtually every cell in response to the detection of intracellular infection. They act as danger signals that alert neighboring cells to the presence of pathogens and induce the local influx of inflammatory cells. Expression of genes that are induced by IFN is commonly used as a surrogate measure of IFN levels in a tissue. In salivary glands and in cells from the peripheral blood of patients with SS there is increased expression of IFN-driven genes[22,23]. High levels of type I IFN may contribute to the pathophysiology of SS by mediating the recruitment, activation, and differentiation of inflammatory cells, by promoting the secretion of other cytokines such as B cell activating factor by monocytes, and by inducing the expression of pro-apoptotic molecules (e.g., Fas and FasL)[24]. In the context of SS, high levels of type I IFN are probably released locally as a result of cellular injury mediated by viruses and perhaps other non-infectious agents, and may also result from the detection of nucleic acid-containing immune complexes[25]. Acinar production of these cytokines may promote local inflammation, whereas systemic production may facilitate the inflammatory response through their pro-inflammatory action in immune cells. For these reasons, therapeutic interventions targeting the type I IFN signaling pathway may be beneficial to patients with elevated type I IFN status. Currently two anti-IFN-α monoclonal antibodies, sifalimumab and rontalizumab are being tested in systemic lupus erythematosus (SLE) patients, but there are still no trials in SS[26].
CD4 T cells that are primed in the presence of IL-12 become Th1 cells. They promote inflammation through the production of IFN-γ and TNF-α, powerful cytokines that affect the behavior of macrophages, T cells, and B cells[27]. Early work reported that T cell differentiation in patients with SS was skewed towards a Th1 response because serum levels of IFN-γ were high and Th1 cells were more abundant in the peripheral blood of patients with SS than in healthy controls[28,29].
The pathogenic capacity of IFN-γ is further supported by work in NOD mice where genetic deletion of this cytokine or its receptor prevented the development of an anti-salivary gland autoimmune response. Of note, IFN-γ deletion was also associated with decreased apoptosis of acinar cells, suggesting that Th1 cells might also damage salivary glands through this mechanism[30].
Th17 cells represent potent pro-inflammatory CD4 T cells that promote the infiltration of innate and adaptive immune cells into tissues by producing cytokines (i.e., IL-17A, IL-17F, IL-22) that act mainly on epithelial and endothelial cells by inducing the production of chemotactic molecules[31]. Th17 cells are generated when naïve CD4 T cells are primed in the presence of TGF-β and pro-inflammatory cytokines such as IL-1, IL-6, and IL-21, and have been reported to be involved in the pathogenesis of several autoimmune diseases, including SLE[32]. IL-17 and other Th17-related cytokines have been found in high levels in the saliva and in salivary gland tissue of patients and mice with SS[33,34], suggesting the presence of a Th17-type inflammatory response.
Retinoic acid-related orphan receptor gamma (ROR-γ) is a transcription factor that is necessary for the development and function of Th17 cells[35]. Transgenic over-expression of ROR-γ led to the development of a severe spontaneous sialadenitis driven by the infiltration of salivary glands by CD4 T cells[36]. Interestingly, salivary gland-infiltrating CD4 T cells in ROR-γ transgenic mice produced not only IL-17, but also IFN-γ, IL-4, IL-21, and other cytokines. In fact, IL-17 was not necessary for disease development since genetic deletion of IL17a did not modify the frequency or pathology of the disease[36].
IL-6 is increased in tears, saliva and serum of PSS patients[37]. This cytokine has been associated with B cell hyperactivity and promotes STAT-3 activation. In Sle1.Yaa mice that develop a lupus- and Sjögren’s-like syndrome, deficiency of IL-6 ameliorated the autoantibody production and salivary gland inflammation[38]. Currently IL-6 blocking therapy (tocilizumab) is being evaluated in a phase III randomized controlled trial in PSS[39].
IL-23, which stabilizes the phenotype of Th17 cells, is also significantly increased at both the protein and the mRNA levels in the salivary glands of patients with PSS[40,41]. Clinical trials using anti-IL-23 (ustekinumab, briakinumab, tildrakizumab and guselkumab) have been performed in psoriasis, yet there is still no information in PSS[42].
The importance of IL-17 in the development of SS has been shown in mouse models. Inhibition of IL-17 activity by expression of a soluble IL-17R:Fc fusion protein restrained the development of SS in C57BL/6.NOD-Aec1Aec2 mice[43]. The use of anti-IL-17 monoclonal antibodies (ixekizumab and secukinumab) or its receptor IL-17RA (brodalumab) are being evaluated in rheumatoid arthritis and psoriatic arthritis, but trials in SS using these treatments are lacking[42]. However the use of anti-IL-6 receptor (tocilizumab)[44] or a fusion protein of the extracellular domain of CTLA-4 and human IgG1 (abatacept) significantly decreased the abundance of Th17 cells in the peripheral blood of patients with PSS[45].
IL-22 is produced by Th17 and NK cells. IL-22 is over-expressed in the serum and salivary glands of patients with PSS and correlates with hyposalivation, the presence of autoantibodies and the focus score[46]. The IL-22 axis seems to be functionally dependent on IL-18 signaling and both IL-18 and IL-22/IL-22R1 are over-expressed in PSS patients with non-Hodgkin lymphoma[47]. The potential role of blocking IL-22 in SS pathogenesis remains to be evaluated in mouse models and humans.
IL-21 has pleiotropic effects promotes the differentiation, proliferation, and survival of B and T cells. IL-21 exerts pro-inflammatory functions by inducing the expression of IL-6 and ROR-γ and thus contributing to the generation of Th17 cells and attenuating Treg induction. Patients with PSS have elevated serum IL-21 levels and expression of IL-21 at lymphocytic foci and periductal areas of minor salivary gland biopsies has been documented[48]. Moreover, a genetic polymorphism located upstream of IL-21 (IL2-IL21 intergenic region) was associated with PSS in a Latin-American population[49]. The suppression of local IL-21 expression at the submandibular glands using an shRNA-encoding lentivirus reduced the lymphocyte infiltration and improved salivary gland function in NOD mice[50]. Nowadays there is only a phase I study of a monoclonal anti-IL-21 in humans, so further research is needed[51].
Th2 cytokines are also produced in the glands of patients and mice with SS. Evidence indicates that contrary to Th1 and Th17 cells that instigate local inflammation, Th2 cells participate in the pathogenesis of SS by facilitating the autoantibody response.
IL-4 is present in minor salivary gland biopsies of patients with PSS[7]. In mouse models, it participates in the IgM to IgG1 isotype switch via the JAK/STAT6 signal transduction pathway. NOD mice deficient in IL-4 or in STAT6 still develop focal salivary gland infiltrates, but they fail to produce anti-muscarinic acetylcholine type-3 receptor antibodies of IgG1 isotype and therefore exocrine gland dysfunction is avoided[52,53]. A salivary proteomic biomarker profile study found IL-4 as part of a 4-plex and 6-plex biomarker signatures in PSS[54]. A study reported a similar distribution of IL-4RA genetic variants among PSS and healthy controls. However, the haplotype ARSPRV was significantly more frequent among patients with parotid gland enlargement and positive immunological parameters, suggesting that differential response to IL-4 in epithelial tissue might affect disease phenotype[55]. The potential role of blocking IL-4 in PSS remains to be evaluated.
IL-13 mRNA is present in salivary gland of patients with PSS[15], and SS patients with antiSSA/Ro antibodies had significantly higher IL-13 levels than patients without this autoantibody[56]. Blockade of IL-13 activity in the ID3-KO mice model improved salivary gland function[57]. Recently anrukinzumab, a humanized anti-IL-13 antibody, was evaluated in ulcerative colitis[58], however there are not trials in PSS patients.
IL-10 mRNA expression is increased in salivary glands of PSS patients[59]. In a transgenic murine model, IL-10 induced lymphocytic infiltration and apoptosis of glandular cells[60]. The use of anti–IL-10 mAb administration has been only explored in a small open study of lupus patients with improvement of disease activity[61]. Conversely, IL-10 has also been considered as an anti-inflammatory agent. For instance, IL-10 is produced by regulatory B cells that are increased in clinical inactive PSS patients[62]. Thus future research to elucidate the complex function of IL-10 is needed.
Several cytokines contribute to the pathogenesis of SS (e.g., IFN-γ, IL-4, IL-6, IL-21) signal through Janus kinases (JAKs) and signal transducer and activator of transcription (STATs) transcription factors. The activity of the JAK-STAT pathway is negatively regulated by suppressors of cytokine signaling proteins. JAK/STAT kinase inhibitors suppress the inflammatory cytokine function and stimulate the production of anti-inflammatory cytokines such as IL-10[63]. A pan-Janus kinase inhibitor with a higher affinity for JAK1 and 3 (tofacitinib) is currently available and has been used successfully in patients with rheumatoid arthritis[64].
Apoptosis of epithelial cells from exocrine glands represents an important phenomenon in the pathogenesis of SS. The increased rate of acinar cell death has direct consequences on the production of saliva and tears, the most frequent clinical complaint of patients with SS. Moreover, it may represent a key local factor responsible for the recruitment of inflammatory cells that amplify the damage to the glandular tissue.
Although most studies have found abnormally high levels of apoptosis in ductal and acinar epithelial cells in salivary glands of patients with primary SS[65], it has been difficult to determine whether increased local apoptosis and release of glandular antigens initiates the local autoimmune response in susceptible patients, or whether self-reactive T cells infiltrate the glands guided by their antigen specificity and cause apoptosis in susceptible cells (e.g., cells that express FasL)[66].
Epithelial cells from salivary glands of patients with SS are susceptible to apoptosis. Because they express high levels of both Fas and FasL, it has been proposed that apoptosis may be triggered in them in an autocrine or paracrine manner as well as in response to glandular infiltration of Fas-bearing T cells[65].
The IgG fraction of sera from patients with SS has the capacity to penetrate living A-253 cells (a human salivary gland cell line) and trigger caspase activation and apoptosis, raising the possibility that autoantibodies produced in SS may contribute to the pathogenesis of the disease by inducing death of epithelial cells[67].
B cell hyperactivity and the presence of high titers of autoantibodies are common phenomena in patients with SS. Germinal center formation is observed in the glandular tissue of up to 25% of the patients with SS, usually in patients with severe disease[68]. Not surprisingly, patients with germinal centers have higher titers of autoantibodies (e.g., rheumatoid factor, anti-Ro) and a higher risk for development of lymphoma[68].
How local B cell activation and proliferation contributes to the pathogenesis of SS is not well understood[69]. B cells may produce cytokines, including IL-6 and IL-10 that affect the activation and function of infiltrating immune cells. Also, they may act as antigen presenting cells, thereby amplifying the autoimmune response. Finally, autoantibodies may contribute to acinar cell dysfunction and death by affecting cholinergic signaling and by inducing apoptosis[67].
Patients with SS develop an autoimmune response that has systemic and local components. The clinical manifestations of the disease are the consequence of pathological processes that result from both autoimmune responses. Exocrine gland infiltration and the development of a local chronic inflammatory response is probably primarily responsible for the destruction of the acinar cells and consequently for the development of sicca symptoms. This immune response is complex and comprised of activated T cells and B cells and cannot be attributed to a single cell type and a unique differentiation program. A more thorough understanding of disease pathogenesis, including information obtained in animal models, will allow us to better understand which therapies may be more useful and may stop the destruction of the glandular tissue that leads to the irreversible changes that affect most patients with SS.
P- Reviewer: Chen WX, Kwon HJ, Lichtor T, Nagata T S- Editor: Qiu S L- Editor: A E- Editor: Jiao XK
| 1. | Ramos-Casals M, Solans R, Rosas J, Camps MT, Gil A, Del Pino-Montes J, Calvo-Alen J, Jiménez-Alonso J, Micó ML, Beltrán J. Primary Sjögren syndrome in Spain: clinical and immunologic expression in 1010 patients. Medicine (Baltimore). 2008;87:210-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 387] [Cited by in RCA: 354] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 2. | Kramer JM. Early events in Sjögren’s Syndrome pathogenesis: the importance of innate immunity in disease initiation. Cytokine. 2014;67:92-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 3. | Singh N, Cohen PL. The T cell in Sjogren’s syndrome: force majeure, not spectateur. J Autoimmun. 2012;39:229-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 129] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 4. | Youinou P. Sjögren’s syndrome: a quintessential B cell-induced autoimmune disease. Joint Bone Spine. 2008;75:1-2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 5. | Cornec D, Saraux A, Devauchelle-Pensec V, Clodic C, Pers JO. The future of B cell-targeted therapies in Sjögren’s syndrome. Immunotherapy. 2013;5:639-646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 6. | Saraux A. The point on the ongoing B-cell depleting trials currently in progress over the world in primary Sjögren’s syndrome. Autoimmun Rev. 2010;9:609-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 7. | Moriyama M, Hayashida JN, Toyoshima T, Ohyama Y, Shinozaki S, Tanaka A, Maehara T, Nakamura S. Cytokine/chemokine profiles contribute to understanding the pathogenesis and diagnosis of primary Sjögren’s syndrome. Clin Exp Immunol. 2012;169:17-26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 78] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 8. | Barone F, Bombardieri M, Manzo A, Blades MC, Morgan PR, Challacombe SJ, Valesini G, Pitzalis C. Association of CXCL13 and CCL21 expression with the progressive organization of lymphoid-like structures in Sjögren’s syndrome. Arthritis Rheum. 2005;52:1773-1784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 198] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 9. | Hernández-Molina G, Michel-Peregrina M, Hernández-Ramírez DF, Sánchez-Guerrero J, Llorente L. Chemokine saliva levels in patients with primary Sjögren’s syndrome, associated Sjögren’s syndrome, pre-clinical Sjögren’s syndrome and systemic autoimmune diseases. Rheumatology (Oxford). 2011;50:1288-1292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 10. | Hasegawa H, Inoue A, Kohno M, Muraoka M, Miyazaki T, Terada M, Nakayama T, Yoshie O, Nose M, Yasukawa M. Antagonist of interferon-inducible protein 10/CXCL10 ameliorates the progression of autoimmune sialadenitis in MRL/lpr mice. Arthritis Rheum. 2006;54:1174-1183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 50] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 11. | Kramer JM, Klimatcheva E, Rothstein TL. CXCL13 is elevated in Sjögren’s syndrome in mice and humans and is implicated in disease pathogenesis. J Leukoc Biol. 2013;94:1079-1089. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 92] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 12. | Hernandez-Molina G, Burkhardt AM, Lima G, Zlotnik A, Betanzos JL, Bahena S, Llorente L. Absence of salivary CCL28 in primary Sjögren’s syndrome. Rheumatol Int. 2015;35:1431-1434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 13. | Furuzawa-Carballeda J, Sánchez-Guerrero J, Betanzos JL, Enriquez AB, Avila-Casado C, Llorente L, Hernández-Molina G. Differential cytokine expression and regulatory cells in patients with primary and secondary Sjögren’s syndrome. Scand J Immunol. 2014;80:432-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 14. | Jin JO, Yu Q. T Cell-Associated Cytokines in the Pathogenesis of Sjögren's Syndrome. J Clin Cell Immunol. 2013;S:11742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 15. | Mitsias DI, Tzioufas AG, Veiopoulou C, Zintzaras E, Tassios IK, Kogopoulou O, Moutsopoulos HM, Thyphronitis G. The Th1/Th2 cytokine balance changes with the progress of the immunopathological lesion of Sjogren’s syndrome. Clin Exp Immunol. 2002;128:562-568. [PubMed] |
| 16. | Pertovaara M, Antonen J, Hurme M. Th2 cytokine genotypes are associated with a milder form of primary Sjogren’s syndrome. Ann Rheum Dis. 2006;65:666-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 17. | van Woerkom JM, Kruize AA, Wenting-van Wijk MJ, Knol E, Bihari IC, Jacobs JW, Bijlsma JW, Lafeber FP, van Roon JA. Salivary gland and peripheral blood T helper 1 and 2 cell activity in Sjögren’s syndrome compared with non-Sjögren’s sicca syndrome. Ann Rheum Dis. 2005;64:1474-1479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 77] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 18. | Oxholm P, Daniels TE, Bendtzen K. Cytokine expression in labial salivary glands from patients with primary Sjögren’s syndrome. Autoimmunity. 1992;12:185-191. [PubMed] |
| 19. | Yamada A, Arakaki R, Kudo Y, Ishimaru N. Targeting IL-1 in Sjögren’s syndrome. Expert Opin Ther Targets. 2013;17:393-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 20. | Vijmasi T, Chen FY, Chen YT, Gallup M, McNamara N. Topical administration of interleukin-1 receptor antagonist as a therapy for aqueous-deficient dry eye in autoimmune disease. Mol Vis. 2013;19:1957-1965. [PubMed] |
| 21. | Norheim KB, Harboe E, Gøransson LG, Omdal R. Interleukin-1 inhibition and fatigue in primary Sjögren’s syndrome--a double blind, randomised clinical trial. PLoS One. 2012;7:e30123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 112] [Cited by in RCA: 125] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 22. | Emamian ES, Leon JM, Lessard CJ, Grandits M, Baechler EC, Gaffney PM, Segal B, Rhodus NL, Moser KL. Peripheral blood gene expression profiling in Sjögren’s syndrome. Genes Immun. 2009;10:285-296. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 222] [Cited by in RCA: 193] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 23. | Hjelmervik TO, Petersen K, Jonassen I, Jonsson R, Bolstad AI. Gene expression profiling of minor salivary glands clearly distinguishes primary Sjögren’s syndrome patients from healthy control subjects. Arthritis Rheum. 2005;52:1534-1544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 278] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 24. | Hall JC, Rosen A. Type I interferons: crucial participants in disease amplification in autoimmunity. Nat Rev Rheumatol. 2010;6:40-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 201] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 25. | Lövgren T, Eloranta ML, Kastner B, Wahren-Herlenius M, Alm GV, Rönnblom L. Induction of interferon-alpha by immune complexes or liposomes containing systemic lupus erythematosus autoantigen- and Sjögren’s syndrome autoantigen-associated RNA. Arthritis Rheum. 2006;54:1917-1927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 181] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 26. | Yao Y, Liu Z, Jallal B, Shen N, Rönnblom L. Type I interferons in Sjögren’s syndrome. Autoimmun Rev. 2013;12:558-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 103] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 27. | O’Shea JJ, Paul WE. Mechanisms underlying lineage commitment and plasticity of helper CD4+ T cells. Science. 2010;327:1098-1102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1113] [Cited by in RCA: 1028] [Article Influence: 68.5] [Reference Citation Analysis (0)] |
| 28. | Hooks JJ, Moutsopoulos HM, Geis SA, Stahl NI, Decker JL, Notkins AL. Immune interferon in the circulation of patients with autoimmune disease. N Engl J Med. 1979;301:5-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 677] [Cited by in RCA: 679] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 29. | Hagiwara E, Pando J, Ishigatsubo Y, Klinman DM. Altered frequency of type 1 cytokine secreting cells in the peripheral blood of patients with primary Sjögren’s syndrome. J Rheumatol. 1998;25:89-93. [PubMed] |
| 30. | Cha S, Brayer J, Gao J, Brown V, Killedar S, Yasunari U, Peck AB. A dual role for interferon-gamma in the pathogenesis of Sjogren’s syndrome-like autoimmune exocrinopathy in the nonobese diabetic mouse. Scand J Immunol. 2004;60:552-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 128] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 31. | Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu Rev Immunol. 2009;27:485-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3754] [Cited by in RCA: 3792] [Article Influence: 237.0] [Reference Citation Analysis (0)] |
| 32. | Apostolidis SA, Crispín JC, Tsokos GC. IL-17-producing T cells in lupus nephritis. Lupus. 2011;20:120-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 93] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 33. | Sakai A, Sugawara Y, Kuroishi T, Sasano T, Sugawara S. Identification of IL-18 and Th17 cells in salivary glands of patients with Sjögren’s syndrome, and amplification of IL-17-mediated secretion of inflammatory cytokines from salivary gland cells by IL-18. J Immunol. 2008;181:2898-2906. [PubMed] |
| 34. | Nguyen CQ, Hu MH, Li Y, Stewart C, Peck AB. Salivary gland tissue expression of interleukin-23 and interleukin-17 in Sjögren’s syndrome: findings in humans and mice. Arthritis Rheum. 2008;58:734-743. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 241] [Cited by in RCA: 231] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 35. | Sun Z, Unutmaz D, Zou YR, Sunshine MJ, Pierani A, Brenner-Morton S, Mebius RE, Littman DR. Requirement for RORgamma in thymocyte survival and lymphoid organ development. Science. 2000;288:2369-2373. [PubMed] |
| 36. | Iizuka M, Tsuboi H, Matsuo N, Asashima H, Hirota T, Kondo Y, Iwakura Y, Takahashi S, Matsumoto I, Sumida T. A crucial role of RORγt in the development of spontaneous Sialadenitis-like Sjögren’s syndrome. J Immunol. 2015;194:56-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 37. | Tishler M, Yaron I, Geyer O, Shirazi I, Naftaliev E, Yaron M. Elevated tear interleukin-6 levels in patients with Sjögren syndrome. Ophthalmology. 1998;105:2327-2329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 95] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 38. | Maier-Moore JS, Horton CG, Mathews SA, Confer AW, Lawrence C, Pan Z, Coggeshall KM, Farris AD. Interleukin-6 deficiency corrects nephritis, lymphocyte abnormalities, and secondary Sjögren’s syndrome features in lupus-prone Sle1.Yaa mice. Arthritis Rheumatol. 2014;66:2521-2531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 39. | University Hospital, Strasbourg , France . Efficacy of tocilizumab in primary Sjögren’s Syndrome (ETAP). In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). Available from: http//clinicaltrials.gov/show/NCT01782235 NLM Identifier: NCT01782235. |
| 40. | Ciccia F, Guggino G, Rizzo A, Ferrante A, Raimondo S, Giardina A, Dieli F, Campisi G, Alessandro R, Triolo G. Potential involvement of IL-22 and IL-22-producing cells in the inflamed salivary glands of patients with Sjogren’s syndrome. Ann Rheum Dis. 2012;71:295-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 131] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 41. | Mieliauskaite D, Dumalakiene I, Rugiene R, Mackiewicz Z. Expression of IL-17, IL-23 and their receptors in minor salivary glands of patients with primary Sjögren’s syndrome. Clin Dev Immunol. 2012;2012:187258. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 42. | Lubberts E. The IL-23-IL-17 axis in inflammatory arthritis. Nat Rev Rheumatol. 2015;11:415-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 278] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 43. | Nguyen CQ, Yin H, Lee BH, Chiorini JA, Peck AB. IL17: potential therapeutic target in Sjögren’s syndrome using adenovirus-mediated gene transfer. Lab Invest. 2011;91:54-62. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 63] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 44. | Samson M, Audia S, Janikashvili N, Ciudad M, Trad M, Fraszczak J, Ornetti P, Maillefert JF, Miossec P, Bonnotte B. Brief report: inhibition of interleukin-6 function corrects Th17/Treg cell imbalance in patients with rheumatoid arthritis. Arthritis Rheum. 2012;64:2499-2503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 284] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 45. | Scarsi M, Zanotti C, Chiarini M, Imberti L, Piantoni S, Frassi M, Tincani A, Airò P. Reduction of peripheral blood T cells producing IFN-γ and IL-17 after therapy with abatacept for rheumatoid arthritis. Clin Exp Rheumatol. 2014;32:204-210. [PubMed] |
| 46. | Lavoie TN, Stewart CM, Berg KM, Li Y, Nguyen CQ. Expression of interleukin-22 in Sjögren’s syndrome: significant correlation with disease parameters. Scand J Immunol. 2011;74:377-382. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 47. | Ciccia F, Guggino G, Rizzo A, Bombardieri M, Raimondo S, Carubbi F, Cannizzaro A, Sireci G, Dieli F, Campisi G. Interleukin (IL)-22 receptor 1 is over-expressed in primary Sjogren’s syndrome and Sjögren-associated non-Hodgkin lymphomas and is regulated by IL-18. Clin Exp Immunol. 2015;181:219-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 48. | Kwok SK, Lee J, Yu D, Kang KY, Cho ML, Kim HR, Ju JH, Lee SH, Park SH, Kim HY. A pathogenetic role for IL-21 in primary Sjögren syndrome. Nat Rev Rheumatol. 2015;11:368-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 49. | Maiti AK, Kim-Howard X, Viswanathan P, Guillén L, Rojas-Villarraga A, Deshmukh H, Direskeneli H, Saruhan-Direskeneli G, Cañas C, Tobön GJ. Confirmation of an association between rs6822844 at the Il2-Il21 region and multiple autoimmune diseases: evidence of a general susceptibility locus. Arthritis Rheum. 2010;62:323-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 66] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 50. | Liu H, Liu G, Gong L, Zhang Y, Jiang G. Local suppression of IL-21 in submandibular glands retards the development of Sjögren’s syndrome in non-obese diabetic mice. J Oral Pathol Med. 2012;41:728-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 51. | Hua F, Comer GM, Stockert L, Jin B, Nowak J, Pleasic-Williams S, Wunderlich D, Cheng J, Beebe JS. Anti-IL21 receptor monoclonal antibody (ATR-107): Safety, pharmacokinetics, and pharmacodynamic evaluation in healthy volunteers: a phase I, first-in-human study. J Clin Pharmacol. 2014;54:14-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 52. | Gao J, Killedar S, Cornelius JG, Nguyen C, Cha S, Peck AB. Sjögren’s syndrome in the NOD mouse model is an interleukin-4 time-dependent, antibody isotype-specific autoimmune disease. J Autoimmun. 2006;26:90-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 88] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 53. | Nguyen CQ, Gao JH, Kim H, Saban DR, Cornelius JG, Peck AB. IL-4-STAT6 signal transduction-dependent induction of the clinical phase of Sjögren’s syndrome-like disease of the nonobese diabetic mouse. J Immunol. 2007;179:382-390. [PubMed] |
| 54. | Delaleu N, Mydel P, Kwee I, Brun JG, Jonsson MV, Jonsson R. High fidelity between saliva proteomics and the biologic state of salivary glands defines biomarker signatures for primary Sjögren’s syndrome. Arthritis Rheumatol. 2015;67:1084-1095. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 76] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 55. | Ramos-Casals M, Font J, Brito-Zeron P, Trejo O, García-Carrasco M, Lozano F. Interleukin-4 receptor alpha polymorphisms in primary Sjögren’s syndrome. Clin Exp Rheumatol. 2004;22:374. [PubMed] |
| 56. | Spadaro A, Rinaldi T, Riccieri V, Taccari E, Valesini G. Interleukin-13 in autoimmune rheumatic diseases: relationship with the autoantibody profile. Clin Exp Rheumatol. 2002;20:213-216. [PubMed] |
| 57. | Mahlios J, Zhuang Y. Contribution of IL-13 to early exocrinopathy in Id3-/- mice. Mol Immunol. 2011;49:227-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 58. | Reinisch W, Panés J, Khurana S, Toth G, Hua F, Comer GM, Hinz M, Page K, O’Toole M, Moorehead TM. Anrukinzumab, an anti-interleukin 13 monoclonal antibody, in active UC: efficacy and safety from a phase IIa randomised multicentre study. Gut. 2015;64:894-900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 129] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 59. | Ohyama Y, Nakamura S, Matsuzaki G, Shinohara M, Hiroki A, Fujimura T, Yamada A, Itoh K, Nomoto K. Cytokine messenger RNA expression in the labial salivary glands of patients with Sjögren’s syndrome. Arthritis Rheum. 1996;39:1376-1384. [PubMed] |
| 60. | Saito I, Haruta K, Shimuta M, Inoue H, Sakurai H, Yamada K, Ishimaru N, Higashiyama H, Sumida T, Ishida H. Fas ligand-mediated exocrinopathy resembling Sjögren’s syndrome in mice transgenic for IL-10. J Immunol. 1999;162:2488-2494. [PubMed] |
| 61. | Llorente L, Richaud-Patin Y, García-Padilla C, Claret E, Jakez-Ocampo J, Cardiel MH, Alcocer-Varela J, Grangeot-Keros L, Alarcón-Segovia D, Wijdenes J. Clinical and biologic effects of anti-interleukin-10 monoclonal antibody administration in systemic lupus erythematosus. Arthritis Rheum. 2000;43:1790-1800. [PubMed] |
| 62. | Furuzawa-Carballeda J, Hernández-Molina G, Lima G, Rivera-Vicencio Y, Férez-Blando K, Llorente L. Peripheral regulatory cells immunophenotyping in primary Sjögren’s syndrome: a cross-sectional study. Arthritis Res Ther. 2013;15:R68. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 63. | Sundberg TB, Xavier RJ, Schreiber SL, Shamji AF. Small-molecule control of cytokine function: new opportunities for treating immune disorders. Curr Opin Chem Biol. 2014;23:23-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 64. | O’Shea JJ, Plenge R. JAK and STAT signaling molecules in immunoregulation and immune-mediated disease. Immunity. 2012;36:542-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 692] [Cited by in RCA: 875] [Article Influence: 67.3] [Reference Citation Analysis (0)] |
| 65. | Manganelli P, Fietta P. Apoptosis and Sjögren syndrome. Semin Arthritis Rheum. 2003;33:49-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 115] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 66. | Polihronis M, Tapinos NI, Theocharis SE, Economou A, Kittas C, Moutsopoulos HM. Modes of epithelial cell death and repair in Sjögren’s syndrome (SS). Clin Exp Immunol. 1998;114:485-490. [PubMed] |
| 67. | Sisto M, Lisi S, Castellana D, Scagliusi P, D’Amore M, Caprio S, Scagliusi A, Acquafredda A, Panaro MA, Mitolo V. Autoantibodies from Sjögren’s syndrome induce activation of both the intrinsic and extrinsic apoptotic pathways in human salivary gland cell line A-253. J Autoimmun. 2006;27:38-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 50] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 68. | Risselada AP, Looije MF, Kruize AA, Bijlsma JW, van Roon JA. The role of ectopic germinal centers in the immunopathology of primary Sjögren’s syndrome: a systematic review. Semin Arthritis Rheum. 2013;42:368-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 136] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 69. | Youinou P, Pers JO. Disturbance of cytokine networks in Sjögren’s syndrome. Arthritis Res Ther. 2011;13:227. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 105] [Article Influence: 7.5] [Reference Citation Analysis (0)] |