Peer-review started: July 1, 2014
First decision: September 28, 2014
Revised: October 23, 2014
Accepted: November 17, 2014
Article in press: November 19, 2014
Published online: March 27, 2015
Processing time: 273 Days and 12.6 Hours
Immune regulation of aggressive tumor growth is often outpaced by tumor up-regulation of ligands that inhibit effector immune responses through the activation of immune checkpoints. A few of such checkpoints include programmed death-1 (PD-1), cytotoxic T lymphocyte associated antigen-4 (CTLA-4), lymphocyte activation gene-3, T-cell immunoglobulin and mucin protein-3, Glucocorticoid-induced TNFR family-related receptor (GITR), and killer cell immunoglobulin like receptor. With the exception of GITR, after binding to their respective ligands these checkpoints induce down-modulation of immune responses to prevent autoimmunity. However, such immune mechanisms are co-opted by tumors to allow rapid tumor cell proliferation. Pre-clinical studies in antibody blockade of PD-1 and CTLA-4 have led to promising augmentation of effector immune responses in murine tumor models, and human antibodies against PD-1 and CTLA-4 alone or in combination have demonstrated tumor regression in clinical trials. The development of immune checkpoint blockade as a potential future immunotherapy has led to increasing interest in combining treatment modalities. Combination checkpoint blockade with chemotherapy and radiation therapy has shown synergistic effects in pre-clinical and clinical studies, and combination checkpoint blockade with bacterial vaccine vectors have produced increased effector immune responses in pre-clinical models. The future of immune checkpoint blockade may be as a powerful adjuvant alongside the current standard of care.
Core tip: Aggressive cancer growth is often characterized by tumor expression of molecules that co-opt effective immune responses through immune checkpoints. Clinical blockade of checkpoints programmed death-1 and cytotoxic T lymphocyte associated antigen-4 and has spurred the discovery of a number of immune checkpoints that may be inhibited in anticancer therapy. The clinical successes of checkpoint blockade have led to increasing interest in combining treatment modalities. Combination checkpoint blockade with chemoradiation has shown synergistic effects, and checkpoint blockade with bacterial vaccine vectors have produced increased immune responses in pre-clinical models. The future of immune checkpoint blockade may be as a powerful adjuvant alongside the current standard of care.
- Citation: Patel MA, Kim JE, Ruzevick J, Lim M. Present and future of immune checkpoint blockade: Monotherapy to adjuvant approaches. World J Immunol 2015; 5(1): 1-15
- URL: https://www.wjgnet.com/2219-2824/full/v5/i1/1.htm
- DOI: https://dx.doi.org/10.5411/wji.v5.i1.1
Aggressive tumors develop molecular mechanisms to survive in harsh host environments and proliferate in regions of chronic inflammation[1]. Tumor release of factors that promote angiogenesis and disrupt nearby tissue architecture promotes invasion and metastatic spread. The central role of the immune system is to destroy foreign antigens, including those expressed as a result of aberrant cell growth[2]. Proliferating cancer cells may go immunologically unnoticed by the systemic immune response because of tumor cell expression of molecules that inhibit immune effector cells or deplete the tumor microenvironment of key factors for immune cell survival[3,4]. While initial tumor growth is detected and destroyed by robust innate and adaptive immune responses, dormant tumor cells are left behind that are in equilibrium with effector cells of the adaptive immune response[4-7]. Tumor cells will up-regulate ligands and release soluble factors that will inhibit cytotoxic T-lymphocyte activity, and tumor expression of such molecules results in cancer escape from the immune response and proliferation of tumor cells[3,4]. This process of immune-mediated editing of tumor cells, equilibrium of tumor growth maintained by antigen-specific immune responses, and ultimate escape of the tumor from immune surveillance is termed the “Three E’s” of cancer immunoediting[3,7]. Recent advances in immunotherapy have sought to intervene in this process of tumor immune escape[8].
Immune checkpoints are cell surface receptors expressed on a variety of immune cells that, under normal circumstances, prevent peripheral autoimmunity during inflammatory responses[9]. Poorly immunogenic cancer cells can express the ligands of such immune checkpoints, resulting in immune effector inhibition in the tumor microenvironment. Blockade of such immune checkpoints attempts to block the inhibitory interaction between tumor cells and T-cells and promote tumor-specific T-cell activation. This review will discuss the cellular pathways of immune checkpoints, pre-clinical and clinical studies in checkpoint blockade, and the potential future of immune checkpoint inhibition as an adjuvant to chemotherapy, radiation therapy, and bacterial vaccine vectors.
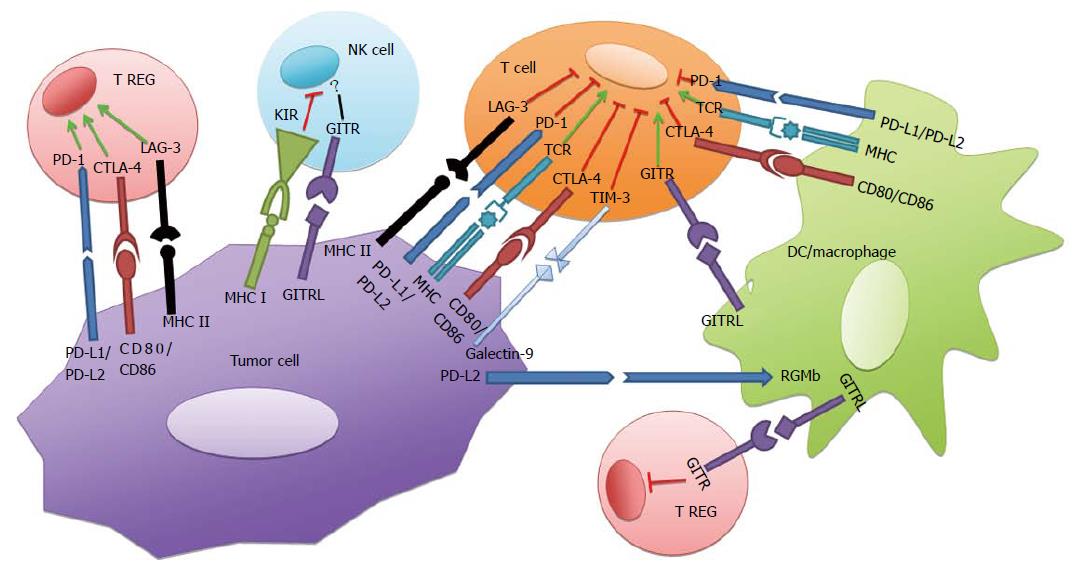
While immune checkpoints are known to down-regulate immune effector cells, studies suggest that each checkpoint induces immune inactivation through discrete cellular mechanisms[10-13]. Immune checkpoint receptor-ligand interactions are not limited to T-lymphocytes and tumors, however. Checkpoint receptors are expressed on a variety of immune cells, including natural killer (NK) cells and cells derived from the myeloid lineage. Checkpoint signaling in these cells is implicated in infection, prevention of autoimmunity, and tumor immune evasion. The following sections will focus on immune checkpoints in T-lymphocytes and their involvement in tumor-lymphocyte interactions. Checkpoints programmed death-1 (PD-1), cytotoxic T-lymphocyte antigen-4 (CTLA-4), lymphocyte activation gene-3 (LAG-3), and T-cell immunoglobulin and mucin protein-3 (TIM-3) are a few of a number of down-regulators of T-cell function.
PD-1 is a transmembrane protein that transmits inhibitory signals upon engagement with its ligands programmed death ligand-1 and -2 (PD-L1, PD-L2) and is responsible for down-regulating T-cell activation[3,11,12,14]. PD-1 is largely expressed in activated T-cells in the periphery and prevents destruction of host tissues expressing PD-L1 during inflammatory responses[15]. In addition to host epithelial cells, PD-L1 is constitutively expressed in myeloid cells, professional antigen presenting cells (APCs), lymphoid cells, and cancer cells, while PD-L2 expression is inducible and largely limited to APCs[12,16,17]. Notably, PD-L2 also binds to repulsive guidance molecule b, and this interaction is responsible for respiratory tolerance[18]. Cell signaling through PD-1 plays a significant role in preventing autoimmunity, as evidenced by the development of lupus-like glomerulonephritis and arthritis, cardiomyopathy, as well as increased levels of IgG2b, IgG3 and IgA in PD-1-/- knockout mice[19-21]. The structure of PD-1 includes the cytoplasmic structural motifs immunoreceptor tyrosine-based inhibitor motif and immunoreceptor tyrosine-based switch motif (ITSM); upon ligand binding to PD-1, ITSM recruits phosphatases SHP-1 and SHP-2 which are capable of inhibiting downstream kinases that in turn inhibit T-cell proliferation, cytokine release, and cytotoxic function[4-7,22] (Figure 1). In addition, PD-1 ligation results in mitigated phosphorylation of the ZAP70/CD3ζ signalosome, resulting in downregulation of the T-cell receptor signaling pathway and subsequent T-cell activation[1,4,23]. Finally, PD-1 has been shown to downmodulate IFN-γ production as evidenced by relatively increased IFN-γ levels after PD-1/PD-L blockade[2-4,20]. IFN-γ is a marker of the Th1 phenotype as well the activation of effector CD8+ T-cells, and decreased IFN-γ production may reflect a depressed immune response.
Similar to PD-1, CTLA-4 downregulates T-cell effector function, although the action of CTLA-4 is manifested largely in naïve, resting T-cells and occurs by a pathway distinct from PD-1[3,4,24]. CTLA-4 is a co-inhibitory receptor, competing with the co-stimulatory receptor CD28 for their shared ligands B7-1 (CD80) and B7-2 (CD86)[4-7,25] (Figure 1). By sequestering CD80/CD86, CTLA-4 is capable of inhibiting T-cell receptor (TCR) function while preventing CD28 from binding to its ligands and promoting TCR-mediated T-cell activation. Importantly, CTLA-4 blockade was the first of the immune checkpoints to exhibit anti-tumor immunity in vivo, which provided evidence for the activity of CTLA-4 in promoting tumorigenesis through cancer immune escape[3,5-7,26,27]. The purpose of CTLA-4 is to check T-cell activation by attenuating T-helper cell activity and up-regulating the immunosuppressive activity of Tregs. Tregs are CD4+ lymphocytes that down-regulate immune effector function, typically to prevent autoimmunity. In the tumor microenvironment, cancer cells may up-regulate Treg activity through ligand-receptor interactions, leading to the inactivation of effector T-cell responses. The critical role of CTLA-4 in T-cell homeostasis is demonstrated by significant lymphocyte-mediated multi-organ destruction in CTLA-4-/- knockout mice, of a magnitude more severe than in PD-1 deficient mice and occurring earlier in life[3,9,24,28,29]. While the signaling pathway by which CTLA-4 inhibits TCR is still unclear, studies show that the cytoplasmic domain of CTLA-4 activates SHP-2 and phosphatase PP2A, resulting in inhibition of kinases ZAP-70, LCK, and FYN that are specific to the TCR and mediate T-cell activation[9-13,30,31].
Responsible for down-regulating effector T-cell responses by promoting Treg-mediated immune suppression, LAG-3 is a CD4 receptor homologue of the Ig superfamily expressed in activated NK and T-cells (Figure 1)[3,10-14,32]. Like CD4, LAG-3 interacts with the major histocompatibility complex class II (MHCII) but is not responsible for MHCII-restriction in CD4-independent lymphocytes[3,11,12,14,15,33]. Instead, LAG-3 regulates antigen-specific lymphocyte responses through its activity in CD4+CD25+ regulatory T cells[12,15-17,34]. Increased lymphocytic expansion was observed in LAG-3 deficient T-cells stimulated with staphylococcal enterotoxin B (SEB), and LAG-3 deficient T-cells produced higher levels of IFN-γ and IL-2 after SEB stimulation[12,16,17,19-21,35]. As a result of its involvement in Treg function, LAG-3 promotes self-antigen tolerance and prevents autoimmunity. This is evidenced by the finding that LAG-3-deficient non-obese diabetic (NOD) mice develop an accelerated insulitis with extensive CD4+ and CD8+ pancreatic infiltration, suggested a role for LAG-3 in the regulation of activated CD4+ and CD8+ lymphocyte responses[19-21,32,36,37]. Unlike in PD-1 and CTLA-4 deficient mice, LAG-3 deficiency does not produce severe or obvious autoimmunity, and while the LAG-3 signaling pathway is still unclear, it does not appear that there is major overlap between the LAG-3 and PD-1 pathways[4-7,22,38]. Importantly, LAG-3 blockade combined with vaccines has shown increased antitumor immunity mediated by activated CD8+ T-cells. LAG-3 inhibition of influenza hemagglutinin (HA)-specific CD8+ T-cells combined with recombinant vaccinia virus expressing wild-type HA protein in a mouse model expressing influenza HA as self antigen and modified as tumor antigen resulted in increased intratumoral CD8+ activation as well as increased levels of antigen-specific CD8+ cells as well as tumor parenchyma destruction[39]. Such evidence suggests a role for LAG-3 blockade in future anticancer therapies[40].
T-cell immunoglobulin and mucin protein-3 downregulates Th1 immune responses in the periphery and prevents autoimmunity after binding to its ligand galectin-9 (Figure 1)[41]. In addition to certain tumors, TIM-3 is expressed on T-cells and nerve cells[42]. Binding of TIM-3 to its ligand induces phosphorylation of a tyrosine residue on the cytoplasmic tail of TIM-3 by interleukin inducible T cell kinase, resulting in downstream inhibition of Th1 effector function[43]. TIM-3 blockade in vivo has resulted in autoimmune disease including encephalomyelitis as well as increased activation of macrophages, possibly resulting from released inhibition on Th1 mediated immune responses[44]. Similar to LAG-3, TIM-3 promotes self-antigen tolerance demonstrated by the observation that TIM-3 blockade results in accelerated diabetes in NOD mice, possibly by reducing Treg activity in attenuating the Th1 immune response[45]. TIM-3 blockade has also shown promise as a potential anticancer therapy, as it results in an increased ratio of CD8+ to CD4+ tumor infiltrating lymphocytes in a murine colon cancer model, especially in cases of tumor regression, as well as increased IFN-γ production by T-cells; similar results and tumor suppression are observed after anti-TIM-3 therapy in murine BALB/c colon adenocarcinoma and fibrosarcoma models[46]. Additional studies are needed to clearly define the TIM-3 signaling pathway and its immunomodulatory effects.
Unlike the immune checkpoints mentioned thus far, Glucocorticoid-induced TNFR family-related receptor (GITR) is a co-stimulatory immune checkpoint that is expressed on CD4+ and CD8+ T-lymphocytes as well as Tregs, NK cells, dendritic cells (DC) and monocytes, and binds to its ligand expressed on APCs and endothelial cells (Figure 1)[47-49]. While GITR is expressed at a low-level on resting CD4+ and CD8+ T-cells, it is temporarily upregulated 24-72 h after an immune stimulus; conversely, GITR is constitutively expressed on Tregs[48,49]. GITR ligation results in an augmented effector T-cell response and resistance to activation induced cell death as well as to the suppressive effects of Tregs[50-52]. The ligation of GITR by agonist antibody DTA-1 has shown to cause Treg lineage instability through loss of FoxP3 and results in increased cytotoxic T-lymphocyte to Treg ratios[51]. Ligation of GITR, whether via an agonist antibody or GITRL-expressing DC vaccine, has resulted in murine melanoma regression and increased intratumoral effector T-cell to Treg ratios as a consequence of impairment of Treg infiltration into the tumor microenvironment and instability caused by loss of FoxP3[51]. The immune activating effects of GITR ligation are dependent on GITR expression in both effector T-cells as well as Tregs[51]. Preclinical studies of GITR in combination with other immunotherapies have demonstrated promising antitumor effects. In fibrosarcoma-bearing mice, co-administration of anti-CTLA-4 and anti-GITR antibodies led to synergistic tumor eradication mediated by tumor-specific CD4+ and CD8+ T-cells[53]. Similarly, combination adoptive T-cell therapy and anti-GITR antibody administration results in increased effector responses as measured by IFN-γ, TNFα and CD107a in a murine fibrosarcoma model[54]. While the precise mechanism of GITR ligation and its downstream consequences have yet to be defined, GITR modulation through agonist antibodies or GITRL expressing DC vaccines may be an efficacious avenue in immune checkpoint antitumor therapy.
Killer cell immunoglobulin like receptor (KIR) is an immune checkpoint that primarily regulates NK cell activation (Figure 1). Cytolytic CD56dimCD16+ NK cells express KIR which is specific for the HLA class I allele, and tumor infiltrating NK cells expressing KIR have been found to be downregulated by tumor-KIR interactions[55,56]. The precise intracellular mechanisms of KIR are as yet unclear, but KIR genes and their respective HLA class I ligands have been identified in non-small cell lung cancer (NSCLC) and kidney cancer patients, providing a potential role for NK cells in antitumor immune responses[57]. One study found that a high percentage of NSCLC patients had KIR-expressing NK cells compared to controls (P = 0.0004), and those NK cells were dysfunctional in terms of effector cytokine expression and overall cytotoxicity[58]. The involvement of KIR in tumor mediated immune suppression has led to the development of KIR-blocking human monoclonal antibody Lirilumab, which is currently being tested in combination with human anti-PD-1 and anti-CTLA-4 antibodies (NCT01750580, NCT01714739). Discovery of additional immune checkpoints on cells outside of the T-cell subset has led to increasing opportunities in overcoming tumor mediated immune suppression by blocking tumor-cell interactions involving a variety of immune cells. NK cells may become the next target for immune checkpoint blockade.
The first steps to understanding the role of PD-1 and its ligands in tumor survival was the characterization of PD-L1 expression almost exclusively on cancer cells[59]. Dong and colleagues showed that PD-L1 expression on tumor cell lines resulted in increased apoptosis of antigen-specific T-cells in vitro and in vivo, and that the apoptotic effect was mediated by multiple T-cell receptors including PD-1[59]. Early studies showed that tumor PD-L1 expression rendered tumor cells less susceptible to cytotoxic T-lymphocyte activity, and the interruption of the interaction between PD-1 and PD-L1 could re-establish tumor susceptibility to immune mediated cytotoxicity[60]. This discovery established the potential for PD-1 or PD-L1 blockade as a means toward increased antitumor efficacy by cancer therapeutics. Iwai and colleagues further confirmed the dependence of some aggressive and poorly immunogenic tumors on immune checkpoint pathways to evade immune responses. Their group transgenically expressed PD-L1 in a murine myeloma cell line and demonstrated decreased cell lysis in vitro by cytotoxic T-cells compared to tumor cells that did not express PD-L1[60]. When grown in syngeneic mice and treated with anti-PD-L1 antibody, murine myeloma cell lines expressing PD-L1 had transiently diminished growth; the same tumors did not grow in PD-1 deficient mice[60]. Blank et al[61] further characterized tumor resistance to CD8+ T-cell effector function by exposing PD-L1-expressing cancer cell lines to PD-1-deficient effector T-cells. Compared to wild-type, PD-1 deficient lymphocytes had increased proliferative, cytotoxic, and cytokine-producing activity, and this phenomenon is reproducible in vivo with anti-PD-L1 antibody in the effector phase[61]. This finding illustrated that if PD-1 expressed by lymphocytes could be blocked such that it could no longer interact with its ligand, and if this state essentially mimicked PD-1 deficiency as in the study by Blank et al[61], then future therapeutics could reverse immune inhibition in the tumor microenvironment and allow the host immune response to eliminate tumor. Confirming this concept, Hirano and colleagues demonstrated that antibody blockade of either PD-1 or PD-L1 interrupts tumor immune evasion by reversing resistance to lymphocyte effector function[62]. Together, these studies provided pre-clinical evidence that the PD-1/PD-L1 axis is crucial to tumor evasion of the immune response and blockade of this interaction is an important opportunity for suppressing tumor growth in vivo.
Food and Drug Administration approval for CTLA-4 occurred as preclinical studies were beginning to show that PD-1 blockade could be another opportunity for anti-cancer immunotherapy. The discovery that PD-1 and CTLA-4 signal through distinct and synergistic pathways naturally led to testing both in combination checkpoint blockade in preclinical tumor models[20,23]. In a murine B16 melanoma model, Curran and colleagues showed that combination anti-CTLA-4 and anti-PD-1 therapy with concurrent Flt3-ligand (Fvax) vaccine resulted in a 50% tumor rejection rate, compared to 25% with anti-PD-1 and 10% with anti-CTLA-4 each with Fvax[63]. Notably, combination blockade increased the ratio of effector T-cells to regulatory T-cells and myeloid derived stem cells (MDSCs) in the tumor microenvironment, resulting in greater local T-cell activation and anti-tumor inflammation. This study provided a mechanism for the efficacy of combination therapy centering on the importance of the effector T-cell response and down-regulation of inhibitory cell lines like Tregs and MDSCs in tumor elimination. Duraiswamy et al[64] elucidated the mechanism for increased effector T-cell activity and tumor rejection after anti-PD-1 and anti-CTLA-4 combination blockade in a murine colon cancer model[64]. Lymphocytes that express both PD-1 and CTLA-4 exhibited cellular dysfunction in their inability to produce cytokines or proliferate, while simultaneous antibody blockade of both co-receptors reversed T-cell dysfunction including inhibition of Treg function[64]. Similarly, in a murine glioblastoma (GBM) model, Wainwright et al[65] found that combination inhibition of indoleamine 2,3-dioxygenase, CTLA-4 and PD-1 resulted in decreased intratumoral Tregs with increased T-cell activation and long-term survival in tumor-bearing mice. Together, these studies emphasize the greater utility of PD-1 and CTLA-4 combination blockade relative to single checkpoint blockade in cancer immunotherapy, and they show that tumor regression occurs via effector lymphocyte activation. Inhibition of both T-cell co-receptors fully allows activation and proliferation of antigen-specific anti-tumor T-cells and prevents Treg suppression, resulting in significant suppression of tumor growth in animal models.
The first immune checkpoint inhibitor to be FDA approved for clinical use, anti-CTLA-4 antibody, or Ipilimumab, has been tested in a variety of cancer types with an emphasis on melanoma therapy. One of the earliest clinical trials testing anti-CTLA-4 therapy was done in metastatic melanoma, in which 14 patients were dosed with 3 mg/kg of Ipilimumab alongside gp100 vaccine and 21% experienced objective response, with two patients having complete regression[66]. This study also demonstrated the serious adverse autoimmune events associated with anti-CTLA-4 therapy, as 43% of patients experienced grade 3 or 4 adverse effects including enterocolitis and hypophysitis. Ipilimumab therapy for advanced metastatic melanoma continued to be tested in phase 2 and 3 clinical trials with encouraging tumor regression, but immune-related adverse events persisted in each study. In their phase II dose ranging study of 217 participants, Wolchok et al[67] established that the best overall response rate (BORR) of 11.1% was achieved with 10 mg/kg Ipilimumab, as compared to 4.2% with 3 mg/kg and 0% for 0.3 mg/kg. O’Day et al[68] applied the 10 mg/kg dosing scheme in pretreated advanced melanoma, and they observed a BORR of 5.8%, a disease control rate of 27%, and a median improved overall survival (OS) of 10.2 mo; 22.3% experienced immune related adverse events in this cohort[68]. Even in heavily pretreated melanoma, treatment with 10 mg/kg Ipilimumab resulted in promising disease control rates at 24 and 60 wk of 29.6% and 15%, respectively, with a one year survival rate of 34.8%, providing evidence that immune checkpoint inhibitors could still be used as a last resort in treatment resistant disease[69]. Robert et al[70] corroborated these results and found that even in pre-treated melanoma with disease progression, 75% treated with Ipilimumab alone experienced disease regression[70]. Brain metastasis did not preclude individuals from disease control with Ipilimumab, and in one study 18% of those neurologically asymptomatic from intracranial metastasis experienced overall disease control[71]. Importantly, Ipilimumab-induced disease regression in melanoma was durable, as an analysis of three phase II trials demonstrated a four year survival rate of 19.7%-28.4% at a dose of 10 mg/kg[72]. In a recent phase III trial of 676 individuals with metastatic melanoma treated with Ipilimumab alone, 25% survived at two and three years and only 8% experienced drug related toxicity[73,74]. The aforementioned clinical studies are summarized in Table 1.
| Ref. | Phase | Cancer type | No. of patients | Dose (mg/kg) | Outcome |
| McDermott et al[73] | 3 | Melanoma | 676 | 3 | 3-yr survival rate 25% |
| Hodi et al[74] | 3 | Melanoma | 676 | 3 | BORR: 10.9% |
| Robert et al[70] | 3 | Melanoma | 676 | 3 | BORR: 37.5% |
| Margolin et al[71] | 2 | Melanoma with brain metastasis | 93 | 10 | 1Cohort A: 18% disease control 2Cohort B: 5% disease control |
| Di Giacomo et al[69] | N/A | Melanoma | 27 | 10 | 2-yr survival rate 23.5% |
| Yang et al[78] | 2 | Pancreatic adenocarcinoma | 27 | 3 | No responders |
| O’Day et al[68] | 2 | Melanoma | 155 | 10 | BORR: 5.8% |
| Wolchok et al[67] | 2 | Melanoma | 217 | 10 | BORR: 11.1% |
| Yang et al[78] | 2 | RCC | 40 | 3 | PR: 12.5% |
| Phan et al[66] | 1 | Melanoma | 14 | 3 | OR: 21% |
As a result of encouraging clinical trial outcomes, a number of studies have ensued attempting to define markers of response to Ipilimumab therapy. Hamid and colleagues found that there is a significant association between clinical activity of Ipilimumab and indoleamine 2,3-dioxygenase levels (P = 0.012), FoxP3 expression (P = 0.014), as well as increased tumor infiltrating lymphocytes at three weeks after initiating therapy (P = 0.005)[75]. Moreover, there is a significant increase in expression of immune-related genes such as immunoglobulins, granzyme B, perforin-1, granulysin, and CD8 beta subunit and a decrease in expression of melanoma and cancer genes after therapy with Ipilimumab[75]. Moreover, Weber et al[76] in a study of humoral responses after Ipilimumab found that serologic activity against antigen NY-ESO-1 increased up to five fold at 12 wk in 10%-33% of individuals, and there were increased antibody levels against antigens p53, SSX2, MAGE-A4 and Melan-A[76]. Furthermore, they found significantly increased activated CD4 and CD8 and memory CD4 cells but not FoxP3 Treg or CD8 cells after 4 wk of Ipilimumab therapy[76]. While some had speculated that HLA status may affect response to anti-CTLA-4 therapy, Wolchok et al[77] found that HLA status had no association with overall survival or adverse events.
Antitumor responses with Ipilimumab are not limited to melanoma therapy; clinical responses have been observed in renal cell carcinoma (RCC), non-Hodgkin lymphoma, and prostate cancer, but such responses were not observable in pancreatic ductal adenocarcinoma[78-82]. Overall, Ipilimumab has demonstrated that immune checkpoint blockade alone can produce modest tumor regression in a number of solid tumors with some grade 3 and 4 immune related adverse events. As a result, a number of groups have begun to test other checkpoint molecules that could be blocked clinically to have a greater impact on antitumor immunity with similar or more limited side effect profiles.
Anti-PD-1 antibodies Nivolumab and Lambrolizumab have begun to show promising results in melanoma clinical trials. In their phase I trial of anti-PD-1 antibody at a dose of 0.1-10 mg/kg in 296 patients, Topalian and colleagues reported cumulative response rates of 28% with melanoma, 27% with RCC, and 18% with NSCLC[83]. Responses were durable with 64.5% responding at one year[83]. Notably, 36% with PD-L1 positive cancers also had an objective response (P = 0.006), although those with PD-L1 negative tumor were not precluded from responding[83]. In a follow-up study, Lipson et al[84] confirmed the durability of response to anti-PD-1 therapy by reporting that in patients who experienced objective responses, all continued to have a complete response after three years, except for one patient with melanoma who had a partial response that was stable for 16 mo off therapy, and whose recurrent disease was successfully treated with anti-PD-1 re-induction therapy. In a phase I study of advanced pretreated melanoma, Weber et al[85] observed a 25% RECIST 1.1 response rate and found that increased peripheral Tregs and decreased antigen specific T-cell were associated with progression, while PD-L1 staining was associated with a response to Nivolumab therapy, but not all who responded stained PD-L1 positive. Moreover, high pre-treatment levels of MART-1 and NY-ESO-1 specific CD8+ T-lymphocytes were associated with progression. In a study of Lambrolizumab in advanced melanoma, Hamid and colleagues observed an overall response rate of 38% with the highest response being 52% at a dose of 10 mg/kg[75]. Finally, there has been some investigation into blockade of the PD-1 ligand, PD-L1, as a more effective anti-tumor therapy. A phase I study in 207 patients with an array of advanced solid cancers treated with anti-PD-L1 antibody observed an objective response in 17.3% with melanoma, 11.7% with RCC, 10.2% with NSCLC and 6% with ovarian cancer[86]. Expression of PD-L1 on the tumor cell surface seemed to correlate with response to anti-PD-L1 therapy. These clinical studies are summarized in Table 2.
| Ref. | Phase | Cancer type | No. of patients | Antibody type | Outcome |
| Hamid et al[75] | 2 | Melanoma | 135 | Lambrolizumab | RECIST: 1.1; RR: 38% (95%CI: 25-44) |
| Weber et al[85] | 1 | Melanoma | 90 | Nivolumab | RECIST: 1.1; RR: 25% |
| Brahmer et al[86] | 1 | Advanced cancers | 207 | Anti-PD-L1 | OR: 6%-17% |
| Topalian et al[83] | 1 | Advanced cancers | 296 | Nivolumab | OR: 18%-36% |
Checkpoint blockade monotherapy with anti-PD-1 and anti-CTLA-4 antibodies have shown durable, though modest, tumor regression, but with the understanding that the two checkpoints function through discrete intracellular pathways, their clinical use together in double checkpoint blockade appeared to be a potentially additive or even synergistic therapy. Wolchok and colleagues produced encouraging results in their phase I trial of advanced melanoma treated with 1 mg/kg Nivolumab and 3 mg/kg Ipilimumab concurrently[87]. At maximum tolerated doses, 53% of patients had an objective response with an overall tumor regression of greater than 80% for all patients. Importantly, the side effect profile of combination therapy was similar to monotherapy and was largely reversible, indicating that patients may be treated and any side effects managed on an outpatient basis with corticosteroids. As a consequence of such promising clinical results, a number of clinical trials studying Nivolumab and Ipilumab in combination are currently ongoing (Table 3). These include studies of advanced stage and untreated melanoma, renal cell carcinoma, metastatic colon carcinoma, and recurrent glioblastoma involving dose escalation as well as sequential versus concurrent therapy. Whether combination checkpoint blockade extends to other solid tumors is yet to be seen, but this period certainly marks the advent of combination checkpoint blockade as a potential future anti-cancer therapy. It is important to note that there may be significant adverse events associated with immune checkpoint blockade, including enterocolitis and hypophysitis, among other immune related adverse events[67].
| Identifier | Phase | Cancer type | Checkpoint antibodies | Status |
| NCT02060188 | 2 | Microsatellite High (MSI-H) Colon Cancer | Nivolumab, ipilimumab | Recruiting |
| NCT01454102 | 1 | NSCLC | Nivolumab, ipilimumab | Recruiting |
| NCT01472081 | 1 | RCC | Nivolumab, ipilimumab | Recruiting |
| NCT01024231 | 1b | Melanoma | MDX-1106, ipilimumab | Active |
| NCT01928394 | 1/2 | TNBC, GC, PC, SCLC, BC | Nivolumab, ipilimumab | Recruiting |
| NCT02017717 | 2b | GBM | Nivolumab, ipilimumab | Recruiting |
| NCT01927419 | 2 | Melanoma | Nivolumab, ipilimumab | Active |
| NCT01844505 | 3 | Melanoma | Nivolumab, ipilimumab | Active |
| NCT01592370 | 1 | Hematologic malignancy | Nivolumab, ipilimumab | Recruiting |
| NCT01783938 | 2 | Melanoma | Nivolumab1, ipilimumab | Recruiting |
Immunotherapy with checkpoint blockade provides an opportunity for future adjuvant therapy with other treatment modalities, including.
The emergence of checkpoint blockade as a putative therapy for malignant neoplasms has raised the possibility of combination cytotoxic and immunotherapeutic regimens as avenues for tumor eradication (Table 4). Chronic pro-cancer inflammation mediated by cell-intrinsic somatic mutations and cell-extrinsic pathways such as vascular proliferation has historically prevented total tumor eradication by chemotherapy[1]. Immune checkpoint blockade may reprogram the inflammatory tumor microenvironment by harnessing active tumor-specific effector and memory CD4+ and CD8+ T-cells and thereby shifting tumor-associated inflammation from a pro-cancer to an anticancer state[1]. A number of pre-clinical and clinical studies have combined various cytotoxic therapies with checkpoint inhibitors with promising results.
| Ref. | Phase | Cancer type | Treatment combination | Outcome |
| Robert et al[70] | 3 | Melanoma | Ipilimumab + dacarbazine | 3-yr survival 20.8% (P < 0.001) |
| Lynch et al[94] | 2 | NSCLC | Ipilimumab + paclitaxel, carboplatin (phased) | irPFS HR: 0.72, P = 0.05 |
| Reck et al[95] | 2 | SCLC | Ipilimumab + paclitaxel, carboplatin (phased) | irPFS HR: 0.64, P = 0.03 |
| Hersh et al[90] | 2 | Melanoma | Ipilimumab + dacarbazine | ORR: 14.3% (95%CI: 4.8-30.3) |
Anti-CTLA-4 antibody combined with chemotherapy in murine models of lung cancer has demonstrated synergistic effects. Lesterhuis et al[88] found that 60% of mice receiving anti-CTLA-4 antibody and gemcitabine had complete tumor regression, compared to 13% in those receiving anti-CTLA-4 alone and 8% in those receiving gemcitabine alone. Importantly, this combination effect was timing dependent, with greatest tumor regression observed in mice receiving concomitant anti-CTLA-4 and gemcitabine. In a murine mesothelioma model, anti-CTLA-4 antibody administered between cisplatin dosing intervals inhibited tumor regrowth and prolonged survival in mice receiving anti-CTLA-4 plus cisplatin therapy compared to cisplatin alone (38 d vs 30 d, P = 0.0139)[89]. Moreover, combination anti-CTLA-4 plus cisplatin therapy produced increased intratumoral CD4+ and CD8+ lymphocytic infiltrate and increased expression of markers of lymphocyte activation including interleukin-2 (IL-2), IFN-γ, granzyme B and perforin[89].
In line with pre-clinical findings, clinical studies in metastatic melanoma have shown improved disease control with combination Ipilimumab and chemotherapy. In a phase II study of Ipilimumab and dacarbazine in metastatic melanoma, those who received combination therapy had an objective response rate (ORR) of 14.3% vs 5.4% in those who received dacarbazine plus placebo, and a median OS of 14.3 mo vs 11.4 mo[90]. Those who received combination therapy in this study did experience moderately increased immune mediated adverse events compared to the placebo arm (65% vs 53.8%)[90]. A phase III study of untreated metastatic melanoma had similar findings of OS in those receiving Ipilimumab plus dacarbazine (11.2 mo vs 9.1 mo) with durable improvements in survival rates at three years (20.8% vs 12.2%, P < 0.001). Combination checkpoint blockade plus chemotherapy has even shown disease control in advanced melanoma with brain metastasis, with Ipilimumab plus fontemustine producing disease control in 50% with intracranial disease and in 46.5% with stage IV disease without brain metastasis[91]. Retrospective studies have shown that even regional chemotherapy in melanoma followed by Ipilimumab has produced higher complete rates than IL-2 alone (33% vs 0%, P = 0.021)[92]. Importantly, combination anti-CTLA-4 in melanoma not only improves tumor regression, but Ipilimumab plus dacarbazine has demonstrated increasing quality-adjusted survival over time compared to dacarbazine alone, with a difference of 3.28 mo at 4 years (P = 0.0074)[93].
The benefits of Ipilimumab combined with chemotherapy are not limited to melanoma, but have been observed in NSCLC and small cell lung cancer (SCLC) as well. In a phase II trial of stage IIIB/IV NSCLC treated with Ipilimumab plus paclitaxel and carboplatin, phased Ipilimumab with chemotherapy improved immune-related progression free survival (irPFS) compared to concurrent Ipilimumab and control treatments (5.7, 5.5, 4.6 mo, respectively), as well as PFS (5.1, 4.1, 4.2 mo, respectively) and median OS (12.2, 9.7, 8.3 mo, respectively)[94]. Similarly, in a phase II study of SCLC treated with Iplimumab plus paclitaxel and carboplatin, only phased Ipilimumab improved irPFS compared to the control [hazard ratio (HR) = 0.64, P = 0.03], but without any improvement in PFS or OS[95].
While a number of clinical studies show a potential adjunctive role for anti-CTLA-4 therapy with chemotherapy, combination anti-PD-1 antibody with cytotoxic regimens are just entering pre-clinical and clinical studies. Hasan et al[96] demonstrated that doxorubicin down-regulates surface PD-L1 expression in breast cancer, leading to anti-apoptotic effects through intracellular regulation by PD-L1 of apoptotic machinery[96]. Their findings emphasize the need to further elucidate the cell-level interactions between chemotherapy, PD-1 and PD-L1 in cancers being targeted by anti-PD-1 therapy. Moreover, case reports have shown severe hypersensitivity reactions including autoimmune demyelinating polyneuropathy in those who received anti-PD-1 therapy followed by vemurafenib[97]. The mechanism behind such adverse events is unclear, but it is evident that the safety of combining anti-PD-1 with cytotoxic therapies must be established. Currently, ongoing phase I trial Checkmate 012 (NCT01454102) is recruiting those with stage IIIB/IV NSCLC for treatment with Nivolumab in combination with a number of chemotherapeutic regimes.
Given what studies have shown regarding combination Ipilimumab and chemotherapy, it is worthwhile pursuing such combination regimens with other checkpoint inhibitors in pre-clinical and clinical settings. It is important to maintain that cytotoxic therapy is successful only if the host is able to mount an appropriate immune response to counter procancer inflammation and immune resistance of cancer cells; in those whose cancers are particularly aggressive because of up-regulation of immune checkpoint ligands, checkpoint inhibitor therapy provides the opportunity to combat tumor regrowth and allow cytotoxic therapy to fully eradicate tumor[98].
Radiation therapy produces effects locally and systemically to promote tumor regression. Locally, tumor cell destruction through apoptotic pathways is closely interlinked with increased inflammatory changes that recruit antitumor immune responses. Ionizing radiation induces DNA damage that initiates a cascade of intracellular and extracellular events. Intracellularly, caspases inactivate proteins responsible for DNA damage recognition and repair. Extracellularly, a number of “stress signals” are expressed that attract immune-mediated cell death[99,100]. The expressed stress signals along with tumor antigens released by damaged tissue not only promote direct tumor destruction through cell mediated immunity, but provide an immunogenic substrate for an increased inflammatory response[2,101-107]. Early studies showed that T-cells were involved in tumor regression in irradiated mice, and it is now understood that radiation therapy modulates the tumor microenvironment through increased cytokine expression and antigen expression[108-111].
In effect, radiation therapy recreates an immunogenic tumor microenvironment in the place of one that was immunosuppressive.
Systemically, radiation therapy may promote tumor regression in distant sites through the abscopal effect. The abscopal effect is an immune-mediated phenomenon, involving dendritic cell presentation of dying tumor cell antigens to antigen-specific T-cells, resulting in activation of anti-tumor T-cells that may destroy tumor at peripheral sites[112]. Such a systemic effect of local radiation therapy has been observed in mammary carcinoma in mice and human hepatic carcinoma after radiation of bony metastases as well as adenocarcinoma[113-115]. Such observations provide promise for future combination therapies involving immunotherapy and local radiation therapy to destroy even metastatic disease.
The non-redundancy of immune checkpoint signaling and the ability of radiation therapy to enhance tumor immunogenicity provide powerful opportunities for combination multi-checkpoint blockade and adjuvant radiotherapy[116]. Inhibitory checkpoint co-receptors have been shown to be co-expressed on effector T-cells, and at least PD-1 and CTLA-4 are known to signal through distinct and synergistic pathways[20,23,117,118]. Similar to a vaccine, radiation therapy may prime an inflammatory response that induces tumor expression of inhibitory co-receptor cognate ligands which can subsequently be blocked by checkpoint blockade; in addition, radiation causes local tumor destruction, increased immune activity, and an abscopal effect[3,115]. For these reasons, inhibitory immune checkpoint blockade and local radiotherapy are promising components of anti-cancer combination therapy. As discussed, radiation therapy induces the expression of a number of “danger signals”, or cytokines and ligands expressed by tumor cells under stress. This stress response is highly immunogenic, and when combined with antibodies that block inhibitory interactions between tumor and T-cell, have the potential to produce significant anti-tumor immune responses. Irradiation of tumor cells up-regulates the retinoid acid early inducible-1 (RAE-1) ligand, which binds to receptor NKG2D on CD8+ T-cells and is critical in the cytotoxic lymphocyte effector response[119]. Another such danger signal released after tumor cell irradiation is CXCL16, which is a chemokine that recruits effector T-cells to the area of inflammation[120]. Sensitizing T-cells within the tumor microenvironment to tumor antigens produces an ideal environment for checkpoint blockade, as evidenced by synergistic responses in metastatic murine breast cancer regression after CTLA-4 blockade combined with radiotherapy[121]. Moreover, preclinical evidence shows that fractionated radiation may be superior to single dose radiation in producing the optimal degree of local inflammation to adequately sensitize T-cells for CTLA-4 blockade[122]. The synergism between radiation therapy and checkpoint blockade extends to PD-1 inhibition, with significantly increased survival observed in a mouse model of GBM treated with combination anti-PD-1 antibody and radiation therapy compared to either treatment alone[123]. Radiation has been found to up-regulate PD-L1 on tumor cells, and when radiation is combined with antibody-mediated PD-L1 blockade, cytotoxic T-cell activity is increased and MDSC’s suppressing effect on T-cells is inhibited[124]. Clearly, preclinical models show that radiation therapy is a potent up-regulator of immune activity in the tumor microenvironment, and has the potential to synergize with checkpoint blockade by sensitizing T-cells to tumor antigens.
Promising outcomes in pre-clinical studies in combination checkpoint blockade of CTLA-4 and PD-1 and combination radiation therapy and CTLA-4 have prompted a slew of clinical trials in an array of solid tumor types. Following from the success of pre-clinical studies combining radiation and anti-CTLA-4 therapy, Slovin and colleagues conducted a phase I/II dose escalation study of Ipilimumab and radiotherapy in castration-resistant prostate cancer (NCT00323882)[125]. At a maximum dose of 10 mg/kg Ipilimumab and 8 Gy of radiation per lesion, 26% of patients experienced grade 3 or 4 adverse events, six out of 33 patients had stable disease and one patient experienced a complete remission. There are now two phase III studies of radiotherapy and Ipilimumab in castration-resistant prostate cancer underway. In addition, a number of phase I and II trials studying combination Ipilimumab and radiation therapy in a variety of solid tumors are currently recruiting or underway, including colon cancer, NSCLC, triple negative breast cancer, melanoma, rectal carcinoma, head and neck cancer, cervical cancer, and metastatic melanoma to the brain. These trials differ in the mode of radiation therapy, varying from stereotactic radiosurgery to whole brain radiation to brachytherapy, and some compare fractionation to single dose. It will be interesting to see whether clinical outcomes correlate to the encouraging findings of pre-clinical studies combining checkpoint blockade and radiation.
Attenuated strains of Listeria monocytogenes has been developed with deletion of the internalin B and actin A genes that are capable of expressing tumor-associated antigens[126]. These strains have been used as tumor vaccination vectors and have been shown to elicit tumor-specific Th1 CD8+ immune responses in murine breast cancer[127-129]. Moreover, Olino et al[126] have developed such an attenuated strain expressing antigen AH1 of murine colorectal cancer line CT26; they found that this vaccine successfully treated 90% of mice with hepatic CRC metastases via a strong tumor-specific CD8+ response with central and effector memory T-cell generation[126]. Of note, this vaccine down-modulated PD-1 and had variable effects on CTLA-4 on tumor-infiltrating lymphocytes[126]. Given that the anti-tumor effects mediated by Listeria vaccines are largely CD8+ mediated, there may be a role for combination Listeria and anti-CTLA-4 therapy, as CTLA-4 is expressed on effector T-cells. In a murine model of Listeria infection, anti-CTLA-4 therapy after priming with attenuated Listeria resulted in an augmented immune responses when mice were re-infected with virulent Listeria[130]. Anti-CTLA-4 therapy resulted in increased numbers of CD4+ and CD8+ T-cells specific for Listeria antigen, and upon re-infection produced more rapid bacterial clearance. Such data indicates that combination Listeria and anti-CTLA-4 therapy could have similar immune effects in a tumor model, priming the immune system with a tumor-antigen expressing Listeria vaccine vector and augmenting the resultant Th1 immune response with anti-CTLA-4 antibody. Combination immune checkpoint blockade and bacterial tumor vaccine therapy is a promising future therapeutic that is worth exploring in murine cancer models.
In the field of passive immunotherapies, immune checkpoint blockade has garnered significant attention as an efficacious component of anticancer therapy. Anti-CTLA-4 and anti-PD-1 monotherapies have demonstrated significant cancer regression in a number of solid tumor types, but combination blockade of both immune checkpoints has resulted in significant tumor regression[87]. The future of checkpoint blockade may be as an adjuvant to chemotherapy and radiotherapy, augmenting the immune response to destroy even therapy resistant tumor. Research in bacterial vaccine vectors is still in its early stages, but murine cancer models demonstrate robust antigen-specific cytotoxic T-cell responses to Listeria monocytogenes vector priming, which can be combined with checkpoint blockade to further augment anti-tumor responses[126,130]. In addition, checkpoint receptor expression in other immune cells, such as NK cells, provides opportunities for inducing anti-tumor immune responses mediated by cells other than T-lymphocytes. KIR is such a checkpoint expressed primarily on NK cells that, when blocked, may prevent downregulation of NK cell activation; moreover, LAG-3 is also expressed in NK cells and other antigen presenting cells[131]. Thus, the future of immune checkpoint blockade may expand to include modulation of many types of immune effector cells in addition to T-cells. The paradigm of anticancer therapy is now making space for immunotherapies, with checkpoint blockade showing great promise for future therapeutics.
P- Reviewer: Antonelli A, Shou ZF S- Editor: Ji FF L- Editor: A E- Editor: Jiao XK
| 1. | Coussens LM, Zitvogel L, Palucka AK. Neutralizing tumor-promoting chronic inflammation: a magic bullet? Science. 2013;339:286-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 849] [Cited by in RCA: 865] [Article Influence: 72.1] [Reference Citation Analysis (0)] |
| 2. | Tesniere A, Apetoh L, Ghiringhelli F, Joza N, Panaretakis T, Kepp O, Schlemmer F, Zitvogel L, Kroemer G. Immunogenic cancer cell death: a key-lock paradigm. Curr Opin Immunol. 2008;20:504-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 236] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 3. | Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9936] [Cited by in RCA: 10338] [Article Influence: 795.2] [Reference Citation Analysis (34)] |
| 4. | Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science. 2011;331:1565-1570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4947] [Cited by in RCA: 4538] [Article Influence: 324.1] [Reference Citation Analysis (0)] |
| 5. | Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 2002;3:991-998. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3425] [Cited by in RCA: 3519] [Article Influence: 153.0] [Reference Citation Analysis (0)] |
| 6. | Dunn GP, Old LJ, Schreiber RD. The immunobiology of cancer immunosurveillance and immunoediting. Immunity. 2004;21:137-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1843] [Cited by in RCA: 2013] [Article Influence: 95.9] [Reference Citation Analysis (0)] |
| 7. | Dunn GP, Old LJ, Schreiber RD. The three Es of cancer immunoediting. Annu Rev Immunol. 2004;22:329-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1859] [Cited by in RCA: 2009] [Article Influence: 95.7] [Reference Citation Analysis (0)] |
| 8. | Topalian SL, Weiner GJ, Pardoll DM. Cancer immunotherapy comes of age. J Clin Oncol. 2011;29:4828-4836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 319] [Cited by in RCA: 386] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 9. | Nirschl CJ, Drake CG. Molecular pathways: coexpression of immune checkpoint molecules: signaling pathways and implications for cancer immunotherapy. Clin Cancer Res. 2013;19:4917-4924. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 221] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 10. | Chen L. Co-inhibitory molecules of the B7-CD28 family in the control of T-cell immunity. Nat Rev Immunol. 2004;4:336-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 910] [Cited by in RCA: 990] [Article Influence: 47.1] [Reference Citation Analysis (0)] |
| 11. | Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677-704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3785] [Cited by in RCA: 4137] [Article Influence: 243.4] [Reference Citation Analysis (0)] |
| 12. | Nishimura H, Nose M, Hiai H, Minato N, Honjo T. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity. 1999;11:141-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1873] [Cited by in RCA: 1994] [Article Influence: 76.7] [Reference Citation Analysis (0)] |
| 13. | Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, Fitz LJ, Malenkovich N, Okazaki T, Byrne MC. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192:1027-1034. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3572] [Cited by in RCA: 4049] [Article Influence: 162.0] [Reference Citation Analysis (0)] |
| 14. | Keir ME, Liang SC, Guleria I, Latchman YE, Qipo A, Albacker LA, Koulmanda M, Freeman GJ, Sayegh MH, Sharpe AH. Tissue expression of PD-L1 mediates peripheral T cell tolerance. J Exp Med. 2006;203:883-895. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 839] [Cited by in RCA: 984] [Article Influence: 51.8] [Reference Citation Analysis (0)] |
| 15. | Butte MJ, Keir ME, Phamduy TB, Sharpe AH, Freeman GJ. Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses. Immunity. 2007;27:111-122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1404] [Cited by in RCA: 1384] [Article Influence: 76.9] [Reference Citation Analysis (0)] |
| 16. | Nishimura H, Minato N, Nakano T, Honjo T. Immunological studies on PD-1 deficient mice: implication of PD-1 as a negative regulator for B cell responses. Int Immunol. 1998;10:1563-1572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 370] [Cited by in RCA: 378] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 17. | Nishimura H, Okazaki T, Tanaka Y, Nakatani K, Hara M, Matsumori A, Sasayama S, Mizoguchi A, Hiai H, Minato N. Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science. 2001;291:319-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1292] [Cited by in RCA: 1400] [Article Influence: 58.3] [Reference Citation Analysis (0)] |
| 18. | Xiao Y, Yu S, Zhu B, Bedoret D, Bu X, Francisco LM, Hua P, Duke-Cohan JS, Umetsu DT, Sharpe AH. RGMb is a novel binding partner for PD-L2 and its engagement with PD-L2 promotes respiratory tolerance. J Exp Med. 2014;211:943-959. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 19. | Chemnitz JM, Parry RV, Nichols KE, June CH, Riley JL. SHP-1 and SHP-2 associate with immunoreceptor tyrosine-based switch motif of programmed death 1 upon primary human T cell stimulation, but only receptor ligation prevents T cell activation. J Immunol. 2004;173:945-954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 775] [Cited by in RCA: 889] [Article Influence: 42.3] [Reference Citation Analysis (0)] |
| 20. | Parry RV, Chemnitz JM, Frauwirth KA, Lanfranco AR, Braunstein I, Kobayashi SV, Linsley PS, Thompson CB, Riley JL. CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms. Mol Cell Biol. 2005;25:9543-9553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1248] [Cited by in RCA: 1489] [Article Influence: 74.5] [Reference Citation Analysis (0)] |
| 21. | Riley JL. PD-1 signaling in primary T cells. Immunol Rev. 2009;229:114-125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 619] [Cited by in RCA: 614] [Article Influence: 38.4] [Reference Citation Analysis (0)] |
| 22. | Sheppard KA, Fitz LJ, Lee JM, Benander C, George JA, Wooters J, Qiu Y, Jussif JM, Carter LL, Wood CR. PD-1 inhibits T-cell receptor induced phosphorylation of the ZAP70/CD3zeta signalosome and downstream signaling to PKCtheta. FEBS Lett. 2004;574:37-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 497] [Cited by in RCA: 615] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 23. | Blazar BR, Carreno BM, Panoskaltsis-Mortari A, Carter L, Iwai Y, Yagita H, Nishimura H, Taylor PA. Blockade of programmed death-1 engagement accelerates graft-versus-host disease lethality by an IFN-gamma-dependent mechanism. J Immunol. 2003;171:1272-1277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 272] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 24. | Chuang E, Fisher TS, Morgan RW, Robbins MD, Duerr JM, Vander Heiden MG, Gardner JP, Hambor JE, Neveu MJ, Thompson CB. The CD28 and CTLA-4 receptors associate with the serine/threonine phosphatase PP2A. Immunity. 2000;13:313-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 238] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 25. | Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996;271:1734-1736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2582] [Cited by in RCA: 2809] [Article Influence: 96.9] [Reference Citation Analysis (0)] |
| 26. | Tivol EA, Borriello F, Schweitzer AN, Lynch WP, Bluestone JA, Sharpe AH. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity. 1995;3:541-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2109] [Cited by in RCA: 2189] [Article Influence: 73.0] [Reference Citation Analysis (0)] |
| 27. | Waterhouse P, Penninger JM, Timms E, Wakeham A, Shahinian A, Lee KP, Thompson CB, Griesser H, Mak TW. Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4. Science. 1995;270:985-988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2111] [Cited by in RCA: 2136] [Article Influence: 71.2] [Reference Citation Analysis (0)] |
| 28. | Rudd CE, Taylor A, Schneider H. CD28 and CTLA-4 coreceptor expression and signal transduction. Immunol Rev. 2009;229:12-26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 702] [Cited by in RCA: 660] [Article Influence: 41.3] [Reference Citation Analysis (0)] |
| 29. | Marengère LE, Waterhouse P, Duncan GS, Mittrücker HW, Feng GS, Mak TW. Regulation of T cell receptor signaling by tyrosine phosphatase SYP association with CTLA-4. Science. 1996;272:1170-1173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 419] [Cited by in RCA: 391] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 30. | Triebel F, Jitsukawa S, Baixeras E, Roman-Roman S, Genevee C, Viegas-Pequignot E, Hercend T. LAG-3, a novel lymphocyte activation gene closely related to CD4. J Exp Med. 1990;171:1393-1405. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 506] [Cited by in RCA: 683] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 31. | Baixeras E, Huard B, Miossec C, Jitsukawa S, Martin M, Hercend T, Auffray C, Triebel F, Piatier-Tonneau D. Characterization of the lymphocyte activation gene 3-encoded protein. A new ligand for human leukocyte antigen class II antigens. J Exp Med. 1992;176:327-337. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 247] [Cited by in RCA: 317] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 32. | Miyazaki T, Dierich A, Benoist C, Mathis D. LAG-3 is not responsible for selecting T helper cells in CD4-deficient mice. Int Immunol. 1996;8:725-729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 27] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 33. | Huang CT, Workman CJ, Flies D, Pan X, Marson AL, Zhou G, Hipkiss EL, Ravi S, Kowalski J, Levitsky HI. Role of LAG-3 in regulatory T cells. Immunity. 2004;21:503-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 791] [Cited by in RCA: 946] [Article Influence: 45.0] [Reference Citation Analysis (0)] |
| 34. | Workman CJ, Vignali DA. The CD4-related molecule, LAG-3 (CD223), regulates the expansion of activated T cells. Eur J Immunol. 2003;33:970-979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 245] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 35. | Bettini M, Szymczak-Workman AL, Forbes K, Castellaw AH, Selby M, Pan X, Drake CG, Korman AJ, Vignali DA. Cutting edge: accelerated autoimmune diabetes in the absence of LAG-3. J Immunol. 2011;187:3493-3498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 128] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 36. | Blackburn SD, Shin H, Haining WN, Zou T, Workman CJ, Polley A, Betts MR, Freeman GJ, Vignali DA, Wherry EJ. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat Immunol. 2009;10:29-37. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1703] [Cited by in RCA: 1642] [Article Influence: 102.6] [Reference Citation Analysis (0)] |
| 37. | Woo SR, Turnis ME, Goldberg MV, Bankoti J, Selby M, Nirschl CJ, Bettini ML, Gravano DM, Vogel P, Liu CL. Immune inhibitory molecules LAG-3 and PD-1 synergistically regulate T-cell function to promote tumoral immune escape. Cancer Res. 2012;72:917-927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 949] [Cited by in RCA: 1290] [Article Influence: 92.1] [Reference Citation Analysis (0)] |
| 38. | Grosso JF, Kelleher CC, Harris TJ, Maris CH, Hipkiss EL, De Marzo A, Anders R, Netto G, Getnet D, Bruno TC. LAG-3 regulates CD8+ T cell accumulation and effector function in murine self- and tumor-tolerance systems. J Clin Invest. 2007;117:3383-3392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 39. | Goldberg MV, Drake CG. LAG-3 in Cancer Immunotherapy. Curr Top Microbiol Immunol. 2011;344:269-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 173] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 40. | Zhu C, Anderson AC, Schubart A, Xiong H, Imitola J, Khoury SJ, Zheng XX, Strom TB, Kuchroo VK. The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity. Nat Immunol. 2005;6:1245-1252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1334] [Cited by in RCA: 1589] [Article Influence: 79.5] [Reference Citation Analysis (0)] |
| 41. | Gielen AW, Lobell A, Lidman O, Khademi M, Olsson T, Piehl F. Expression of T cell immunoglobulin- and mucin-domain-containing molecules-1 and -3 (TIM-1 and -3) in the rat nervous and immune systems. J Neuroimmunol. 2005;164:93-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 42. | van de Weyer PS, Muehlfeit M, Klose C, Bonventre JV, Walz G, Kuehn EW. A highly conserved tyrosine of Tim-3 is phosphorylated upon stimulation by its ligand galectin-9. Biochem Biophys Res Commun. 2006;351:571-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 158] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 43. | Monney L, Sabatos CA, Gaglia JL, Ryu A, Waldner H, Chernova T, Manning S, Greenfield EA, Coyle AJ, Sobel RA. Th1-specific cell surface protein Tim-3 regulates macrophage activation and severity of an autoimmune disease. Nature. 2002;415:536-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1124] [Cited by in RCA: 1291] [Article Influence: 56.1] [Reference Citation Analysis (0)] |
| 44. | Sánchez-Fueyo A, Tian J, Picarella D, Domenig C, Zheng XX, Sabatos CA, Manlongat N, Bender O, Kamradt T, Kuchroo VK. Tim-3 inhibits T helper type 1-mediated auto- and alloimmune responses and promotes immunological tolerance. Nat Immunol. 2003;4:1093-1101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 519] [Cited by in RCA: 577] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 45. | Ngiow SF, von Scheidt B, Akiba H, Yagita H, Teng MW, Smyth MJ. Anti-TIM3 antibody promotes T cell IFN-γ-mediated antitumor immunity and suppresses established tumors. Cancer Res. 2011;71:3540-3551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 382] [Cited by in RCA: 457] [Article Influence: 32.6] [Reference Citation Analysis (0)] |
| 46. | Schaer DA, Hirschhorn-Cymerman D, Wolchok JD. Targeting tumor-necrosis factor receptor pathways for tumor immunotherapy. J Immunother Cancer. 2014;2:7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 94] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 47. | Nocentini G, Giunchi L, Ronchetti S, Krausz LT, Bartoli A, Moraca R, Migliorati G, Riccardi C. A new member of the tumor necrosis factor/nerve growth factor receptor family inhibits T cell receptor-induced apoptosis. Proc Natl Acad Sci USA. 1997;94:6216-6221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 320] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 48. | Nocentini G, Ronchetti S, Petrillo MG, Riccardi C. Pharmacological modulation of GITRL/GITR system: therapeutic perspectives. Br J Pharmacol. 2012;165:2089-2099. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 64] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 49. | Schaer DA, Murphy JT, Wolchok JD. Modulation of GITR for cancer immunotherapy. Curr Opin Immunol. 2012;24:217-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 110] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 50. | Cohen AD, Diab A, Perales MA, Wolchok JD, Rizzuto G, Merghoub T, Huggins D, Liu C, Turk MJ, Restifo NP. Agonist anti-GITR antibody enhances vaccine-induced CD8(+) T-cell responses and tumor immunity. Cancer Res. 2006;66:4904-4912. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 172] [Cited by in RCA: 164] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 51. | Cohen AD, Schaer DA, Liu C, Li Y, Hirschhorn-Cymmerman D, Kim SC, Diab A, Rizzuto G, Duan F, Perales MA. Agonist anti-GITR monoclonal antibody induces melanoma tumor immunity in mice by altering regulatory T cell stability and intra-tumor accumulation. PLoS One. 2010;5:e10436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 52. | Schaer DA, Budhu S, Liu C, Bryson C, Malandro N, Cohen A, Zhong H, Yang X, Houghton AN, Merghoub T. GITR pathway activation abrogates tumor immune suppression through loss of regulatory T cell lineage stability. Cancer Immunol Res. 2013;1:320-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 124] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 53. | Ko K, Yamazaki S, Nakamura K, Nishioka T, Hirota K, Yamaguchi T, Shimizu J, Nomura T, Chiba T, Sakaguchi S. Treatment of advanced tumors with agonistic anti-GITR mAb and its effects on tumor-infiltrating Foxp3+CD25+CD4+ regulatory T cells. J Exp Med. 2005;202:885-891. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 418] [Cited by in RCA: 425] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 54. | Imai N, Ikeda H, Tawara I, Wang L, Wang L, Nishikawa H, Kato T, Shiku H. Glucocorticoid-induced tumor necrosis factor receptor stimulation enhances the multifunctionality of adoptively transferred tumor antigen-specific CD8+ T cells with tumor regression. Cancer Sci. 2009;100:1317-1325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 55. | Jacobs R, Hintzen G, Kemper A, Beul K, Kempf S, Behrens G, Sykora KW, Schmidt RE. CD56bright cells differ in their KIR repertoire and cytotoxic features from CD56dim NK cells. Eur J Immunol. 2001;31:3121-3127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 56. | Carrega P, Morandi B, Costa R, Frumento G, Forte G, Altavilla G, Ratto GB, Mingari MC, Moretta L, Ferlazzo G. Natural killer cells infiltrating human nonsmall-cell lung cancer are enriched in CD56 bright CD16(-) cells and display an impaired capability to kill tumor cells. Cancer. 2008;112:863-875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 291] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 57. | Al Omar S, Middleton D, Marshall E, Porter D, Xinarianos G, Raji O, Field JK, Christmas SE. Associations between genes for killer immunoglobulin-like receptors and their ligands in patients with solid tumors. Hum Immunol. 2010;71:976-981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 46] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 58. | Al Omar SY, Marshall E, Middleton D, Christmas SE. Increased killer immunoglobulin-like receptor expression and functional defects in natural killer cells in lung cancer. Immunology. 2011;133:94-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 45] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 59. | Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8:793-800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2838] [Cited by in RCA: 3539] [Article Influence: 153.9] [Reference Citation Analysis (0)] |
| 60. | Iwai Y, Ishida M, Tanaka Y, Okazaki T, Honjo T, Minato N. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci USA. 2002;99:12293-12297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2103] [Cited by in RCA: 2453] [Article Influence: 106.7] [Reference Citation Analysis (0)] |
| 61. | Blank C, Brown I, Peterson AC, Spiotto M, Iwai Y, Honjo T, Gajewski TF. PD-L1/B7H-1 inhibits the effector phase of tumor rejection by T cell receptor (TCR) transgenic CD8+ T cells. Cancer Res. 2004;64:1140-1145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 575] [Cited by in RCA: 610] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 62. | Hirano F, Kaneko K, Tamura H, Dong H, Wang S, Ichikawa M, Rietz C, Flies DB, Lau JS, Zhu G. Blockade of B7-H1 and PD-1 by monoclonal antibodies potentiates cancer therapeutic immunity. Cancer Res. 2005;65:1089-1096. [PubMed] |
| 63. | Curran MA, Montalvo W, Yagita H, Allison JP. PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proc Natl Acad Sci USA. 2010;107:4275-4280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1270] [Cited by in RCA: 1490] [Article Influence: 99.3] [Reference Citation Analysis (0)] |
| 64. | Duraiswamy J, Kaluza KM, Freeman GJ, Coukos G. Dual blockade of PD-1 and CTLA-4 combined with tumor vaccine effectively restores T-cell rejection function in tumors. Cancer Res. 2013;73:3591-3603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 470] [Cited by in RCA: 560] [Article Influence: 46.7] [Reference Citation Analysis (0)] |
| 65. | Wainwright DA, Chang AL, Dey M, Balyasnikova IV, Kim CK, Tobias A, Cheng Y, Kim JW, Qiao J, Zhang L. Durable therapeutic efficacy utilizing combinatorial blockade against IDO, CTLA-4, and PD-L1 in mice with brain tumors. Clin Cancer Res. 2014;20:5290-5301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 467] [Cited by in RCA: 462] [Article Influence: 42.0] [Reference Citation Analysis (0)] |
| 66. | Phan GQ, Yang JC, Sherry RM, Hwu P, Topalian SL, Schwartzentruber DJ, Restifo NP, Haworth LR, Seipp CA, Freezer LJ. Cancer regression and autoimmunity induced by cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma. Proc Natl Acad Sci USA. 2003;100:8372-8377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1225] [Cited by in RCA: 1186] [Article Influence: 53.9] [Reference Citation Analysis (0)] |
| 67. | Wolchok JD, Neyns B, Linette G, Negrier S, Lutzky J, Thomas L, Waterfield W, Schadendorf D, Smylie M, Guthrie T. Ipilimumab monotherapy in patients with pretreated advanced melanoma: a randomised, double-blind, multicentre, phase 2, dose-ranging study. Lancet Oncol. 2010;11:155-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 862] [Cited by in RCA: 913] [Article Influence: 57.1] [Reference Citation Analysis (0)] |
| 68. | O’Day SJ, Maio M, Chiarion-Sileni V, Gajewski TF, Pehamberger H, Bondarenko IN, Queirolo P, Lundgren L, Mikhailov S, Roman L. Efficacy and safety of ipilimumab monotherapy in patients with pretreated advanced melanoma: a multicenter single-arm phase II study. Ann Oncol. 2010;21:1712-1717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 387] [Cited by in RCA: 401] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 69. | Di Giacomo AM, Danielli R, Calabrò L, Bertocci E, Nannicini C, Giannarelli D, Balestrazzi A, Vigni F, Riversi V, Miracco C. Ipilimumab experience in heavily pretreated patients with melanoma in an expanded access program at the University Hospital of Siena (Italy). Cancer Immunol Immunother. 2011;60:467-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 75] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 70. | Robert C, Schadendorf D, Messina M, Hodi FS, O’Day S. Efficacy and safety of retreatment with ipilimumab in patients with pretreated advanced melanoma who progressed after initially achieving disease control. Clin Cancer Res. 2013;19:2232-2239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 127] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 71. | Margolin K, Ernstoff MS, Hamid O, Lawrence D, McDermott D, Puzanov I, Wolchok JD, Clark JI, Sznol M, Logan TF. Ipilimumab in patients with melanoma and brain metastases: an open-label, phase 2 trial. Lancet Oncol. 2012;13:459-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 797] [Cited by in RCA: 850] [Article Influence: 65.4] [Reference Citation Analysis (0)] |
| 72. | Wolchok JD, Weber JS, Maio M, Neyns B, Harmankaya K, Chin K, Cykowski L, de Pril V, Humphrey R, Lebbé C. Four-year survival rates for patients with metastatic melanoma who received ipilimumab in phase II clinical trials. Ann Oncol. 2013;24:2174-2180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 144] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 73. | McDermott D, Haanen J, Chen TT, Lorigan P, O’Day S. Efficacy and safety of ipilimumab in metastatic melanoma patients surviving more than 2 years following treatment in a phase III trial (MDX010-20). Ann Oncol. 2013;24:2694-2698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 163] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 74. | Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711-723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10799] [Cited by in RCA: 11775] [Article Influence: 785.0] [Reference Citation Analysis (0)] |
| 75. | Hamid O, Schmidt H, Nissan A, Ridolfi L, Aamdal S, Hansson J, Guida M, Hyams DM, Gómez H, Bastholt L. A prospective phase II trial exploring the association between tumor microenvironment biomarkers and clinical activity of ipilimumab in advanced melanoma. J Transl Med. 2011;9:204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 460] [Cited by in RCA: 444] [Article Influence: 31.7] [Reference Citation Analysis (0)] |
| 76. | Weber JS, Hamid O, Chasalow SD, Wu DY, Parker SM, Galbraith S, Gnjatic S, Berman D. Ipilimumab increases activated T cells and enhances humoral immunity in patients with advanced melanoma. J Immunother. 2012;35:89-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 99] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 77. | Wolchok JD, Weber JS, Hamid O, Lebbé C, Maio M, Schadendorf D, de Pril V, Heller K, Chen TT, Ibrahim R. Ipilimumab efficacy and safety in patients with advanced melanoma: a retrospective analysis of HLA subtype from four trials. Cancer Immun. 2010;10:9. [PubMed] |
| 78. | Yang JC, Hughes M, Kammula U, Royal R, Sherry RM, Topalian SL, Suri KB, Levy C, Allen T, Mavroukakis S. Ipilimumab (anti-CTLA4 antibody) causes regression of metastatic renal cell cancer associated with enteritis and hypophysitis. J Immunother. 2007;30:825-830. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 561] [Cited by in RCA: 532] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 79. | Ansell SM, Hurvitz SA, Koenig PA, LaPlant BR, Kabat BF, Fernando D, Habermann TM, Inwards DJ, Verma M, Yamada R. Phase I study of ipilimumab, an anti-CTLA-4 monoclonal antibody, in patients with relapsed and refractory B-cell non-Hodgkin lymphoma. Clin Cancer Res. 2009;15:6446-6453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 262] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 80. | Sridhar T, Gore A, Boiangiu I, Machin D, Symonds RP. Concomitant (without adjuvant) temozolomide and radiation to treat glioblastoma: a retrospective study. Clin Oncol (R Coll Radiol). 2009;21:19-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 81. | Santegoets SJ, Stam AG, Lougheed SM, Gall H, Scholten PE, Reijm M, Jooss K, Sacks N, Hege K, Lowy I. T cell profiling reveals high CD4+CTLA-4 + T cell frequency as dominant predictor for survival after prostate GVAX/ipilimumab treatment. Cancer Immunol Immunother. 2013;62:245-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 75] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 82. | Kwek SS, Dao V, Roy R, Hou Y, Alajajian D, Simko JP, Small EJ, Fong L. Diversity of antigen-specific responses induced in vivo with CTLA-4 blockade in prostate cancer patients. J Immunol. 2012;189:3759-3766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 65] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 83. | Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443-2454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8900] [Cited by in RCA: 9899] [Article Influence: 761.5] [Reference Citation Analysis (0)] |
| 84. | Lipson EJ, Sharfman WH, Drake CG, Wollner I, Taube JM, Anders RA, Xu H, Yao S, Pons A, Chen L. Durable cancer regression off-treatment and effective reinduction therapy with an anti-PD-1 antibody. Clin Cancer Res. 2013;19:462-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 389] [Cited by in RCA: 432] [Article Influence: 33.2] [Reference Citation Analysis (0)] |
| 85. | Weber JS, Kudchadkar RR, Yu B, Gallenstein D, Horak CE, Inzunza HD, Zhao X, Martinez AJ, Wang W, Gibney G. Safety, efficacy, and biomarkers of nivolumab with vaccine in ipilimumab-refractory or -naive melanoma. J Clin Oncol. 2013;31:4311-4318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 420] [Cited by in RCA: 446] [Article Influence: 37.2] [Reference Citation Analysis (0)] |
| 86. | Brahmer JR, Drake CG, Wollner I, Powderly JD, Picus J, Sharfman WH, Stankevich E, Pons A, Salay TM, McMiller TL. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol. 2010;28:3167-3175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2330] [Cited by in RCA: 2414] [Article Influence: 160.9] [Reference Citation Analysis (0)] |
| 87. | Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, Segal NH, Ariyan CE, Gordon RA, Reed K. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369:122-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3192] [Cited by in RCA: 3320] [Article Influence: 276.7] [Reference Citation Analysis (0)] |
| 88. | Lesterhuis WJ, Salmons J, Nowak AK, Rozali EN, Khong A, Dick IM, Harken JA, Robinson BW, Lake RA. Synergistic effect of CTLA-4 blockade and cancer chemotherapy in the induction of anti-tumor immunity. PLoS One. 2013;8:e61895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 89. | Wu L, Yun Z, Tagawa T, Rey-McIntyre K, de Perrot M. CTLA-4 blockade expands infiltrating T cells and inhibits cancer cell repopulation during the intervals of chemotherapy in murine mesothelioma. Mol Cancer Ther. 2012;11:1809-1819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 62] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 90. | Hersh EM, O’Day SJ, Powderly J, Khan KD, Pavlick AC, Cranmer LD, Samlowski WE, Nichol GM, Yellin MJ, Weber JS. A phase II multicenter study of ipilimumab with or without dacarbazine in chemotherapy-naïve patients with advanced melanoma. Invest New Drugs. 2011;29:489-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 230] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 91. | Di Giacomo AM, Ascierto PA, Pilla L, Santinami M, Ferrucci PF, Giannarelli D, Marasco A, Rivoltini L, Simeone E, Nicoletti SV. Ipilimumab and fotemustine in patients with advanced melanoma (NIBIT-M1): an open-label, single-arm phase 2 trial. Lancet Oncol. 2012;13:879-886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 227] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 92. | Jiang BS, Beasley GM, Speicher PJ, Mosca PJ, Morse MA, Hanks B, Salama A, Tyler DS. Immunotherapy following regional chemotherapy treatment of advanced extremity melanoma. Ann Surg Oncol. 2014;21:2525-2531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 93. | Sherrill B, Wang J, Kotapati S, Chin K. Q-TWiST analysis comparing ipilimumab/dacarbazine vs placebo/dacarbazine for patients with stage III/IV melanoma. Br J Cancer. 2013;109:8-13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 94. | Lynch TJ, Bondarenko I, Luft A, Serwatowski P, Barlesi F, Chacko R, Sebastian M, Neal J, Lu H, Cuillerot JM. Ipilimumab in combination with paclitaxel and carboplatin as first-line treatment in stage IIIB/IV non-small-cell lung cancer: results from a randomized, double-blind, multicenter phase II study. J Clin Oncol. 2012;30:2046-2054. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 722] [Cited by in RCA: 810] [Article Influence: 62.3] [Reference Citation Analysis (0)] |
| 95. | Reck M, Bondarenko I, Luft A, Serwatowski P, Barlesi F, Chacko R, Sebastian M, Lu H, Cuillerot JM, Lynch TJ. Ipilimumab in combination with paclitaxel and carboplatin as first-line therapy in extensive-disease-small-cell lung cancer: results from a randomized, double-blind, multicenter phase 2 trial. Ann Oncol. 2013;24:75-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 413] [Cited by in RCA: 494] [Article Influence: 38.0] [Reference Citation Analysis (0)] |
| 96. | Hasan A, Ghebeh H, Lehe C, Ahmad R, Dermime S. Therapeutic targeting of B7-H1 in breast cancer. Expert Opin Ther Targets. 2011;15:1211-1225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 97. | Johnson DB, Wallender EK, Cohen DN, Likhari SS, Zwerner JP, Powers JG, Shinn L, Kelley MC, Joseph RW, Sosman JA. Severe cutaneous and neurologic toxicity in melanoma patients during vemurafenib administration following anti-PD-1 therapy. Cancer Immunol Res. 2013;1:373-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 83] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 98. | Zitvogel L, Kepp O, Kroemer G. Immune parameters affecting the efficacy of chemotherapeutic regimens. Nat Rev Clin Oncol. 2011;8:151-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 474] [Cited by in RCA: 524] [Article Influence: 37.4] [Reference Citation Analysis (0)] |
| 99. | Watters D. Molecular mechanisms of ionizing radiation-induced apoptosis. Immunol Cell Biol. 1999;77:263-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 94] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 100. | de la Cruz-Merino L, Illescas-Vacas A, Grueso-López A, Barco-Sánchez A, Míguez-Sánchez C. Radiation for Awakening the Dormant Immune System, a Promising Challenge to be Explored. Front Immunol. 2014;5:102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 101. | Skoberne M, Beignon AS, Bhardwaj N. Danger signals: a time and space continuum. Trends Mol Med. 2004;10:251-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 80] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 102. | Kessler B, Hudrisier D, Schroeter M, Tschopp J, Cerottini JC, Luescher IF. Peptide modification or blocking of CD8, resulting in weak TCR signaling, can activate CTL for Fas- but not perforin-dependent cytotoxicity or cytokine production. J Immunol. 1998;161:6939-6946. [PubMed] |
| 103. | Gasser S, Orsulic S, Brown EJ, Raulet DH. The DNA damage pathway regulates innate immune system ligands of the NKG2D receptor. Nature. 2005;436:1186-1190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 962] [Cited by in RCA: 1051] [Article Influence: 52.6] [Reference Citation Analysis (0)] |
| 104. | Obeid M, Tesniere A, Panaretakis T, Tufi R, Joza N, van Endert P, Ghiringhelli F, Apetoh L, Chaput N, Flament C. Ecto-calreticulin in immunogenic chemotherapy. Immunol Rev. 2007;220:22-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 172] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 105. | Larsson M, Fonteneau JF, Bhardwaj N. Dendritic cells resurrect antigens from dead cells. Trends Immunol. 2001;22:141-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 133] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 106. | Kim JY, Son YO, Park SW, Bae JH, Chung JS, Kim HH, Chung BS, Kim SH, Kang CD. Increase of NKG2D ligands and sensitivity to NK cell-mediated cytotoxicity of tumor cells by heat shock and ionizing radiation. Exp Mol Med. 2006;38:474-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 149] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 107. | Apetoh L, Ghiringhelli F, Tesniere A, Obeid M, Ortiz C, Criollo A, Mignot G, Maiuri MC, Ullrich E, Saulnier P. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med. 2007;13:1050-1059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2084] [Cited by in RCA: 2417] [Article Influence: 134.3] [Reference Citation Analysis (0)] |
| 108. | Stone HB, Peters LJ, Milas L. Effect of host immune capability on radiocurability and subsequent transplantability of a murine fibrosarcoma. J Natl Cancer Inst. 1979;63:1229-1235. [PubMed] |
| 109. | Shiao SL, Coussens LM. The tumor-immune microenvironment and response to radiation therapy. J Mammary Gland Biol Neoplasia. 2010;15:411-421. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 108] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 110. | Friedman EJ. Immune modulation by ionizing radiation and its implications for cancer immunotherapy. Curr Pharm Des. 2002;8:1765-1780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 151] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 111. | Robbins PF, Lu YC, El-Gamil M, Li YF, Gross C, Gartner J, Lin JC, Teer JK, Cliften P, Tycksen E. Mining exomic sequencing data to identify mutated antigens recognized by adoptively transferred tumor-reactive T cells. Nat Med. 2013;19:747-752. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 895] [Cited by in RCA: 885] [Article Influence: 73.8] [Reference Citation Analysis (0)] |
| 112. | Demaria S, Ng B, Devitt ML, Babb JS, Kawashima N, Liebes L, Formenti SC. Ionizing radiation inhibition of distant untreated tumors (abscopal effect) is immune mediated. Int J Radiat Oncol Biol Phys. 2004;58:862-870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 867] [Cited by in RCA: 1049] [Article Influence: 50.0] [Reference Citation Analysis (0)] |
| 113. | Formenti SC, Friedman K, Chao K, Adams S, Fenton-Kerimian M, Donach ME, Demaria S. Abscopal Response in Irradiated Patients: Results of a Proof of Principle Trial. IJROBP. 2008;72:S6-S7. [RCA] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 114. | Formenti SC, Demaria S. Systemic effects of local radiotherapy. Lancet Oncol. 2009;10:718-726. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 815] [Cited by in RCA: 769] [Article Influence: 48.1] [Reference Citation Analysis (0)] |
| 115. | Demaria S, Bhardwaj N, McBride WH, Formenti SC. Combining radiotherapy and immunotherapy: a revived partnership. Int J Radiat Oncol Biol Phys. 2005;63:655-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 283] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 116. | Formenti SC, Demaria S. Combining radiotherapy and cancer immunotherapy: a paradigm shift. J Natl Cancer Inst. 2013;105:256-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 660] [Cited by in RCA: 808] [Article Influence: 67.3] [Reference Citation Analysis (0)] |
| 117. | Baitsch L, Legat A, Barba L, Fuertes Marraco SA, Rivals JP, Baumgaertner P, Christiansen-Jucht C, Bouzourene H, Rimoldi D, Pircher H. Extended co-expression of inhibitory receptors by human CD8 T-cells depending on differentiation, antigen-specificity and anatomical localization. PLoS One. 2012;7:e30852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 118. | Duraiswamy J, Freeman G, Coukos G. Replenish the source within: Rescuing tumor-infiltrating lymphocytes by double checkpoint blockade. Oncoimmunology. 2013;2:e25912. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 119. | Demaria S, Pilones KA, Formenti SC, Dustin ML. Exploiting the stress response to radiation to sensitize poorly immunogenic tumors to anti-CTLA-4 treatment. Oncoimmunology. 2013;2:e23127. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 120. | Matsumura S, Wang B, Kawashima N, Braunstein S, Badura M, Cameron TO, Babb JS, Schneider RJ, Formenti SC, Dustin ML. Radiation-induced CXCL16 release by breast cancer cells attracts effector T cells. J Immunol. 2008;181:3099-3107. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 581] [Cited by in RCA: 561] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 121. | Demaria S, Kawashima N, Yang AM, Devitt ML, Babb JS, Allison JP, Formenti SC. Immune-mediated inhibition of metastases after treatment with local radiation and CTLA-4 blockade in a mouse model of breast cancer. Clin Cancer Res. 2005;11:728-734. [PubMed] |
| 122. | Dewan MZ, Galloway AE, Kawashima N, Dewyngaert JK, Babb JS, Formenti SC, Demaria S. Fractionated but not single-dose radiotherapy induces an immune-mediated abscopal effect when combined with anti-CTLA-4 antibody. Clin Cancer Res. 2009;15:5379-5388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1020] [Cited by in RCA: 1312] [Article Influence: 82.0] [Reference Citation Analysis (0)] |
| 123. | Zeng J, See AP, Phallen J, Jackson CM, Belcaid Z, Ruzevick J, Durham N, Meyer C, Harris TJ, Albesiano E. Anti-PD-1 blockade and stereotactic radiation produce long-term survival in mice with intracranial gliomas. Int J Radiat Oncol Biol Phys. 2013;86:343-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 585] [Cited by in RCA: 704] [Article Influence: 58.7] [Reference Citation Analysis (0)] |
| 124. | Deng L, Liang H, Burnette B, Beckett M, Darga T, Weichselbaum RR, Fu YX. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest. 2014;124:687-695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 125. | Slovin SF, Higano CS, Hamid O, Tejwani S, Harzstark A, Alumkal JJ, Scher HI, Chin K, Gagnier P, McHenry MB. Ipilimumab alone or in combination with radiotherapy in metastatic castration-resistant prostate cancer: results from an open-label, multicenter phase I/II study. Ann Oncol. 2013;24:1813-1821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 436] [Cited by in RCA: 433] [Article Influence: 36.1] [Reference Citation Analysis (0)] |
| 126. | Olino K, Wada S, Edil BH, Pan X, Meckel K, Weber W, Slansky J, Tamada K, Lauer P, Brockstedt D. Tumor-associated antigen expressing Listeria monocytogenes induces effective primary and memory T-cell responses against hepatic colorectal cancer metastases. Ann Surg Oncol. 2012;19 Suppl 3:S597-S607. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 127. | Kim SH, Castro F, Paterson Y, Gravekamp C. High efficacy of a Listeria-based vaccine against metastatic breast cancer reveals a dual mode of action. Cancer Res. 2009;69:5860-5866. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 173] [Cited by in RCA: 154] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 128. | Seavey MM, Pan ZK, Maciag PC, Wallecha A, Rivera S, Paterson Y, Shahabi V. A novel human Her-2/neu chimeric molecule expressed by Listeria monocytogenes can elicit potent HLA-A2 restricted CD8-positive T cell responses and impact the growth and spread of Her-2/neu-positive breast tumors. Clin Cancer Res. 2009;15:924-932. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 45] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 129. | Sinnathamby G, Lauer P, Zerfass J, Hanson B, Karabudak A, Krakover J, Secord AA, Clay TM, Morse MA, Dubensky TW. Priming and activation of human ovarian and breast cancer-specific CD8+ T cells by polyvalent Listeria monocytogenes-based vaccines. J Immunother. 2009;32:856-869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 130. | Rowe JH, Johanns TM, Ertelt JM, Lai JC, Way SS. Cytotoxic T-lymphocyte antigen 4 blockade augments the T-cell response primed by attenuated Listeria monocytogenes resulting in more rapid clearance of virulent bacterial challenge. Immunology. 2009;128:e471-e478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 131. | Creelan BC. Update on immune checkpoint inhibitors in lung cancer. Cancer Control. 2014;21:80-89. [PubMed] |