Peer-review started: June 10, 2014
First decision: July 10, 2014
Revised: November 18, 2014
Accepted: February 4, 2015
Article in press: February 9, 2015
Published online: March 27, 2015
Processing time: 294 Days and 20.7 Hours
Mycobacterium tuberculosis (Mtb) is a pathogen that is widely distributed geographically and continues to be a major threat to world health. Bacterial virulence factors, nutritional state, host genetic condition and immune response play an important role in the evolution of the infection. The genetically diverse Mtb strains from different lineages have been shown to induce variable immune system response. The modern and ancient lineages strains induce different cytokines patterns. The immunity to Mtb depends on Th1-cell activity [interferon-γ (IFN-γ), interleukin-12 (IL-12) and tumor necrosis factor-α (TNF-α)]. IL-1β directly kills Mtb in murine and human macrophages. IL-6 is a requirement in host resistance to Mtb infection. IFN-γ, TNF-α, IL-12 and IL-17 are participants in Mycobacterium-induced granuloma formation. Other regulating proteins as IL-27 and IL-10 can prevent extensive immunopathology. CXCL 8 enhances the capacity of the neutrophil to kill Mtb. CXCL13 and CCL19 have been identified as participants in the formation of granuloma and control the Mtb infection. Treg cells are increased in patients with active tuberculosis (TB) but decrease with anti-TB treatment. The increment of these cells causes down- regulation of adaptive immune response facilitating the persistence of the bacterial infection. Predominance of Th2 phenotype cytokines increases the severity of TB. The evolution of the Mtb infection will depend of the cytokines network and of the influence of other factors aforementioned.
Core tip: Cytokines are proteins that can alter the behavior or properties of the cell itself or of another cell. These proteins are involved in the immunopathology of different diseases. Study of the cytokines in Mycobacterium tuberculosis infection is very important. They participate in the establishment, persistence and evolution of the infection. The intricate complexity of these regulating proteins stimulate the investigation to the search of more effective treatments that permit the eradication of a disease as tuberculosis which is one of the leading causes of mortality and morbidity worldwide despite efforts made by the scientific community.
-
Citation: Romero-Adrian TB, Leal-Montiel J, Fernández G, Valecillo A. Role of cytokines and other factors involved in the
Mycobacterium tuberculosis infection. World J Immunol 2015; 5(1): 16-50 - URL: https://www.wjgnet.com/2219-2824/full/v5/i1/16.htm
- DOI: https://dx.doi.org/10.5411/wji.v5.i1.16
Tuberculosis (TB), caused by Mycobacterium tuberculosis (Mtb) is one of the leading causes of mortality and morbidity in different age groups throughout the world, especially in developing countries. The World Health Organization reported an incidence of 8.6 million cases of TB globally. Most of the estimated number of cases occurred in South-East Asia (29%), African (27%) and Western Pacific (19%) regions. India and China alone accounted for 26% and 12% of total cases, respectively. An estimated 1.1 million (13%) of the 8.6 million people who developed TB were HIV-positive. About 75% of these cases were in the African Region[1]. The latent form of Mtb, that represent one-third of the global population, can reactivate years after a primary infection when host immunity declines[2]. In Venezuela the TB prevalence in Warao children was 3190/100.000[3].
Mtb enters the body almost exclusively by the airway (95%). Mtb is usually located in the lungs, causing pulmonary TB, but in a variable proportion, can spread through the blood and it produces extra pulmonary tuberculosis, with involvement of the lymph nodes, pleura, genitourinary system, meninges and peritoneum[4].
Pulmonary TB is the main clinical form of the disease and is classified into primary and post-primary (or reactivation). The primary pulmonary TB is due to initial infection with tuberculous bacillus. The location of the primary focus is sub pleural in the mid lung segment. In these primary foci infiltration of lymphocytes, monocytes (MNs) and macrophages (MAs) occur. MAs engulf the bacilli and reach the hilar and mediastinal lymph nodes and occasionally supraclavicular or retroperitoneal causing lymphadenopathy. The injuries of the parenchyma and lymph nodes are resolved spontaneously with calcifications radiographically visible. The post-primary TB is due to endogenous reactivation of the bacillus present in residual foci located in the pulmonary apexes, kidney and/or adrenal glands, which were controlled at the time and remained dormant for many years[4].
Investigations appoint that TB pathogenesis can be divided in four events: inhalation of Mtb, inflammatory cell recruitment, control of mycobacteria proliferation and post primary TB. Mycobacteria persistence is associated to failure in the immune vigilance; reactivation of the disease, nearby bronchial damage and spreading of the Mtb to other areas of the lungs[4-9]. It has been shown that whereas 90% of infected individuals will remain latently infected without clinical symptom, 10% of the individuals infected with Mtb will develop active disease[10].
In developing TB, many factors participate, such as: (1) virulence of Mtb strain; (2) Mechanisms of Mtb Evasion; (3) Host genetic; (4) the coexistence environmental factors such as poverty, malnutrition and overcrowding, facilitate infection; and (5) immune response[11,12].
In this review, we discuss all the factors related with immune response and the participation of cells and regulating proteins in the Mtb infection. Also, overall information about the pathological-mechanisms inherent to the behavior of cytokines which allow explaining the clinical manifestations, the evolution of the disease and the resistance to drugs among other aspects, represent a substantial contribution to the knowledge of TB.
Many models of animals have been utilized for the study and understanding of TB, such as: Mice[13], rabbits[14], guinea pigs[15], and Nonhuman Primates[16,17]. In addition, studies in vivo e in vitro in human have provided important insights.
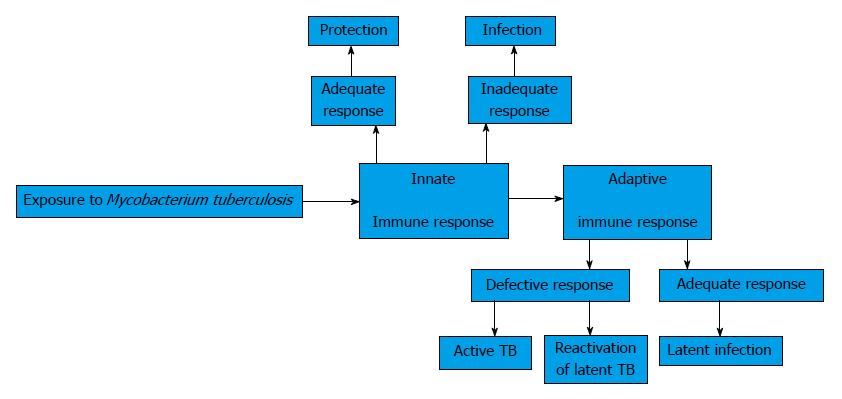
These investigations and other have shown that the balance between host immunity and bacterial evasion strategies among other factors determine the control in vivo of Mtb. Innate and adaptive immune responses are important for the eradication of the microorganism Figure 1. Pathogen recognition receptors, Toll-like receptors, Nucleotide Oligomerization Domain (NOD)-like receptors, and C-type lectins, have all been implicated in recognition of mycobacteria and in the initiation of the cytokines response. Adaptive immunity is triggered when the bacterial infection eludes the innate defense mechanisms[18]. Gallegos et al[19] and Urdahl et al[20] have suggested that TB bacterium reside in an immune-privileged site during the earliest stages of infection. Mycobacteria invade the host’s pulmonary alveoli, where adaptive immunity is activated. Mtb is initially phagocytized by macrophages, where the bacterium is able to survive[21]. Infected macrophages secrete Tumor necrosis factor-α (TNF-α) to recruit CD4+ and CD8+ T cells to the site of infection[22] where they realize effector functions. In turn, cytokines as Interferon-γ (IFN-γ) cause the activation of macrophages[23]. Bacteria are mainly killed by activated macrophages and by cytotoxic functions of activated CD4+ and CD8+ T cells or by TNF-α induced apoptosis of infected macrophages. The balance between pro-inflammatory and anti-inflammatory cytokines regulates the effectiveness of the immune response and tissue damage. Recent studies have demonstrated a role for B lymphocytes towards protection against mycobacterial infections[24]. These lymphocytes form evident aggregates in the lungs of tuberculous humans, non-human primates and mice, which show features of germinal center B cells. These cells can regulate the T cell response, cytokine production and the level of granulomatous reaction[25].
Granulomas form when an intracellular pathogen or its constituents cannot be totally eliminated. These consist of a central core of infected macrophages. The core can include multinucleated giant cells surrounded by epithelial cells. Other cell types are recruited such as: DCs, lymphocytes and fibroblasts. The collagen as element of extracellular matrix integrates the granuloma. This circumscribes the infectious process and also affects immunopathologically sites, where located[26,27].
Matrix metalloproteinases-9 (MMP-9) from epithelial cells initiates recruitment of monocytes to the developing granuloma. During reactivation, granulomas become caseating and necrotic, and the increment of MMP-1 secretion from macrophages allows the degradation of collagen and tissue destruction, which culminates in Mtb released into the airways. Experimental studies have revealed the importance of metalloproteinases. Mice treated with an inhibitor of MMPs delayed the formation of granuloma or these were smaller with more collagen. The exact mechanisms by which this balance is achieved, and how it breaks down are unknown. After many years, the organisms emerge from latency to develop post primary tuberculosis that produces cavities in the lungs where the proliferation of large numbers of bacteria occurs and the cough that the patients present facilitate transmission to new hosts[26,27].
Many cells take place in the immune response in the Mtb infection. Also, the regulating proteins that secrete these cells form a complex network that traduces pathological change in cells and tissues. All this determines an active and latent form of the infection which depends of the evolution in time[4].
Neutrophils are among the earliest cells to migrate to the site of Mtb infection and evidence exists that these phagocytes participate in the granulomatous reaction[28,29]. Increased neutrophil infiltration has been associated with excessive lung pathology and with poor bacillary control in genetically susceptible mice[30,31]. It has been proposed that neutrophilia is indicative of failed Th1 immunity in response to the use and challenge with aerosol Mtb[32]. There is also evidence suggesting that interaction of Mtb with neutrophils increment DCs migration to the draining lymph nodes thereby promoting the initiation of adaptive immune response in an aerogenic tuberculous infection[33].
Researchers have evaluated the significance of neutrophils in the protection against Mtb and conflicting results have yielded[28,30,34-39]. The role of these professional phagocytes in TB is yet to be clearly defined. However, the roles of neutrophils in development of immune response to Mtb could depend on the characteristics of the site of immunological reaction and the level of neutrophilia, as well as other immune system factors[40].
Mtb-induced neutrophil extracellular traps (NETs) were found to be reactive oxygen species and phagocytosis dependent. NETs binding heat shock protein 72 (Hsp72) or recombinant Hsp72 were able to trigger cytokine release from macrophages. NETs can participate in the innate response and influence the immune regulation[41].
The macrophages are activated for many stimuli in the course of an immune reaction and are important in the innate and adaptive immune response. Special attention has been given to macrophages and dendritic cells during the Mtb infection. In the pathogenesis of TB, macrophages are the first-line of defense as the TB bacilli enter the airways[42-45]. Mtb has several mechanisms for persisting in human tissues[46-48]. The necrosis of Mtb-infected macrophages is considered as the dominant form of cell death instead of apoptosis[47,49,50].
Mtb also promotes its replication by inhibiting the apoptosis of infected macrophages[51]. Apoptosis-associated biomarkers, rather than inflammatory cytokines, are independent factors in predicting active TB. Among the apoptosis-associated biomarkers, Decoy receptor 3 (DcR3) seems to be the most associated with immune cells[52-54]. It has the potential to discriminate between latent and active TB. If 99% of active TB cases can be identified by DcR3 plus PGE2, these both will be useful as a screening criterion[55].
Researchers have demonstrated that Mtb suppresses the pyroptosis by macrophages, and possibly in dendritic cells. Pyroptosis is a cell death that is accompanied by the release of pro- inflammatory cytokines from the dying cells and attracts an innate response to the site of infection. This mechanism is different to apoptosis and necrosis[56].
A study has demonstrated that culture conditions can promote or limit replication of the bacteria in macrophages. Also, the cytokines had different effects depending on: the cell period (differentiation or activation), time (early or late) of exposure, concentration of the cytokines, and the magnitude of the microbial challenge. The authors had demonstrated that 40% human plasma, under 5%-10% oxygen, and the involvement of granulocyte macrophage colony-stimulating factor (GM-CSF), TNF-α, followed by IFN-γ, limit the replication of the bacteria in macrophages. However, if fetal bovine serum is used, 20% oxygen, GM-CSF, higher concentrations of regulating proteins, and there is premature exposure of IFN-γ, the control of the infection by phagocytic cells is lost. Even, GM-CSF and/or TNF-α contributed with the most successful cellular differentiation, whereas IFN-γ and TNF-α allowed for the best activation. The new culture method will favor the study of antimicrobial mechanisms of human macrophages[57].
Mature dendritic cells (mDCs) are antigen presenting cells. DCs capture Ags of Mtb and transport it to the lymph nodes for T cell priming and T helper type 1 polarization because they are important secretors of Interleukin-12 (IL-12) after bacterial stimulation. In contrast, macrophages realize their microbicidal function in the granuloma because they are more efficient in killing intracellular Mtb[58,59] and for the maintenance of the Th1 polarity. The IL-12-secreting DCs are considered as the bridge between innate and adaptive immunity in TB, with important implications for DCs-based vaccine designed strategies[60,61]. However; the increment of Cortisol affects significantly the functions of Mtb-induced DCs. It has demonstrated a cross-regulation between adrenal steroids and the function of antigen-presenting cells in TB[62].
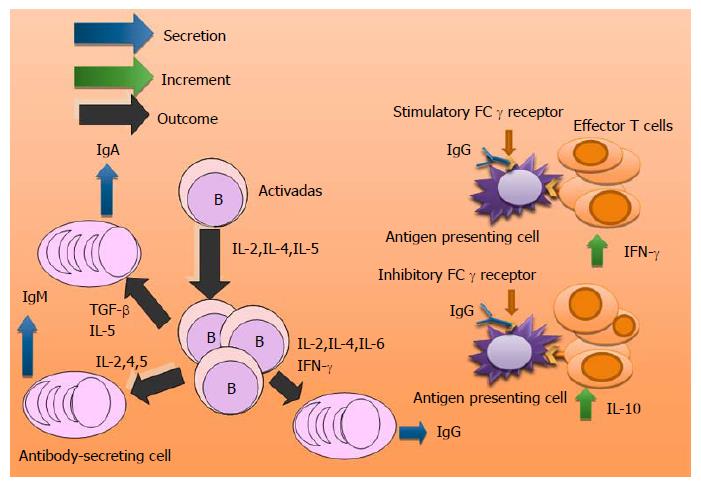
B cells contribute to adaptive immunity by secreting antibodies. Studies have shown that the administration of an Mtb high dose in aerosol[63] or intravenous[64] provoke in B cell-deficient mice higher bacterial loads compared to control mice. However, low dose does not alter lung bacterial burden[65-68].
The lungs of Mtb-infected B cell-deficient mice display exacerbated inflammation, with enhanced neutrophil recruitment[63]. Experimental evidence suggests that humoral immunity plays a role in the regulation of the Th1 response in TB[69]. It has recently been reported that a subset of B cells (CD19+, CD1d+, CD5+) in the blood of humans with tuberculous infection can suppress pro-inflammatory Th17 phenotype[70].
The lung neutrophilia and enhanced Th17 response seen in Mtb-infected B cell-deficient mice could be reversed by passive immune serum therapy, increasing the possibility that immunoglobulins may contribute to the regulation of some immune system cells. Researchers have demonstrated that B cells are required for the development of optimal protective anti-TB immunity upon BCG vaccination by regulating the IL-17/neutrophilic response[40]. The presence of antibody to Ag85 in the sera of TB patients has been associated with a good prognosis[71]. However, studies of B cell immunodeficiency in both humans[72,73] and mice[66,67] have questioned whether these lymphocytes impart a protective effect against Mtb.
Investigators based in their results appoint that the participation of B lymphocytes in tuberculous infection is phase-specific. These cells participant in the granuloma formation during the acute infection maintain the local response against Mtb and prevent reactivation of the disease during its evolution[29,63,74].
A more recent study has demonstrated that when antibodies interact with stimulatory FCγ receptors of the antigen presenting cells enhance the Th1 response (Predominance of IFN-γ) which controls the infection. Interaction of the antibodies with inhibitory FCγ receptors compromises the anti-bacterial immunity (Predominance of IL-10)[24].There exist immunopathological differences in each case[69]. Other researchers have revealed that the immunity to Mtb can be modulated by B cells “in an organ specific manner” with the participation of cytokine production and macrophage activation[75] (Figure 2).
Armed effector T cells are crucial to almost all adaptive immune response. Alterations of the Th cells functions conduce to inefficient clearance of pathogens and can cause inflammation and autoimmunity[76].
The reasons for the impaired Mtb-specific T cell function in active tuberculosis remain controversial. Patients with mutations in Th-1 cytokine signaling pathways such as IFN-γ and IL-12 (a p40 and p35 heterodimer) are susceptible to overwhelming infection with Mtb[77,78]. Impaired Th1 lymphocyte response in HIV infection also produces ineffective immunity to Mtb[79]. Several observations suggest that the Th2 cytokines, IL-4 and IL-10, are associated with LTB infection, reactivation and advanced TB[80,81].
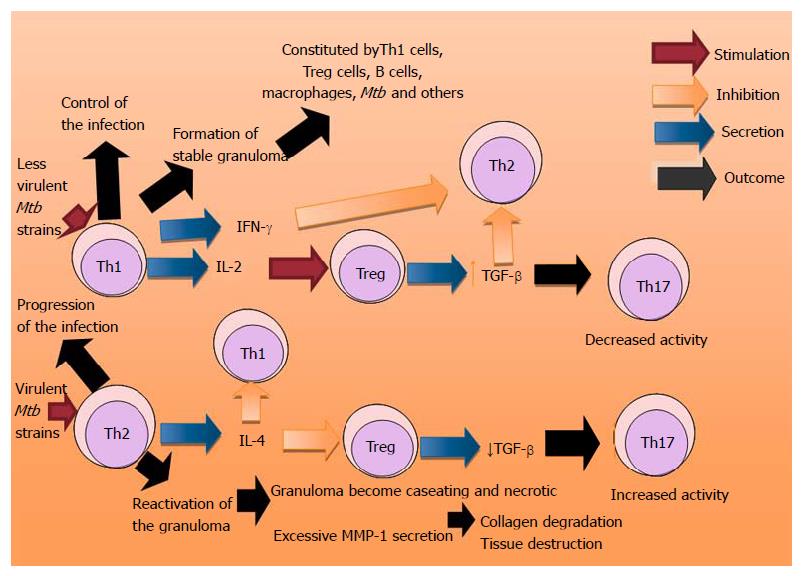
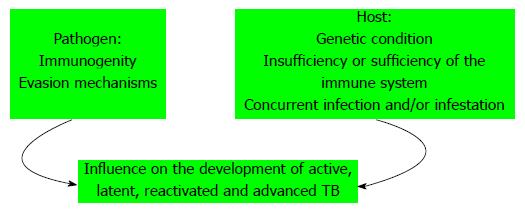
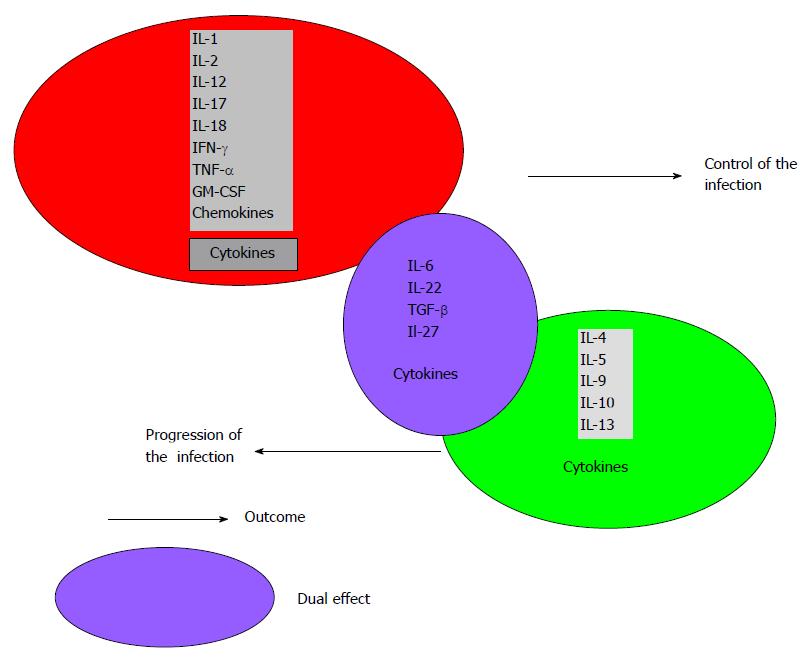
Patients with extrapulmonary disease have immune responses in vitro suggestive of Th1 response, whereas patients with miliary/disseminated disease have a suggestive Th2 response[82]. There are several lines of evidence suggesting that overexpression of Th2 cytokines increases the severity of TB, including observations that virulent Mtb strains preferentially induce Th2 cytokines expression, whereas less virulent strains induce Th1 cytokines, including IFN-γ and TNF-α[83-85] (Figure 3). There are many factors that can change the immune response in different pathologies, such as: the etiological agent and its immunogenicity, evasion mechanisms of the pathogen, the type of pathology, the phase of the clinical entity, concurrent infections and infestations, the host genetic condition and the sufficiency or insufficiency of the immune system among others[86] (Figure 4). Thereon, authors express that coincident hookworm infection exerts a profound inhibitory effect on protective Th1 and Th17 response in latent TB and may predispose toward the development of active TB in humans[87].
In the antigenic presentation the function of MHC class II molecules is to present peptides generated in the intracellular vesicles of B cells, macrophages, and other antigen-presenting cells to CD4 T cells. CD4+ T cells are required for the control of intracellular Mtb. The depletion of CD4 T cells increases in quantitative form the bacterial burden associated with MHC II (+/+) cells but not MHC II (-/-) cells[88].
There is no doubt that immunity to Mtb depends on Th1-cell activity (IFN-γ and IL-12 and the production of tumoral necrosis factor-α), but Th1 immunity alone is not sufficient to protect the host from Mtb infection, development of the disease, or dissemination[8]. For other authors, active TB is characterized by a profound and prolonged suppression of Mtb-specific T cell responses, as evidenced by decreased production of the Th1 phenotype cytokines as IL-2 and IFN-γ[89-93]. Overproduction of immunosuppressive cytokines (IL-10 and Transforming growth factor-β) by mononuclear phagocytes has been implicated in decreased T cell function during TB[94-97]. Other studies are controversial with respect to IFN-γ serum levels. These reported in active TB levels significantly higher than in patients during anti- TB therapy, in patients after treatment, in contact and in healthy control. Also, they observed the increment of IL-10, IL-6 and decrease of IL-4[98].
The predominance of Th1 phenotype plays a relevant role in immunity to TB in children. The children are more prone to developing extrapulmonary manifestations of TB than adults. Pediatric TB is characterized by diminished Th1, Th2 and Th17 phenotype cytokines, which favor the development of neurologic TB, suggesting a crucial role for these cytokines in protection against pediatric tuberculosis. Among children with extrapulmonary TB, those with neurologic involvement exhibited a more significantly diminished Ag-driven IFN-γ and IL-17 production[99].
Investigations have implicated Regulatory T cells (Treg) in the pathogenesis of Mtb infection. The induced Treg cells (iTreg) are differentiated from naïve T cells in the presence of Transforming growth factor β following T cell receptor (TCR) stimulation. These cells produce large amounts of IL-10 and Transforming growth factor-β[100,101]. Unlike Th1, Th2 or Th17 cells, iTreg displays immune-suppressive activity with minimal antigen specificity[102]. Tregs are increased in the peripheral blood of active TB patients compared with M. bovis BCG vaccinated healthy donors. This agrees with recent reports in humans[103] and in the murine TB model[104,105]. It has been demonstrated that Treg cells proliferate and accumulate at sites of infection, and have the capacity to suppress immune responses[105]. Circulating Treg cells in the peripheral blood declined progressively by anti-TB treatment[106].
During the initial T cell response to Mtb infection, the pathogen induces the expansions of Treg cells that delay the onset of adaptive immunity, suggesting that Mtb has sequestered Treg to allow that the bacterium replicate endlessly in the lungs until T cells finally arrive[107]. The increase of these cells causes down- regulation of adaptive immune response facilitating the persistence of bacterial infections. The induction of Treg by Mtb can be an evasive mechanism of the bacterium that permits its replication[108]. Studies have appreciated in active TB infection high levels of circulating Treg cells which inhibit the Th1 response but not the Th17, facilitating the bacterial replication and tissue damage. The presence of persisting immune activation and high frequencies of Treg lymphocytes may reflect immune dysregulation that predisposes individuals to clinical tuberculosis, specifically to extrapulmonary TB[109,110].
CD8 +T cells secrete preformed perforins and granzimes that act over the target cells to die via apoptosis. A study has demonstrated reduced numbers of CTLs expressing low levels of perforin and granulysin, correlated with an elevated frequency of FoxP3+ Treg cells inside of the granulomas. Also, there are high levels of transforming growth factor-β that produce active immunosuppression at the local infection site. These results suggest that an imbalance in the proportion of effector T cells to Treg cells, present at the site of infection, may contribute to the establishment of TB infection[111]. A recent study has identified a mycobacterial protein and peptide recognized by γδ T cells isolated from pulmonary tuberculosis patients. The activated γδ T cells exhibited cytolytic effector function against BCG-infected cells and played a role in the recruitment and activation of other immune cells involved in the antibacterial response[112].
Studies reveal that cytokines network is formed with the participation of the regulating proteins and different subset of cells to achieve control, persistence and severity of TB (Figure 3).
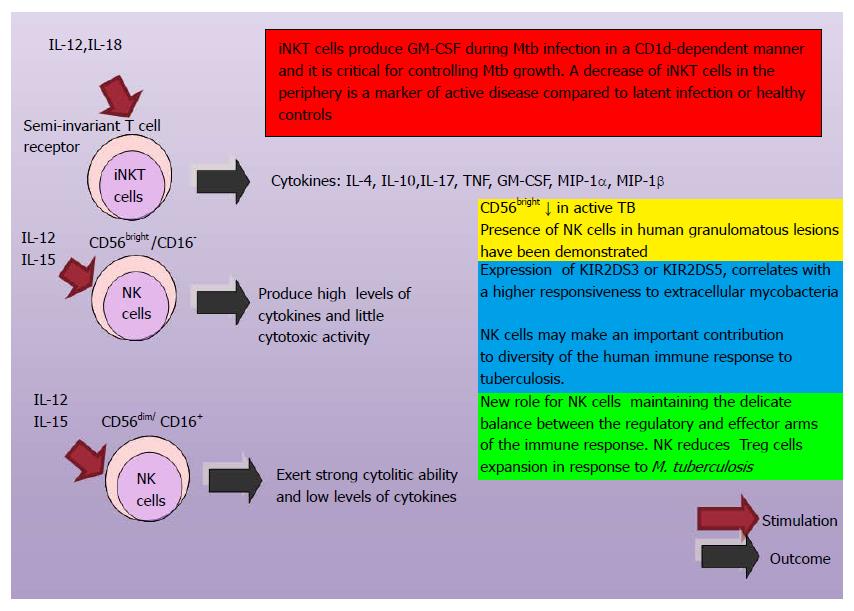
Natural killer cells (NK) are important components of innate immune system and mediate resistance against intracellular pathogens. Their cytotoxicity is modulated by a wide variety of cell surface receptors. Both inhibitory and activating receptors encoded by killer immunoglobulin-like receptors (KIR) genes bind to HLA ligands to control the activation NK. Not much is known about KIR genes and their influence on the pathogenesis with Mtb infection. Activating genes KIR2DS1, KIR2DS5 and inhibitory genes KIR3DL1, KIR2DL3 conferred susceptibility towards TB either individually or in haplotype combinations[113]. A study demonstrated that the aerosol infection with Mtb, permit the expansion of the NK cell within the lungs, the expression of markers of activation, and the production of IFN-γ and perforin. These authors appoint that the depletion of NK cells did not affected the bacterial load. Redundant biological actions may be involved[114].
Before, was thought that the memory-like responses were limited to adaptive immunity. Recently, has been demonstrated that NK cells have the capacity for memory-like responses. Three steps have been proposed in the participation of NK cells for the control of infectious processes: initial infection, resolution of inflammation and new inflammatory challenge. An in vivo adoptive transfer system was used to determine the NK cell immune memory property. Cytokines secreted by macrophages and dendritic cells induced the production of IFN-γ by NK cells. IFN-γ active CPAs and the naive NK cells transform into memory like NK cells which will be prepared for a new infection and an effective control of the intracellular pathogens such as Mtb[115-117]. Investigations have demonstrated that human CD45RO+ NK cells from pleural fluid cells (PFCs) of tuberculous patients express a “memory-like” phenotype that may have an important role in the defense against infection by Mtb[118] (Figure 5).
Virulent Mtb strains that infect multinuclear osteoclasts present an intracellular rapid growth and an osteolytic response, rather than inflammation. Also, highly-fused multinucleated osteoclasts incapacitated the production of cytokines and chemokines[119]. A study reveals that Mtb produces a protein called chaperonin Cpn60.1 which stimulates the human and murine monocytes cytokines sintesis. Also, it is a potent inhibitor of osteoclastogenesis both in vitro and in vivo and is considered a potential cure for osteoporosis[120].
Cytokines are proteins that participate in regulating the immune system in physiological entities such as pregnancy[121] and other pathologies: bacterial[86,108], viral[122,123], parasitic[124,125], allergic[126,127], rheumatologic[128,129] and neoplastic[130,131], and in deficiencies of Vitamin A and iron[132-134]. Their synergistic, antagonistic, redundant and pleiotropic biological effects can affect or not the immune response against Mtb. The cytokines can be regulated for the control of the immune system and the maintenance of homeostasis[86].
Study has provided important details on the Mtb lineage-specific patterns of growth and cytokine induction. The lineage 2 Mtb strains induce low levels of TNF-α and IL12p40, lineage 3 strains induce high levels of TNF-α, but low levels of IL12p40 and the lineage 4 strains induce high levels of both cytokines[135]. The Modern lineages (lineages 2, 3 and 4) induce lower levels of proinflammatory cytokines when compared with ancient lineages (lineages 1, 5 and 6)[136]. The variability of the immune post challenge response with the strains of those lineages, establishes that studies realized without knowledge of the participant strain, conduces to controversial investigative results.
Strains of Mtb influence in the immune response and the evolution of the disease depending of their virulence. Strains of the modern or ancient Beijing (Bj) genotype, as well as the Euro-American lineage, have been used for the induction of ex-vivo cytokine production by PBMCs in healthy individuals. Regarding this, researchers have demonstrated that modern and ancient Mtb Beijing genotypes induced different cytokine patterns[137].
Every cytokine, based in its biological actions and interactions with elements of the immune system and other factors, will have a relevant effect in the control or eradication of the Mtb infection (Figure 6).
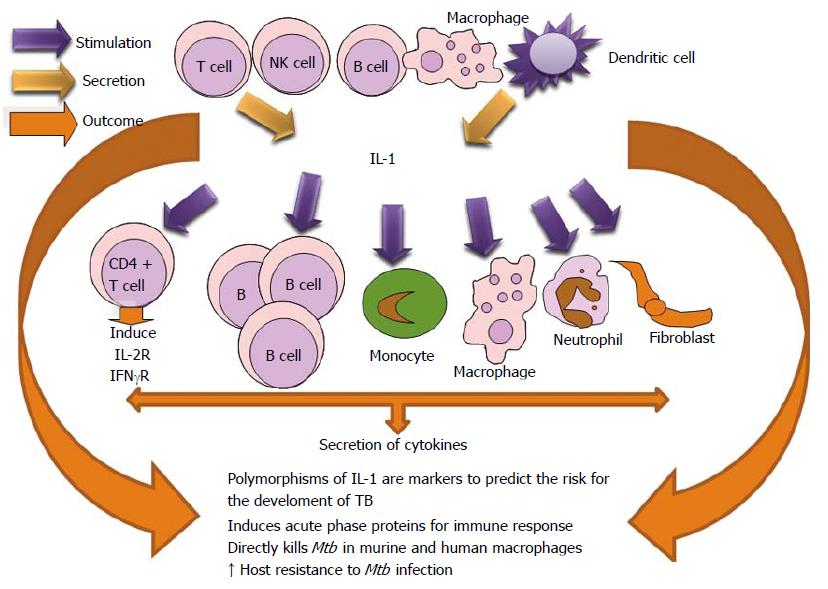
IL-1β directly kills Mtb in murine and human macro phages and promotes the recruitment of anti-microbial effector molecules. Also, it augments the TNF-α and Tumoral necrosis factor receptor-1 (TNFR1) cell surface expression and results in activation of caspase-3[138,139]. Vitamin D1, 25-dihydroxyvitamin D (1,25D) directly stimulates IL1B gene transcription which is important for macrophage response to Mtb infection[140].
Pro-IL-1β maturation is dependent on the NOD-like receptor 3 (NLRP3) inflammasome. IL-1β, in combination with 1,25D, leads to the control of mycobacterial proliferation in the macrophage. 1,25D deficiency is seen in patients with active tuberculosis. This vitamin generally boosts infection-stimulated cytokine/chemokine responses and increases its role in innate immune regulation in humans[141]. Researchers have appreciated a correlation between vitamin D deficiency and TB susceptibility[142,143].
IL-1β is important for host resistance to Mtb infection. It has been demonstrated by the significantly reduced survival of IL-1β-/- or IL1R-/- mice after infection[144-147]. Investigations realized in infected infants have shown reduced levels of IL-1 and the affectation of its productive capacity demonstrated immune vulnerability to TB in this population[148-150]. A role for IL-1 in human immunity against TB is supported by several studies showing an association between polymorphisms in the IL-1 or IL-1 receptor genes and host resistance[151-154]. The polymorphisms of IL-1β and IL-10 genes may be valuable markers to predict the risk for the development of TB in household contacts[155]. Studies reveal that mice lacking the signaling adaptor molecule utilized by most membrane-bound TLR (MyD88) are extremely susceptible to TB[156-158]. Significant secretion of IL-1β was detected from macrophages cocultured with NETs from Mtb-activated neutrophils[41] (Figure 7).
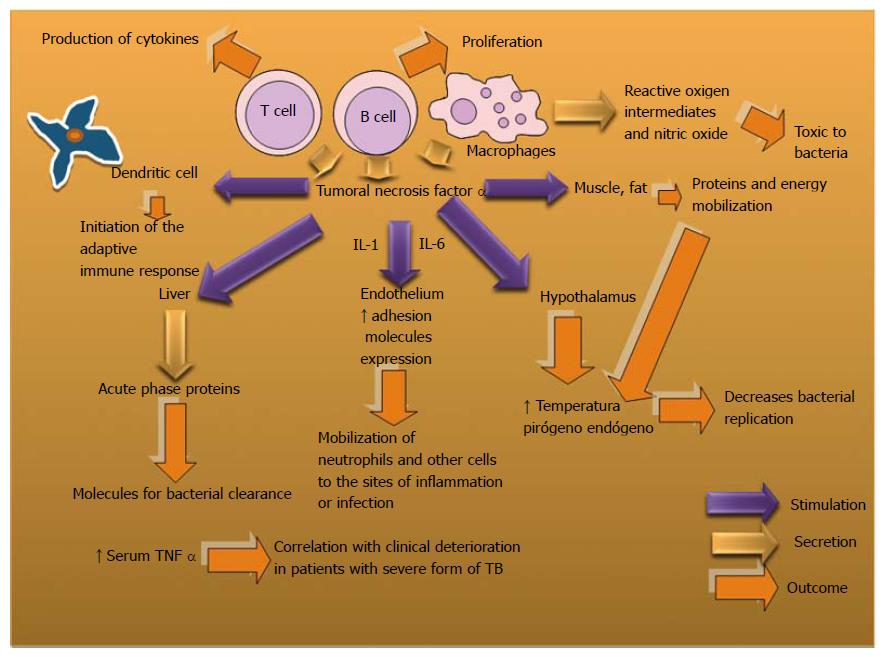
TNFα is produced by the Th1, some Th2, and some CTL phenotypes. It induces nitric oxide production and activates microvascular endothelium among other biological actions. It is a cytokine whose deregulated expression may cause immunopathology[159,160]. However, in countries with a high incidence of TB, the biological therapy with anti-TNF-α has been associated with immunosuppression, reactivation of latent TB[161-163] and a risk of new Mtb infection[164,165].
The exacerbation of TB occurs with the breakdown of Granuloma which has an important role in the host protection against mycobacterial infections[166-168]. However, the host immunity can decline and provide chance to reactivate the latent form in the granulomas which can be a niche where mycobacteria might persist[169]. Mtb induces exacerbated inflammatory responses associated to important tissue lesions and dissemination of the bacilli into the airways[170].
Dysregulated TNF expression has been associated with defective host immunity due to excessive or inefficient inflammation[171]. In HIV patients with pulmonary TB, a clinical trial combining TNF inhibitors with anti-TB drugs showed that TNF inhibitors can be safely administrated during TB treatment and, in addition, higher responses to TB treatment were observed in the group of Enbrel (etanercept, soluble TNFR2-Fc) treated patients[172].
Mtb chemotherapy may be more efficient in the presence of a TNF inhibitor to clear bacilli and reduce lung pathology, which may be considered in acute and chronic Mtb infection[173]. However, for other authors the neutralization of TNF α produces disseminated disease in acute and latent Mtb infection without alterations in the granuloma structure in a cynomolgus macaque model[174]. TNF expression is necessary for controlling Mtb infection in vivo and TNF neutralization in monkeys and humans correlates with an increased risk of reactivation of latent tuberculosis[161,174].
Infection of human alveolar macrophages by Mtb has been reported to be sufficient to induce apoptosis mediated by TNF-α in an autocrine/paracrine manner and proinflammatory cytokines directly or indirectly modulated apoptotic response depending on the degree of virulence of the strain[175]. It has been observed that serum TNF α and Malondialdehyde (MDA) measure ments may play an important role in the evaluation of the inflammatory phenomena in TB[176]. Also, a positive correlation was found between an increase in serum TNF-α levels and clinical deterioration in patients with a severe form of TB[177] (Figure 8).
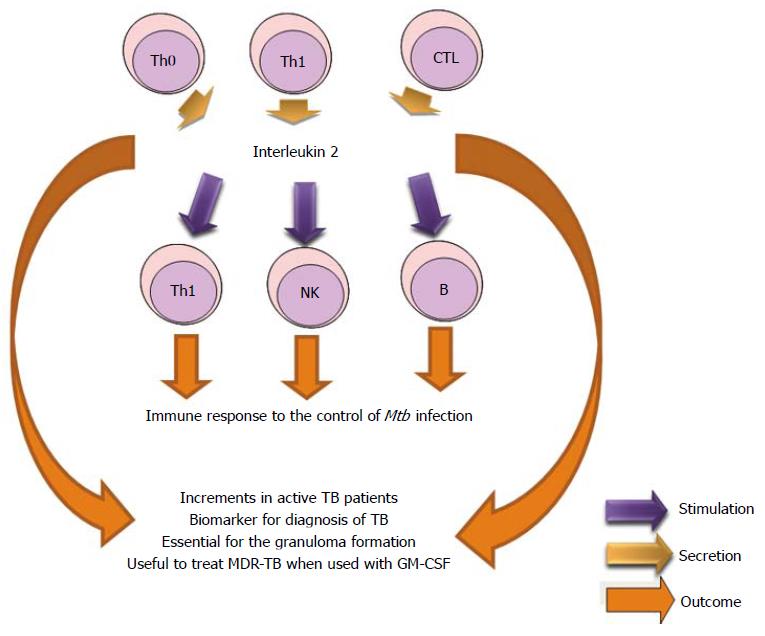
IL-2 is produced by Th0, Th1 and some CTL. It stimulates growth of B, T and NK cells and is essential for cellular immunity and granuloma formation in Mtb infection. The IL-2 liberation is stimulated by TB-specific antigens and was significantly higher in TB patients than in healthy controls and suggested that IL-2 could be a potential biomarker for diagnosing TB[178-180]. The detection of IL-2 and IFN-γ permits to discriminate between active and latent tuberculosis when compared with controls[181]. Another study did not appreciate the utility of the IL-2 as a diagnostic biomarker for TB infection due to its low amount released[182]. Studies have reported that IL-2 and IL-9 expressions are elevated in PBMC (Stimulated by ESAT-6) from TB patients[183,184]. Researches demonstrated that immunotherapy with both IL-2 and GM-CSF may be useful to treat multidrug resistant tuberculosis (MDR-TB). Mice receiving immunotherapy developed fewer lesions in the lungs compared with mice receiving antibacterial therapy alone[185] (Figure 9).
IL-4 is produced by lymphocytes Th2 and activated mast cells. This cytokine stimulates the IgG4 and IgE isotype change, acts as an autocrine growth factor for Th2 lymphocytes, also, inhibit the development of Th1 and Th17 lymphocytes and participates in the activation of macrophages[76,186].
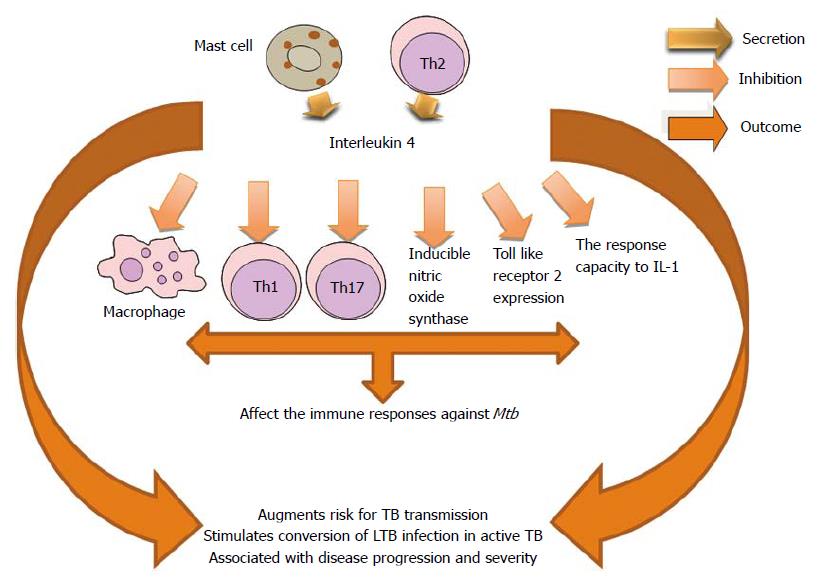
Increased production of the Th2 cytokine (IL-4) by bronchoalveolar lavage cells (BAL) is a strong risk factor for TB transmission in South African patients. Increasing IL-4 was associated with BAL PMNs and negatively associated with BAL lymphocytes. IL-4 has been implicated in conversion of LTB infection to active TB[187]. IL-4 has been postulated as key in TB pathogenesis, especially with its ability to down-regulate inducible nitric oxide synthase, Toll-like receptor 2, and macrophage activation[188].
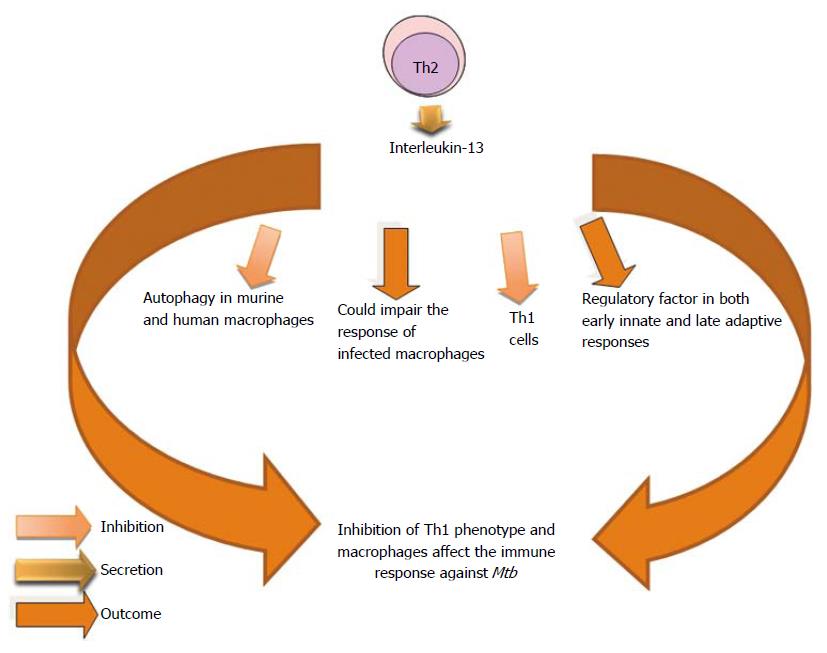
Clinical studies involving patients with latent TB show a clear correlation between the intensity of a Th2 response and the risk of developing active disease and in particular a direct correlation between the level of IL-4 messenger RNA and disease severity[189]. The induction of IL-4 production by DCs generated by BCG-infected monocytes could explain the failure of the BCG vaccine to prevent pulmonary TB[190]. It has been demonstrated that high levels of IL-4 were associated with disease progression in TB-susceptible families when there is lack of IFN-γ expansion. Resistant families have overrepresentation of IFN-γ +874 A allele and an increment of IFN-γ secreting cells[191]. Authors agree in relation to the worsening of the host immune response to Mtb due to the effects of IL-4[191,192]. However, the mechanisms of inhibition may be different. Inhibition of autophagy through the autocrine secretion of IL-4 and/or IL-13 by infected macrophages could allow that the bacteria gain a foothold previous to the formation of a protective granuloma[193]. This effect on the autophagy has been demonstrated in murine and human macrophages[194] (Figure 10).
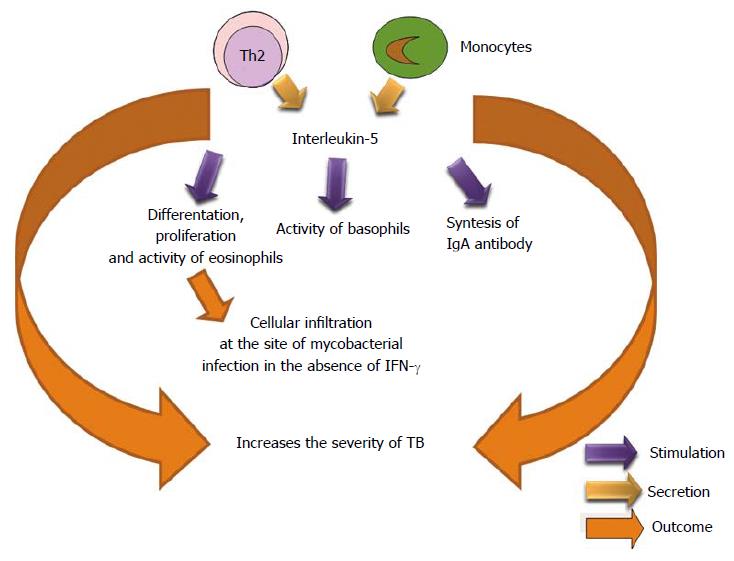
IL-5 is produced by Th2 phenotype. It activates to eosinophil and stimulates its growth and differentiation. Also, it stimulates proliferation of lymphocytes and synthesis of IgA antibody[195,196]. This regulating protein may be a factor in the reduction of Mtb-specific T cell responses within coinfected (SIV/Mtb) individuals. Researchers found that neutralizing IL-5 in coinfected monocytes partially restored normal T cell TNF production[197]. The presence of eosinophils in the cellular infiltration at the site of mycobacterial infection strongly suggests that increased levels of IL-5 are produced in vivo during Mycobacterium bovis bacillus Calmette Guérin (BCG) infection in the absence of IFN-γ signaling[198,199] (Figure 11).
IL-6, IL-1 and TNF-α, are important inductors of the acute-phase response. These cytokines are termed endogenous pyrogens because they cause fever and derive from an endogenous source rather than from bacterial components. IL-6, together with the other cytokines aforementioned, has effect on hepatocytes, Bone-marrow, endothelium, hypothalamus, fat, muscles and DCs[200].
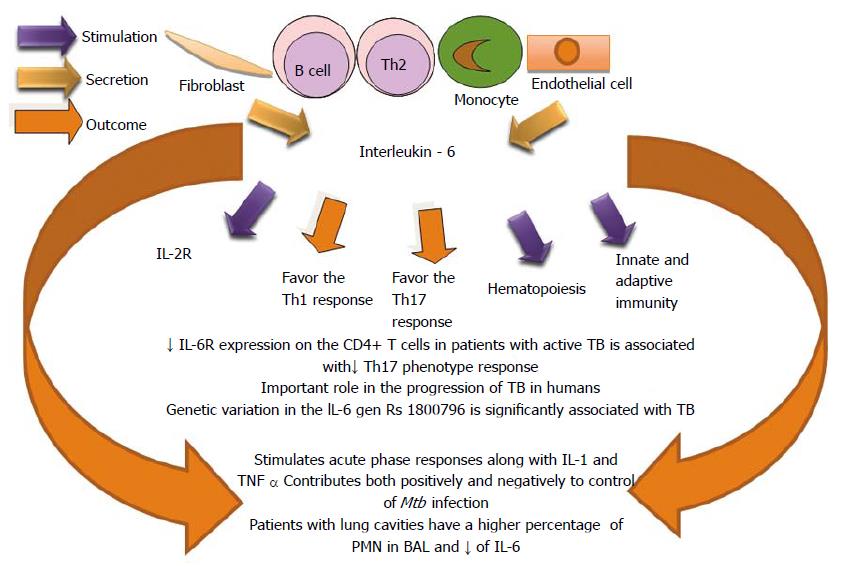
It has been reported that IL-6 plays an important role in protection against murine Mtb infection[201,202] due to the influence of the CD4+ T cells response[203]. Mtb-infected IL-6-deficient animals show an impaired Th1 response and increased bacterial loads, indicating a requirement for IL-6 in host resistance to Mtb infection[201,204]. IL-6 down regulates macrophage microbicidal activity and IL-6 inhibits the production of INF-α and promotes in vitro growth of Mycobacterium avium[205,206]. IL-6 secreted by Mtb-infected macrophages suppresses the responses of uninfected macrophages to IFN-γ[207]. Increased levels of IL-6 in the lungs, along with increased levels of IL-1β and IL-11, is significantly correlated with tuberculosis progression in genetically susceptible mice[208]. Together, these mice studies indicate that IL-6 may play multiple roles and contribute both positively and negatively to host control of Mtb infection.
IL-6 has been shown to contribute to the differentiation and activation of cells of the immune response and others not related with this system[209]. It is associated with the pathogenesis of many chronic inflammatory diseases, including tuberculosis[208,210,211]. Genetic variants in IL-6/IL-6R have been linked to the susceptibility to the severity of a wide range of diseases, such as: respiratory tract infection, asthma, meningococcal disease, chronic hepatitis C virus infection and rheumatoid arthritis[212-216]. A rare genetic variation in the IL-6 gene, rs1800796, is significantly associated with tuberculosis disease in the Chinese Han population[217]. Down regulation of IL-6R expression on CD4 T cells in patients with active pulmonary TB is associated with decreased of Th17 phenotype response, suggesting a role for IL-6 in the progression of TB in humans[217-219].
Lung parenchyma can be destroyed during active TB and it provokes immunological alterations for controlling the infection. Patients with radiographically advanced TB showed an increase of the inducible protein-10 (IP-10) and IL-6 production by BAL cells and these are biomarkers of non-cavitary TB. This may reflect an effective Th1 immune response for controlling TB and for attenuating the tuberculous lung destruction. The patients with lung cavities had a higher percentage of polymorphonuclear neutrophils (PMN) in BAL as well as lower IP-10 and IL-6 compared to those without cavities. Also, was demonstrated a negative association between IP-10 and PMN of BAL[220] (Figure 12).
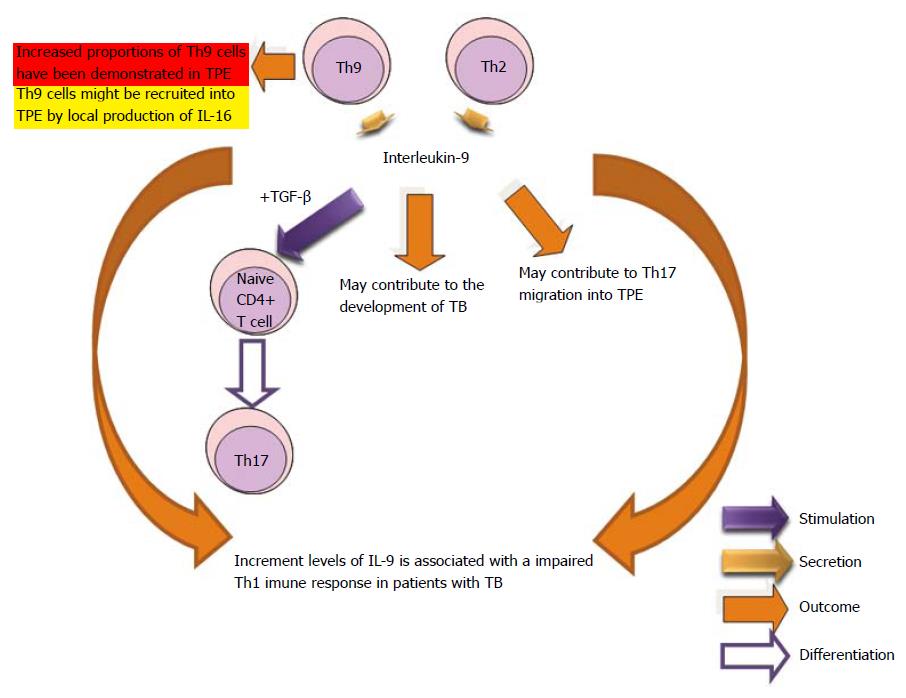
It is known that Th9 phenotype cells secrete the regulating proteins IL-9 and IL-10. Th9 cells are involved in the intestinal responses to helminths which were thought to be mediated only by the Th2 phenotype. Studies have demonstrated that the increased expression of IL-9 may contribute to the development of TB and it is associated with an impaired Th1 immune response in patients with tuberculosis[184,221]. Th9 cells with the phenotype of effector memory cells were found in tuberculous pleural effusion as compared with blood. Pleural mesothelial cells were able to function as antigen-presenting cells to stimulate Th9 cell differentiation. Further investigations are needed to reveal the function of this type of cells and their products in pathogen clearance and inflammatory diseases[76,222] (Figure 13).
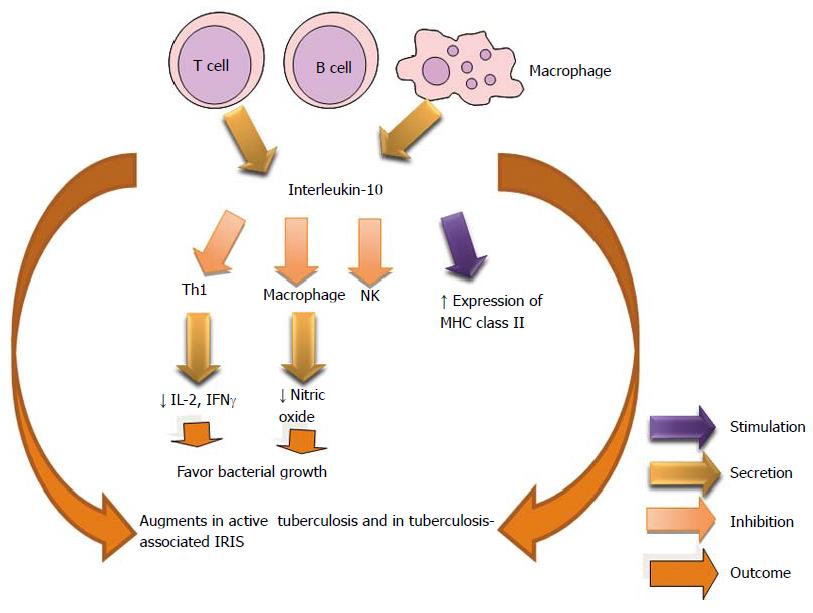
IL-10 is an immunosuppressive cytokine that is produced by Th0, Th1, Th2 and T regs phenotypes among other cellular types. It inhibits Th1, augment MHC class II and gene knock-out of this regulating protein cause inflammatory bowel disease. IL-10 and transforming growth factor-beta (TGF-β) restrict T effector cell response[223]. Increase in CD4+CD25+ FoxP3+ cells has been shown to decrease Th1 cell responses in patients with TB[224]. IL-10 has been reported to modulate the innate and adaptive immune responses, potentially creating a favorable environment for the persistence of microbes, intracellular pathogens, and chronic infections[225]. The increased ability of macrophages to produce IL-10 when stimulated with Toll-like receptor ligands is also associated with an increased tendency to develop primary progressive tuberculosis[226]. Production of IL-10 has also been reported to be higher in patients who had active TB, compared with tuberculin skin test responders[227].
IL-10 plays an important role in Mtb infection, where the cytokine has shown to reduce the immunity. IL-10+ T cells with immunosuppressive properties are present in anergic TB patients[228]. IL-10 decrease the macrophage activity in the Mycobacterium avium infection and the administration of monoclonal anti-IL-10 diminishes bacterial growth in the spleen[229]. IL-10 helps maintain mycobacterial infections[230]. It has been demonstrated in vivo that the production of IL-10 reactivates the chronic pulmonary tuberculosis[231]. The heterogeneity of macrophages may be determinant in disease outcome in intracellular bacterial infection as type I and II macrophages have opposite effects in the cellular immunity[232].
Study revealed an increase in the transcript levels of IL-10 and IL-22 in tuberculosis-associated immune reconstitution inflammatory syndrome (IRIS) patients, compared with non-IRIS controls. The serum samples showed statistically significant high concentrations of IL-10 and IL-22 cytokines in tuberculosis-IRIS patients[233]. Two forms of TB-IRIS are recognized: paradoxical and unmasking. The first manifests with new or recurrent TB symptomatology and second with an exaggerated and unusually inflammation. Both forms have occurred during the early anti retroviral therapy[234] (Figure 14).
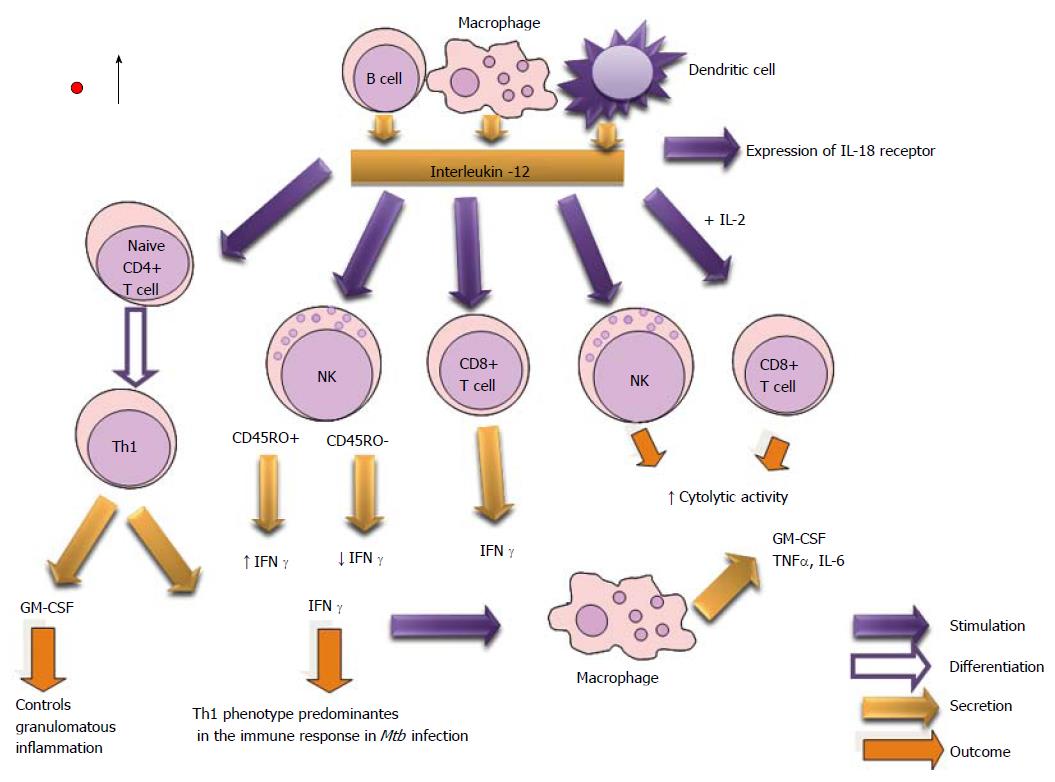
IL-12 is crucial for optimal differentiation and main tenance of IFN-γ-secreting antigen-specific Th1 cells[235,236], and in controlling mycobacterial infections in mice and men[237,238]. The increase of IL-12p40 production by BAL cells in sputum of patients with radiographically advanced TB reveals less effective immune control and more complications. It has been demonstrated that IL-12 receptor deficiency is found in healthy individuals with mycobacterial infections[77,187].
Parenteral administration of IL-12p70 to Mtb-infected IL-12p40-deficient mice restores CD4+ T-cell production of IFNγ and control of bacterial growth in the lungs and spleen, whereas these effects are lost when administration of IL-12p70 is discontinued[235].
Researchers appoint that IL-12 enhanced the expression of granzyme B, activation inducer molecule (CD69), IL-2 receptor α chain (CD25), Natural Killer Group 2D (NKG2D), IL-12 receptors β1 and β2 on CD45RO+ NK cells from pleural fluid cells (PFCs) from tuberculous patients. Also, they have demonstrated that CD45RO+ NK cells produced significantly more IFN-γ and were more cytotoxic compared with CD45RO− NK cells from PFCs when stimulated with 12 IL-12. The activity of NK cells is associated with early resistance against M. tuberculosis infection[118,239] (Figure 15).
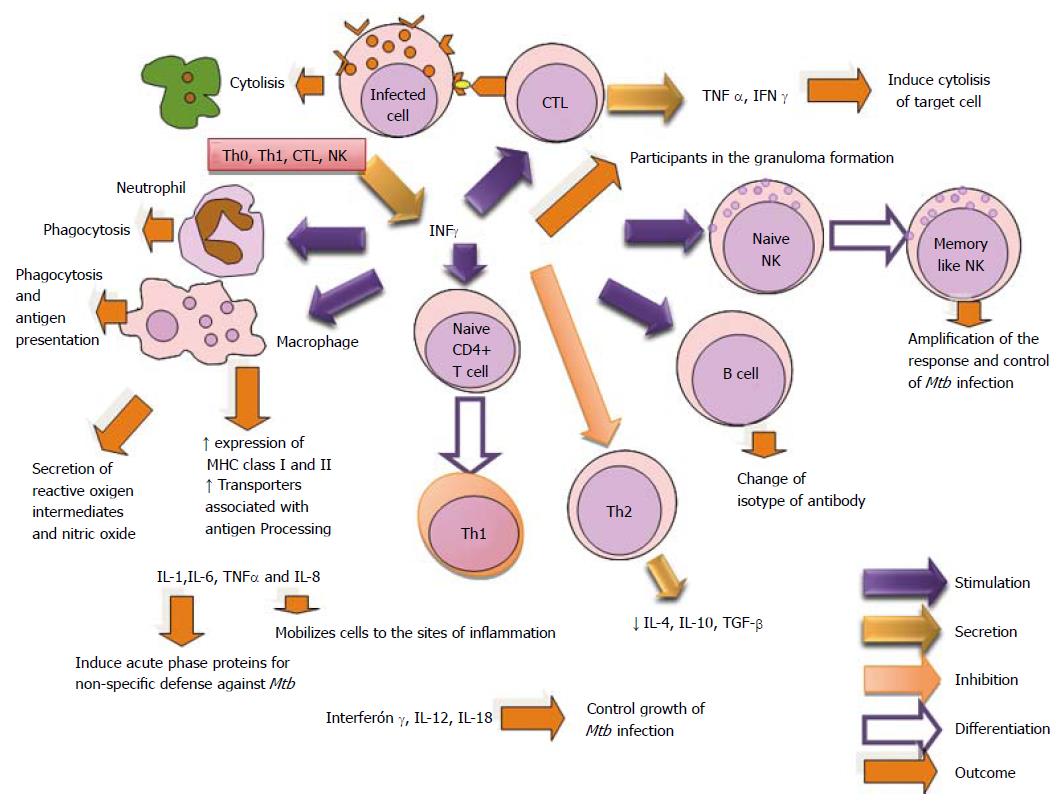
Another pro-inflammatory cytokine is IFN-γ which is secreted by Th1, CTL and NK cells. It participates in the synthesis of IgG2a, inhibits the phenotype Th2, activates NK cells and augment MHC class I and II. Also, the gen knock-out produces susceptibility to mycobacteria. IFN-γ has been shown to be an important mediator of macrophage activation involved in the control of a number of intracellular pathogens[240-247].
The active tuberculosis is 5-10 times more frequent in infants than adults. Also, the children have higher rates of severe disseminated disease. It has been shown that infant T cells are less capable of transforming into IFN-γ -producing T cells[244]. IFN-γ stimulated responses are lowered in TB, while the expression of Suppressor of Cytokine Signaling (SOCS) molecules-1 and 3 and CD4+CD25+FoxP3+T regulatory cells are increased[247]. The enhanced susceptibility to mycobacterial infection of IFN-γ knockout mice[246,247], and of patients with genetic defects in IL-12/ IFN-γ pathway[248], provides strong evidence that IFN-γ is required in defense against Mtb.
BCG is a licensed vaccine in use that mediates immune protection through the production of IFN-γ by CD4 T cells, which activates macrophages to kill Mtb. However, some recent studies have reported a lack of correlation between IFN-γ production by CD4 cells and BCG-induced immune protection[249]. IFN-γ is necessary for the control of TB[23,250] and has been the focus of multiple coinfection studies. These studies conclude that HIV reduces IFN-γ production by Mtb-specific CD4 T cells in the periphery and airway[251-254].
Study reveals that dehydroepiandrosterone increments the antigen-specific T-cell proliferation and IFN-γ production induced by Mtb-stimulated DC. The adrenal axis is important in the modulation of the DCs function in the context of TB[62,255]. Mice deficient in IFN-γ (IFN-γ-/-) or the IFN-γ receptor (IFN-γR-/-) are extremely susceptible to infection with tuberculosis-causing organisms[23].
Murine macrophage studies show that IFN-γ induces Mtb killing[23,255] but when IFN-γ gene has been disrupted is unable to contain or control sublethal dose of Mtb[256].
The secretion of IFN-γ and, to a lesser extent, of IL-17 by CD4 (+) T cells plays a major role both in protection and immunopathology[257]. But the effect of IFN-γ in human macrophages remains controversial[258,259]. Regard this, some researchers have shown that IFNγ activates human macrophages to become tumoricidal and leishmanicidal but enhances replication of macrophage-associated mycobacteria[260]. For others the IFN-γ-mediated anti mycobacterial activity requires specific in vitro conditions for human macrophages, such as physiological O2 levels and the presence of the GM-CSF[57]. Extracellular trap formation and mycobacterial aggregation are IFN-γ-inducible events and require the ESX-1 secretion system. In the absence of ESX-1, IFN-γ does not restore any extracellular trap formation, mycobacterial aggregation, or macrophage necrosis[261]. Therefore, the monitoring of the in vivo and in vitro metabolic activity of both slow-growing and fast-growing mycobacteria, using methods as chronoamperometry and chronopotentiometry is of great importance[262].
Th1 phenotype plays a relevant role in the formation of granulomas. IFNγ is the most characteristic cytokine produced by armed Th1 cells (Figure 16).
IL-13 is produced by T lymphocytes and exerts its biological functions on B cells and monocytes, and inhibits pro- inflammatory cytokines production. It upregulated MHC class II expression, also, promotes IgE class switching. This cytokine is a key regulator of the extracellular matrix, and is redundant with IL-4. Concentrations of IL-13 were found to be significantly higher in fast responders to antimycobacterial treatment than in slow responders in the fifth week of treatment. The role of IL-13 in Mtb infection is not well defined. IL-13 abrogates autophagy-mediated killing of Mtb in human and murine macrophages[194]. However, IL-13 has modulated the resistance to a number of intracellular pathogens including Leishmania major, L. mexicana and Listeria monocytogenes. The elevated level of IL-13 observed in fast responders compared with slow responders may suggest their better resistance to the infection, although the mechanisms for the IL-13 effect are not yet clear[263]. IL-13 can be substituted for IL-4 in several physiological responses. However, the presence of IL-13 inhibits the action of IL-4 on Mtb-induced IL-8 secretion but does not affect the inhibition of IL-8 secretion by IL-10[264].
IL-4 and IL-13 are well recognized as activating distinct signaling cascades[83,265-268]. IL-13 inhibits IFN-γ-induced autophagy, but this process is independent of protein kinase B (AKT); instead it is dependent of the signal traducer and activator of transcription 6. Autophagy is a major intracellular pathway for the lysosomal degradation. Therefore, IL-13 could specifically impair the response of infected macrophages[194].
IL-13 released by macrophages infected with virulent strains of Mtb[83,85] act in an autocrine manner to inhibit the autophagic process. Treatment of Mtb-infected macrophages with either IL-4 or IL-13 promotes the intracellular survival of the bacteria[194] (Figure 17).
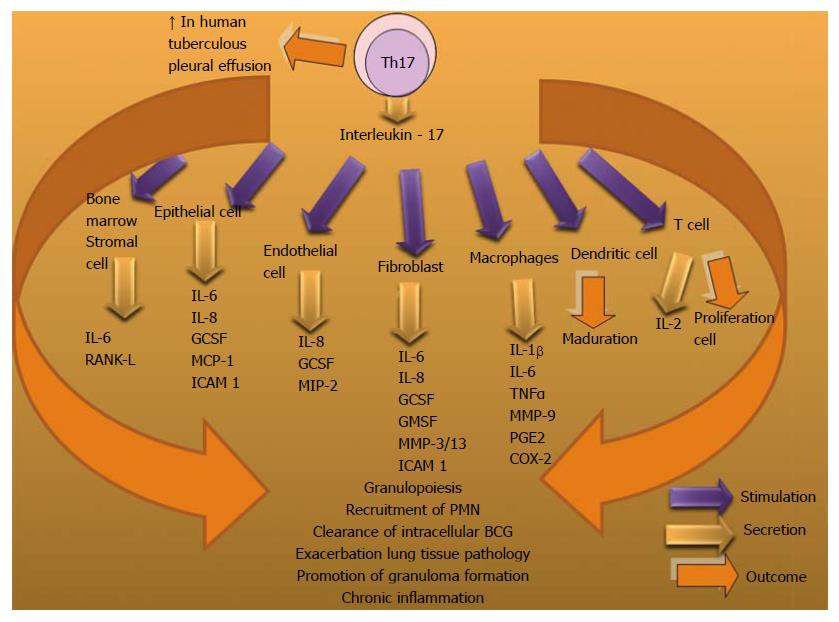
Generation of human Th17 cells is dependent on IL-23[269,270], IL-1β[269,271,272], TGFβ[270] and IL-6[272]. IL-17A is a cytokine that participates as an immunomodulator in chronic immunological diseases such as: rheumatoid arthritis and inflammatory bowel disease. It can control the pathological mechanisms in the Mtb infection through the dysregulating cytokines and chemokines production and promoting granuloma formation. It has been observed that IL-17A significantly enhanced the clearance of intracellular Bacillus Calmette-Guérin (BCG) by macrophages through nitric oxide (NO) -dependent killing mechanism[273].
During the initial stages of infection IL-17 acts on different types of cells and stimulates the secretion of antimicrobial peptides, granulocyte colony-stimulating factor (G-CSF) and cysteine X cysteine (CXC) chemokines. As DCs migrate to the lymph node, both Th1 and Th17 cell are differentiated. Chemokines in the infected lung promote recruitment of protective cells and a mononuclear granuloma is formed, where IL-23 and IL-6 are highly expressed. IL-17-producing cells accumulate in high numbers in the lungs and immunopathological consequences develop[274].
Other investigations appoint that the involvement of Th17 cells remains to be clarified in relation to TB. Researchers demonstrated that Mtb-specific Th17 cells are undetectable in peripheral blood and BALs from TB patients[275].
It has been demonstrated that IL-17 plays an important role in the recruitment of neutrophils to the site of inflammation[275-278], including the airways during infection[279,280]. This cytokine is produced by a variety of host cells, including myeloid cells[281], invariant natural killer (iNK) T cells[282], NK cells[283,284], γδ T cells[285-287] and Th17 cells[288]. IL-17 can downregulate IL-10 production and modulate the Th1 response, which has been demonstrated in models immunized with BCG[289]. This vaccination induces Th17 cells that populate the lungs of immunized mice. Th17 cells recruit Th1 cells to the site of infection to restrict mycobacterial growth, upon challenge with Mtb[290]. IL-17 can promote tissue damage during Mtb infection[274,291] and in the context of other infectious and autoimmune diseases[276,277,292-294]. An increment of IL-17 production is associated with increased neutrophil recruitment and exacerbated lung tissue pathology after repeated BCG vaccinations[295].
B cells can optimize BCG-elicited Th1 immunity by regulating the IL-17/neutrophil response[296]. In human tuberculous pleural effusion (TPE) Th17 cells and regulatory T cells (Tregs) have been found to be increased. Th17 cells were significantly increased in TPE due to local generation and differentiation stimulated by IL-1β and/or IL-6. CD39+Tregs might participate in the suppression of local immune responses by inhibiting Th17 phenotype[297] (Figure 18).
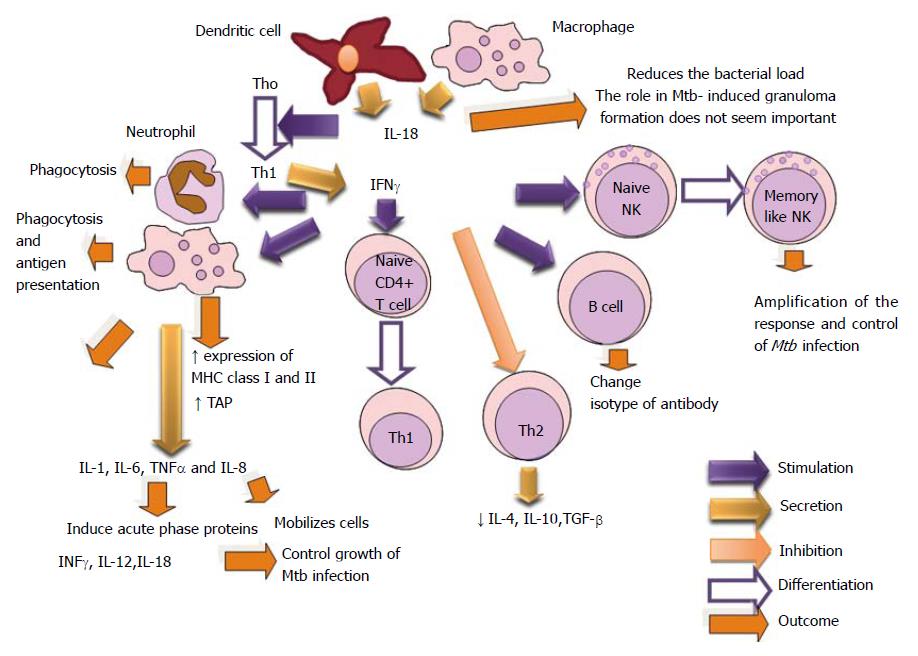
Interleukin- 18 (IL-18) was designed as an IFN-γ-inducing factor, which induces IFN-γ production by splenocytes, hepatic lymphocytes, and type 1 T helper (Th1) cell clones. Its biological actions appear to be similar to those of IL-12[298-300].
Mtb infection in the absence of IL-18 diminishes the Th1 phenotype response. Also, IL-17, chemokines as: CXCL-1 and CXCL-2 cause PMN influx, which exacerbates the immunopathology in IL-18 KO mice. This reveals its immune protective role against Mtb[301]. It has been demonstrated that treatment with exogenous IL-18 reduced the bacterial load. This finding does not agree with the studies that have demonstrated that the sizes of the granulomatous lesions in IFN-γ-KO and TNF-α-KO mice infected with Mtb were not reduced significantly by recombinant IFN-γ or TNF-α[302].
The inflammatory lesions in IL-18-KO mice were no more severe than those observed in IFN-γ-KO[23,257], TNF-α-KO mice[302] and IL-12-KO[303]. Therefore, IL-18 does not seem to play a role in Mtb-induced granuloma formation (Figure 19).
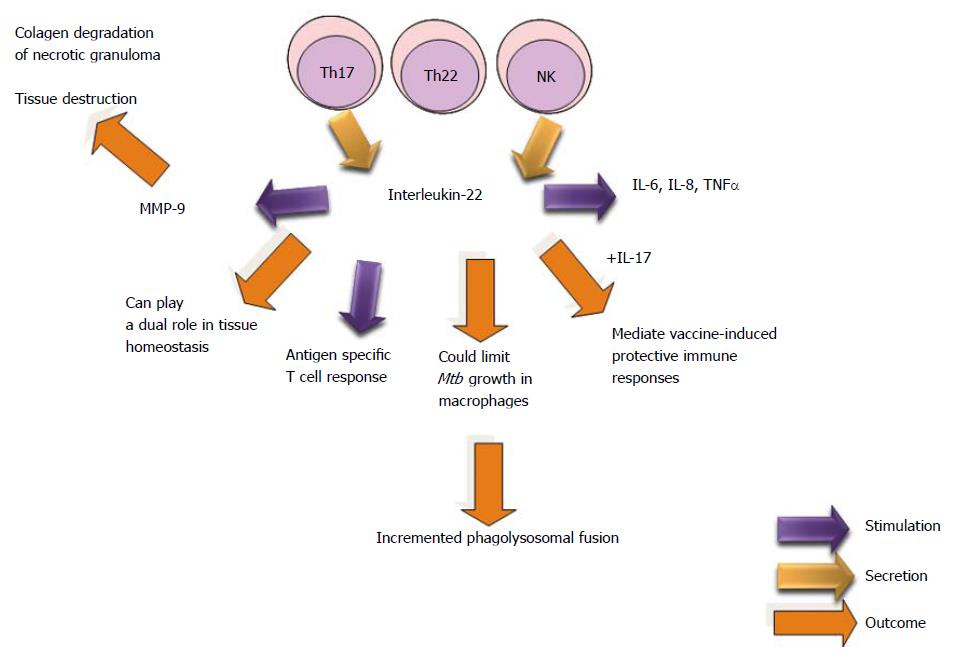
IL-22, a member of the IL-10 family, is mainly produced by T and NK cells[304,305]. It is considered to be produced by Th17 cells in an IL-23-dependent manner[306,307] or by a private T cell lineage termed Th22[308,309].
Previously, it had been shown that IL-22 produced by NK cells in humans and CD4+ T cells in macaques could limit Mtb growth in macrophages by increasing phagolysosomal fusion. However, IL-22 can play a dual role in tissue homeostasis depending on the cytokine microenvironment where it is induced[310,311].
Study suggests that NK1.1+ cell-derived IL-22 contributes to vaccine-induced protective immunity but not to the primary immune response to Mtb, as is the case for IL-17. IL-17 and IL-22 appear to mediate vaccine-induced protective immune responses; however, different mechanisms are involved. IL-17 induces local chemokine production, which leads to optimal priming of T-cells[306], whereas IL-22 inhibits expansion of induced Tregs and enhances antigen-specific T-cell responses, resulting in a reduced bacillary burden after challenge with Mtb virulent strains (H37Rv). The mechanisms through which IL-22 inhibits Treg expansion and enhances T-cell responses remain uncertain[310].
IL-22 levels in pericardial fluid correlated positively with MMP-9, an enzyme known to degrade the pulmonary extracellular matrix. Levels of MMP-9 in blood are associated with severity of TB disease[312].
Researchers reported significantly higher IL-22 levels in BAL fluid from patients with pulmonary TB, compared with healthy controls. Also, levels of IL-22 in pleural effusion and pericardial effusions from TB patients were readily detectable in most[313].
It has been detected IL-22-producing T cells in lung tissue sections and granulomas of Mtb infected macaques[314]. However, the treatment of Mtb infected mice with neutralizing anti-IL-22 antibodies did not affect pathology, granuloma formation or bacterial burdens in the lung[315] (Figure 20).
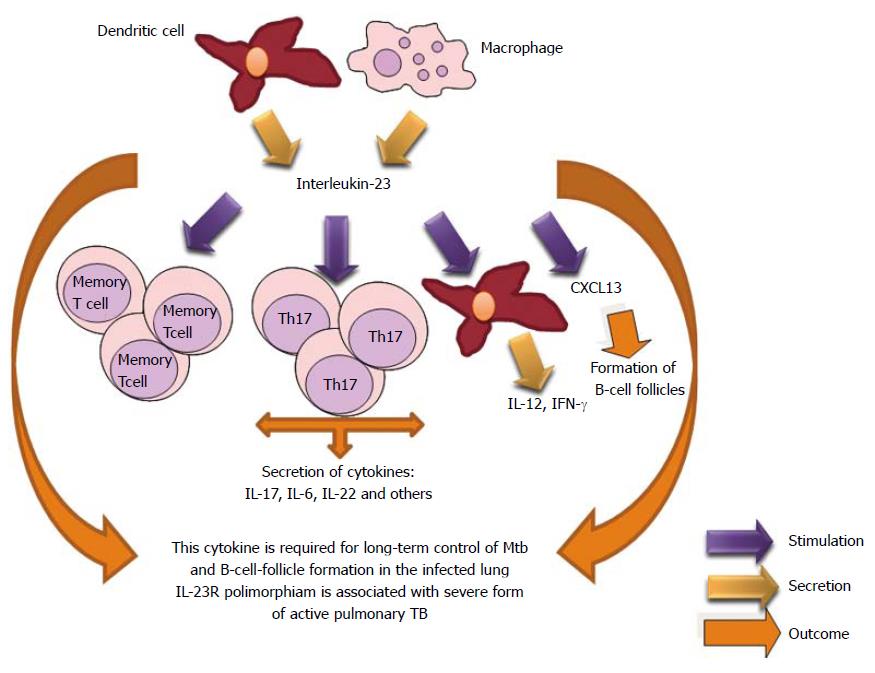
IL-23 is a new IL-12 family member. IL-23 mediates its activity through IL-23R. IL-23 is a heterodimeric cytokine composed of a p19 subunit and a p40 subunit. Also, stimulates the proliferation of Th17 cells, a phenotype which produces inflammatory cytokines such as IL-17, TNFα, and IL-6[316,317]. IL-23 is necessary for the expression of IL-17A and IL-22 in the lung. The absence of IL-23 affects the expression of CXCL13 (B cell chemoattractant) within Mtb-induced lymphocyte follicles in the lungs and its deficiency is associated with increased T cells around the vessels in the lungs of studied mice[318]. The absence of homeostatic chemokines delays the protective immunity and granuloma formation[319]. Study has demonstrated that the IL23R (Arg381Gln) functional polymorphism is associated with an increased risk of development of a severe form of active pulmonary TB. Further studies are necessary[320] (Figure 21).
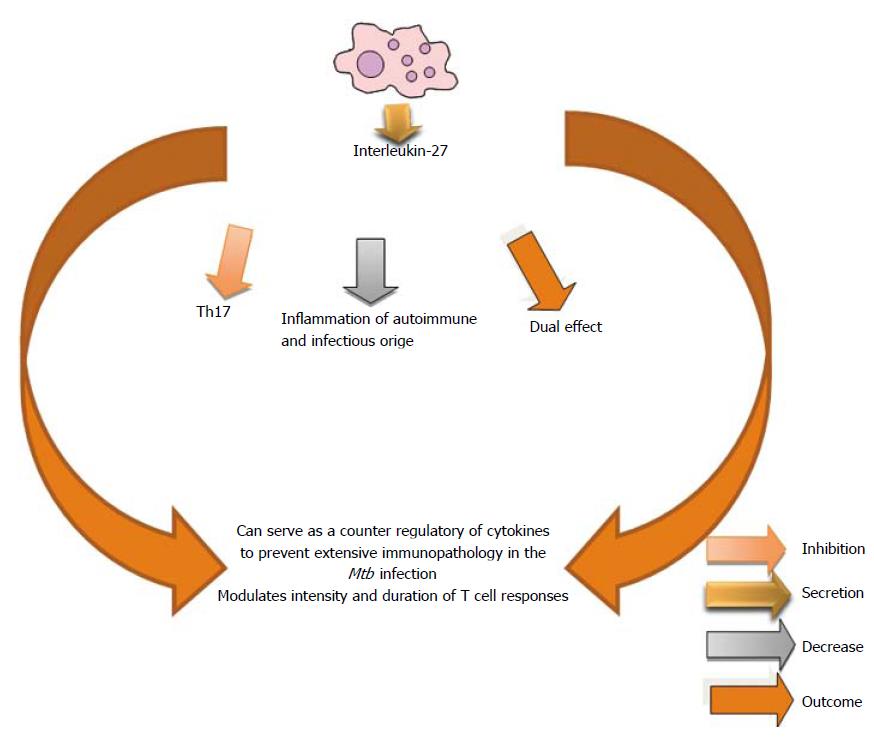
IL-27 is a member of the IL-12 family. IL-27 is an important inhibitory cytokine for the Th17 differentiation and limits inflammation of autoimmune and infectious origin[321-323]. Although the role of this cytokine is still not well understood in TB, two different studies have shown that IL-27R signaling has detrimental effects for the control of Mtb in the mouse model[324,325]. IL-27 can serve as a counter-regulatory of cytokines to prevent extensive immunopathology by keeping cellular responses in control. This cytokine can modulate the intensity and duration of many classes of T cell responses[326] (Figure 22).
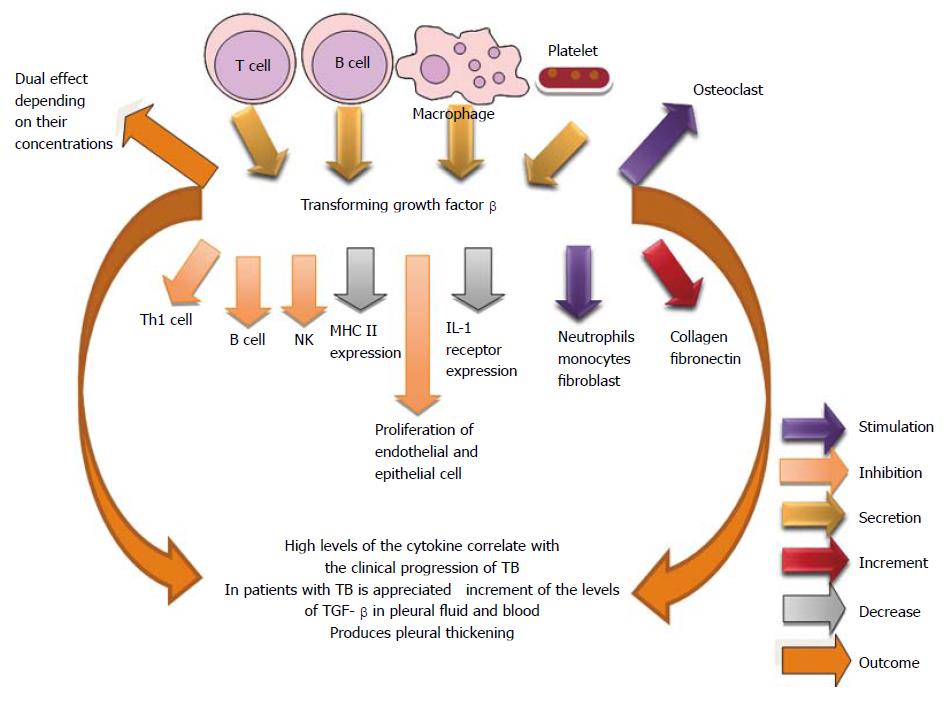
TGF-β is a key mediator in the immunopathogenesis of TB because it is able to affect quantitative and qualitatively other cytokines, such as IL-1β and TNF-α, and modulate the functions of T lymphocytes and macrophages[327-330]. In pleural tuberculosis, the excessive production of TGF-β is believed to be related to the clinical progression of the disease, particularly in the physiopathology of pleural thickening. TGF-β possesses proinflammatory activity in low concentrations (pleural tuberculosis and healthy contacts of tuberculosis carriers) and anti-inflammatory activity in high concentrations (pulmonary tuberculosis)[329]. It has observed increased levels of TGF-β in the pleural fluid and blood of tuberculosis patients. Although higher TGF-β levels were observed in the pleuropulmonary form, there was no statistical significance when compared to the levels in patients with pleural disease[331]. Pediatric TB is associated with elevated plasma levels of TGF-β, IL-21, and IL-23, which reveal an important role in the disease pathogenesis[332] (Figure 23).
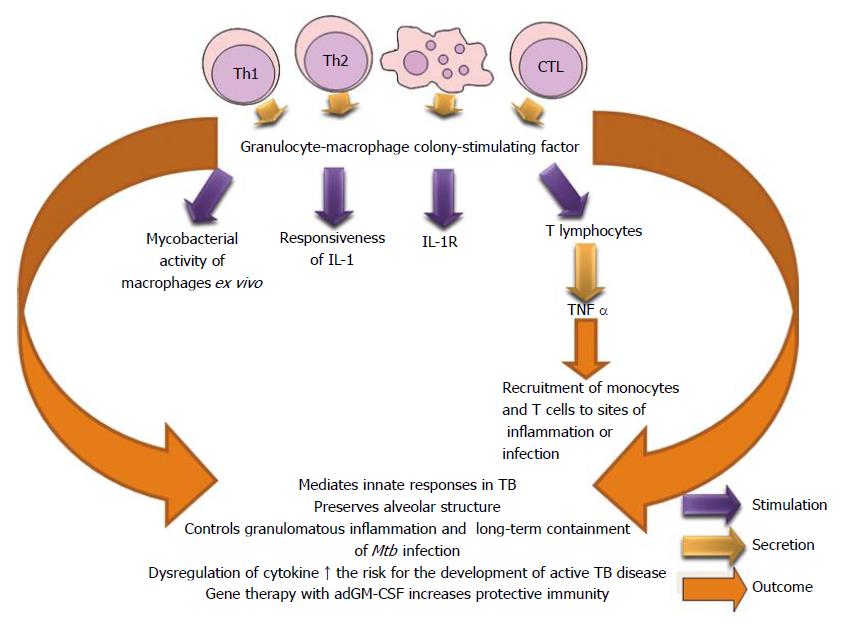
GM-CSF is produced by Th1, Th2 and CTL. This cytokine augments the production of granulocytes, macrophages and dendritic cells. GM-CSF and IL-3 stimulate the production of new macrophages when those cytokines act on primary hematopoietic cells of the bone marrow. GM-CSF has a fundamental role in a balanced innate host defense against tuberculosis by its role in preserving the integrity of alveolar epithelial cells and in regulating macrophages and dendritic cells to facilitate containment of virulent mycobacteria in pulmonary granulomas. Prolonged dys-regulation of GM-CSF expression favors the development of pulmonary tuberculosis in immuno-competent individuals[333]. Impaired GM-CSF signaling determines a defective innate activity in alveolar macrophages and allows high susceptibility to lung infections[334].
Invariant natural killer T (iNKT) cells produced GM-CSF in vitro and in vivo in a cluster of differentiation molecule 1 d (CD1d)-dependent manner during Mtb infection, and GM-CSF were both necessary and sufficient to control Mtb growth. GM-CSF has a potential role in T cell immunity against Mtb[335]. GM-CSF and/or TNF-α contributed with the most successful cellular differentiation and appoint that the culture conditions can limit or favor the replication of the bacteria in macrophages[57].
GM-CSF is an important cytokine in the immune protection against Mtb and gene therapy with recombinant adenoviruses encoding granulocyte-macrophage colony-stimulating factor increased protective immunity when administered in a model of progressive disease, and when used to prevent reactivation of latent infection or transmission[336] (Figure 24).
Chemokines belong to a large family of proteins called chemotactic cytokines and have an average molecular mass of 8-14 kDa. They can mediate the constitutive recruitment of leukocytes from the blood into tissues[337,338].
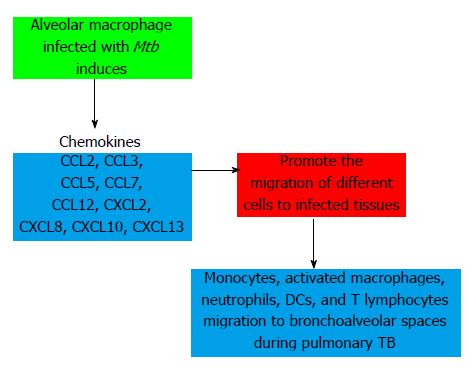
CCL2 (monocyte chemoattractant protein, MCP-1) promotes polarization to Th2 phenotype, resulting in a defective control of Mtb infection. Serum levels have been associated with TB disease activity and treatment response. CCL-3 [macrophage inflammatory protein 1α (MIP-1α)] mobilizes more Th1 than Th2 cells. CCL5 (Regulated upon Activation, Normal T cells Expressed and Secreted, RANTES) is important in the recruitment of Th1 cells to form lymphocyte-enriched granulomas and it has a relevant role in early response of IFNγ producing Tcells. CCL7 (monocyte chemoattractant protein-3, MCP-3) mobilizes phagocytic cells, NK cells and T Lymphocytes. This chemokine has been found elevated in bronchoalveolar lavage fluid and biopsy specimens of subjects with pulmonary tuberculosis. CCL12 (monocyte chemoattractant protein-5, MCP-5) is chemotactic for eosinophils, monocytes, and T and B lymphocytes. CXCL2 (GRO-β) mobilizes neutrophils and fibroblasts. CXCL8 (IL-8) increases the capacity of the neutrophil to kill Mtb among other functions and CXCL10 (IFN-γ induced protein, IP-10) is a good marker for monitoring of the treatment in adults with active TB[337,338].
IL-8 (CXCL8) is a strong neutrophil, monocytes and T-cells chemoattractant[339,340]. TB patients presented increased of CXCL8 levels in plasma and bronchoalveolar lavage fluids[341,342] which activates phagocytes as neutrophilis[343]. These cells are found in abundantly in the sputum of TB patients and are persistently recruited to sites of chronic mycobacterial infection[344,345]. Researchers have demonstrated that Mycobacterial infection of alveolar epithelial cells induce the secretion of CXCL8 and IL-6, but not secretion of the monocyte chemotactic CCL2 or pro-inflammatory TNF-α[346].
Study reveals that up-regulation of CCL18 and IL-10 in macrophages by Mtb may be involved in the recruitment of naïve Tcells in association with local suppressive immunity against intracellular pathogen[347]. The relationship between mycobacterial antigen-induced IFN-γ and CXCL9 may play a role in determining disease severity in TB[348]. CCL20 attracts immature dendritic cells and down-regulates the characteristic production of reactive oxygen species induced by Mtb in monocytes which may affect the activity of the cells. This chemokine inhibits apoptosis mediated by the mycobacteria[349]. CCL22 might be capable of inducing the migration of Tregs to the pleural space of the patients with TPE[350].
It has proposed a model in relation to the formation of granuloma and the participation of chemokines for host defense during Mtb infection. Alveolar macrophages uptake Mtb and secrete chemokines. Other cells such as epithelial cells and fibroblasts also produce chemoattractant proteins. This chemokine cascade causes an initial recruitment of neutrophils and monocytes. Meanwhile, lung DCs infected by Mtb increase the expression of CCR7 and migrate in response to chemokines expressed within lymphoid organs to polarize T cells into phenotypes productors of cytokines. CXCL13 and CCL19 may then mediate correct spatial localization of immune cells to form granulomas and mediate the control of Mtb[351] (Figure 25). The complexity of the network of cytokines and chemokines is observed by a recent study which shows that patients with coinfection HIV/Mtb have similar pattern of regulating proteins. Anti TB treatment significantly improves the level of pro-inflammatory cytokines (Th1 phenotype) and chemokines but does not restore the immune response in HIV-positive patients[352].
The existence of lineages, sublineages, strains and substrains reveal the complexity of Mtb and hence differences in the behavior of the immune system and the evolution of the disease. There is evidence suggesting some strains of Mtb may result in higher rates of disease progression, treatment failure, and relapse. The presence of persisting immune activation and high frequencies of Treg lymphocytes may reflect immune dysregulation that predisposes individuals to clinical tuberculosis, specifically to extrapulmonary tuberculosis. There is no doubt that immunity to Mtb depends on Th1-cell activity (IFN-γ and IL-12 and the production of TNF-α), but Th1 immunity alone is not sufficient to protect the host from Mtb infection, development of the disease, or dissemination. CD8+ T and γδ T cells exhibit cytolytic effector functions in the Mtb infection which amplifies the response. Studies of B cell immunodeficiency in both humans and mice have questioned whether these lymphocytes impart a protective effect against Mtb.
Recently, has been demonstrated that NK cells have the capacity for memory-like responses which permit greater control of the bacterial infection. IFN-γ, TNF-α, IL-12 and IL-17 are important participants in Mycobacterium-induced granuloma formation. Cytokines produced by Th17 phenotype enhance the clearance of intracellular Bacillus Calmette-Guérin (BCG) by macrophages.
IL-27 and IL-10 can serve as counter-regulatory of the cytokines to prevent extensive immunopathology by keeping anti-bacterial cellular response in control. The excessive production of TGF-β is believed to be related to the clinical progression of the disease, particularly, in the physiopathology of pleural thickening. Some chemokines may mediate correct spatial localization of immune cells to form granulomas and mediate the control of Mtb and others are also involved in the migration of different cells to infected tissues. As appreciated, the immune system is an extensive network and its final outcome is based in biological actions of cytokines and the participation of the factors aforementioned.
P- Reviewer: Boonsarngsuk V, Drain P S- Editor: Song XX L- Editor: A E- Editor: Jiao XK
| 1. | World Health Organization. Global tuberculosis control 2013. [Accessed on August 12]. Geneva: WHO. Available from: http: //www.who.int/tb/publications/global_report/2011/gtbr11_full.pdf. |
| 2. | Ernst JD. The immunological life cycle of tuberculosis. Nat Rev Immunol. 2012;12:581-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 353] [Cited by in RCA: 410] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 3. | González N, De Cubeddu L, de Waard JH, Fandiño C, Fernández de Larrea C, López D, Maldonado A, Ocaña Y, Hernández E, Ortega R. [Study of immune response in Warao children from communities with high tuberculosis prevalence]. Invest Clin. 2003;44:303-318. [PubMed] |
| 4. | Frieden TR, Sterling TR, Munsiff SS, Watt CJ, Dye C. Tuberculosis. Lancet. 2003;362:887-899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 636] [Cited by in RCA: 582] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 5. | Roper WH, Waring JJ. Primary serofibrinous pleural effusion in military personnel. Am Rev Tuberc. 1955;71:616-634. [PubMed] |
| 6. | Rieder HL, Kelly GD, Bloch AB, Cauthen GM, Snider DE. Tuberculosis diagnosed at death in the United States. Chest. 1991;100:678-681. [PubMed] |
| 7. | Abdi-Liae Z, Moradnejad P, Alijani N, Khazraiyan H, Mansoori S, Mohammadi N. Disseminated tuberculosis in an AIDS/HIV-infected patient. Acta Med Iran. 2013;51:587-589. [PubMed] |
| 8. | Zuñiga J, Torres-García D, Santos-Mendoza T, Rodriguez-Reyna TS, Granados J, Yunis EJ. Cellular and humoral mechanisms involved in the control of tuberculosis. Clin Dev Immunol. 2012;2012:193923. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 97] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 9. | Hossain MM, Norazmi MN. Pattern recognition receptors and cytokines in Mycobacterium tuberculosis infection--the double-edged sword? Biomed Res Int. 2013;2013:179174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 89] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 10. | Murray CJ, Styblo K, Rouillon A. Tuberculosis in developing countries: burden, intervention and cost. Bull Int Union Tuberc Lung Dis. 1990;65:6-24. [PubMed] |
| 11. | Barnes PF, Cave MD. Molecular epidemiology of tuberculosis. N Engl J Med. 2003;349:1149-1156. [PubMed] |
| 12. | Caws M, Thwaites G, Dunstan S, Hawn TR, Lan NT, Thuong NT, Stepniewska K, Huyen MN, Bang ND, Loc TH. The influence of host and bacterial genotype on the development of disseminated disease with Mycobacterium tuberculosis. PLoS Pathog. 2008;4:e1000034. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 340] [Cited by in RCA: 364] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 13. | Orme IM. The mouse as a useful model of tuberculosis. Tuberculosis (Edinb). 2003;83:112-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 73] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 14. | Nedeltchev GG, Raghunand TR, Jassal MS, Lun S, Cheng QJ, Bishai WR. Extrapulmonary dissemination of Mycobacterium bovis but not Mycobacterium tuberculosis in a bronchoscopic rabbit model of cavitary tuberculosis. Infect Immun. 2009;77:598-603. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 47] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 15. | McMurray DN. Hematogenous reseeding of the lung in low-dose, aerosol-infected guinea pigs: unique features of the host-pathogen interface in secondary tubercles. Tuberculosis (Edinb). 2003;83:131-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 59] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 16. | Chen CY, Huang D, Wang RC, Shen L, Zeng G, Yao S, Shen Y, Halliday L, Fortman J, McAllister M. A critical role for CD8 T cells in a nonhuman primate model of tuberculosis. PLoS Pathog. 2009;5:e1000392. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 183] [Cited by in RCA: 217] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 17. | Larsen MH, Biermann K, Chen B, Hsu T, Sambandamurthy VK, Lackner AA, Aye PP, Didier P, Huang D, Shao L. Efficacy and safety of live attenuated persistent and rapidly cleared Mycobacterium tuberculosis vaccine candidates in non-human primates. Vaccine. 2009;27:4709-4717. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 82] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 18. | Torrado E, Cooper AM. Cytokines in the balance of protection and pathology during mycobacterial infections. Adv Exp Med Biol. 2013;783:121-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 49] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 19. | Gallegos AM, Pamer EG, Glickman MS. Delayed protection by ESAT-6-specific effector CD4+ T cells after airborne M. tuberculosis infection. J Exp Med. 2008;205:2359-2368. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 144] [Cited by in RCA: 148] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 20. | Urdahl KB, Shafiani S, Ernst JD. Initiation and regulation of T-cell responses in tuberculosis. Mucosal Immunol. 2011;4:288-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 188] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 21. | Manabe YC, Bishai WR. Latent Mycobacterium tuberculosis-persistence, patience, and winning by waiting. Nat Med. 2000;6:1327-1329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 196] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 22. | Flynn JL, Chan J. Tuberculosis: latency and reactivation. Infect Immun. 2001;69:4195-4201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 312] [Cited by in RCA: 309] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 23. | Flynn JL, Chan J, Triebold KJ, Dalton DK, Stewart TA, Bloom BR. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J Exp Med. 1993;178:2249-2254. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1855] [Cited by in RCA: 1920] [Article Influence: 60.0] [Reference Citation Analysis (0)] |
| 24. | Maglione PJ, Chan J. How B cells shape the immune response against Mycobacterium tuberculosis. Eur J Immunol. 2009;39:676-686. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 151] [Cited by in RCA: 145] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 25. | Kozakiewicz L, Phuah J, Flynn J, Chan J. The role of B cells and humoral immunity in Mycobacterium tuberculosis infection. Adv Exp Med Biol. 2013;783:225-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 108] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 26. | Hunter RL. Pathology of post primary tuberculosis of the lung: an illustrated critical review. Tuberculosis (Edinb). 2011;91:497-509. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 195] [Cited by in RCA: 163] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 27. | Salgame P. MMPs in tuberculosis: granuloma creators and tissue destroyers. J Clin Invest. 2011;121:1686-1688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 54] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 28. | Seiler P, Aichele P, Bandermann S, Hauser AE, Lu B, Gerard NP, Gerard C, Ehlers S, Mollenkopf HJ, Kaufmann SH. Early granuloma formation after aerosol Mycobacterium tuberculosis infection is regulated by neutrophils via CXCR3-signaling chemokines. Eur J Immunol. 2003;33:2676-2686. [PubMed] |
| 29. | Tsai MC, Chakravarty S, Zhu G, Xu J, Tanaka K, Koch C, Tufariello J, Flynn J, Chan J. Characterization of the tuberculous granuloma in murine and human lungs: cellular composition and relative tissue oxygen tension. Cell Microbiol. 2006;8:218-232. [PubMed] |
| 30. | Eruslanov EB, Lyadova IV, Kondratieva TK, Majorov KB, Scheglov IV, Orlova MO, Apt AS. Neutrophil responses to Mycobacterium tuberculosis infection in genetically susceptible and resistant mice. Infect Immun. 2005;73:1744-1753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 221] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 31. | Keller C, Hoffmann R, Lang R, Brandau S, Hermann C, Ehlers S. Genetically determined susceptibility to tuberculosis in mice causally involves accelerated and enhanced recruitment of granulocytes. Infect Immun. 2006;74:4295-4309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 126] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 32. | Nandi B, Behar SM. Regulation of neutrophils by interferon-γ limits lung inflammation during tuberculosis infection. J Exp Med. 2011;208:2251-2262. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 246] [Cited by in RCA: 282] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 33. | Blomgran R, Ernst JD. Lung neutrophils facilitate activation of naive antigen-specific CD4+ T cells during Mycobacterium tuberculosis infection. J Immunol. 2011;186:7110-7119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 179] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 34. | Appelberg R, Castro AG, Gomes S, Pedrosa J, Silva MT. Susceptibility of beige mice to Mycobacterium avium: role of neutrophils. Infect Immun. 1995;63:3381-3387. [PubMed] |
| 35. | Feng CG, Kaviratne M, Rothfuchs AG, Cheever A, Hieny S, Young HA, Wynn TA, Sher A. NK cell-derived IFN-gamma differentially regulates innate resistance and neutrophil response in T cell-deficient hosts infected with Mycobacterium tuberculosis. J Immunol. 2006;177:7086-7093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 172] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 36. | Fulton SA, Reba SM, Martin TD, Boom WH. Neutrophil-mediated mycobacteriocidal immunity in the lung during Mycobacterium bovis BCG infection in C57BL/6 mice. Infect Immun. 2002;70:5322-5327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 69] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 37. | Martineau AR, Newton SM, Wilkinson KA, Kampmann B, Hall BM, Nawroly N, Packe GE, Davidson RN, Griffiths CJ, Wilkinson RJ. Neutrophil-mediated innate immune resistance to mycobacteria. J Clin Invest. 2007;117:1988-1994. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 316] [Cited by in RCA: 296] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 38. | Pedrosa J, Saunders BM, Appelberg R, Orme IM, Silva MT, Cooper AM. Neutrophils play a protective nonphagocytic role in systemic Mycobacterium tuberculosis infection of mice. Infect Immun. 2000;68:577-583. [PubMed] |
| 39. | Zhang X, Majlessi L, Deriaud E, Leclerc C, Lo-Man R. Coactivation of Syk kinase and MyD88 adaptor protein pathways by bacteria promotes regulatory properties of neutrophils. Immunity. 2009;31:761-771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 249] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 40. | Kozakiewicz L, Chen Y, Xu J, Wang Y, Dunussi-Joannopoulos K, Ou Q, Flynn JL, Porcelli SA, Jacobs WR, Chan J. B cells regulate neutrophilia during Mycobacterium tuberculosis infection and BCG vaccination by modulating the interleukin-17 response. PLoS Pathog. 2013;9:e1003472. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 95] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 41. | Braian C, Hogea V, Stendahl O. Mycobacterium tuberculosis- induced neutrophil extracellular traps activate human macrophages. J Innate Immun. 2013;5:591-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 115] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 42. | Matucci A, Maggi E, Vultaggio A. Cellular and humoral immune responses during tuberculosis infection: useful knowledge in the era of biological agents. J Rheumatol Suppl. 2014;91:17-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 43. | Raja A. Immunology of tuberculosis. Indian J Med Res. 2004;120:213-232. [PubMed] |
| 44. | Rom WN, Schluger N, Law K, Condos R, Zhang Y, Weiden M, Harkin T, Tchou-Wong KM. Human host response to Mycobacterium tuberculosis. Schweiz Med Wochenschr. 1995;125:2178-2185. [PubMed] |
| 45. | Jo EK. Mycobacterial interaction with innate receptors: TLRs, C-type lectins, and NLRs. Curr Opin Infect Dis. 2008;21:279-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 127] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 46. | Noss EH, Pai RK, Sellati TJ, Radolf JD, Belisle J, Golenbock DT, Boom WH, Harding CV. Toll-like receptor 2-dependent inhibition of macrophage class II MHC expression and antigen processing by 19-kDa lipoprotein of Mycobacterium tuberculosis. J Immunol. 2001;167:910-918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 333] [Cited by in RCA: 326] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 47. | Chen M, Gan H, Remold HG. A mechanism of virulence: virulent Mycobacterium tuberculosis strain H37Rv, but not attenuated H37Ra, causes significant mitochondrial inner membrane disruption in macrophages leading to necrosis. J Immunol. 2006;176:3707-3716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 205] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 48. | Jung SB, Yang CS, Lee JS, Shin AR, Jung SS, Son JW, Harding CV, Kim HJ, Park JK, Paik TH. The mycobacterial 38-kilodalton glycolipoprotein antigen activates the mitogen-activated protein kinase pathway and release of proinflammatory cytokines through Toll-like receptors 2 and 4 in human monocytes. Infect Immun. 2006;74:2686-2696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 118] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 49. | Torrado E, Robinson RT, Cooper AM. Cellular response to mycobacteria: balancing protection and pathology. Trends Immunol. 2011;32:66-72. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 52] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 50. | Park JS, Tamayo MH, Gonzalez-Juarrero M, Orme IM, Ordway DJ. Virulent clinical isolates of Mycobacterium tuberculosis grow rapidly and induce cellular necrosis but minimal apoptosis in murine macrophages. J Leukoc Biol. 2006;79:80-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 106] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 51. | Blomgran R, Desvignes L, Briken V, Ernst JD. Mycobacterium tuberculosis inhibits neutrophil apoptosis, leading to delayed activation of naive CD4 T cells. Cell Host Microbe. 2012;11:81-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 144] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 52. | Lin WW, Hsieh SL. Decoy receptor 3: a pleiotropic immunomodulator and biomarker for inflammatory diseases, autoimmune diseases and cancer. Biochem Pharmacol. 2011;81:838-847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 127] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 53. | You RI, Chang YC, Chen PM, Wang WS, Hsu TL, Yang CY, Lee CT, Hsieh SL. Apoptosis of dendritic cells induced by decoy receptor 3 (DcR3). Blood. 2008;111:1480-1488. [PubMed] |
| 54. | Chang YC, Chen TC, Lee CT, Yang CY, Wang HW, Wang CC, Hsieh SL. Epigenetic control of MHC class II expression in tumor-associated macrophages by decoy receptor 3. Blood. 2008;111:5054-5063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 100] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 55. | Shu CC, Wu MF, Hsu CL, Huang CT, Wang JY, Hsieh SL, Yu CJ, Lee LN, Yang PC. Apoptosis-associated biomarkers in tuberculosis: promising for diagnosis and prognosis prediction. BMC Infect Dis. 2013;13:45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 56. | Danelishvili L, Bermudez LE. Analysis of pyroptosis in bacterial infection. Methods Mol Biol. 2013;1004:67-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 57. | Vogt G, Nathan C. In vitro differentiation of human macrophages with enhanced antimycobacterial activity. J Clin Invest. 2011;121:3889-3901. [RCA] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 87] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 58. | Bodnar KA, Serbina NV, Flynn JL. Fate of Mycobacterium tuberculosis within murine dendritic cells. Infect Immun. 2001;69:800-809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 150] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 59. | Hickman SP, Chan J, Salgame P. Mycobacterium tuberculosis induces differential cytokine production from dendritic cells and macrophages with divergent effects on naive T cell polarization. J Immunol. 2002;168:4636-4642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 143] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 60. | Tascon RE, Soares CS, Ragno S, Stavropoulos E, Hirst EM, Colston MJ. Mycobacterium tuberculosis-activated dendritic cells induce protective immunity in mice. Immunology. 2000;99:473-480. [PubMed] |
| 61. | Demangel C, Bean AG, Martin E, Feng CG, Kamath AT, Britton WJ. Protection against aerosol Mycobacterium tuberculosis infection using Mycobacterium bovis Bacillus Calmette Guérin-infected dendritic cells. Eur J Immunol. 1999;29:1972-1979. [PubMed] |
| 62. | Angerami M, Suarez G, Pascutti MF, Salomon H, Bottasso O, Quiroga MF. Modulation of the phenotype and function of Mycobacterium tuberculosis-stimulated dendritic cells by adrenal steroids. Int Immunol. 2013;25:405-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 63. | Maglione PJ, Xu J, Chan J. B cells moderate inflammatory progression and enhance bacterial containment upon pulmonary challenge with Mycobacterium tuberculosis. J Immunol. 2007;178:7222-7234. [PubMed] |
| 64. | Vordermeier HM, Venkataprasad N, Harris DP, Ivanyi J. Increase of tuberculous infection in the organs of B cell-deficient mice. Clin Exp Immunol. 1996;106:312-316. [PubMed] |
| 65. | Bosio CM, Gardner D, Elkins KL. Infection of B cell-deficient mice with CDC 1551, a clinical isolate of Mycobacterium tuberculosis: delay in dissemination and development of lung pathology. J Immunol. 2000;164:6417-6425. [PubMed] |
| 66. | Johnson CM, Cooper AM, Frank AA, Bonorino CB, Wysoki LJ, Orme IM. Mycobacterium tuberculosis aerogenic rechallenge infections in B cell-deficient mice. Tuber Lung Dis. 1997;78:257-261. [PubMed] |
| 67. | Turner J, Frank AA, Brooks JV, Gonzalez-Juarrero M, Orme IM. The progression of chronic tuberculosis in the mouse does not require the participation of B lymphocytes or interleukin-4. Exp Gerontol. 2001;36:537-545. [PubMed] |
| 68. | Hooper LV, Gordon JI. Commensal host-bacterial relationships in the gut. Science. 2001;292:1115-1118. [PubMed] |
| 69. | Maglione PJ, Xu J, Casadevall A, Chan J. Fc gamma receptors regulate immune activation and susceptibility during Mycobacterium tuberculosis infection. J Immunol. 2008;180:3329-3338. [PubMed] |
| 70. | Zhang M, Zheng X, Zhang J, Zhu Y, Zhu X, Liu H, Zeng M, Graner MW, Zhou B, Chen X. CD19(+)CD1d(+)CD5(+) B cell frequencies are increased in patients with tuberculosis and suppress Th17 responses. Cell Immunol. 2012;274:89-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 90] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 71. | Sánchez-Rodríguez C, Estrada-Chávez C, García-Vigil J, Laredo-Sánchez F, Halabe-Cherem J, Pereira-Suárez A, Mancilla R. An IgG antibody response to the antigen 85 complex is associated with good outcome in Mexican Totonaca Indians with pulmonary tuberculosis. Int J Tuberc Lung Dis. 2002;6:706-712. [PubMed] |
| 72. | Casanova JL, Abel L. Genetic dissection of immunity to mycobacteria: the human model. Annu Rev Immunol. 2002;20:581-620. [PubMed] |
| 73. | Doffinger R, Patel S, Kumararatne DS. Human immunodeficiencies that predispose to intracellular bacterial infections. Curr Opin Rheumatol. 2005;17:440-446. [PubMed] |
| 74. | Ulrichs T, Kosmiadi GA, Trusov V, Jörg S, Pradl L, Titukhina M, Mishenko V, Gushina N, Kaufmann SH. Human tuberculous granulomas induce peripheral lymphoid follicle-like structures to orchestrate local host defence in the lung. J Pathol. 2004;204:217-228. [PubMed] |
| 75. | Torrado E, Fountain JJ, Robinson RT, Martino CA, Pearl JE, Rangel-Moreno J, Tighe M, Dunn R, Cooper AM. Differential and site specific impact of B cells in the protective immune response to Mycobacterium tuberculosis in the mouse. PLoS One. 2013;8:e61681. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 76. | Wan YY, Flavell RA. How diverse--CD4 effector T cells and their functions. J Mol Cell Biol. 2009;1:20-36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 153] [Cited by in RCA: 142] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 77. | Altare F, Durandy A, Lammas D, Emile JF, Lamhamedi S, Le Deist F, Drysdale P, Jouanguy E, Döffinger R, Bernaudin F. Impairment of mycobacterial immunity in human interleukin-12 receptor deficiency. Science. 1998;280:1432-1435. [PubMed] |
| 78. | Jouanguy E, Lamhamedi-Cherradi S, Altare F, Fondanèche MC, Tuerlinckx D, Blanche S, Emile JF, Gaillard JL, Schreiber R, Levin M. Partial interferon-gamma receptor 1 deficiency in a child with tuberculoid bacillus Calmette-Guérin infection and a sibling with clinical tuberculosis. J Clin Invest. 1997;100:2658-2664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 279] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 79. | Selwyn PA, Hartel D, Lewis VA, Schoenbaum EE, Vermund SH, Klein RS, Walker AT, Friedland GH. A prospective study of the risk of tuberculosis among intravenous drug users with human immunodeficiency virus infection. N Engl J Med. 1989;320:545-550. [PubMed] |
| 80. | Seah GT, Scott GM, Rook GA. Type 2 cytokine gene activation and its relationship to extent of disease in patients with tuberculosis. J Infect Dis. 2000;181:385-389. [PubMed] |
| 81. | Gong JH, Zhang M, Modlin RL, Linsley PS, Iyer D, Lin Y, Barnes PF. Interleukin-10 downregulates Mycobacterium tuberculosis-induced Th1 responses and CTLA-4 expression. Infect Immun. 1996;64:913-918. [PubMed] |
| 82. | Wilsher ML, Hagan C, Prestidge R, Wells AU, Murison G. Human in vitro immune responses to Mycobacterium tuberculosis. Tuber Lung Dis. 1999;79:371-377. [PubMed] |
| 83. | Freeman S, Post FA, Bekker LG, Harbacheuski R, Steyn LM, Ryffel B, Connell ND, Kreiswirth BN, Kaplan G. Mycobacterium tuberculosis H37Ra and H37Rv differential growth and cytokine/chemokine induction in murine macrophages in vitro. J Interferon Cytokine Res. 2006;26:27-33. [PubMed] |
| 84. | Surewicz K, Aung H, Kanost RA, Jones L, Hejal R, Toossi Z. The differential interaction of p38 MAP kinase and tumor necrosis factor-alpha in human alveolar macrophages and monocytes induced by Mycobacterium tuberculois. Cell Immunol. 2004;228:34-41. [PubMed] |
| 85. | Sun YJ, Lim TK, Ong AK, Ho BC, Seah GT, Paton NI. Tuberculosis associated with Mycobacterium tuberculosis Beijing and non-Beijing genotypes: a clinical and immunological comparison. BMC Infect Dis. 2006;6:105. [PubMed] |
| 86. | Romero-Adrián TB, Leal-Montiel J, Monsalve-Castillo F, Mengual-Moreno E, McGregor EG, Perini L, Antúnez A. Helicobacter pylori: bacterial factors and the role of cytokines in the immune response. Curr Microbiol. 2010;60:143-155. [PubMed] |
| 87. | George PJ, Anuradha R, Kumaran PP, Chandrasekaran V, Nutman TB, Babu S. Modulation of mycobacterial-specific Th1 and Th17 cells in latent tuberculosis by coincident hookworm infection. J Immunol. 2013;190:5161-5168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 88. | Srivastava S, Ernst JD. Cutting edge: Direct recognition of infected cells by CD4 T cells is required for control of intracellular Mycobacterium tuberculosis in vivo. J Immunol. 2013;191:1016-1020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 110] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 89. | Hirsch CS, Toossi Z, Othieno C, Johnson JL, Schwander SK, Robertson S, Wallis RS, Edmonds K, Okwera A, Mugerwa R. Depressed T-cell interferon-gamma responses in pulmonary tuberculosis: analysis of underlying mechanisms and modulation with therapy. J Infect Dis. 1999;180:2069-2073. [PubMed] |
| 90. | Toossi Z, Kleinhenz ME, Ellner JJ. Defective interleukin 2 production and responsiveness in human pulmonary tuberculosis. J Exp Med. 1986;163:1162-1172. [PubMed] |
| 91. | Huygen K, Van Vooren JP, Turneer M, Bosmans R, Dierckx P, De Bruyn J. Specific lymphoproliferation, gamma interferon production, and serum immunoglobulin G directed against a purified 32 kDa mycobacterial protein antigen (P32) in patients with active tuberculosis. Scand J Immunol. 1988;27:187-194. [PubMed] |
| 92. | Zhang M, Gong J, Iyer DV, Jones BE, Modlin RL, Barnes PF. T cell cytokine responses in persons with tuberculosis and human immunodeficiency virus infection. J Clin Invest. 1994;94:2435-2442. [PubMed] |
| 93. | Torres M, Mendez-Sampeiro P, Jimenez-Zamudio L, Teran L, Camarena A, Quezada R, Ramos E, Sada E. Comparison of the immune response against Mycobacterium tuberculosis antigens between a group of patients with active pulmonary tuberculosis and healthy household contacts. Clin Exp Immunol. 1994;96:75-78. [PubMed] |
| 94. | Diniz LM, Zandonade E, Dietze R, Pereira FE, Ribeiro-Rodrigues R. Short report: do intestinal nematodes increase the risk for multibacillary leprosy? Am J Trop Med Hyg. 2001;65:852-854. [PubMed] |
| 95. | Hirsch CS, Hussain R, Toossi Z, Dawood G, Shahid F, Ellner JJ. Cross-modulation by transforming growth factor beta in human tuberculosis: suppression of antigen-driven blastogenesis and interferon gamma production. Proc Natl Acad Sci USA. 1996;93:3193-3198. [PubMed] |
| 96. | Gong JH, Zhang M, Modlin RL, Iyer D, Lin Y, and Barnes P F. Interleukin-10 downregulates Mycobacterium tuberculosis-induced Th1 responses and CTLA-4 expression. Infect Immun. 1996;64:913-918. |
| 97. | Hirsch CS, Ellner JJ, Blinkhorn R, Toossi Z. In vitro restoration of T cell responses in tuberculosis and augmentation of monocyte effector function against Mycobacterium tuberculosis by natural inhibitors of transforming growth factor beta. Proc Natl Acad Sci USA. 1997;94:3926-3931. [PubMed] |
| 98. | Verbon A, Juffermans N, Van Deventer SJ, Speelman P, Van Deutekom H, Van Der Poll T. Serum concentrations of cytokines in patients with active tuberculosis (TB) and after treatment. Clin Exp Immunol. 1999;115:110-113. [PubMed] |
| 99. | Kumar NP, Anuradha R, Suresh R, Ganesh R, Shankar J, Kumaraswami V, Nutman TB, Babu S. Suppressed type 1, type 2, and type 17 cytokine responses in active tuberculosis in children. Clin Vaccine Immunol. 2011;18:1856-1864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 100. | Weiner HL. Induction and mechanism of action of transforming growth factor-beta-secreting Th3 regulatory cells. Immunol Rev. 2001;182:207-214. [PubMed] |
| 101. | Stassen M, Fondel S, Bopp T, Richter C, Müller C, Kubach J, Becker C, Knop J, Enk AH, Schmitt S. Human CD25+ regulatory T cells: two subsets defined by the integrins alpha 4 beta 7 or alpha 4 beta 1 confer distinct suppressive properties upon CD4+ T helper cells. Eur J Immunol. 2004;34:1303-1311. [PubMed] |
| 102. | Vieira PL, Christensen JR, Minaee S, O’Neill EJ, Barrat FJ, Boonstra A, Barthlott T, Stockinger B, Wraith DC, O’Garra A. IL-10-secreting regulatory T cells do not express Foxp3 but have comparable regulatory function to naturally occurring CD4+CD25+ regulatory T cells. J Immunol. 2004;172:5986-5993. [PubMed] |
| 103. | Singh A, Dey AB, Mohan A, Sharma PK, Mitra DK. Foxp3+ regulatory T cells among tuberculosis patients: impact on prognosis and restoration of antigen specific IFN-γ producing T cells. PLoS One. 2012;7:e44728. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 70] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 104. | Quinn KM, McHugh RS, Rich FJ, Goldsack LM, de Lisle GW, Buddle BM, Delahunt B, Kirman JR. Inactivation of CD4+ CD25+ regulatory T cells during early mycobacterial infection increases cytokine production but does not affect pathogen load. Immunol Cell Biol. 2006;84:467-474. [PubMed] |
| 105. | Scott-Browne JP, Shafiani S, Tucker-Heard G, Ishida-Tsubota K, Fontenot JD, Rudensky AY, Bevan MJ, Urdahl KB. Expansion and function of Foxp3-expressing T regulatory cells during tuberculosis. J Exp Med. 2007;204:2159-2169. [PubMed] |
| 106. | Ribeiro-Rodrigues R, Resende Co T, Rojas R, Toossi Z, Dietze R, Boom WH, Maciel E, Hirsch CS. A role for CD4+CD25+ T cells in regulation of the immune response during human tuberculosis. Clin Exp Immunol. 2006;144:25-34. [PubMed] |
| 107. | Larson RP, Shafiani S, Urdahl KB. Foxp3(+) regulatory T cells in tuberculosis. Adv Exp Med Biol. 2013;783:165-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 108. | Romero- Adrián T, Leal- Montiel J. Helicobacter pylori infection: Regulatory T cells and participation in the immune response. Jundishapur J Microbiol. 2013;6:e5183. [RCA] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 109. | Marin ND, París SC, Vélez VM, Rojas CA, Rojas M, García LF. Regulatory T cell frequency and modulation of IFN-gamma and IL-17 in active and latent tuberculosis. Tuberculosis (Edinb). 2010;90:252-261. [PubMed] |
| 110. | de Almeida AS, Fiske CT, Sterling TR, Kalams SA. Increased frequency of regulatory T cells and T lymphocyte activation in persons with previously treated extrapulmonary tuberculosis. Clin Vaccine Immunol. 2012;19:45-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 111. | Rahman S, Gudetta B, Fink J, Granath A, Ashenafi S, Aseffa A, Derbew M, Svensson M, Andersson J, Brighenti SG. Compartmentalization of immune responses in human tuberculosis: few CD8+ effector T cells but elevated levels of FoxP3+ regulatory t cells in the granulomatous lesions. Am J Pathol. 2009;174:2211-2224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 87] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 112. | Xi X, Han X, Li L, Zhao Z. Identification of a new tuberculosis antigen recognized by γδ T cell receptor. Clin Vaccine Immunol. 2013;20:530-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 113. | Pydi SS, Sunder SR, Venkatasubramanian S, Kovvali S, Jonnalagada S, Valluri VL. Killer cell immunoglobulin like receptor gene association with tuberculosis. Hum Immunol. 2013;74:85-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 114. | Junqueira-Kipnis AP, Kipnis A, Jamieson A, Juarrero MG, Diefenbach A, Raulet DH, Turner J, Orme IM. NK cells respond to pulmonary infection with Mycobacterium tuberculosis, but play a minimal role in protection. J Immunol. 2003;171:6039-6045. [PubMed] |
| 115. | Cooper MA, Colonna M, Yokoyama WM. Hidden talents of natural killers: NK cells in innate and adaptive immunity. EMBO Rep. 2009;10:1103-1110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 96] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 116. | Cooper MA, Yokoyama WM. Memory-like responses of natural killer cells. Immunol Rev. 2010;235:297-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 117. | Cooper MA, Elliott JM, Keyel PA, Yang L, Carrero JA, Yokoyama WM. Cytokine-induced memory-like natural killer cells. Proc Natl Acad Sci USA. 2009;106:1915-1919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 505] [Cited by in RCA: 623] [Article Influence: 38.9] [Reference Citation Analysis (0)] |
| 118. | Fu X, Liu Y, Li L, Li Q, Qiao D, Wang H, Lao S, Fan Y, Wu C. Human natural killer cells expressing the memory-associated marker CD45RO from tuberculous pleurisy respond more strongly and rapidly than CD45RO− natural killer cells following stimulation with interleukin-12. Immunology. 2011;134:41-49. [RCA] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 119. | Hoshino A, Hanada S, Yamada H, Mii S, Takahashi M, Mitarai S, Yamamoto K, Manome Y. Mycobacterium tuberculosis escapes from the phagosomes of infected human osteoclasts reprograms osteoclast development via dysregulation of cytokines and chemokines. Pathog Dis. 2014;70:28-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 120. | Winrow VR, Mesher J, Meghji S, Morris CJ, Maguire M, Fox S, Coates AR, Tormay P, Blake DR, Henderson B. The two homologous chaperonin 60 proteins of Mycobacterium tuberculosis have distinct effects on monocyte differentiation into osteoclasts. Cell Microbiol. 2008;10:2091-2104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 121. | Romero-Adrián T, Ruiz A, Molina-Vílchez R, Estévez J, Atencio R. Interleukin-2 receptor serum concentrations in normal pregnancy and pre-eclampsia. Invest Clin. 2002;43:73-78. [PubMed] |
| 122. | Monsalve F, Romero-A T, Estévez J, Costa L, Callejas D. [Serum levels of soluble CD30 molecule in hepatitis B virus infection]. Rev Med Chil. 2001;129:1248-1252. [PubMed] |
| 123. | Monsalve-De Castillo F, Romero TA, Estévez J, Costa LL, Atencio R, Porto L, Callejas D. Concentrations of cytokines, soluble interleukin-2 receptor, and soluble CD30 in sera of patients with hepatitis B virus infection during acute and convalescent phases. Clin Diagn Lab Immunol. 2002;9:1372-1375. [PubMed] |
| 124. | Gomes JA, Molica AM, Keesen TS, Morato MJ, de Araujo FF, Fares RC, Fiuza JA, Chaves AT, Pinheiro V, Nunes Mdo C. Inflammatory mediators from monocytes down-regulate cellular proliferation and enhance cytokines production in patients with polar clinical forms of Chagas disease. Hum Immunol. 2014;75:20-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 125. | Kima PE, Soong L. Interferon gamma in leishmaniasis. Front Immunol. 2013;4:156. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 82] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 126. | García E, Duarte S, Calderón C, González JM, Cuéllar A, Gómez A, Halpert E, Rodríguez A. Expression of IL-10, IL-4 and IFN-γ in active skin lesions of children with papular urticarial. Biomedica. 2011;31:525-531. [RCA] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 127. | Tang XQ, Sun WP, Xu HB, Liu WB, Wang TS, Liu HJ. The changes in the levels of IL-6, IL-17, and IL-21 in the acute stage of childhood asthma. Clin Lab. 2013;59:1381-1387. [PubMed] |
| 128. | Brkic Z, Corneth OB, van Helden-Meeuwsen CG, Dolhain RJ, Maria NI, Paulissen SM, Davelaar N, van Hamburg JP, van Daele PL, Dalm VA. T-helper 17 cell cytokines and interferon type I: partners in crime in systemic lupus erythematosus? Arthritis Res Ther. 2014;16:R62. [PubMed] |
| 129. | Shen H, Xia L, Lu J. Interleukin-4 in rheumatoid arthritis patients with interstitial lung disease: a pilot study. Indian J Med Res. 2013;138:919-921. [PubMed] |
| 130. | Malekzadeh M, Dehaghani AS, Ghaderi A, Doroudchi M. IL-17A is elevated in sera of patients with poorly differentiated ovarian papillary serous cystadenocarcinoma. Cancer Biomark. 2013;13:417-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 131. | Souza JM, Matias BF, Rodrigues CM, Murta EF, Michelin MA. IL-17 and IL-22 serum cytokine levels in patients with squamous intraepithelial lesion and invasive cervical carcinoma. Eur J Gynaecol Oncol. 2013;34:466-468. [PubMed] |
| 132. | Leal JY, Castejón HV, Romero T, Ortega P, Gómez G, Amaya D, Estévez J. [Serum values of cytokines in children with vitamin A deficiency disorders]. Invest Clin. 2004;45:243-256. [PubMed] |
| 133. | Leal JY, Castejón HV, Romero T, Ortega P, Gómez G, Amaya D, Estévez J. [Serum levels of interferon-gamma and interleukine-10 in anemic children with vitamin A deficiency]. Arch Latinoam Nutr. 2006;56:329-334. [PubMed] |
| 134. | Leal JY, Romero T, Ortega P, Amaya D. [Serum values of interleukin-10, gamma-interferon and vitamin A in female adolescents]. Invest Clin. 2007;48:317-326. [PubMed] |
| 135. | Sarkar R, Lenders L, Wilkinson KA, Wilkinson RJ, Nicol MP. Modern lineages of Mycobacterium tuberculosis exhibit lineage-specific patterns of growth and cytokine induction in human monocyte-derived macrophages. PLoS One. 2012;7:e43170. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 67] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 136. | Portevin D, Gagneux S, Comas I, Young D. Human macrophage responses to clinical isolates from the Mycobacterium tuberculosis complex discriminate between ancient and modern lineages. PLoS Pathog. 2011;7:e1001307. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 233] [Cited by in RCA: 213] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 137. | van Laarhoven A, Mandemakers JJ, Kleinnijenhuis J, Enaimi M, Lachmandas E, Joosten LA, Ottenhoff TH, Netea MG, van Soolingen D, van Crevel R. Low induction of proinflammatory cytokines parallels evolutionary success of modern strains within the Mycobacterium tuberculosis Beijing genotype. Infect Immun. 2013;81:3750-3756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 138. | Jayaraman P, Sada-Ovalle I, Nishimura T, Anderson AC, Kuchroo VK, Remold HG, Behar SM. IL-1β promotes antimicrobial immunity in macrophages by regulating TNFR signaling and caspase-3 activation. J Immunol. 2013;190:4196-4204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 177] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 139. | Krishnan N, Robertson BD, Thwaites G. Pathways of IL-1β secretion by macrophages infected with clinical Mycobacterium tuberculosis strains. Tuberculosis (Edinb). 2013;93:538-547. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 140. | Master SS, Rampini SK, Davis AS, Keller C, Ehlers S, Springer B, Timmins GS, Sander P, Deretic V. Mycobacterium tuberculosis prevents inflammasome activation. Cell Host Microbe. 2008;3:224-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 308] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 141. | Verway M, Bouttier M, Wang TT, Carrier M, Calderon M, An BS, Devemy E, McIntosh F, Divangahi M, Behr MA. Vitamin D induces interleukin-1β expression: paracrine macrophage epithelial signaling controls M. tuberculosis infection. PLoS Pathog. 2013;9:e1003407. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 152] [Cited by in RCA: 180] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 142. | Koh GC, Hawthorne G, Turner AM, Kunst H, Dedicoat M. Tuberculosis incidence correlates with sunshine: an ecological 28-year time series study. PLoS One. 2013;8:e57752. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 69] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 143. | White JH. Vitamin D signaling, infectious diseases, and regulation of innate immunity. Infect Immun. 2008;76:3837-3843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 260] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 144. | Mayer-Barber KD, Barber DL, Shenderov K, White SD, Wilson MS, Cheever A, Kugler D, Hieny S, Caspar P, Núñez G. Caspase-1 independent IL-1beta production is critical for host resistance to mycobacterium tuberculosis and does not require TLR signaling in vivo. J Immunol. 2010;184:3326-3330. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 400] [Cited by in RCA: 370] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 145. | Fremond CM, Togbe D, Doz E, Rose S, Vasseur V, Maillet I, Jacobs M, Ryffel B, Quesniaux VF. IL-1 receptor-mediated signal is an essential component of MyD88-dependent innate response to Mycobacterium tuberculosis infection. J Immunol. 2007;179:1178-1189. [PubMed] |
| 146. | Juffermans NP, Florquin S, Camoglio L, Verbon A, Kolk AH, Speelman P, van Deventer SJ, van Der Poll T. Interleukin-1 signaling is essential for host defense during murine pulmonary tuberculosis. J Infect Dis. 2000;182:902-908. [PubMed] |
| 147. | Yamada H, Mizumo S, Horai R, Iwakura Y, Sugawara I. Protective role of interleukin-1 in mycobacterial infection in IL-1 alpha/beta double-knockout mice. Lab Invest. 2000;80:759-767. [PubMed] |
| 148. | Kollmann TR, Levy O, Montgomery RR, Goriely S. Innate immune function by Toll-like receptors: distinct responses in newborns and the elderly. Immunity. 2012;37:771-783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 373] [Cited by in RCA: 437] [Article Influence: 36.4] [Reference Citation Analysis (0)] |
| 149. | Nguyen M, Leuridan E, Zhang T, De Wit D, Willems F, Van Damme P, Goldman M, Goriely S. Acquisition of adult-like TLR4 and TLR9 responses during the first year of life. PLoS One. 2010;5:e10407. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 118] [Cited by in RCA: 125] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 150. | Lisciandro JG, Prescott SL, Nadal-Sims MG, Devitt CJ, Pomat W, Siba PM, Tulic MC, Holt PG, Strickland D, van den Biggelaar AH. Ontogeny of Toll-like and NOD-like receptor-mediated innate immune responses in Papua New Guinean infants. PLoS One. 2012;7:e36793. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 151. | Bellamy R, Ruwende C, Corrah T, McAdam KP, Whittle HC, Hill AV. Assessment of the interleukin 1 gene cluster and other candidate gene polymorphisms in host susceptibility to tuberculosis. Tuber Lung Dis. 1998;79:83-89. [PubMed] |
| 152. | Gomez LM, Camargo JF, Castiblanco J, Ruiz-Narváez EA, Cadena J, Anaya JM. Analysis of IL1B, TAP1, TAP2 and IKBL polymorphisms on susceptibility to tuberculosis. Tissue Antigens. 2006;67:290-296. [PubMed] |
| 153. | Awomoyi AA, Charurat M, Marchant A, Miller EN, Blackwell JM, McAdam KP, Newport MJ. Polymorphism in IL1B: IL1B-511 association with tuberculosis and decreased lipopolysaccharide-induced IL-1beta in IFN-gamma primed ex-vivo whole blood assay. J Endotoxin Res. 2005;11:281-286. [PubMed] |
| 154. | Wilkinson RJ, Patel P, Llewelyn M, Hirsch CS, Pasvol G, Snounou G, Davidson RN, Toossi Z. Influence of polymorphism in the genes for the interleukin (IL)-1 receptor antagonist and IL-1beta on tuberculosis. J Exp Med. 1999;189:1863-1874. [PubMed] |
| 155. | Meenakshi P, Ramya S, Shruthi T, Lavanya J, Mohammed HH, Mohammed SA, Vijayalakshmi V, Sumanlatha G. Association of IL-1β +3954 C/T and IL-10-1082 G/A cytokine gene polymorphisms with susceptibility to tuberculosis. Scand J Immunol. 2013;78:92-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 156. | Sugawara I, Yamada H, Mizuno S, Takeda K, Akira S. Mycobacterial infection in MyD88-deficient mice. Microbiol Immunol. 2003;47:841-847. [PubMed] |
| 157. | Scanga CA, Bafica A, Feng CG, Cheever AW, Hieny S, Sher A. MyD88-deficient mice display a profound loss in resistance to Mycobacterium tuberculosis associated with partially impaired Th1 cytokine and nitric oxide synthase 2 expression. Infect Immun. 2004;72:2400-2404. [PubMed] |
| 158. | Fremond CM, Yeremeev V, Nicolle DM, Jacobs M, Quesniaux VF, Ryffel B. Fatal Mycobacterium tuberculosis infection despite adaptive immune response in the absence of MyD88. J Clin Invest. 2004;114:1790-1799. [PubMed] |
| 159. | Bekker LG, Moreira AL, Bergtold A, Freeman S, Ryffel B, Kaplan G. Immunopathologic effects of tumor necrosis factor alpha in murine mycobacterial infection are dose dependent. Infect Immun. 2000;68:6954-6961. [PubMed] |
| 160. | Feldmann M. Development of anti-TNF therapy for rheumatoid arthritis. Nat Rev Immunol. 2002;2:364-371. [PubMed] |
| 161. | Keane J, Gershon S, Wise RP, Mirabile-Levens E, Kasznica J, Schwieterman WD, Siegel JN, Braun MM. Tuberculosis associated with infliximab, a tumor necrosis factor alpha-neutralizing agent. N Engl J Med. 2001;345:1098-1104. [PubMed] |
| 162. | Ehlers S. Why does tumor necrosis factor targeted therapy reactivate tuberculosis? J Rheumatol Suppl. 2005;74:35-39. [PubMed] |
| 163. | Garcia I, Olleros ML, Quesniaux VF, Jacobs M, Allie N, Nedospasov SA, Szymkowski DE, Ryffel B. Roles of soluble and membrane TNF and related ligands in mycobacterial infections: effects of selective and non-selective TNF inhibitors during infection. Adv Exp Med Biol. 2011;691:187-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 164. | Sichletidis L, Settas L, Spyratos D, Chloros D, Patakas D. Tuberculosis in patients receiving anti-TNF agents despite chemoprophylaxis. Int J Tuberc Lung Dis. 2006;10:1127-1132. [PubMed] |
| 165. | Bruns H, Meinken C, Schauenberg P, Härter G, Kern P, Modlin RL, Antoni C, Stenger S. Anti-TNF immunotherapy reduces CD8+ T cell-mediated antimicrobial activity against Mycobacterium tuberculosis in humans. J Clin Invest. 2009;119:1167-1177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 238] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 166. | Chakravarty SD, Zhu G, Tsai MC, Mohan VP, Marino S, Kirschner DE, Huang L, Flynn J, Chan J. Tumor necrosis factor blockade in chronic murine tuberculosis enhances granulomatous inflammation and disorganizes granulomas in the lungs. Infect Immun. 2008;76:916-926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 102] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 167. | Egen JG, Rothfuchs AG, Feng CG, Winter N, Sher A, Germain RN. Macrophage and T cell dynamics during the development and disintegration of mycobacterial granulomas. Immunity. 2008;28:271-284. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 285] [Cited by in RCA: 275] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 168. | Saunders BM, Britton WJ. Life and death in the granuloma: immunopathology of tuberculosis. Immunol Cell Biol. 2007;85:103-111. [PubMed] |
| 169. | Silva Miranda M, Breiman A, Allain S, Deknuydt F, Altare F. The tuberculous granuloma: an unsuccessful host defence mechanism providing a safety shelter for the bacteria? Clin Dev Immunol. 2012;2012:139127. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 146] [Cited by in RCA: 167] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 170. | Russell DG. Mycobacterium tuberculosis and the intimate discourse of a chronic infection. Immunol Rev. 2011;240:252-268. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 232] [Cited by in RCA: 215] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 171. | Tobin DM, Roca FJ, Oh SF, McFarland R, Vickery TW, Ray JP, Ko DC, Zou Y, Bang ND, Chau TT. Host genotype-specific therapies can optimize the inflammatory response to mycobacterial infections. Cell. 2012;148:434-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 469] [Cited by in RCA: 445] [Article Influence: 34.2] [Reference Citation Analysis (0)] |
| 172. | Wallis RS, Kyambadde P, Johnson JL, Horter L, Kittle R, Pohle M, Ducar C, Millard M, Mayanja-Kizza H, Whalen C. A study of the safety, immunology, virology, and microbiology of adjunctive etanercept in HIV-1-associated tuberculosis. AIDS. 2004;18:257-264. [PubMed] |
| 173. | Bourigault ML, Vacher R, Rose S, Olleros ML, Janssens JP, Quesniaux VF, Garcia I. Tumor necrosis factor neutralization combined with chemotherapy enhances Mycobacterium tuberculosis clearance and reduces lung pathology. Am J Clin Exp Immunol. 2013;2:124-134. [PubMed] |
| 174. | Lin PL, Myers A, Smith L, Bigbee C, Bigbee M, Fuhrman C, Grieser H, Chiosea I, Voitenek NN, Capuano SV. Tumor necrosis factor neutralization results in disseminated disease in acute and latent Mycobacterium tuberculosis infection with normal granuloma structure in a cynomolgus macaque model. Arthritis Rheum. 2010;62:340-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 153] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 175. | Ciaramella A, Cavone A, Santucci MB, Garg SK, Sanarico N, Bocchino M, Galati D, Martino A, Auricchio G, D’Orazio M. Induction of apoptosis and release of interleukin-1 beta by cell wall-associated 19-kDa lipoprotein during the course of mycobacterial infection. J Infect Dis. 2004;190:1167-1176. [PubMed] |
| 176. | Kulkarni R, Deshpande A, Saxena R, Saxena K. A study of serum malondialdehyde and cytokine in tuberculosis patients. J Clin Diagn Res. 2013;7:2140-2142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 177. | Bekker LG, Maartens G, Steyn L, Kaplan G. Selective increase in plasma tumor necrosis factor-alpha and concomitant clinical deterioration after initiating therapy in patients with severe tuberculosis. J Infect Dis. 1998;178:580-584. [PubMed] |
| 178. | Kellar KL, Gehrke J, Weis SE, Mahmutovic-Mayhew A, Davila B, Zajdowicz MJ, Scarborough R, LoBue PA, Lardizabal AA, Daley CL. Multiple cytokines are released when blood from patients with tuberculosis is stimulated with Mycobacterium tuberculosis antigens. PLoS One. 2011;6:e26545. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 61] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 179. | Frahm M, Goswami ND, Owzar K, Hecker E, Mosher A, Cadogan E, Nahid P, Ferrari G, Stout JE. Discriminating between latent and active tuberculosis with multiple biomarker responses. Tuberculosis (Edinb). 2011;91:250-256. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 117] [Cited by in RCA: 114] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 180. | Lighter-Fisher J, Peng CH, Tse DB. Cytokine responses to QuantiFERON® peptides, purified protein derivative and recombinant ESAT-6 in children with tuberculosis. Int J Tuberc Lung Dis. 2010;14:1548-1555. [PubMed] |
| 181. | Biselli R, Mariotti S, Sargentini V, Sauzullo I, Lastilla M, Mengoni F, Vanini V, Girardi E, Goletti D, D’ Amelio R. Detection of interleukin-2 in addition to interferon-gamma discriminates active tuberculosis patients, latently infected individuals, and controls. Clin Microbiol Infect. 2010;16:1282-1284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 95] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 182. | Ruhwald M, Petersen J, Kofoed K, Nakaoka H, Cuevas LE, Lawson L, Squire SB, Eugen-Olsen J, Ravn P. Improving T-cell assays for the diagnosis of latent TB infection: potential of a diagnostic test based on IP-10. PLoS One. 2008;3:e2858. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 82] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 183. | Wu B, Huang C, Kato-Maeda M, Hopewell PC, Daley CL, Krensky AM, Clayberger C. Messenger RNA expression of IL-8, FOXP3, and IL-12beta differentiates latent tuberculosis infection from disease. J Immunol. 2007;178:3688-3694. [PubMed] |
| 184. | Wu B, Huang C, Kato-Maeda M, Hopewell PC, Daley CL, Krensky AM, Clayberger C. IL-9 is associated with an impaired Th1 immune response in patients with tuberculosis. Clin Immunol. 2008;126:202-210. |
| 185. | Zhang Y, Liu J, Wang Y, Xian Q, Shao L, Yang Z, Wang X. Immunotherapy using IL-2 and GM-CSF is a potential treatment for multidrug-resistant Mycobacterium tuberculosis. Sci China Life Sci. 2012;55:800-806. [PubMed] |
| 186. | Van Dyken SJ, Locksley RM. Interleukin-4- and interleukin-13-mediated alternatively activated macrophages: roles in homeostasis and disease. Annu Rev Immunol. 2013;31:317-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 425] [Cited by in RCA: 539] [Article Influence: 44.9] [Reference Citation Analysis (0)] |
| 187. | Nolan A, Fajardo E, Huie ML, Condos R, Pooran A, Dawson R, Dheda K, Bateman E, Rom WN, Weiden MD. Increased production of IL-4 and IL-12p40 from bronchoalveolar lavage cells are biomarkers of Mycobacterium tuberculosis in the sputum. PLoS One. 2013;8:e59461. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 188. | Rook GA, Hernandez-Pando R, Dheda K, Teng Seah G. IL-4 in tuberculosis: implications for vaccine design. Trends Immunol. 2004;25:483-488. [PubMed] |
| 189. | Ordway DJ, Costa L, Martins M, Silveira H, Amaral L, Arroz MJ, Ventura FA, Dockrell HM. Increased Interleukin-4 production by CD8 and gammadelta T cells in health-care workers is associated with the subsequent development of active tuberculosis. J Infect Dis. 2004;190:756-766. [PubMed] |
| 190. | Martino A, Sacchi A, Sanarico N, Spadaro F, Ramoni C, Ciaramella A, Pucillo LP, Colizzi V, Vendetti S. Dendritic cells derived from BCG-infected precursors induce Th2-like immune response. J Leukoc Biol. 2004;76:827-834. [PubMed] |
| 191. | Hussain R, Talat N, Ansari A, Shahid F, Hasan Z, Dawood G. Endogenously activated interleukin-4 differentiates disease progressors and non-progressors in tuberculosis susceptible families: a 2-year biomarkers follow-up study. J Clin Immunol. 2011;31:913-923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 192. | Morris KR, Lutz RD, Bai X, McGibney MT, Cook D, Ordway D, Chan ED. Suppression of IFNgamma+mycobacterial lipoarabinomannan-induced NO by IL-4 is due to decreased IRF-1 expression. Tuberculosis (Edinb). 2009;89:294-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 193. | Harris J, Master SS, De Haro SA, Delgado M, Roberts EA, Hope JC, Keane J, Deretic V. Th1-Th2 polarisation and autophagy in the control of intracellular mycobacteria by macrophages. Vet Immunol Immunopathol. 2009;128:37-43. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 52] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 194. | Harris J, De Haro SA, Master SS, Keane J, Roberts EA, Delgado M, Deretic V. T helper 2 cytokines inhibit autophagic control of intracellular Mycobacterium tuberculosis. Immunity. 2007;27:505-517. [PubMed] |
| 195. | Huffnagle GB, Boyd MB, Street NE, Lipscomb MF. IL-5 is required for eosinophil recruitment, crystal deposition, and mononuclear cell recruitment during a pulmonary Cryptococcus neoformans infection in genetically susceptible mice (C57BL/6). J Immunol. 1998;160:2393-2400. [PubMed] |
| 196. | Morikawa K, Oseko F, Morikawa S, Imai K, Sawada M. Recombinant human IL-5 augments immunoglobulin generation by human B lymphocytes in the presence of IL-2. Cell Immunol. 1993;149:390-401. [PubMed] |
| 197. | Diedrich CR, Mattila JT, Flynn JL. Monocyte-derived IL-5 reduces TNF production by Mycobacterium tuberculosis-specific CD4 T cells during SIV/M. tuberculosis coinfection. J Immunol. 2013;190:6320-6328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 198. | Erb KJ, Kirman J, Delahunt B, Moll H, Le Gros G. Infection of mice with Mycobacterium bovis-BCG induces both Th1 and Th2 immune responses in the absence of interferon-gamma signalling. Eur Cytokine Netw. 1999;10:147-154. [PubMed] |
| 199. | Murray PJ, Young RA, Daley GQ. Hematopoietic remodeling in interferon-gamma-deficient mice infected with mycobacteria. Blood. 1998;91:2914-2924. [PubMed] |
| 200. | Dinarello CA. Cytokines as endogenous pyrogens. J Infect Dis. 1999;179 Suppl 2:S294-S304. [PubMed] |
| 201. | Ladel CH, Blum C, Dreher A, Reifenberg K, Kopf M, Kaufmann SH. Lethal tuberculosis in interleukin-6-deficient mutant mice. Infect Immun. 1997;65:4843-4849. [PubMed] |
| 202. | Appelberg R. Protective role of interferon gamma, tumor necrosis factor alpha and interleukin-6 in Mycobacterium tuberculosis and M. avium infections. Immunobiology. 1994;191:520-525. [PubMed] |
| 203. | Dienz O, Rincon M. The effects of IL-6 on CD4 T cell responses. Clin Immunol. 2009;130:27-33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 427] [Cited by in RCA: 402] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 204. | Saunders BM, Frank AA, Orme IM, Cooper AM. Interleukin-6 induces early gamma interferon production in the infected lung but is not required for generation of specific immunity to Mycobacterium tuberculosis infection. Infect Immun. 2000;68:3322-3326. [PubMed] |
| 205. | Schindler R, Mancilla J, Endres S, Ghorbani R, Clark SC, Dinarello CA. Correlations and interactions in the production of interleukin-6 (IL-6), IL-1, and tumor necrosis factor (TNF) in human blood mononuclear cells: IL-6 suppresses IL-1 and TNF. Blood. 1990;75:40-47. [PubMed] |
| 206. | Shiratsuchi H, Johnson JL, Ellner JJ. Bidirectional effects of cytokines on the growth of Mycobacterium avium within human monocytes. J Immunol. 1991;146:3165-3170. [PubMed] |
| 207. | Nagabhushanam V, Solache A, Ting LM, Escaron CJ, Zhang JY, Ernst JD. Innate inhibition of adaptive immunity: Mycobacterium tuberculosis-induced IL-6 inhibits macrophage responses to IFN-gamma. J Immunol. 2003;171:4750-4757. [PubMed] |
| 208. | Lyadova IV, Tsiganov EN, Kapina MA, Shepelkova GS, Sosunov VV, Radaeva TV, Majorov KB, Shmitova NS, van den Ham HJ, Ganusov VV. In mice, tuberculosis progression is associated with intensive inflammatory response and the accumulation of Gr-1 cells in the lungs. PLoS One. 2010;5:e10469. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 66] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 209. | Kishimoto T, Hibi M, Murakami M, Narazaki M, Saito M, Taga T. The molecular biology of interleukin 6 and its receptor. Ciba Found Symp. 1992;167:5-16; discussion 16-23. [PubMed] |
| 210. | Barnes TC, Anderson ME, Moots RJ. The many faces of interleukin-6: the role of IL-6 in inflammation, vasculopathy, and fibrosis in systemic sclerosis. Int J Rheumatol. 2011;2011:721608. [PubMed] |
| 211. | Law K, Weiden M, Harkin T, Tchou-Wong K, Chi C, Rom WN. Increased release of interleukin-1 beta, interleukin-6, and tumor necrosis factor-alpha by bronchoalveolar cells lavaged from involved sites in pulmonary tuberculosis. Am J Respir Crit Care Med. 1996;153:799-804. [PubMed] |
| 212. | Cussigh A, Falleti E, Fabris C, Bitetto D, Cmet S, Fontanini E, Bignulin S, Fornasiere E, Fumolo E, Minisini R. Interleukin 6 promoter polymorphisms influence the outcome of chronic hepatitis C. Immunogenetics. 2011;63:33-41. [PubMed] |
| 213. | Rantala A, Lajunen T, Juvonen R, Silvennoinen-Kassinen S, Peitso A, Vainio O, Saikku P, Leinonen M. Association of IL-6 and IL-6R gene polymorphisms with susceptibility to respiratory tract infections in young Finnish men. Hum Immunol. 2011;72:63-68. [PubMed] |
| 214. | Balding J, Healy CM, Livingstone WJ, White B, Mynett-Johnson L, Cafferkey M, Smith OP. Genomic polymorphic profiles in an Irish population with meningococcaemia: is it possible to predict severity and outcome of disease? Genes Immun. 2003;4:533-540. [PubMed] |
| 215. | Ferreira MA, Matheson MC, Duffy DL, Marks GB, Hui J, Le Souëf P, Danoy P, Baltic S, Nyholt DR, Jenkins M. Identification of IL6R and chromosome 11q13.5 as risk loci for asthma. Lancet. 2011;378:1006-1014. [PubMed] |
| 216. | Lamas JR, Rodríguez-Rodríguez L, Varadé J, López-Romero P, Tornero-Esteban P, Abasolo L, Urcelay E, Fernández-Gutiérrez B. Influence of IL6R rs8192284 polymorphism status in disease activity in rheumatoid arthritis. J Rheumatol. 2010;37:1579-1581. [PubMed] |
| 217. | Zhang G, Zhou B, Wang W, Zhang M, Zhao Y, Wang Z, Yang L, Zhai J, Feng CG, Wang J. A functional single-nucleotide polymorphism in the promoter of the gene encoding interleukin 6 is associated with susceptibility to tuberculosis. J Infect Dis. 2012;205:1697-1704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 218. | Shey MS, Randhawa AK, Bowmaker M, Smith E, Scriba TJ, de Kock M, Mahomed H, Hussey G, Hawn TR, Hanekom WA. Single nucleotide polymorphisms in toll-like receptor 6 are associated with altered lipopeptide- and mycobacteria-induced interleukin-6 secretion. Genes Immun. 2010;11:561-572. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 56] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 219. | Chen X, Zhang M, Liao M, Graner MW, Wu C, Yang Q, Liu H, Zhou B. Reduced Th17 response in patients with tuberculosis correlates with IL-6R expression on CD4+ T Cells. Am J Respir Crit Care Med. 2010;181:734-742. [PubMed] |
| 220. | Nolan A, Condos R, Huie ML, Dawson R, Dheda K, Bateman E, Rom WN, Weiden MD. Elevated IP-10 and IL-6 from bronchoalveolar lavage cells are biomarkers of non-cavitary tuberculosis. Int J Tuberc Lung Dis. 2013;17:922-927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 221. | Herrera MT, Torres M, Nevels D, Perez-Redondo CN, Ellner JJ, Sada E, Schwander SK. Compartmentalized bronchoalveolar IFN-gamma and IL-12 response in human pulmonary tuberculosis. Tuberculosis (Edinb). 2009;89:38-47. [PubMed] |
| 222. | Ye ZJ, Yuan ML, Zhou Q, Du RH, Yang WB, Xiong XZ, Zhang JC, Wu C, Qin SM, Shi HZ. Differentiation and recruitment of Th9 cells stimulated by pleural mesothelial cells in human Mycobacterium tuberculosis infection. PLoS One. 2012;7:e31710. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 63] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 223. | Shevach EM. Mechanisms of foxp3+ T regulatory cell-mediated suppression. Immunity. 2009;30:636-645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1257] [Cited by in RCA: 1345] [Article Influence: 84.1] [Reference Citation Analysis (0)] |
| 224. | Guyot-Revol V, Innes JA, Hackforth S, Hinks T, Lalvani A. Regulatory T cells are expanded in blood and disease sites in patients with tuberculosis. Am J Respir Crit Care Med. 2006;173:803-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 324] [Cited by in RCA: 328] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 225. | Mege JL, Meghari S, Honstettre A, Capo C, Raoult D. The two faces of interleukin 10 in human infectious diseases. Lancet Infect Dis. 2006;6:557-569. [PubMed] |
| 226. | Redford PS, Boonstra A, Read S, Pitt J, Graham C, Stavropoulos E, Bancroft GJ, O’Garra A. Enhanced protection to Mycobacterium tuberculosis infection in IL-10-deficient mice is accompanied by early and enhanced Th1 responses in the lung. Eur J Immunol. 2010;40:2200-2210. [PubMed] |
| 227. | Vankayalapati R, Wizel B, Weis SE, Klucar P, Shams H, Samten B, Barnes PF. Serum cytokine concentrations do not parallel Mycobacterium tuberculosis-induced cytokine production in patients with tuberculosis. Clin Infect Dis. 2003;36:24-28. [PubMed] |
| 228. | Boussiotis VA, Tsai EY, Yunis EJ, Thim S, Delgado JC, Dascher CC, Berezovskaya A, Rousset D, Reynes JM, Goldfeld AE. IL-10-producing T cells suppress immune responses in anergic tuberculosis patients. J Clin Invest. 2000;105:1317-1325. [PubMed] |
| 229. | Denis M, Ghadirian E. IL-10 neutralization augments mouse resistance to systemic Mycobacterium avium infections. J Immunol. 1993;151:5425-5430. [PubMed] |
| 230. | Murray PJ, Wang L, Onufryk C, Tepper RI, Young RA. T cell-derived IL-10 antagonizes macrophage function in mycobacterial infection. J Immunol. 1997;158:315-321. [PubMed] |
| 231. | Turner J, Gonzalez-Juarrero M, Ellis DL, Basaraba RJ, Kipnis A, Orme IM, Cooper AM. In vivo IL-10 production reactivates chronic pulmonary tuberculosis in C57BL/6 mice. J Immunol. 2002;169:6343-6351. [PubMed] |
| 232. | Verreck FA, de Boer T, Langenberg DM, Hoeve MA, Kramer M, Vaisberg E, Kastelein R, Kolk A, de Waal-Malefyt R, Ottenhoff TH. Human IL-23-producing type 1 macrophages promote but IL-10-producing type 2 macrophages subvert immunity to (myco)bacteria. Proc Natl Acad Sci USA. 2004;101:4560-4565. [PubMed] |
| 233. | Tadokera R, Wilkinson KA, Meintjes GA, Skolimowska KH, Matthews K, Seldon R, Rangaka MX, Maartens G, Wilkinson RJ. Role of the interleukin 10 family of cytokines in patients with immune reconstitution inflammatory syndrome associated with HIV infection and tuberculosis. J Infect Dis. 2013;207:1148-1156. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 234. | Meintjes G, Rabie H, Wilkinson RJ, Cotton MF. Tuberculosis-associated immune reconstitution inflammatory syndrome and unmasking of tuberculosis by antiretroviral therapy. Clin Chest Med. 2009;30:797-810, x. [PubMed] |
| 235. | Feng CG, Jankovic D, Kullberg M, Cheever A, Scanga CA, Hieny S, Caspar P, Yap GS, Sher A. Maintenance of pulmonary Th1 effector function in chronic tuberculosis requires persistent IL-12 production. J Immunol. 2005;174:4185-4192. [PubMed] |
| 236. | Leepiyasakulchai C, Taher C, Chuquimia OD, Mazurek J, Söderberg-Naucler C, Fernández C, Sköld M. Infection rate and tissue localization of murine IL-12p40-producing monocyte-derived CD103(+) lung dendritic cells during pulmonary tuberculosis. PLoS One. 2013;8:e69287. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 237. | Cooper AM, Kipnis A, Turner J, Magram J, Ferrante J, Orme IM. Mice lacking bioactive IL-12 can generate protective, antigen-specific cellular responses to mycobacterial infection only if the IL-12 p40 subunit is present. J Immunol. 2002;168:1322-1327. [PubMed] |
| 238. | Filipe-Santos O, Bustamante J, Chapgier A, Vogt G, de Beaucoudrey L, Feinberg J, Jouanguy E, Boisson-Dupuis S, Fieschi C, Picard C. Inborn errors of IL-12/23- and IFN-gamma-mediated immunity: molecular, cellular, and clinical features. Semin Immunol. 2006;18:347-361. [PubMed] |
| 239. | Bermudez LE, Young LS. Natural killer cell-dependent mycobacteriostatic and mycobactericidal activity in human macrophages. J Immunol. 1991;146:265-270. [PubMed] |
| 240. | Belosevic M, Davis CE, Meltzer MS, Nacy CA. Regulation of activated macrophage antimicrobial activities. Identification of lymphokines that cooperate with IFN-gamma for induction of resistance to infection. J Immunol. 1988;141:890-896. [PubMed] |
| 241. | Kiderlen AF, Kaufmann SH, Lohmann-Matthes ML. Protection of mice against the intracellular bacterium Listeria monocytogenes by recombinant immune interferon. Eur J Immunol. 1984;14:964-967. [PubMed] |
| 242. | Murray HW, Rubin BY, Rothermel CD. Killing of intracellular Leishmania donovani by lymphokine-stimulated human mononuclear phagocytes. Evidence that interferon-gamma is the activating lymphokine. J Clin Invest. 1983;72:1506-1510. [PubMed] |
| 243. | Orme IM, Miller ES, Roberts AD, Furney SK, Griffin JP, Dobos KM, Chi D, Rivoire B, Brennan PJ. T lymphocytes mediating protection and cellular cytolysis during the course of Mycobacterium tuberculosis infection. Evidence for different kinetics and recognition of a wide spectrum of protein antigens. J Immunol. 1992;148:189-196. [PubMed] |
| 244. | Vanden Driessche K, Persson A, Marais BJ, Fink PJ, Urdahl KB. Immune vulnerability of infants to tuberculosis. Clin Dev Immunol. 2013;2013:781320. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 245. | Masood KI, Rottenberg ME, Salahuddin N, Irfan M, Rao N, Carow B, Islam M, Hussain R, Hasan Z. Expression of M. tuberculosis-induced suppressor of cytokine signaling (SOCS) 1, SOCS3, FoxP3 and secretion of IL-6 associates with differing clinical severity of tuberculosis. BMC Infect Dis. 2013;13:13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 246. | Dalton DK, Pitts-Meek S, Keshav S, Figari IS, Bradley A, Stewart TA. Multiple defects of immune cell function in mice with disrupted interferon-gamma genes. Science. 1993;259:1739-1742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1314] [Cited by in RCA: 1365] [Article Influence: 42.7] [Reference Citation Analysis (0)] |
| 247. | Kaufmann SH. Protection against tuberculosis: cytokines, T cells, and macrophages. Ann Rheum Dis. 2002;61 Suppl 2:ii54-ii58. [PubMed] |
| 248. | Qiu L, Huang D, Chen CY, Wang R, Shen L, Shen Y, Hunt R, Estep J, Haynes BF, Jacobs WR. Severe tuberculosis induces unbalanced up-regulation of gene networks and overexpression of IL-22, MIP-1alpha, CCL27, IP-10, CCR4, CCR5, CXCR3, PD1, PDL2, IL-3, IFN-beta, TIM1, and TLR2 but low antigen-specific cellular responses. J Infect Dis. 2008;198:1514-1519. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 82] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 249. | Abebe F. Is interferon-gamma the right marker for bacille Calmette-Guérin-induced immune protection? The missing link in our understanding of tuberculosis immunology. Clin Exp Immunol. 2012;169:213-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 75] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 250. | Onwubalili JK, Scott GM, Robinson JA. Deficient immune interferon production in tuberculosis. Clin Exp Immunol. 1985;59:405-413. [PubMed] |
| 251. | Geldmacher C, Ngwenyama N, Schuetz A, Petrovas C, Reither K, Heeregrave EJ, Casazza JP, Ambrozak DR, Louder M, Ampofo W. Preferential infection and depletion of Mycobacterium tuberculosis-specific CD4 T cells after HIV-1 infection. J Exp Med. 2010;207:2869-2881. [PubMed] |
| 252. | Geldmacher C, Schuetz A, Ngwenyama N, Casazza JP, Sanga E, Saathoff E, Boehme C, Geis S, Maboko L, Singh M. Early depletion of Mycobacterium tuberculosis-specific T helper 1 cell responses after HIV-1 infection. J Infect Dis. 2008;198:1590-1598. [PubMed] |
| 253. | Condos R, Rom WN, Weiden M. Lung-specific immune response in tuberculosis. Int J Tuberc Lung Dis. 2000;4:S11-S17. [PubMed] |
| 254. | Bonecini-Almeida Mda G, Werneck-Barroso E, Carvalho PB, de Moura CP, Andrade EF, Hafner A, Carvalho CE, Ho JL, Kritski AL, Morgado MG. Functional activity of alveolar and peripheral cells in patients with human acquired immunodeficiency syndrome and pulmonary tuberculosis. Cell Immunol. 1998;190:112-120. [PubMed] |
| 255. | Denis M. Interferon-gamma-treated murine macrophages inhibit growth of tubercle bacilli via the generation of reactive nitrogen intermediates. Cell Immunol. 1991;132:150-157. [PubMed] |
| 256. | Cooper AM, Dalton DK, Stewart TA, Griffin JP, Russell DG, Orme IM. Disseminated tuberculosis in interferon gamma gene-disrupted mice. J Exp Med. 1993;178:2243-2247. [PubMed] |
| 257. | Palma C, Schiavoni G, Abalsamo L, Mattei F, Piccaro G, Sanchez M, Fernandez C, Singh M, Gabriele L. Mycobacterium tuberculosis PstS1 amplifies IFN-γ and induces IL-17/IL-22 responses by unrelated memory CD4+ T cells via dendritic cell activation. Eur J Immunol. 2013;43:2386-2397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 258. | Warwick-Davies J, Dhillon J, O’Brien L, Andrew PW, Lowrie DB. Apparent killing of Mycobacterium tuberculosis by cytokine-activated human monocytes can be an artefact of a cytotoxic effect on the monocytes. Clin Exp Immunol. 1994;96:214-217. [PubMed] |
| 259. | Rook GA, Steele J, Ainsworth M, Champion BR. Activation of macrophages to inhibit proliferation of Mycobacterium tuberculosis: comparison of the effects of recombinant gamma-interferon on human monocytes and murine peritoneal macrophages. Immunology. 1986;59:333-338. [PubMed] |
| 260. | Douvas GS, Looker DL, Vatter AE, Crowle AJ. Gamma interferon activates human macrophages to become tumoricidal and leishmanicidal but enhances replication of macrophage-associated mycobacteria. Infect Immun. 1985;50:1-8. [PubMed] |
| 261. | Wong K-W, Jacobs WR, Jr . Mycobacterium tuberculosis Exploits Human Interferon γ to Stimulate Macrophage Extracellular Trap Formation and Necrosis. J Infect Dis. 2013;208:109-119. |
| 262. | Rodríguez J, Ramírez AS, Suárez MF, Soto CY. Electrochemical monitoring of the metabolic activity of mycobacteria in culture. Antonie Van Leeuwenhoek. 2012;102:193-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 263. | Wynn TA. IL-13 effector functions. Annu Rev Immunol. 2003;21:425-456. [PubMed] |
| 264. | Ameixa C, Friedland JS. Down-regulation of interleukin-8 secretion from Mycobacterium tuberculosis-infected monocytes by interleukin-4 and -10 but not by interleukin-13. Infect Immun. 2001;69:2470-2476. [PubMed] |
| 265. | Keegan AD, Johnston JA, Tortolani PJ, McReynolds LJ, Kinzer C, O’Shea JJ, Paul WE. Similarities and differences in signal transduction by interleukin 4 and interleukin 13: analysis of Janus kinase activation. Proc Natl Acad Sci USA. 1995;92:7681-7685. [PubMed] |
| 266. | Wang LM, Keegan AD, Paul WE, Heidaran MA, Gutkind JS, Pierce JH. IL-4 activates a distinct signal transduction cascade from IL-3 in factor-dependent myeloid cells. EMBO J. 1992;11:4899-4908. [PubMed] |
| 267. | Welham MJ, Learmonth L, Bone H, Schrader JW. Interleukin-13 signal transduction in lymphohemopoietic cells. Similarities and differences in signal transduction with interleukin-4 and insulin. J Biol Chem. 1995;270:12286-12296. [PubMed] |
| 268. | Mijatovic T, Kruys V, Caput D, Defrance P, Huez G. Interleukin-4 and -13 inhibit tumor necrosis factor-alpha mRNA translational activation in lipopolysaccharide-induced mouse macrophages. J Biol Chem. 1997;272:14394-14398. [PubMed] |
| 269. | van Beelen AJ, Zelinkova Z, Taanman-Kueter EW, Muller FJ, Hommes DW, Zaat SA, Kapsenberg ML, de Jong EC. Stimulation of the intracellular bacterial sensor NOD2 programs dendritic cells to promote interleukin-17 production in human memory T cells. Immunity. 2007;27:660-669. [PubMed] |
| 270. | Volpe E, Servant N, Zollinger R, Bogiatzi SI, Hupé P, Barillot E, Soumelis V. A critical function for transforming growth factor-beta, interleukin 23 and proinflammatory cytokines in driving and modulating human T(H)-17 responses. Nat Immunol. 2008;9:650-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 697] [Cited by in RCA: 756] [Article Influence: 44.5] [Reference Citation Analysis (0)] |
| 271. | Wilson NJ, Boniface K, Chan JR, McKenzie BS, Blumenschein WM, Mattson JD, Basham B, Smith K, Chen T, Morel F. Development, cytokine profile and function of human interleukin 17-producing helper T cells. Nat Immunol. 2007;8:950-957. [PubMed] |
| 272. | Acosta-Rodriguez EV, Napolitani G, Lanzavecchia A, Sallusto F. Interleukins 1beta and 6 but not transforming growth factor-beta are essential for the differentiation of interleukin 17-producing human T helper cells. Nat Immunol. 2007;8:942-949. [PubMed] |
| 273. | Ling WL, Wang LJ, Pong JC, Lau AS, Li JC. A role for interleukin-17A in modulating intracellular survival of Mycobacterium bovis bacillus Calmette-Guérin in murine macrophages. Immunology. 2013;140:323-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 274. | Torrado E, Cooper AM. IL-17 and Th17 cells in tuberculosis. Cytokine Growth Factor Rev. 2010;21:455-462. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 255] [Cited by in RCA: 244] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 275. | Perreau M, Rozot V, Welles HC, Belluti-Enders F, Vigano S, Maillard M, Dorta G, Mazza-Stalder J, Bart PA, Roger T. Lack of Mycobacterium tuberculosis-specific interleukin-17A-producing CD4+ T cells in active disease. Eur J Immunol. 2013;43:939-948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 276. | Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu Rev Immunol. 2009;27:485-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3754] [Cited by in RCA: 3787] [Article Influence: 236.7] [Reference Citation Analysis (0)] |
| 277. | Miossec P, Kolls JK. Targeting IL-17 and TH17 cells in chronic inflammation. Nat Rev Drug Discov. 2012;11:763-776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 857] [Cited by in RCA: 1085] [Article Influence: 83.5] [Reference Citation Analysis (0)] |
| 278. | Ouyang W, Kolls JK, Zheng Y. The biological functions of T helper 17 cell effector cytokines in inflammation. Immunity. 2008;28:454-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1353] [Cited by in RCA: 1338] [Article Influence: 78.7] [Reference Citation Analysis (0)] |
| 279. | Laan M, Cui ZH, Hoshino H, Lötvall J, Sjöstrand M, Gruenert DC, Skoogh BE, Lindén A. Neutrophil recruitment by human IL-17 via C-X-C chemokine release in the airways. J Immunol. 1999;162:2347-2352. [PubMed] |
| 280. | Ye P, Rodriguez FH, Kanaly S, Stocking KL, Schurr J, Schwarzenberger P, Oliver P, Huang W, Zhang P, Zhang J. Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense. J Exp Med. 2001;194:519-527. [PubMed] |
| 281. | Li L, Huang L, Vergis AL, Ye H, Bajwa A, Narayan V, Strieter RM, Rosin DL, Okusa MD. IL-17 produced by neutrophils regulates IFN-gamma-mediated neutrophil migration in mouse kidney ischemia-reperfusion injury. J Clin Invest. 2010;120:331-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 366] [Cited by in RCA: 398] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 282. | Michel ML, Keller AC, Paget C, Fujio M, Trottein F, Savage PB, Wong CH, Schneider E, Dy M, Leite-de-Moraes MC. Identification of an IL-17-producing NK1.1(neg) iNKT cell population involved in airway neutrophilia. J Exp Med. 2007;204:995-1001. [PubMed] |
| 283. | Passos ST, Silver JS, O’Hara AC, Sehy D, Stumhofer JS, Hunter CA. IL-6 promotes NK cell production of IL-17 during toxoplasmosis. J Immunol. 2010;184:1776-1783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 174] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 284. | Cella M, Fuchs A, Vermi W, Facchetti F, Otero K, Lennerz JK, Doherty JM, Mills JC, Colonna M. A human natural killer cell subset provides an innate source of IL-22 for mucosal immunity. Nature. 2009;457:722-725. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1031] [Cited by in RCA: 1050] [Article Influence: 65.6] [Reference Citation Analysis (0)] |
| 285. | Sutton CE, Lalor SJ, Sweeney CM, Brereton CF, Lavelle EC, Mills KH. Interleukin-1 and IL-23 induce innate IL-17 production from gammadelta T cells, amplifying Th17 responses and autoimmunity. Immunity. 2009;31:331-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1240] [Cited by in RCA: 1280] [Article Influence: 80.0] [Reference Citation Analysis (0)] |
| 286. | Ito Y, Usui T, Kobayashi S, Iguchi-Hashimoto M, Ito H, Yoshitomi H, Nakamura T, Shimizu M, Kawabata D, Yukawa N. Gamma/delta T cells are the predominant source of interleukin-17 in affected joints in collagen-induced arthritis, but not in rheumatoid arthritis. Arthritis Rheum. 2009;60:2294-2303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 142] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 287. | Lockhart E, Green AM, Flynn JL. IL-17 production is dominated by gammadelta T cells rather than CD4 T cells during Mycobacterium tuberculosis infection. J Immunol. 2006;177:4662-4669. [PubMed] |
| 288. | Cua DJ, Tato CM. Innate IL-17-producing cells: the sentinels of the immune system. Nat Rev Immunol. 2010;10:479-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1091] [Cited by in RCA: 1243] [Article Influence: 82.9] [Reference Citation Analysis (0)] |
| 289. | Gopal R, Lin Y, Obermajer N, Slight S, Nuthalapati N, Ahmed M, Kalinski P, Khader SA. IL-23-dependent IL-17 drives Th1-cell responses following Mycobacterium bovis BCG vaccination. Eur J Immunol. 2012;42:364-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 140] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 290. | Khader SA, Bell GK, Pearl JE, Fountain JJ, Rangel-Moreno J, Cilley GE, Shen F, Eaton SM, Gaffen SL, Swain SL. IL-23 and IL-17 in the establishment of protective pulmonary CD4+ T cell responses after vaccination and during Mycobacterium tuberculosis challenge. Nat Immunol. 2007;8:369-377. [PubMed] |
| 291. | Desvignes L, Ernst JD. Interferon-gamma-responsive nonhematopoietic cells regulate the immune response to Mycobacterium tuberculosis. Immunity. 2009;31:974-985. [PubMed] |
| 292. | Diveu C, McGeachy MJ, Cua DJ. Cytokines that regulate autoimmunity. Curr Opin Immunol. 2008;20:663-668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 71] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 293. | Jovanovic DV, Di Battista JA, Martel-Pelletier J, Jolicoeur FC, He Y, Zhang M, Mineau F, Pelletier JP. IL-17 stimulates the production and expression of proinflammatory cytokines, IL-beta and TNF-alpha, by human macrophages. J Immunol. 1998;160:3513-3521. [PubMed] |
| 294. | Eddens T, Kolls JK. Host defenses against bacterial lower respiratory tract infection. Curr Opin Immunol. 2012;24:424-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 77] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 295. | Cruz A, Fraga AG, Fountain JJ, Rangel-Moreno J, Torrado E, Saraiva M, Pereira DR, Randall TD, Pedrosa J, Cooper AM. Pathological role of interleukin 17 in mice subjected to repeated BCG vaccination after infection with Mycobacterium tuberculosis. J Exp Med. 2010;207:1609-1616. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 191] [Cited by in RCA: 205] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 296. | Hamilton T, Li X, Novotny M, Pavicic PG, Datta S, Zhao C, Hartupee J, Sun D. Cell type- and stimulus-specific mechanisms for post-transcriptional control of neutrophil chemokine gene expression. J Leukoc Biol. 2012;91:377-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 297. | Ye ZJ, Zhou Q, Du RH, Li X, Huang B, Shi HZ. Imbalance of Th17 cells and regulatory T cells in tuberculous pleural effusion. Clin Vaccine Immunol. 2011;18:1608-1615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 298. | Kohno K, Kataoka J, Ohtsuki T, Suemoto Y, Okamoto I, Usui M, Ikeda M, Kurimoto M. IFN-gamma-inducing factor (IGIF) is a costimulatory factor on the activation of Th1 but not Th2 cells and exerts its effect independently of IL-12. J Immunol. 1997;158:1541-1550. [PubMed] |
| 299. | Matsui K, Yoshimoto T, Tsutsui H, Hyodo Y, Hayashi N, Hiroishi K, Kawada N, Okamura H, Nakanishi K, Higashino K. Propionibacterium acnes treatment diminishes CD4+ NK1.1+ T cells but induces type I T cells in the liver by induction of IL-12 and IL-18 production from Kupffer cells. J Immunol. 1997;159:97-106. [PubMed] |
| 300. | Okamura H, Tsutsi H, Komatsu T, Yutsudo M, Hakura A, Tanimoto T, Torigoe K, Okura T, Nukada Y, Hattori K. Cloning of a new cytokine that induces IFN-gamma production by T cells. Nature. 1995;378:88-91. [PubMed] |
| 301. | Schneider BE, Korbel D, Hagens K, Koch M, Raupach B, Enders J, Kaufmann SH, Mittrücker HW, Schaible UE. A role for IL-18 in protective immunity against Mycobacterium tuberculosis. Eur J Immunol. 2010;40:396-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 87] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 302. | Kaneko H, Yamada H, Mizuno S, Udagawa T, Kazumi Y, Sekikawa K, Sugawara I. Role of tumor necrosis factor-alpha in Mycobacterium-induced granuloma formation in tumor necrosis factor-alpha-deficient mice. Lab Invest. 1999;79:379-386. [PubMed] |
| 303. | Cooper AM, Magram J, Ferrante J, Orme IM. Interleukin 12 (IL-12) is crucial to the development of protective immunity in mice intravenously infected with mycobacterium tuberculosis. J Exp Med. 1997;186:39-45. [PubMed] |
| 304. | Wolk K, Sabat R. Interleukin-22: a novel T- and NK-cell derived cytokine that regulates the biology of tissue cells. Cytokine Growth Factor Rev. 2006;17:367-380. [PubMed] |
| 305. | Ouyang W, Rutz S, Crellin NK, Valdez PA, Hymowitz SG. Regulation and functions of the IL-10 family of cytokines in inflammation and disease. Annu Rev Immunol. 2011;29:71-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1100] [Cited by in RCA: 1417] [Article Influence: 101.2] [Reference Citation Analysis (0)] |
| 306. | Khader SA, Pearl JE, Sakamoto K, Gilmartin L, Bell GK, Jelley-Gibbs DM, Ghilardi N, deSauvage F, Cooper AM. IL-23 compensates for the absence of IL-12p70 and is essential for the IL-17 response during tuberculosis but is dispensable for protection and antigen-specific IFN-gamma responses if IL-12p70 is available. J Immunol. 2005;175:788-795. [PubMed] |
| 307. | Liang SC, Tan XY, Luxenberg DP, Karim R, Dunussi-Joannopoulos K, Collins M, Fouser LA. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J Exp Med. 2006;203:2271-2279. [PubMed] |
| 308. | Eyerich S, Eyerich K, Pennino D, Carbone T, Nasorri F, Pallotta S, Cianfarani F, Odorisio T, Traidl-Hoffmann C, Behrendt H. Th22 cells represent a distinct human T cell subset involved in epidermal immunity and remodeling. J Clin Invest. 2009;119:3573-3585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 439] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 309. | Zenewicz LA, Flavell RA. Recent advances in IL-22 biology. Int Immunol. 2011;23:159-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 258] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 310. | Dhiman R, Indramohan M, Barnes PF, Nayak RC, Paidipally P, Rao LV, Vankayalapati R. IL-22 produced by human NK cells inhibits growth of Mycobacterium tuberculosis by enhancing phagolysosomal fusion. J Immunol. 2009;183:6639-6645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 140] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 311. | Zeng G, Chen CY, Huang D, Yao S, Wang RC, Chen ZW. Membrane-bound IL-22 after de novo production in tuberculosis and anti-Mycobacterium tuberculosis effector function of IL-22+ CD4+ T cells. J Immunol. 2011;187:190-199. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 60] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 312. | Matthews K, Wilkinson KA, Kalsdorf B, Roberts T, Diacon A, Walzl G, Wolske J, Ntsekhe M, Syed F, Russell J. Predominance of interleukin-22 over interleukin-17 at the site of disease in human tuberculosis. Tuberculosis (Edinb). 2011;91:587-593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 64] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 313. | Scriba TJ, Kalsdorf B, Abrahams DA, Isaacs F, Hofmeister J, Black G, Hassan HY, Wilkinson RJ, Walzl G, Gelderbloem SJ. Distinct, specific IL-17- and IL-22-producing CD4+ T cell subsets contribute to the human anti-mycobacterial immune response. J Immunol. 2008;180:1962-1970. [PubMed] |
| 314. | Yao S, Huang D, Chen CY, Halliday L, Zeng G, Wang RC, Chen ZW. Differentiation, distribution and gammadelta T cell-driven regulation of IL-22-producing T cells in tuberculosis. PLoS Pathog. 2010;6:e1000789. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 67] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 315. | Wilson MS, Feng CG, Barber DL, Yarovinsky F, Cheever AW, Sher A, Grigg M, Collins M, Fouser L, Wynn TA. Redundant and pathogenic roles for IL-22 in mycobacterial, protozoan, and helminth infections. J Immunol. 2010;184:4378-4390. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 115] [Cited by in RCA: 108] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 316. | Hunter CA. New IL-12-family members: IL-23 and IL-27, cytokines with divergent functions. Nat Rev Immunol. 2005;5:521-531. [PubMed] |
| 317. | Kikly K, Liu L, Na S, Sedgwick JD. The IL-23/Th(17) axis: therapeutic targets for autoimmune inflammation. Curr Opin Immunol. 2006;18:670-675. [PubMed] |
| 318. | Khader SA, Guglani L, Rangel-Moreno J, Gopal R, Junecko BA, Fountain JJ, Martino C, Pearl JE, Tighe M, Lin YY. IL-23 is required for long-term control of Mycobacterium tuberculosis and B cell follicle formation in the infected lung. J Immunol. 2011;187:5402-5407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 147] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 319. | Khader SA, Rangel-Moreno J, Fountain JJ, Martino CA, Reiley WW, Pearl JE, Winslow GM, Woodland DL, Randall TD, Cooper AM. In a murine tuberculosis model, the absence of homeostatic chemokines delays granuloma formation and protective immunity. J Immunol. 2009;183:8004-8014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 96] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 320. | Ben-Selma W, Boukadida J. IL23R(Arg381Gln) functional polymorphism is associated with active pulmonary tuberculosis severity. Clin Vaccine Immunol. 2012;19:1188-1192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 321. | Batten M, Li J, Yi S, Kljavin NM, Danilenko DM, Lucas S, Lee J, de Sauvage FJ, Ghilardi N. Interleukin 27 limits autoimmune encephalomyelitis by suppressing the development of interleukin 17-producing T cells. Nat Immunol. 2006;7:929-936. [PubMed] |
| 322. | Stumhofer JS, Laurence A, Wilson EH, Huang E, Tato CM, Johnson LM, Villarino AV, Huang Q, Yoshimura A, Sehy D. Interleukin 27 negatively regulates the development of interleukin 17-producing T helper cells during chronic inflammation of the central nervous system. Nat Immunol. 2006;7:937-945. [PubMed] |
| 323. | Villarino A, Hibbert L, Lieberman L, Wilson E, Mak T, Yoshida H, Kastelein RA, Saris C, Hunter CA. The IL-27R (WSX-1) is required to suppress T cell hyperactivity during infection. Immunity. 2003;19:645-655. [PubMed] |
| 324. | Pearl JE, Khader SA, Solache A, Gilmartin L, Ghilardi N, deSauvage F, Cooper AM. IL-27 signaling compromises control of bacterial growth in mycobacteria-infected mice. J Immunol. 2004;173:7490-7496. [PubMed] |
| 325. | Hölscher C, Hölscher A, Rückerl D, Yoshimoto T, Yoshida H, Mak T, Saris C, Ehlers S. The IL-27 receptor chain WSX-1 differentially regulates antibacterial immunity and survival during experimental tuberculosis. J Immunol. 2005;174:3534-3544. [PubMed] |
| 326. | Hunter CA, Kastelein R. Interleukin-27: balancing protective and pathological immunity. Immunity. 2012;37:960-969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 209] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 327. | Antony VB. Immunological mechanisms in pleural disease. Eur Respir J. 2003;21:539-544. [PubMed] |
| 328. | Olobo JO, Geletu M, Demissie A, Eguale T, Hiwot K, Aderaye G, Britton S. Circulating TNF-alpha, TGF-beta, and IL-10 in tuberculosis patients and healthy contacts. Scand J Immunol. 2001;53:85-91. [PubMed] |
| 329. | Ceyhan BB, Demiralp E, Karakurt ZL, Karakurt S, Sungur M. Transforming growth factor beta-1 level in pleural effusion. Respirology. 2003;8:321-325. [PubMed] |
| 330. | Marino S, Myers A, Flynn JL, Kirschner DE. TNF and IL-10 are major factors in modulation of the phagocytic cell environment in lung and lymph node in tuberculosis: a next-generation two-compartmental model. J Theor Biol. 2010;265:586-598. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 69] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 331. | Antonangelo L, Vargas FS, Puka J, Seiscento M, Acencio MM, Teixeira LR, Terra RM, Sales RK. Pleural tuberculosis: is radiological evidence of pulmonary-associated disease related to the exacerbation of the inflammatory response? Clinics (Sao Paulo). 2012;67:1259-1263. [PubMed] |
| 332. | Pavan Kumar N, Anuradha R, Andrade BB, Suresh N, Ganesh R, Shankar J, Kumaraswami V, Nutman TB, Babu S. Circulating biomarkers of pulmonary and extrapulmonary tuberculosis in children. Clin Vaccine Immunol. 2013;20:704-711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 55] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 333. | Szeliga J, Daniel DS, Yang CH, Sever-Chroneos Z, Jagannath C, Chroneos ZC. Granulocyte-macrophage colony stimulating factor-mediated innate responses in tuberculosis. Tuberculosis (Edinb). 2008;88:7-20. [PubMed] |
| 334. | Greenhill SR, Kotton DN. Pulmonary alveolar proteinosis: a bench-to-bedside story of granulocyte-macrophage colony-stimulating factor dysfunction. Chest. 2009;136:571-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 43] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 335. | Rothchild AC, Jayaraman P, Nunes-Alves C, Behar SM. iNKT cell production of GM-CSF controls Mycobacterium tuberculosis. PLoS Pathog. 2014;10:e1003805. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 99] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 336. | Francisco-Cruz A, Mata-Espinosa D, Estrada-Parra S, Xing Z, Hernández-Pando R. Immunotherapeutic effects of recombinant adenovirus encoding granulocyte-macrophage colony-stimulating factor in experimental pulmonary tuberculosis. Clin Exp Immunol. 2013;171:283-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 337. | Yoshie O, Imai T, Nomiyama H. Chemokines in immunity. Adv Immunol. 2001;78:57-110. [PubMed] |
| 338. | Zlotnik A, Yoshie O. Chemokines: a new classification system and their role in immunity. Immunity. 2000;12:121-127. [PubMed] |
| 339. | Zhang Y, Broser M, Cohen H, Bodkin M, Law K, Reibman J, Rom WN. Enhanced interleukin-8 release and gene expression in macrophages after exposure to Mycobacterium tuberculosis and its components. J Clin Invest. 1995;95:586-592. [PubMed] |
| 340. | Gerszten RE, Garcia-Zepeda EA, Lim YC, Yoshida M, Ding HA, Gimbrone MA, Luster AD, Luscinskas FW, Rosenzweig A. MCP-1 and IL-8 trigger firm adhesion of monocytes to vascular endothelium under flow conditions. Nature. 1999;398:718-723. [PubMed] |
| 341. | Meddows-Taylor S, Martin DJ, Tiemessen CT. Dysregulated production of interleukin-8 in individuals infected with human immunodeficiency virus type 1 and Mycobacterium tuberculosis. Infect Immun. 1999;67:1251-1260. [PubMed] |
| 342. | Pokkali S, Das SD. Augmented chemokine levels and chemokine receptor expression on immune cells during pulmonary tuberculosis. Hum Immunol. 2009;70:110-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 42] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 343. | Nibbering PH, Pos O, Stevenhagen A, Van Furth R. Interleukin-8 enhances nonoxidative intracellular killing of Mycobacterium fortuitum by human granulocytes. Infect Immun. 1993;61:3111-3116. [PubMed] |
| 344. | McCarter YS, Robinson A. Quality evaluation of sputum specimens for mycobacterial culture. Am J Clin Pathol. 1996;105:769-773. [PubMed] |
| 345. | Ribeiro-Rodrigues R, Resende Co T, Johnson JL, Ribeiro F, Palaci M, Sá RT, Maciel EL, Pereira Lima FE, Dettoni V, Toossi Z. Sputum cytokine levels in patients with pulmonary tuberculosis as early markers of mycobacterial clearance. Clin Diagn Lab Immunol. 2002;9:818-823. [PubMed] |
| 346. | Andersson M, Lutay N, Hallgren O, Westergren-Thorsson G, Svensson M, Godaly G. Mycobacterium bovis bacilli Calmette-Guerin regulates leukocyte recruitment by modulating alveolar inflammatory responses. Innate Immun. 2012;18:531-540. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 347. | Ferrara G, Bleck B, Richeldi L, Reibman J, Fabbri LM, Rom WN, Condos R. Mycobacterium tuberculosis induces CCL18 expression in human macrophages. Scand J Immunol. 2008;68:668-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 348. | Hasan Z, Jamil B, Ashraf M, Islam M, Yusuf MS, Khan JA, Hussain R. ESAT6-induced IFNgamma and CXCL9 can differentiate severity of tuberculosis. PLoS One. 2009;4:e5158. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 25] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 349. | Rivero-Lezcano OM, González-Cortés C, Reyes-Ruvalcaba D, Diez-Tascón C. CCL20 is overexpressed in Mycobacterium tuberculosis-infected monocytes and inhibits the production of reactive oxygen species (ROS). Clin Exp Immunol. 2010;162:289-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 350. | Wu C, Zhou Q, Qin XJ, Qin SM, Shi HZ. CCL22 is involved in the recruitment of CD4+CD25 high T cells into tuberculous pleural effusions. Respirology. 2010;15:522-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 351. | Slight SR, Khader SA. Chemokines shape the immune responses to tuberculosis. Cytokine Growth Factor Rev. 2013;24:105-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 61] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 352. | Mihret A, Abebe M, Bekele Y, Aseffa A, Walzl G, Howe R. Impact of HIV co-infection on plasma level of cytokines and chemokines of pulmonary tuberculosis patients. BMC Infect Dis. 2014;14:125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |