Peer-review started: December 9, 2014
First decision: December 26, 2014
Revised: January 14, 2015
Accepted: June 4, 2015
Article in press: June 8, 2015
Published online: July 27, 2015
Processing time: 239 Days and 17.4 Hours
Physiological stress takes place in the endoplasmic reticulum (ER) of cells where activation and up-regulation of genes and proteins are primarily induced to enhance pro-survival mechanisms such as the unfolded protein response (UPR). A dominant protein in the UPR response is the heat shock GRP78 protein. Although GRP78 is primarily located in the ER, under certain conditions it is transported to the cell surface, where it acts as a receptor inducing pathways of cell signaling such as proliferation or apoptosis. In the prolonged chronic stress transportation of the GRP78 from the ER to the cell membrane is a major event where in addition to the presentation of the GRP78 as a receptor to various ligands, it also marks the cells that will proceed to apoptotic pathways. In the normal cell that under stress acquires cell surface GRP78 and in the tumor cell that already presents cell surface GRP78, cell surface GRP78 is an apoptotic flag. The internalization of GRP78 from the cell surface in normal cells by ligands such as peptides will enhance cell survival and alleviate cardiovascular ischemic diseases. The absence of cell surface GRP78 in the tumor cells portends proliferative and metastatic tumors. Pharmacological induction of cell surface GRP78 will induce the process of apoptosis and might be used as a therapeutic modality for cancer treatment.
Core tip: In the prolonged chronic stress transportation of the GRP78 from the endoplasmic reticulum to the cell membrane is a major event where in addition to the presentation of the GRP78 as a receptor to various ligands, it also marks the cells that will proceed to apoptotic pathways. In the normal cell that under stress acquires cell surface GRP78 and in the tumor cell that already presents cell surface GRP78, cell surface GRP78 is an apoptotic flag. This review analyzes the input of cell surface GRP78 on apoptosis in normal and tumor cells.
- Citation: Hardy B, Raiter A. GRP78 expression beyond cellular stress: A biomarker for tumor manipulation. World J Immunol 2015; 5(2): 78-85
- URL: https://www.wjgnet.com/2219-2824/full/v5/i2/78.htm
- DOI: https://dx.doi.org/10.5411/wji.v5.i2.78
Cellular stress response covers a number of molecular changes that cells undergo in reaction to patho-physiological conditions, such as lack of nutrients and oxygen, or exposure to toxins in their micro environment[1,2].
The cellular response to stress aims to protect the cells by either a short or a long term mechanism that minimizes the damage to the cell integrity. Cellular stress responses are primarily located in the endoplasmic reticulum (ER) and are mediated through highly conserved stress proteins such as heat shock proteins, some of which are only activated by stress while others are involved both in stress responses and in normal cellular functioning[2,3].
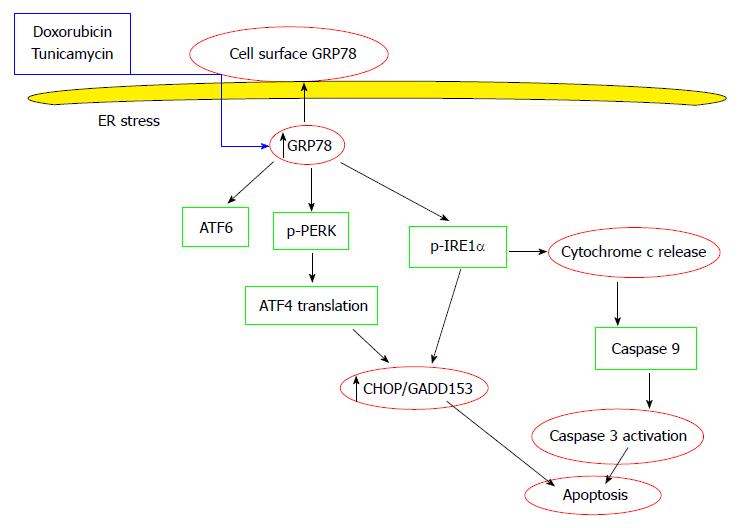
One such mechanism is the unfolded protein response (UPR), an evolutionarily conserved mechanism in which survival or apoptotic pathways are activated[4,5]. UPR is initiated upon the accumulation of unfolded proteins in the ER. The master UPR regulator is the glucose-regulated protein GRP78, a member of the heat shock protein 70 family that functions as a chaperone for the folding, maturation and transport of polypeptides and proteins in the ER[6,7]. GRP78 is also a key member of the UPR. Its primary role is to protect cells from undergoing apoptosis under physiological stress conditions[8]. If the adaptive response fails, apoptotic cell death ensues[9].
As an adaptive response to ER stress, the UPR triggers a set of pathways that results in the activation of inositol-requiring protein1 (IRE1), PKR-like ER kinase (PERK) and setting in motion transcription factor 6 (ATF6)[10]. Activation of these pathways selectively suppresses protein synthesis while promoting the translation of other specific proteins and regulating a variety of UPR target genes expression, including glucose-regulated protein GRP78 and the major pro-apoptotic transcription factor CHOP (also called GADD153)[10].
The induction of GRP78 to enhance protein folding and assembly in the ER leads to an increase in GRP78 in the ER compartment as well as to the promotion of GRP78 re-localization to the cell surface- where it assumes a new function as a receptor for cell-surface signaling[6].
Several possibilities for how GRP78 escapes to the cell surface in tumor cells were suggested[11]. In general, GRP78 trafficking from the ER to the cell surface is not well understood. It was demonstrated that ER stress actively promotes GRP78 localization on the cell surface, however ectopic expression of GRP78 is also able to cause cell surface relocation in the absence of ER stress[11]. There are also conflicting reports of whether GRP78 is expressed on specific tumor cell lines, such as PC-3 prostate cancer cells[12]. It is reasonable that since ER membrane is a source of plasma membrane, this form of GRP78 could be cycled to the cell surface. Studies also suggested that specific cell types may utilize different proteins for transporting GRP78 to the cell surface. For example, the ER transmembrane protein, MTJ-1 is implicated as the GRP78 carrier protein in macrophages[13]. The tumor suppressor Par-4 is reported to be required for GRP78 cell surface localization in PC-3 cells[14].
The present review aims to describe the function and modulation of cell surface GRP78 for the treatment of a number of maladies.
Although Shock protein GRP78 has long been studied as a molecular chaperone in the ER expressed in mammalian cells and has a critical role in cellular integrity, its translocation to the plasma membrane on different cells was recently found to have several implications[6,15].
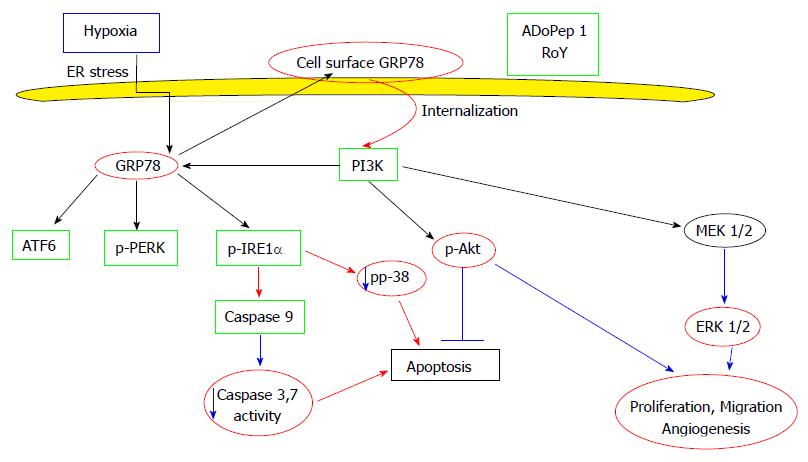
Stress induced mechanisms such as hypoxia that increased the expression of GRP78 on the cell surface was also found to stimulate cell cycle arrest at the G0/G1 phase, resulting in massive cell apoptosis[16]. It is possible therefore that hypoxia induced membrane GRP78 is a trigger for apoptosis.
Therefore, the binding of a peptide or an anti-GRP78 antibody to hypoxia- induced membrane GRP78 might decrease the stress protein on endothelial cell membranes and reduce apoptosis.
This last affirmation was substantially corroborated by the experiments in which cell surface GRP78 binding of peptides RoY, ADoPep1, or an anti GRP78 antibody, inhibited hypoxia-induced apoptosis of endothelial cells and induced proliferation and angiogenesis[17-19].
The addition of ADoPep1 to endothelial cells under hypoxic conditions induced a dramatic decrease in membrane GRP78 after only 15 min, as measured by FACS analysis. This was most likely due to the internalization of the cell surface GRP78 receptor. The internalization of the GRP78 receptor triggered PI3K pathway increasing Akt phosphorylation and MEK pathways including ERK phosphorylation for the activation of the survival/proliferation activity. The implications of specific inhibitors to PI3K and MEK pathways confirmed the specific signaling[18].
The inhibition of apoptosis was initiated by the internalization of the GRP78 receptor and the inhibition of cytochrome c release, caspase 3 activation and decrease of p38 phosphorylation[18-22].
Cell surface GRP78 induction in cultured endothelial cells was triggered by their incubation for 24 h under hypoxic conditions[17-19]. The process of cell surface GRP78 removal from the endothelial cells by peptide binding and its internalization, lead to the inhibition of apoptosis, activation of a survival mechanism, proliferation and the initiation of the angiogenic process[17,23].
Similar to the results obtained with cultured endothelial cells was the outcome of experiments with ischemic (ischemia is the term for the lack of oxygen) diseases.
As for hypoxic conditions, the chronic lack of oxygen in mammalian tissues is the basis for ischemic diseases. An experimental ischemic hind limb model can be obtained by ligation of the femoral artery in one of the mouse hind limbs and comparing it to the non-operated second limb which serves as a control for the ischemic disease of the legs[17-19,24].
Histological sections from the ischemic leg that featured a significant decreased in blood flow, showed a significant increase in GRP78-positive endothelial cells along with the increased number of apoptotic cells and the decrease in number of capillaries[17,18].
A single local administration of the peptide binding GRP78 to the femoral ligated ischemic mouse alleviated the ischemia and restored blood transfusion after 3 wk. Histological analysis of the peptide treated limb demonstrated reduction in GRP78 positive endothelial cells accompanied by proliferation, numerous capillaries and restored blood perfusion[17,18].
Normal cells under normal conditions maintain a homeostasis of GRP78 in the ER where this protein serves as a chaperone for the normal folding and final secretion of glycoprotein[25]. Stress will induce the up-regulation of GRP78, that as stated, aims to protect the cell from undergoing apoptosis[1-4,25]. However, in chronic stress conditions the up-regulation of GRP78 expression that is associated with the expression of cell surface GRP78 on the normal cell will direct the cell to apoptosis, probably in order to protect the organism from secreting abnormal proteins[17-19,26,27].
Chronic stress conditions due to reduced blood flow in atherosclerosis or diabetes patients[28,29], might induce ischemic vascular diseases (IVD) in mammals that might affect the legs, heart and brain[19,30,31]. In pathological conditions such as atherosclerotic lesions of the human aorta and in endothelial cells of the tumor vasculature, cell-surface GRP78 co-localizes with T-cadherin in human umbilical vein endothelial cells (HUVECs). Overexpression of T-cadherin in HUVECs mediated cell survival in a GRP78-dependent fashion by increasing phospho-Akt and phospho-GSK3β and decreasing caspase-3 levels[32].
It has been demonstrated that ER stress associated apoptosis is involved in the pathogenesis of heart failure following acute myocardial infarction (MI)[30].
Ischemia was demonstrated to induce myocardial apoptosis, which results in loss of cardiomyocytes, leading to the impairment of cardiac systolic and diastolic functions[33]. In our studies, cardiomyocytes cultured for 4 h under hypoxic conditions, manifested the increased expression of cell surface GRP78 accompanied by increased apoptosis[33].
Additionally, TUNEL staining indicated apoptosis in cardiomyocytes in the ischemic myocardium model in animals. We have also found increased GRP78 staining near the infarct heart of experimentally- induced MI in mice[33].
Increased GRP78 has previously been reported in heart failure[34], in diabetic cardiomyopathy[29], and in an experimental rat coronary artery occlusion model, where the GRP78 protein level increased after short cycles of ischemia[35]. Cardiomyocytes under these hypoxic conditions manifested apoptosis[35]. As for the endothelial cells, the apoptotic process could be reversed both in cardiomyocytes cultures cells or ischemic heart tissues by the peptide binding cell surface GRP78[33]. Moreover, normal cells studies of ischemia induced increased cell surface GRP78 in neurons and ischemia of the optic nerve were conducted with similar results[36].
Cell surface GRP78 survival and apoptotic pathways in the normal cells are described in Figure 1.
In contrast to the normal cells where cell surface GRP78 were induced by stress conditions, tumor cells were already exposed to cell surface GRP78 to a variable extent[6,21,37]. This was attributed to the tumor microenvironment that is characterized as chronic stress conditions caused by the deprivation of oxygen, glucose and nutrients[38].
The tumor microenvironment induced ER stress response activates the UPR[15,38,39] which has been shown to be up regulated in primary human tumor cells of several origins, including breast[40], lung[41], liver[42], colon[43], prostate[44] and brain[45].
Whether the UPR inhibits tumor growth or protects tumor cells by facilitating their adaptation to stressful conditions within the tumor microenvironment is still under controversy[15].
Permanently up-regulated GRP78 expression was also frequently documented in tumor cell lines and primary clinical samples[8,27]. However, it is not yet clear whether the increase in GRP78 expression facilitating tumor cell survival is achieved by the blockage of pro-apoptotic or the activation of pro-survival pathways[6,7,15].
It has been claimed that due to its pro-survival property in stress response, GRP78 contributes to tumor growth and confers drug resistance onto cancer cells[6,46]. In certain tumors the increase in tumorigenicity and drug resistance has been attributed to the over expression of GRP78[15].
For example, it was reported that increased GRP78 expression in glioblastoma and melanoma promotes cell survival and correlates with poor prognosis[45,47]. High GRP78 levels produced the predicted result in WM266-4 melanoma and MO59J glioblastoma cell lines, reducing cell death in response to stress. However, inducing stable over-expression of GRP78 was accompanied by large changes in UPR activator expression, with reductions in PERK and increased IRE1 in glioblastoma cells but decreased ATF6 in the melanoma cells. The contribution of these changes in UPR activator expression to decreased stress sensitivity is uncertain because GRP78 over-expression in these cells was also accompanied by reduced stress, possibly as a result of the large and unexpected increases in expression of all three UPR activators[48]. In contrast, other studies associated increased GRP78 expression with tumor growth inhibition and a predictor for positive cancer treatments[49]. One such study described GRP78 as a novel positive predictor for breast cancer sensitivity to doxorubicin/taxane-based adjuvant chemotherapy[50]. Increased GRP78 expression was also shown in neuroblastoma that correlated with improved stress sensitivity and prognosis[51]. In addition, the expression of GRP78 correlated with an ameliorated prognosis in lung cancer[52].
A recent study indicated that metabolism deficiency that promotes increase in GRP78 is related to stress induced apoptosis[53]. An explanation to these contradictory reports might suggest that GRP78 has different roles as a sensor of ER stress in tumors.
Besides in the ER, GRP78 was also found to be located in the cytoplasm, mitochondria, nucleus, cell membrane as well as extracellular secretions by tumor cells[6,8].
How GRP78 escapes to the cell surface in tumor cells is not well understood, but it may also involve some specific mechanisms adapted by the tumor cells[6].
Cell surface GRP78 was reported as a receptor to mediate tumor cell signal transduction.
Cell-surface GRP78 was found to be associates with MHC class I, a receptor for the coxsackie A9 and Dengue viruses, and functions as the signaling receptor upon binding to the activated form of the plasma proteinase inhibitor, α2-macroglogulin (α2M*)[54]. Binding of cell-surface GRP78 with α2M* on 1-LN prostate tumor cells induced Akt phosphorylation[54] promoting cell proliferation either by inactivating apoptotic pathways or upregulating activated NFk-Β. Up-regulation of NF-Β augments inactivation of mitogen-activated protein kinase kinase 7 through its binding, to increase levels of growth arrest and DNA-damage-inducible β (GADD45β), thereby preventing JNK-mediated apoptosis. In addition, inactivation of apoptosis signal-regulating kinase (ASK1) by active Akt attenuates downstream JNK-mediated apoptosis[54].
Another interacting protein with GRP78 receptor is the GPI-anchored oncogene Cripto (Cripto-1, teratocarcinoma-derived growth factor 1). Cripto is expressed at high levels in human tumors and is associated with cell proliferation, migration, invasion and tumor angiogenesis via activation of MAPK/ERK and PI3K/Akt. Binding of GRP78 receptor to Cripto was ound to inhibit transforming growth factor-β (TGF-β) signaling and to promote cell proliferation[55].
The Protease-activated receptor 4 also known as coagulation factor II (Par-4) is a tumor suppressor that was also associated with cell-surface GRP78. Binding of Par-4 to GRP78 receptor near its N-terminus elicits the apoptotic pathway by activation the FADD/caspase-8/caspase-3 pathway[14]. On the other hand, Kringle 5 of human plasminogen binding to the N-terminal domain of GRP78 receptor mediates apoptosis of tumor cells involving activation of caspase-7[56].
Cell surface GRP78 has been demonstrated in a large variety of tumor cell types and to variable extent[11,13-15].
Subpopulations of cell surface GRP78 positive and negative were compared in order to analyze and clarify some of the contradictory conclusions on the fate of tumor cells expressing cell surface GRP78 and to elucidate whether cell surface GRP78 positive and negative tumor cells manifest different properties in colorectal cancer and whether these cells are directed to survival or to apoptotic pathways, or to pro or non metastatic directions. Two subpopulations of cell surface GRP78 positive and cell surface negative tumor cells were artificially separated by GRP78 antibody- bound magnetic beads from two different colorectal carcinoma cell lines. The HM7 cell line, a sub-line of the human colon carcinoma LS174T having a higher metastatic tendency and HCT116 cells derived from a human adenomatous polyposis. The results demonstrated that only GRP78 negative cells were highly proliferative, induced significant growth in tumor size and metastasized to the liver. In contrast, GRP78 positive cells manifested reduced proliferation, colony formation, tumor growth and liver metastases. The decreased tumorigenicity of the GRP78 positive subpopulation was abrogated by silencing GRP78 expression[57].
In breast cancer tumors, subtypes are based on the expression of cell surface receptors such as estrogen, progesterone and human epidermal growth factor receptors and tumor cells negative cell surface receptors, usually referred to as luminal and basal like tumors[58]. The luminal subtype with positive receptors has a favorable prognosis while the basal-like tumors with triple-negative receptors exhibit a poor prognosis. In addition to MDAMB468 cells which are basal- like tumor, negative for all 3 receptors were also negative to cell surface GRP78. In contrast, BT474, a representative of the luminal subtype, was also positive for cell surface GRP78 expression[59].
To evaluate the effect of cell surface GRP78 expression on the basal, receptor negative breast cancer cells, cell surface GRP78 was pharmacologically induced by doxorubicin and taxotere. These drugs significantly increased cell surface GRP78 expression on the basal receptor negative breast cancer tumor cells. Increased tumor cell surface GRP78 resulted in a significant decrease in tumorigenicity, reduced tumor growth and an increase in cell apoptosis demonstrating a direct correlation between expressed cell surface GRP78 and apoptosis. In addition, the potential application of doxorubicin and tunicamycin to induce the over-expression of cell surface GRP78 causes a significant increase in stress induced apoptosis in the triple negative tumor cell lines[59]. In a study of breast cancer, it was reported that CHOP/GADD153 over-expression correlates with a significantly lower risk of recurrence in the GRP78-positive subset[49]. It is possible that cell surface GRP78 expression is associated with the induction of the pro-apoptotic factor CHOP/GADD153.
The two major apoptotic pathways recognized as the death receptor (extrinsic) and mitochondrial (intrinsic) pathways play crucial roles in tumor progression as well as resistance to therapeutic strategies. Although the mechanisms that cause the biological selection for a specific mode of cell death remain unclear, it seems probable that the results depend on the intensity of the stress[60]. Pharmacological induction of intrinsic apoptosis was achieved by exogenous agents triggering acute ER stress[57,59].
Additional applications to cell surface GRP78 induction on tumor cells, as a potential target for cancer therapy were suggested[61]. For example, pro-apoptotic moieties or cytotoxic agents were conjugated onto peptides with a high affinity for GRP78 to successfully target and kill cancer cells[62]. Also, an un-conjugated peptidic GRP78 ligand demonstrated toxicity to prostate cancer cell by an extrinsic apoptotic pathway[63]. A human monoclonal IgM antibody against cell surface GRP78 isolated from a cancer patient was found to be capable of inducing lipid accumulation and apoptosis, probably extrinsic, in cancer cells[64].
Cell surface GRP78 survival and apoptotic pathways in the tumor cell are described in Figure 2.
The significance of cell surface GRP78 expression, beyond cellular stress, might be the focus of new therapeutic strategies for ischemic diseases. Pharmacological manipulation of cell surface GRP78 in tumor cells may serve as a new modality for tumor therapy.
The authors wish to thank Mrs. Sara Dominitz for her excellent editorial skills. The authors wish to send their gratitude to our collaborators from the Departments of Cardiology, Gastroenterology and Pathology at the Rabin Medical center, and to Dr. Rinat Yerushalmi from the Davidoff Institute of Oncology.
P- Reviewer: Hori T, Lai ZF, Muntane J, Munoz M S- Editor: Tian YL L- Editor: A E- Editor: Jiao XK
| 1. | Walter P, Ron D. The unfolded protein response: from stress pathway to homeostatic regulation. Science. 2011;334:1081-1086. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3842] [Cited by in RCA: 4537] [Article Influence: 349.0] [Reference Citation Analysis (0)] |
| 2. | Schönthal AH. Endoplasmic reticulum stress: its role in disease and novel prospects for therapy. Scientifica (Cairo). 2012;2012:857516. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 169] [Cited by in RCA: 224] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 3. | Lee AS. Mammalian stress response: induction of the glucose-regulated protein family. Curr Opin Cell Biol. 1992;4:267-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 345] [Cited by in RCA: 344] [Article Influence: 10.4] [Reference Citation Analysis (1)] |
| 4. | Malhotra JD, Kaufman RJ. The endoplasmic reticulum and the unfolded protein response. Semin Cell Dev Biol. 2007;18:716-731. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 802] [Cited by in RCA: 779] [Article Influence: 43.3] [Reference Citation Analysis (0)] |
| 5. | Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8:519-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4586] [Cited by in RCA: 5058] [Article Influence: 281.0] [Reference Citation Analysis (0)] |
| 6. | Ni M, Zhang Y, Lee AS. Beyond the endoplasmic reticulum: atypical GRP78 in cell viability, signalling and therapeutic targeting. Biochem J. 2011;434:181-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 375] [Cited by in RCA: 429] [Article Influence: 30.6] [Reference Citation Analysis (1)] |
| 7. | Lee AS. The glucose-regulated proteins: stress induction and clinical applications. Trends Biochem Sci. 2001;26:504-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 780] [Cited by in RCA: 809] [Article Influence: 33.7] [Reference Citation Analysis (1)] |
| 8. | Li Z, Li Z. Glucose regulated protein 78: a critical link between tumor microenvironment and cancer hallmarks. Biochim Biophys Acta. 2012;1826:13-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 76] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 9. | Han J, Back SH, Hur J, Lin YH, Gildersleeve R, Shan J, Yuan CL, Krokowski D, Wang S, Hatzoglou M. ER-stress-induced transcriptional regulation increases protein synthesis leading to cell death. Nat Cell Biol. 2013;15:481-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1032] [Cited by in RCA: 1329] [Article Influence: 110.8] [Reference Citation Analysis (0)] |
| 10. | Fels DR, Koumenis C. The PERK/eIF2alpha/ATF4 module of the UPR in hypoxia resistance and tumor growth. Cancer Biol Ther. 2006;5:723-728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 267] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 11. | Zhang Y, Liu R, Ni M, Gill P, Lee AS. Cell surface relocalization of the endoplasmic reticulum chaperone and unfolded protein response regulator GRP78/BiP. J Biol Chem. 2010;285:15065-15075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 269] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 12. | Burikhanov R, Zhao Y, Goswami A, Qiu S, Schwarze SR, Rangnekar VM. The tumor suppressor Par-4 activates an extrinsic pathway for apoptosis. Cell. 2009;138:377-388. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 218] [Cited by in RCA: 186] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 13. | Gonzalez-Gronow M, Selim MA, Papalas J, Pizzo SV. GRP78: a multifunctional receptor on the cell surface. Antioxid Redox Signal. 2009;11:2299-2306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 202] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 14. | Lee AS. The Par-4-GRP78 TRAIL, more twists and turns. Cancer Biol Ther. 2009;8:2103-2105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 15. | Vandewynckel YP, Laukens D, Geerts A, Bogaerts E, Paridaens A, Verhelst X, Janssens S, Heindryckx F, Van Vlierberghe H. The paradox of the unfolded protein response in cancer. Anticancer Res. 2013;33:4683-4694. [PubMed] |
| 16. | Li C, Issa R, Kumar P, Hampson IN, Lopez-Novoa JM, Bernabeu C, Kumar S. CD105 prevents apoptosis in hypoxic endothelial cells. J Cell Sci. 2003;116:2677-2685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 125] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 17. | Hardy B, Battler A, Weiss C, Kudasi O, Raiter A. Therapeutic angiogenesis of mouse hind limb ischemia by novel peptide activating GRP78 receptor on endothelial cells. Biochem Pharmacol. 2008;75:891-899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 39] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 18. | Raiter A, Weiss C, Bechor Z, Ben-Dor I, Battler A, Kaplan B, Hardy B. Activation of GRP78 on endothelial cell membranes by an ADAM15-derived peptide induces angiogenesis. J Vasc Res. 2010;47:399-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 19. | Raiter A, Bechor Z, Kleiman M, Leshem-Lev D, Battler A, Hardy B. Angiogenic peptides improve blood flow and promote capillary growth in a diabetic and ischaemic mouse model. Eur J Vasc Endovasc Surg. 2010;40:381-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 20. | Kang JS, Liu C, Derynck R. New regulatory mechanisms of TGF-beta receptor function. Trends Cell Biol. 2009;19:385-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 276] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 21. | Sato M, Yao VJ, Arap W, Pasqualini R. GRP78 signaling hub a receptor for targeted tumor therapy. Adv Genet. 2010;69:97-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 72] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 22. | Schütze S, Schneider-Brachert W. Impact of TNF-R1 and CD95 internalization on apoptotic and antiapoptotic signaling. Results Probl Cell Differ. 2009;49:63-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 23. | Nakamura S, Takizawa H, Shimazawa M, Hashimoto Y, Sugitani S, Tsuruma K, Hara H. Mild endoplasmic reticulum stress promotes retinal neovascularization via induction of BiP/GRP78. PLoS One. 2013;8:e60517. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 24. | Arderiu G, Peña E, Badimon L. Angiogenic microvascular endothelial cells release microparticles rich in tissue factor that promotes postischemic collateral vessel formation. Arterioscler Thromb Vasc Biol. 2015;35:348-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 25. | Pan YX, Ren AJ, Zheng J, Rong WF, Chen H, Yan XH, Wu C, Yuan WJ, Lin L. Delayed cytoprotection induced by hypoxic preconditioning in cultured neonatal rat cardiomyocytes: role of GRP78. Life Sci. 2007;81:1042-1049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 26. | Rao RV, Poksay KS, Castro-Obregon S, Schilling B, Row RH, del Rio G, Gibson BW, Ellerby HM, Bredesen DE. Molecular components of a cell death pathway activated by endoplasmic reticulum stress. J Biol Chem. 2004;279:177-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 122] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 27. | Xu C, Bailly-Maitre B, Reed JC. Endoplasmic reticulum stress: cell life and death decisions. J Clin Invest. 2005;115:2656-2664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1683] [Cited by in RCA: 1840] [Article Influence: 92.0] [Reference Citation Analysis (0)] |
| 28. | Gargalovic PS, Gharavi NM, Clark MJ, Pagnon J, Yang WP, He A, Truong A, Baruch-Oren T, Berliner JA, Kirchgessner TG. The unfolded protein response is an important regulator of inflammatory genes in endothelial cells. Arterioscler Thromb Vasc Biol. 2006;26:2490-2496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 274] [Cited by in RCA: 297] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 29. | Howangyin KY, Silvestre JS. Diabetes mellitus and ischemic diseases: molecular mechanisms of vascular repair dysfunction. Arterioscler Thromb Vasc Biol. 2014;34:1126-1135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 107] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 30. | Ortega A, Roselló-Lletí E, Tarazón E, Molina-Navarro MM, Martínez-Dolz L, González-Juanatey JR, Lago F, Montoro-Mateos JD, Salvador A, Rivera M. Endoplasmic reticulum stress induces different molecular structural alterations in human dilated and ischemic cardiomyopathy. PLoS One. 2014;9:e107635. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 31. | Raghubir R, Nakka VP, Mehta SL. Endoplasmic reticulum stress in brain damage. Methods Enzymol. 2011;489:259-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 32. | Philippova M, Ivanov D, Joshi MB, Kyriakakis E, Rupp K, Afonyushkin T, Bochkov V, Erne P, Resink TJ. Identification of proteins associating with glycosylphosphatidylinositol- anchored T-cadherin on the surface of vascular endothelial cells: role for Grp78/BiP in T-cadherin-dependent cell survival. Mol Cell Biol. 2008;28:4004-4017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 100] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 33. | Hardy B, Raiter A. Peptide-binding heat shock protein GRP78 protects cardiomyocytes from hypoxia-induced apoptosis. J Mol Med (Berl). 2010;88:1157-1167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 34. | Shintani-Ishida K, Nakajima M, Uemura K, Yoshida K. Ischemic preconditioning protects cardiomyocytes against ischemic injury by inducing GRP78. Biochem Biophys Res Commun. 2006;345:1600-1605. [PubMed] |
| 35. | Szegezdi E, Duffy A, O’Mahoney ME, Logue SE, Mylotte LA, O’brien T, Samali A. ER stress contributes to ischemia-induced cardiomyocyte apoptosis. Biochem Biophys Res Commun. 2006;349:1406-1411. [PubMed] |
| 36. | Goldenberg-Cohen N, Raiter A, Gaydar V, Dratviman-Storobinsky O, Goldstein T, Weizman A, Hardy B. Peptide-binding GRP78 protects neurons from hypoxia-induced apoptosis. Apoptosis. 2012;17:278-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 37. | Zhang LH, Zhang X. Roles of GRP78 in physiology and cancer. J Cell Biochem. 2010;110:1299-1305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 137] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 38. | Taddei ML, Giannoni E, Comito G, Chiarugi P. Microenvironment and tumor cell plasticity: an easy way out. Cancer Lett. 2013;341:80-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 189] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 39. | Luo B, Lee AS. The critical roles of endoplasmic reticulum chaperones and unfolded protein response in tumorigenesis and anticancer therapies. Oncogene. 2013;32:805-818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 374] [Cited by in RCA: 456] [Article Influence: 35.1] [Reference Citation Analysis (0)] |
| 40. | Nagelkerke A, Bussink J, Mujcic H, Wouters BG, Lehmann S, Sweep FC, Span PN. Hypoxia stimulates migration of breast cancer cells via the PERK/ATF4/LAMP3-arm of the unfolded protein response. Breast Cancer Res. 2013;15:R2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 165] [Cited by in RCA: 186] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 41. | Sun Q, Hua J, Wang Q, Xu W, Zhang J, Zhang J, Kang J, Li M. Expressions of GRP78 and Bax associate with differentiation, metastasis, and apoptosis in non-small cell lung cancer. Mol Biol Rep. 2012;39:6753-6761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 42. | Malhi H, Kaufman RJ. Endoplasmic reticulum stress in liver disease. J Hepatol. 2011;54:795-809. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 980] [Cited by in RCA: 952] [Article Influence: 68.0] [Reference Citation Analysis (0)] |
| 43. | Guo B, Li Z. Endoplasmic reticulum stress in hepatic steatosis and inflammatory bowel diseases. Front Genet. 2014;5:242. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 61] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 44. | de Ridder G, Ray R, Misra UK, Pizzo SV. Modulation of the unfolded protein response by GRP78 in prostate cancer. Methods Enzymol. 2011;489:245-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 45. | Pyrko P, Schönthal AH, Hofman FM, Chen TC, Lee AS. The unfolded protein response regulator GRP78/BiP as a novel target for increasing chemosensitivity in malignant gliomas. Cancer Res. 2007;67:9809-9816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 313] [Cited by in RCA: 347] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 46. | Schönthal AH. Pharmacological targeting of endoplasmic reticulum stress signaling in cancer. Biochem Pharmacol. 2013;85:653-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 146] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 47. | Hersey P, Zhang XD. Adaptation to ER stress as a driver of malignancy and resistance to therapy in human melanoma. Pigment Cell Melanoma Res. 2008;21:358-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 83] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 48. | Martin S, Lovat PE, Redfern CP. Cell-type variation in stress responses as a consequence of manipulating GRP78 expression in neuroectodermal cells. J Cell Biochem. 2015;116:438-449. [PubMed] |
| 49. | Zheng YZ, Cao ZG, Hu X, Shao ZM. The endoplasmic reticulum stress markers GRP78 and CHOP predict disease-free survival and responsiveness to chemotherapy in breast cancer. Breast Cancer Res Treat. 2014;145:349-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 81] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 50. | Lee E, Nichols P, Groshen S, Spicer D, Lee AS. GRP78 as potential predictor for breast cancer response to adjuvant taxane therapy. Int J Cancer. 2011;128:726-731. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 62] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 51. | Hsu WM, Hsieh FJ, Jeng YM, Kuo ML, Tsao PN, Lee H, Lin MT, Lai HS, Chen CN, Lai DM. GRP78 expression correlates with histologic differentiation and favorable prognosis in neuroblastic tumors. Int J Cancer. 2005;113:920-927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 52. | Uramoto H, Sugio K, Oyama T, Nakata S, Ono K, Yoshimastu T, Morita M, Yasumoto K. Expression of endoplasmic reticulum molecular chaperone Grp78 in human lung cancer and its clinical significance. Lung Cancer. 2005;49:55-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 116] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 53. | Wang M, Kaufman RJ. The impact of the endoplasmic reticulum protein-folding environment on cancer development. Nat Rev Cancer. 2014;14:581-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 716] [Cited by in RCA: 841] [Article Influence: 76.5] [Reference Citation Analysis (0)] |
| 54. | Misra UK, Payne S, Pizzo SV. Ligation of prostate cancer cell surface GRP78 activates a proproliferative and antiapoptotic feedback loop: a role for secreted prostate-specific antigen. J Biol Chem. 2011;286:1248-1259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 58] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 55. | Shani G, Fischer WH, Justice NJ, Kelber JA, Vale W, Gray PC. GRP78 and Cripto form a complex at the cell surface and collaborate to inhibit transforming growth factor beta signaling and enhance cell growth. Mol Cell Biol. 2008;28:666-677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 152] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 56. | Gonzalez-Gronow M, Kaczowka SJ, Payne S, Wang F, Gawdi G, Pizzo SV. Plasminogen structural domains exhibit different functions when associated with cell surface GRP78 or the voltage-dependent anion channel. J Biol Chem. 2007;282:32811-32820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 45] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 57. | Hardy B, Raiter A, Yakimov M, Vilkin A, Niv Y. Colon cancer cells expressing cell surface GRP78 as a marker for reduced tumorigenicity. Cell Oncol (Dordr). 2012;35:345-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 58. | Sotiriou C, Neo SY, McShane LM, Korn EL, Long PM, Jazaeri A, Martiat P, Fox SB, Harris AL, Liu ET. Breast cancer classification and prognosis based on gene expression profiles from a population-based study. Proc Natl Acad Sci USA. 2003;100:10393-10398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1496] [Cited by in RCA: 1422] [Article Influence: 64.6] [Reference Citation Analysis (0)] |
| 59. | Raiter A, Yerushalmi R, Hardy B. Pharmacological induction of cell surface GRP78 contributes to apoptosis in triple negative breast cancer cells. Oncotarget. 2014;5:11452-11463. [PubMed] |
| 60. | Syed DN, Lall RK, Chamcheu JC, Haidar O, Mukhtar H. Involvement of ER stress and activation of apoptotic pathways in fisetin induced cytotoxicity in human melanoma. Arch Biochem Biophys. 2014;563:108-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 61. | Liu R, Li X, Gao W, Zhou Y, Wey S, Mitra SK, Krasnoperov V, Dong D, Liu S, Li D. Monoclonal antibody against cell surface GRP78 as a novel agent in suppressing PI3K/AKT signaling, tumor growth, and metastasis. Clin Cancer Res. 2013;19:6802-6811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 150] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 62. | Miao YR, Eckhardt BL, Cao Y, Pasqualini R, Argani P, Arap W, Ramsay RG, Anderson RL. Inhibition of established micrometastases by targeted drug delivery via cell surface-associated GRP78. Clin Cancer Res. 2013;19:2107-2116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 60] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 63. | Maddalo D, Neeb A, Jehle K, Schmitz K, Muhle-Goll C, Shatkina L, Walther TV, Bruchmann A, Gopal SM, Wenzel W. A peptidic unconjugated GRP78/BiP ligand modulates the unfolded protein response and induces prostate cancer cell death. PLoS One. 2012;7:e45690. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 64. | Rauschert N, Brändlein S, Holzinger E, Hensel F, Müller-Hermelink HK, Vollmers HP. A new tumor-specific variant of GRP78 as target for antibody-based therapy. Lab Invest. 2008;88:375-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 105] [Article Influence: 6.2] [Reference Citation Analysis (0)] |