Revised: May 22, 2014
Accepted: June 20, 2014
Published online: July 27, 2014
Processing time: 116 Days and 7.5 Hours
Hepatitis C virus (HCV) is an important etiologic agent of hepatitis and a major cause of chronic liver infection that often leads to cirrhosis, fibrosis and hepatocellular carcinoma. Although, HCV is a hepatotropic virus, there is strong evidence that HCV could replicate extra-hepatic in the gastrointestinal tissue which could serve as a reservoir for HCV. The outcome of HCV infection depends mainly on the host innate and adaptive immune responses. Innate immunity against HCV includes mainly nuclear factor cells and activation of IFN-related genes. There is an immunologic link between the gut and the liver through a population of T-cells that are capable of homing to both the liver and gut via the portal circulation. However, little is known on the role of Gut immune response in HCV. In this review we discussed the immune regulation of Gut immune cells and its association with HCV pathogenesis, various outcomes of anti-HCV therapy, viral persistence and degree of liver inflammation. Additionally, we investigated the relationship between Gut immune responses to HCV and IL28B genotypes, which were identified as a strong predictor for HCV pathogenesis and treatment outcome after acute infection.
Core tip: Chronic hepatitis C (CHC) is a global worldwide health problem with approximately 200 million people worldwide infected with hepatitis C virus (HCV). It is also a major cause of chronic liver infection that often leads to chronic hepatitis which may progress to cirrhosis, fibrosis and finally hepatocellular carcinoma. In CHC, immune responses play an important role in HCV pathogenesis and responses to therapy. Intra-hepatic immune responses to HCV are highly regulated. There is a clear relationship between hepatic immune responses and mucosal immune response in the gut. Additionally, genetic immunological markers have been proposed to predict response to HCV treatment, and outcome of infection.
- Citation: Hetta HF, Mehta MJ, Shata MTM. Gut immune response in the presence of hepatitis C virus infection. World J Immunol 2014; 4(2): 52-62
- URL: https://www.wjgnet.com/2219-2824/full/v4/i2/52.htm
- DOI: https://dx.doi.org/10.5411/wji.v4.i2.52
Hepatitis C virus (HCV) was first identified by Harvey Alter in 1978 and named non-A, non-B hepatitis[1], and cloned by Houghton in 1986[2]. HCV is a single-stranded, positive sense RNA virus belonging to Hepacivirus group in the family Flaviviridae[3]. There are 6 HCV genotypes. Due to the low fidelity and lack of proofreading of HCV polymerase enzymes used for viral genome amplification, multiple mutations occur within a genotype to produce quasi-species[4,5].
Chronic hepatitis C infection (CHC) is a global worldwide health care problem with an increasing burden year-by-year[6-8]. The World Health Organization estimates that approximately 200 million people worldwide are infected with HCV[9]. It is also a major cause of chronic liver infection that often leads to chronic hepatitis which may progress to cirrhosis, fibrosis and finally hepatocellular carcinoma[3,10].
HCV is one of the most important etiologic agents of post transfusion hepatitis. HCV is usually spread by sharing infected needles with a carrier, from receiving infected blood, and from accidental exposure to infected blood. Some people acquire the infection through non parenteral means that have not been fully defined, but include sexual transmission in persons with high risk behaviors[11]. It is not reported that HCV can spread orally by food, water, breast feeding, or by normal social contact as sneezing, coughing, hugging, sharing eating utensils or drinking glasses[12]. Mother-to-baby transmission is rare and needs a high viremia as found in HIV co-infection[13].
HCV is a single stranded RNA virus which produces negative strand RNA as a replicative intermediate. The HCV genome is about 9.6 kb in length. During HCV replication cycle, one large precursor protein is synthesized from an open reading frame then cleaved to produce 10 proteins including three structural proteins which are Core, two envelope proteins (E1 and E2)[3], and P7 which results from cleavage of E2 protein[14]. The other six proteins that are not in the viral particle called non-structural proteins (NS) including NS2, NS3, NS4A, NS4B, NS5A, and NS5B[3]. Non-structural (NS) proteins are not found in the virion, therefore, presence of NS proteins inside cells suggests that HCV replication occurred in those cells[3]. Replication of HCV involves converting the viral genomic positive strand into a negative strand, and then back to the genomic strand. Thus, the presence of the negative strand strongly suggests that replication[15].
HCV is primarily a hepatotropic virus[15]. However, a broad spectrum of extra-hepatic manifestations may be associated with HCV infection, including mixed cryoglobulinemia, non-Hodgkin’s lymphoma, arthralgia, paresthesia, myalgia, pruritis, cutaneous vasculitis, glomerulonephritis, neuropathy and lymphoproliferative disorders[16,17].
HCV was believed to infect only hepatocytes[3]. However, recent studies have reported HCV infection of other cell types[15,18-21]. In fact, viral replication has been reported in B cells, T cells, monocytes, macrophages, and macrophage-like cells such as Kupffer cells, dendritic cells (DCs), renal cells, thyroid cells, and gastric cells. There is mounting evidence that these cells could represent replicative compartments for the virus[3,22,23]. In addition, it has been proposed that peripheral blood monocytes (PBMC) could be the source of recurrent HCV infection after liver transplantation[24]. Despite these reports, extra-hepatic replication of HCV is still controversial by some investigators. However, the importance of extra-hepatic HCV replication in HCV pathogenesis is clear. Extra-hepatic compartments might serve as reservoirs for HCV, and hence HCV persistence, reactivation after antiviral therapy and also may contribute to the HCV extra-hepatic manifestations[24].
There is a molecular evidence that HCV may infect and replicate in oral mucosa and gastric cells[23]. Moreover, HCV seems to be involved in development of B-cell non-Hodgkin’s lymphoma of the gastric mucosa[25]. Miglioresi et al[26], reported that Gut mucosa may serve as possible reservoir for HCV relapse after viral clearance. They analyzed HCV gastric localization in 15 patients and compared their levels of viremia with the status of HCV in gastric biopsy specimens and PBMCs. In that study, all 15 patients with positive viremia were positive for HCV RNA on Gut tissue and PBMCs. In 2 patients, HCV RNA was positive on serum, negative at Gut biopsy but their PBMCs were positive. Two patients with negative viremia and PBMCs after antiviral treatment were positive for HCV RNA on gastric sample and eventually relapsed (after 6 and 18 wk). The finding of a positive hidden compartment for HCV and simultaneous negative viremia had previously reported in HCV infected liver without detectable viremia[27]. Replication of HCV in gastrointestinal tissue represents a continuous new source as an extra-hepatic reservoir of viral particles for re-infection of hepatocytes[26].
The immune response against HCV involves innate and adaptive immunity[9]. Innate immunity against HCV is mediated by several innate immune effector cells such as NK cells, and activation of the interferons-stimulated genes (ISGs) response[28]. Recent studies have revealed that the IL28B gene locus, which codes for a type III interferon is a critical locus for outcome after acute infection[29], and response to therapy[29,30]. However, HCV may develop several strategies to overcome these responses. For example, viral NS3 and NS4a protease can cause disruption of important components of type I interferon activation cascade through inactivation of several ISGs[31,32].
Adaptive immunity against HCV is mediated by both humoral and cellular immune responses. Most HCV-infected individuals develop antibodies against HCV, regardless of the outcome of infection. Few of these antibodies can neutralize viral particles and may limit viral spread[33]. However, neutralizing antibodies have a limited role in most of the infected patients due to the high replication and mutation rate of HCV[34]. In fact, HCV clearance had been observed in some patients in the absence of neutralising antibodies[35]. Therefore, despite the potential protective role of innate and humoral immunity in the outcome of infection, it is clear that protection and viral clearance depend primarily on cellular adaptive immune responses through a complex interplay between CD4+ and CD8+ T-cell responses[9]. Unfortunately, in some patients, cellular immune responses are inadequate and fail to clear the infection with a subsequent viral persistence[9]. Fully functional virus-specific CD4+T-cell responses are detectable in patients who cleared infection[9,36-38]. The role of HCV-specific CD4+T-cell was further supported by the finding of in vivo depletion of CD4+T cells from HCV-recovered chimpanzees was associated with viral persistence[38]. Moreover, several studies have shown that HCV-specific CD8+T-cells derived from the peripheral blood or liver are functionally impaired and display a reduced ability to proliferate or secrete anti-viral cytokines such as IFN-γ [39-41]. The mechanisms contributing to CD8+T cell exhaustion in HCV are not fully understood, however, it may be partially explained by the intrinsic regulatory pathways such as signals mediated by the inhibitory receptor PD-1[40,42-45] and extrinsic regulatory pathways as regulatory T cells (Treg) or secretion of immunoregulatory cytokines such as IL-10[46-51]. Ultimately, the outcome of HCV infection, viral persistence or clearance, is determined by the host immune response[9,52,53]. Additionally, sustained HCV-specific cytotoxic T cell responses in the liver have been associated with the development of hepatic immunopathology and liver necrosis which may lead to liver cirrhosis[52,53]. The mechanisms that mediate liver inflammation and damage in CHC are not yet fully elucidated[9,54]. One of the potential mechanisms that might modulate HCV-specific immune responses is Treg cells which are a subtype of T cells that play a fundamental role in maintaining immune homeostasis and the balance between the tissue-damaging and protective effects of the immune response[54-56]. It is characterized by the expression of a unique transcription factor Forkhead box protein P3 (FoxP3), which is highly expressed in the nucleus of Treg cells and is generally accepted as the single best marker to quantify Treg cells[53,56-58]. In cases with CHC, it was reported that the frequency of Treg cells were negatively correlated with the degree of necro-inflammatory scores and their frequency is higher than that in healthy individuals[47,59,60]. Thus, Treg cells appear to assist in the maintenance of chronicity by inhibition of anti-HCV immune responses and consequently attenuate the intrahepatic tissue-damaging response to infection[49,53].
The mucosal immune system is considered the first line of defense that reduces the need for elimination of exogenous invading antigens by pro-inflammatory immune response[61]. The mucosal immune system maintains homeostasis through evolution of two layers of adaptive non-inflammatory defense; the first strategy is immune exclusion by secretory IgA (and IgM) antibodies to limit epithelial contact and penetration of invading microorganisms and other potentially dangerous antigens[61], and the second strategy is oral tolerance by development of immunosuppressive mechanisms to inhibit over-reaction against food antigens and commensal bacteria[62]. Oral tolerance depends mainly on the induction of Treg cells in mesenteric lymph nodes to which mucosal DCs carry and present food and commensal microbial antigens[63]. Gut induced tolerance include other suppressive mechanisms to ensure that persistent food allergy is relatively rare[64].
Some pathogens and food antigens could enter the liver via the portal circulation[65] within 2 h of ingestion[66] and presented on liver endothelial cells. The liver is critical in the regulation of immune responses to pathogens entering via portal circulation[67]. It receives 75% of its blood supply from the portal vein, which drains the gut. Oral tolerance is usually lost in case of a portal-systemic shunt, which allows portal blood to bypass the liver and goes directly from the gut to the systemic circulation[67,68].
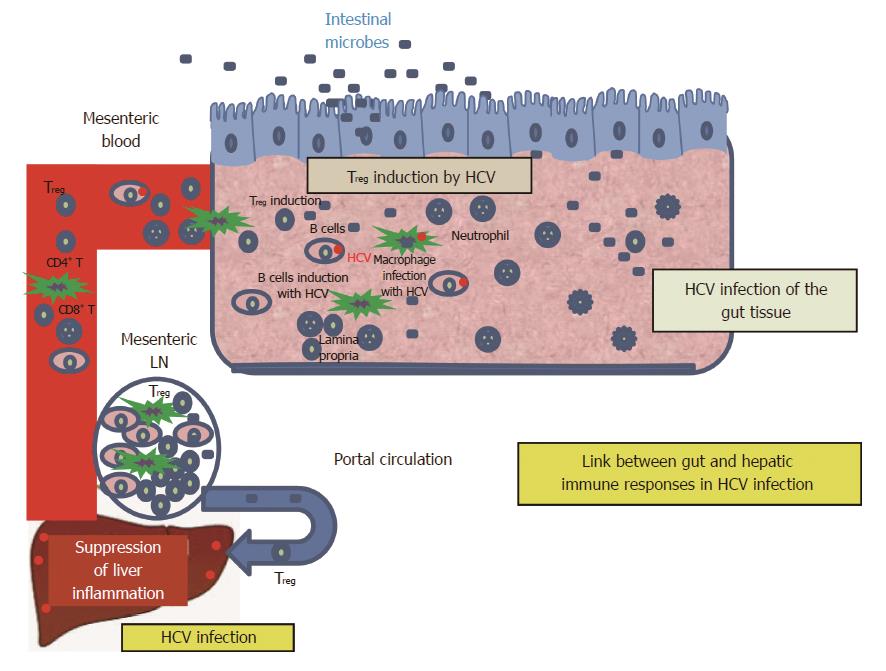
To understand the interactions between the immune responses in the Gut and the liver during HCV infection, we have to dissect the immune responses in each organ. The intestinal immune system can be divided into inductive and effector sites based upon their anatomical and functional properties[61,63]. Inductive sites include the gut-associated lymphoid tissues (GALT) such as Peyer’s patches (PP) and isolated lymphoid follicles and the mesenteric lymph nodes (mLNs). The GALT contains a wide variety of cells, such as Microfold (M) cells, DCs, intraepithelial lymphocytes (IEL), macrophages and Treg cells[61]. The main effector sites of the intestinal immune system are the lamina propria (LP) and epithelium, which harbor large populations of activated T cells and antibody-secreting plasma cells. The LP may also contribute to the induction of tolerance. It is a site of antigen uptake and loading of the migratory DCs that encounter naïve T cells in the mLNs[61]. Antigen are up-taken by absorptive epithelial and M cells in the mucosal inductive sites or directly captured by professional APCs (including DCs, Macrophage and B lymphocytes)[69]. M cells take up molecules and particles from the gut lumen by endocytosis or phagocytosis then sample them to the immune cells. Antigens are transported through M cells by the process of transcytosis. The cell membrane at the base of M cells is folded around lymphocytes and dendritic cells within the Peyer’s patches[69]. M cells present the antigen to conventional CD4+ and CD8+αβ T cells at the inductive site. At the same time, epithelial cells may process and present certain antigens directly to neighboring intraepithelial T cells such as NKT cells and γδ T cells which are T cells with limited repertoire diversity[69]. Naive B and T cells enter GALT and are primed to become memory/effector B and T cells, then migrate from GALT to mesenteric blood and the liver or to the lymph nodes via lymph and then via thoracic duct to peripheral blood for subsequent extravasation at mucosal effector sites. A system of Gut-specific lymphocyte trafficking has been evolved to target lymphocyte to the area of injury or infection through vascular adhesion molecules and chemokines. Thus, the endothelial cells act as a local gatekeeper for mucosal immunity[61]. Under normal physiological conditions enteric antigens are presented to naïve lymphocytes in the draining mesenteric lymph nodes. Lymphocytes activated by gut dendritic cells express a gut-homing phenotype characterized by expression of the chemokine receptor CCR9 and the integrin α4β7 which direct the migration of the activated lymphocytes back to gut tissue where their respective ligands CCL25 and MAdCAM-1 are expressed[67,70]. Lymphocytes that are primed to hepatic antigens acquire expression of adhesion molecules that direct them to traffic to the liver by interacting with molecules expressed on hepatic endothelium such as VAP-1.
Innate immune system in the gut includes the lining epithelium which provides barrier function, mechanical cleaning and defensins which act as chemical antimicrobial factors[71]. The gut mucosa contains a number of other cells as part of the innate immune system, including phagocytic neutrophils and macrophages, DCs, NK cells and mast cells. These cells contribute significantly to host defense against pathogens22 and also initiate adaptive mucosal immune responses[69,72].
The adaptive humoral immune defense at the gut mucosal surfaces is mainly mediated by secretory IgA (sIgA) antibody, which is the ideal antibody for functioning in mucosal secretions due to its resistance to proteases[61]. sIgA plays a protective role against a variety of foreign antigens such as food antigens, toxins, bacteria and viruses[72]. slgA blocks the access of potentially allergenic molecules derived from food or drugs[73]. Because some dietary antigen is clearly absorbed by normal subjects, the importance of sIgA antibody may lie in reducing the amount of antigen that gains access to the lamina propria[73,74]. sIgA can neutralize biologically active antigens as bacteria, toxins, enzymes and viruses. The effectiveness of sIgA as a neutralizing antibody against viruses is shown for example in the responses to oral live-attenuated poliovirus vaccine where protection correlates with levels of secretory antibody[75]. Additionally, sIgA is an efficient agglutinin that can prevent adherence of pathogenic bacteria to the epithelial surfaces and enhance the antibacterial efficiency of other effector immune system; sIgA has bactericidal potential by cooperation with complement and lysozyme and also can act as opsonin. However, the role of sIgA during HCV infection is limited.
The development of IgA immune response against mucosal pathogens and soluble protein antigens is dependent on T helper cells[76]. Mucosal T cells produce large amounts of transforming growth factor (TGF)-β, interleukin (IL)-10 and IL-4 to promote B-cell isotype class switching to IgA[77,78]. Additionally, muco-epithelial cells, and Treg cells are the major sources of TGF-β and IL-10, suggesting that cooperation between neighboring lymphocytes and epithelial cells in the mucosal microenvironment is pivotal to promote B-cell switch to IgA and differentiation into IgA-committed B cells[69].
One of the important cellular immune defense at the gut mucosal surfaces is mainly mediated by cytotoxic T lymphocyte (CTL) responses[69]. It is reported that mucosal CTLs are crucial for the immune clearance of pathogens in several animal models of infection with enteric viruses like Rota virus[79] and intracellular parasites[80]. Besides CTLs, induced IFN-γ producing CD4+ T cells, have been found to be important for mucosal immune defense to both viral and bacterial infections[69].
Gut immune response is controlled by the local microenvironment, the nature of the antigen and the type of APCs. In case of foreign food proteins and non-pathogen antigens, the default pathway for mucosal DCs and other APCs is to generate TH2 and various regulatory T cell types of responses mainly Treg[81], and Th17 cells[82] which usually leads to down-regulatory or active suppression of systemic immunity (oral tolerance). On the other hand, antigens, most pathogens harboring motifs which could bind to Toll-like receptor (TLR), and be sensed by mucosal APCs as ‘danger signals’ and pro-inflammatory conditions in general favor the development of stronger and broader immune responses but do not lead to oral tolerance[81,83,84]. Oral tolerance can be achieved through different mechanisms, including anergy, activation-induced cell death and most important, the induction of regulatory T cells[69,85]. Anergy of antigen-specific T cells has been reported after ingestion of large quantities of soluble proteins[86], and deletion of specific T cells only after mucosal administration of massive, non-physiological antigen doses[87]. Induction of regulatory T cells after mucosal delivery of antigens has been reported and received major attention given the potential of manipulating these regulatory cells as therapeutic agents in immune-mediated diseases[69].
Regulatory T cells includes: (1) CD4+CD45RBlow Tr1 cells that function through the production of IL-10 to suppresses antigen-specific T cell responses and actively down-regulates a pathological immune response[88]; (2) TH3 cell which are CD4+ or CD8+ T cells producing TGF-β with various amounts of interleukin-4 and interleukin-10[89]; and (3) Treg cells, a population of naturally occurring CD4+CD25+ regulatory T cells that suppress proliferation through a cell contact-dependent mechanism[90] followed by cell-contact-independent mechanism mediated by soluble factors such as IL-10 and TGF-β[91]. Induction of tolerance is a contact-dependent mechanism used by naturally occurring CD4+CD25+ Treg to confer suppressive activity upon conventional antigen-specific CD4+ T cells through the expression of the transcription factor Foxp3 and/or the major histocompatibility complex (MHC) class II-binding molecule LAG-3 in such cells[69,91], and inhibit T cell activation via soluble mediators. CD4+CD25+ Treg cells expressing the mucosal α4β7 integrin, when co-cultured with conventional CD4+ T cells, induced Tr1-like IL-10-secreting T cells with strong suppressor activity on effector T cells. While α4β1-positive Treg induced TH3-like TGF-β secreting suppressor T cells[91]. Moreover, intraepithelial CD8+γδ T cells in the small intestine have been involved in mucosal tolerance and are the first T cells to encounter pathogens that have invaded an epithelial surface[92].
Although the liver is capable of generating vigorous immune responses to infections such as hepatitis A and hepatitis E viruses, both of which enter via the gut, it is also characterized by immune tolerance in several settings[93,94]. A vigorous intrahepatic immune response depends on activation of T cells by fully activated DCs within secondary lymphoid tissues whereas direct activation within the liver by resident APCs including endothelial cells and hepatocytes usually results in tolerance[95]. This is logic, as it allows the liver to tolerate soluble food antigens captured by liver endothelial cells and self-antigens on hepatocytes that fail to cause damage whilst responding appropriately to infections that cause injury, inflammation and full activation of DCs[67].
Regulatory T cells as well as NK and CD1-restricted NKT cells seem to contribute to the overall bias of hepatic immune responses toward tolerance. The tolerance microenvironment of the liver may account for the survival of liver allografts and the persistence of certain liver pathogens such as hepatitis viruses[94].
The Gut and the liver share common embryological origins; the liver develops from the ventral floor of the foregut as the liver diverticulum from the undifferentiated gut endoderm[96]. Subsequently, the gut is populated by lymphocyte precursors derived from the developing liver[97] (Figure 1).
There is an immunologic link between the gut and the liver through a population of T-cells that are capable of homing to both the liver and gut via portal circulation[96]. Additionally, the liver is considered an important toleragenic organ for all of foreign proteins we are eating that are probably mediated through the Treg cells, which in turn act as a link between the gut and the liver[67,96]. Most of the infiltrating T-cells in the liver are primed cells suggesting that trafficking of memory T-cells through the liver might contribute to immune surveillance[98]. Evidence, that supports such findings, comes from observations that the gut adhesion molecules and chemokine (such as CCL25) are also detected on liver endothelium[99] providing a mechanism for the recruitment of mucosal lymphocytes to the liver[100].
Evaluation of the gut immune cells for the intrinsic gut-liver immune axis of the shared lymphocytes that recirculate between the gut and liver through the portal circulation may be considered a useful image of the intrahepatic micro-environment during HCV infection. Based on this relationship, the frequency of Treg cells in colonic tissue and its association with the various outcomes of anti-HCV therapy, viral persistence and degree of liver inflammation were examined in our laboratory. Our data indicated that the frequency of colonic Treg in CHC patients is higher than control and our findings are in concordance with previous reports that demonstrated a higher number of FoxP3+Treg cells in the liver of HCV-infected patients compared to healthy control[47,59,60]. These findings support that Treg plays a prominent role in maintaining the balance between tissue damaging and protective effects of immune responses to HCV.
While attempting to limit viral replication, T-cells inadvertently play a pivotal role in limiting hepatic necro-inflammation and subsequent fibrosis[28,101-103] by suppressing HCV-specific immune responses[48]. In our study, we found a significant inverse correlation between the frequency of colonic Treg and liver pathology indicating a role of colonic Treg in controlling the chronic inflammatory response and limit liver damage in CHC infection.
There is still an open question whether Treg cells are protective or harmful in CHC. The effective host anti-HCV immune response may be associated with strong inflammatory reactions and liver damage. To minimize the damage to self, the activation of the immune system also triggers anti-inflammatory pathways through Treg responses. Both inflammatory and anti-inflammatory reactions are normal components of the immune response, which together, fight infections while preventing immunopathology.
Until 2011, the standard of care for chronic hepatitis C patients was combined treatment with Peginterferon (Peg-IFN) and ribavirin (RBV). The combination of Peg-IFN and RBV induced sustained virologic response (SVR) in 40%-50% of genotype 1 and 80% or more in genotype 2 and 3 infections[104-106]. The lack of effective regimens across all genotypes and alternative therapeutics for patients who suffered serious side effects prompted basic science research and numerous clinical trials leading to the development of direct-acting antiviral (DAA) agents. The US Food and Drug Administration approved Telaprevir (TVR) and Boceprevir (BOC) for HCV genotype 1. They inhibit HCV nonstructural protein 3/4A (NS3/4A) serine protease, which is critical for HCV replication. TVR and BOC are approved for use in combination therapies with Peg-IFN-alpha and RBV as they improved SVR rates to 75% and 66% respectively for adult HCV genotype 1 patients with compensated liver cirrhosis[107]. However, these DAAs incur their own set of severe side effects including anemia, rash, and hyperbilirubinemia. New drugs classified as second-wave protease inhibitors, second-generation protease inhibitors, and polymerase inhibitors are being developed and currently undergoing clinical trials[108]. The NS5B polymerase inhibitor, sofosbuvir has been recently approved by the FDA for treatment of hepatitis C genotype 1, 2, and 3 patients[109].
Identifying patients that are likely to achieve SVR versus those that are likely to be non-responders is crucial for disease prognosis, providing optimal therapy, avoiding side effects, and reducing costs associated with Hepatitis C therapy. Since sequencing of the human genome in 2001, advancements along with decrease costs in genotyping technologies have led to investigation of genomic markers associated with a response to Peg-IFN and RBV in patients with chronic hepatitis C. The rs12979860 SNP located on chromosome 19 upstream of the IL-28B gene has been identified as a significant predictor of SVR in HCV Genotype 1 chronically infected patients that underwent standard therapy[110]. The same rs12979860 SNP has the ability to predict natural clearance of the hepatitis C virus[30]. Genotype C/C at the rs12979860 SNP was associated with a higher likelihood of natural clearance and therapy induced clearance of hepatitis C genotype 1, while T/T genotype was the most unfavorable[111]. Studies have confirmed that rs12979860 is the strongest predictor of SVR and can effectively predict response to IFN/RBV based therapy[112]. The mechanisms by which the rs12979860 affects HCV pathogenesis are still unclear. However, it is well-known that the IL-28B gene codes for cytokine IL-28B also known as interferon (IFN) λ -3, which belongs to the type III IFN family. IFN-λ is mainly produced by macrophages and DCs in response to viral proteins and plays an important role in antiviral responses to hepatitis C[30,113]. IFN λ receptors are predominantly expressed on hepatocytes, which may explain its ability to counteract hepatotropic viruses[114]. Therefore, stimulation of IFN λ receptors on hepatocytes by IFN-λ secreted by DCs induces ISGs[115] which have the ability to suppress viral replication and protein synthesis of HCV[116]. Additionally, IFN-λ promotes differentiation of monocyte-derived dendritic cells (DCs) with high PD-L1 expression and further promoted expansion of Treg cells[117] locally and suppressed the inflammatory responses in the liver. Recent data by our laboratory (Hetta et al, 2014 submitted) as well as others[118] identified a correlation between IL28B SNP rs12979860 genotype TT’s and Treg frequencies. The mechanism responsible for elevated Treg in patients with TT genotype may be related to the precise location of rs12979860 in the promoter region of the IL-28B gene. The promoter region plays an important role in gene expression, and the TT genotype might favor increased IL-28B expression in turn resulting in higher Treg frequencies. In support of the relationship between IL-28B phenotypes, Treg frequency, and HCV pathogenesis, recent reports found elevated Treg in acute HCV as a predictor for viral persistence and CHC as well as increased levels of IFN-λ, IL-28, and IL-29 in serum in chronic HCV patients[117].
The association between IL-28B polymorphism and SVR in genotype 2 and 3 infected patients has produced mixed results making its clinical utility less clear. For instance, one study found IL-28B polymorphism to be associated with SVR in patients infected by genotype 2/3 HCV in whom RVR was not achieved[119]. On the other hand, in a study of hepatitis C Genotype 3 infected patients, rs12979860 SNP genotype C/C did not correlate with SVR to PEG-IFN/ribavirin therapy[120]. The majority of studies to this point have focused on IL-28B SNPs in HCV Genotype 1, 2, and 3. The clinical utility of IL-28B testing is probably best served in HCV genotype 1 infected-patients for prediction of outcomes and to limit expenses and side effects associated with IFN-based therapy[110].
P- Reviewer: Dirchwolf M, Narciso-Schiavon JL, Puoti C, Sagnelli E S- Editor: Song XX L- Editor: A E- Editor: Wang CH
| 1. | Alter HJ, Purcell RH, Holland PV, Popper H. Transmissible agent in non-A, non-B hepatitis. Lancet. 1978;1:459-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 246] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 2. | Wang KS, Choo QL, Weiner AJ, Ou JH, Najarian RC, Thayer RM, Mullenbach GT, Denniston KJ, Gerin JL, Houghton M. Structure, sequence and expression of the hepatitis delta (delta) viral genome. Nature. 1986;323:508-514. [PubMed] |
| 3. | Revie D, Salahuddin SZ. Human cell types important for hepatitis C virus replication in vivo and in vitro: old assertions and current evidence. Virol J. 2011;8:346. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 59] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 4. | Simmonds P, Bukh J, Combet C, Deléage G, Enomoto N, Feinstone S, Halfon P, Inchauspé G, Kuiken C, Maertens G. Consensus proposals for a unified system of nomenclature of hepatitis C virus genotypes. Hepatology. 2005;42:962-973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1070] [Cited by in RCA: 1083] [Article Influence: 54.2] [Reference Citation Analysis (0)] |
| 5. | Farci P, Purcell RH. Clinical significance of hepatitis C virus genotypes and quasispecies. Semin Liver Dis. 2000;20:103-126. [PubMed] |
| 6. | Lauer GM, Walker BD. Hepatitis C virus infection. N Engl J Med. 2001;345:41-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2042] [Cited by in RCA: 2025] [Article Influence: 84.4] [Reference Citation Analysis (0)] |
| 7. | Gouda I, Nada O, Ezzat S, Eldaly M, Loffredo C, Taylor C, Abdel-Hamid M. Immunohistochemical detection of hepatitis C virus (genotype 4) in B-cell NHL in an Egyptian population: correlation with serum HCV-RNA. Appl Immunohistochem Mol Morphol. 2010;18:29-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 8. | Frank C, Mohamed MK, Strickland GT, Lavanchy D, Arthur RR, Magder LS, El Khoby T, Abdel-Wahab Y, Aly Ohn ES, Anwar W. The role of parenteral antischistosomal therapy in the spread of hepatitis C virus in Egypt. Lancet. 2000;355:887-891. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 694] [Cited by in RCA: 684] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 9. | Klenerman P, Thimme R. T cell responses in hepatitis C: the good, the bad and the unconventional. Gut. 2012;61:1226-1234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 10. | Kuo G, Choo QL, Alter HJ, Gitnick GL, Redeker AG, Purcell RH, Miyamura T, Dienstag JL, Alter MJ, Stevens CE. An assay for circulating antibodies to a major etiologic virus of human non-A, non-B hepatitis. Science. 1989;244:362-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2495] [Cited by in RCA: 2343] [Article Influence: 65.1] [Reference Citation Analysis (0)] |
| 11. | Alipour A, Rezaianzadeh A, Hasanzadeh J, Rajaeefard A, Davarpanah MA. Sexual Transmission of Hepatitis C Virus Between HIV Infected Subjects and Their Main Heterosexual Partners. Hepat Mon. 2013;13:e13593. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | Mast EE, Alter MJ, Margolis HS. Strategies to prevent and control hepatitis B and C virus infections: a global perspective. Vaccine. 1999;17:1730-1733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 153] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 13. | EASL International Consensus Conference on hepatitis C. Paris, 26-27 February 1999. Consensus statement. J Hepatol. 1999;31 Suppl 1:3-8. [PubMed] |
| 14. | Griffin SD, Beales LP, Clarke DS, Worsfold O, Evans SD, Jaeger J, Harris MP, Rowlands DJ. The p7 protein of hepatitis C virus forms an ion channel that is blocked by the antiviral drug, Amantadine. FEBS Lett. 2003;535:34-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 351] [Cited by in RCA: 347] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 15. | Castillo I, Rodríguez-Iñigo E, Bartolomé J, de Lucas S, Ortíz-Movilla N, López-Alcorocho JM, Pardo M, Carreño V. Hepatitis C virus replicates in peripheral blood mononuclear cells of patients with occult hepatitis C virus infection. Gut. 2005;54:682-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 113] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 16. | Blackard JT, Kemmer N, Sherman KE. Extrahepatic replication of HCV: insights into clinical manifestations and biological consequences. Hepatology. 2006;44:15-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 134] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 17. | Agnello V, De Rosa FG. Extrahepatic disease manifestations of HCV infection: some current issues. J Hepatol. 2004;40:341-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 92] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 18. | Manzin A, Candela M, Paolucci S, Caniglia ML, Gabrielli A, Clementi M. Presence of hepatitis C virus (HCV) genomic RNA and viral replicative intermediates in bone marrow and peripheral blood mononuclear cells from HCV-infected patients. Clin Diagn Lab Immunol. 1994;1:160-163. [PubMed] |
| 19. | Wang JT, Sheu JC, Lin JT, Wang TH, Chen DS. Detection of replicative form of hepatitis C virus RNA in peripheral blood mononuclear cells. J Infect Dis. 1992;166:1167-1169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 134] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 20. | Chang TT, Young KC, Yang YJ, Lei HY, Wu HL. Hepatitis C virus RNA in peripheral blood mononuclear cells: comparing acute and chronic hepatitis C virus infection. Hepatology. 1996;23:977-981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 43] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 21. | Saleh MG, Tibbs CJ, Koskinas J, Pereira LM, Bomford AB, Portmann BC, McFarlane IG, Williams R. Hepatic and extrahepatic hepatitis C virus replication in relation to response to interferon therapy. Hepatology. 1994;20:1399-1404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 63] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 22. | Yan FM, Chen AS, Hao F, Zhao XP, Gu CH, Zhao LB, Yang DL, Hao LJ. Hepatitis C virus may infect extrahepatic tissues in patients with hepatitis C. World J Gastroenterol. 2000;6:805-811. [PubMed] |
| 23. | Carrozzo M, Quadri R, Latorre P, Pentenero M, Paganin S, Bertolusso G, Gandolfo S, Negro F. Molecular evidence that the hepatitis C virus replicates in the oral mucosa. J Hepatol. 2002;37:364-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 72] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 24. | Féray C, Samuel D, Thiers V, Gigou M, Pichon F, Bismuth A, Reynes M, Maisonneuve P, Bismuth H, Bréchot C. Reinfection of liver graft by hepatitis C virus after liver transplantation. J Clin Invest. 1992;89:1361-1365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 206] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 25. | Tursi A, Brandimante G, Chiarelli F, Spagnoli A, Torello M. Detection of HCV RNA in gastric mucosa-associated lymphoid tissue by in situ hybridization: evidence of a new extrahepatic localization of HCV with increased risk of gastric malt lymphoma. Am J Gastroenterol. 2002;97:1802-1806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 26. | Miglioresi L, Riva E, Antonelli G, Russo F, Ricci GL. Localization of hepatitis C virus in gastrointestinal mucosa: a possible reservoir for relapse. Hepatology. 2003;38:775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 27. | McHutchison JG, Poynard T, Esteban-Mur R, Davis GL, Goodman ZD, Harvey J, Ling MH, Garaud JJ, Albrecht JK, Patel K. Hepatic HCV RNA before and after treatment with interferon alone or combined with ribavirin. Hepatology. 2002;35:688-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 89] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 28. | Rehermann B. Interaction between the hepatitis C virus and the immune system. Semin Liver Dis. 2000;20:127-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 98] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 29. | Kelly C, Klenerman P, Barnes E. Interferon lambdas: the next cytokine storm. Gut. 2011;60:1284-1293. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 86] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 30. | Thomas DL, Thio CL, Martin MP, Qi Y, Ge D, O’Huigin C, Kidd J, Kidd K, Khakoo SI, Alexander G. Genetic variation in IL28B and spontaneous clearance of hepatitis C virus. Nature. 2009;461:798-801. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1706] [Cited by in RCA: 1687] [Article Influence: 105.4] [Reference Citation Analysis (0)] |
| 31. | Rehermann B. Hepatitis C virus versus innate and adaptive immune responses: a tale of coevolution and coexistence. J Clin Invest. 2009;119:1745-1754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 394] [Cited by in RCA: 419] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 32. | Walker CM. Adaptive immunity to the hepatitis C virus. Adv Virus Res. 2010;78:43-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 57] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 33. | Pestka JM, Zeisel MB, Bläser E, Schürmann P, Bartosch B, Cosset FL, Patel AH, Meisel H, Baumert J, Viazov S. Rapid induction of virus-neutralizing antibodies and viral clearance in a single-source outbreak of hepatitis C. Proc Natl Acad Sci USA. 2007;104:6025-6030. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 444] [Cited by in RCA: 435] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 34. | Spengler U, Nattermann J. Immunopathogenesis in hepatitis C virus cirrhosis. Clin Sci (Lond). 2007;112:141-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 68] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 35. | Post JJ, Pan Y, Freeman AJ, Harvey CE, White PA, Palladinetti P, Haber PS, Marinos G, Levy MH, Kaldor JM. Clearance of hepatitis C viremia associated with cellular immunity in the absence of seroconversion in the hepatitis C incidence and transmission in prisons study cohort. J Infect Dis. 2004;189:1846-1855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 117] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 36. | Lucas M, Ulsenheimer A, Pfafferot K, Heeg MH, Gaudieri S, Grüner N, Rauch A, Gerlach JT, Jung MC, Zachoval R. Tracking virus-specific CD4+ T cells during and after acute hepatitis C virus infection. PLoS One. 2007;2:e649. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 62] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 37. | Missale G, Bertoni R, Lamonaca V, Valli A, Massari M, Mori C, Rumi MG, Houghton M, Fiaccadori F, Ferrari C. Different clinical behaviors of acute hepatitis C virus infection are associated with different vigor of the anti-viral cell-mediated immune response. J Clin Invest. 1996;98:706-714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 509] [Cited by in RCA: 490] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 38. | Grakoui A, Shoukry NH, Woollard DJ, Han JH, Hanson HL, Ghrayeb J, Murthy KK, Rice CM, Walker CM. HCV persistence and immune evasion in the absence of memory T cell help. Science. 2003;302:659-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 632] [Cited by in RCA: 635] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 39. | Gruener NH, Lechner F, Jung MC, Diepolder H, Gerlach T, Lauer G, Walker B, Sullivan J, Phillips R, Pape GR. Sustained dysfunction of antiviral CD8+ T lymphocytes after infection with hepatitis C virus. J Virol. 2001;75:5550-5558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 407] [Cited by in RCA: 397] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 40. | Nakamoto N, Kaplan DE, Coleclough J, Li Y, Valiga ME, Kaminski M, Shaked A, Olthoff K, Gostick E, Price DA. Functional restoration of HCV-specific CD8 T cells by PD-1 blockade is defined by PD-1 expression and compartmentalization. Gastroenterology. 2008;134:1927-1937, 1937.e1-2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 233] [Cited by in RCA: 233] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 41. | Spangenberg HC, Viazov S, Kersting N, Neumann-Haefelin C, McKinney D, Roggendorf M, von Weizsäcker F, Blum HE, Thimme R. Intrahepatic CD8+ T-cell failure during chronic hepatitis C virus infection. Hepatology. 2005;42:828-837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 120] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 42. | McMahan RH, Golden-Mason L, Nishimura MI, McMahon BJ, Kemper M, Allen TM, Gretch DR, Rosen HR. Tim-3 expression on PD-1+ HCV-specific human CTLs is associated with viral persistence, and its blockade restores hepatocyte-directed in vitro cytotoxicity. J Clin Invest. 2010;120:4546-4557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 260] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 43. | Radziewicz H, Ibegbu CC, Fernandez ML, Workowski KA, Obideen K, Wehbi M, Hanson HL, Steinberg JP, Masopust D, Wherry EJ. Liver-infiltrating lymphocytes in chronic human hepatitis C virus infection display an exhausted phenotype with high levels of PD-1 and low levels of CD127 expression. J Virol. 2007;81:2545-2553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 355] [Cited by in RCA: 380] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 44. | Bengsch B, Seigel B, Ruhl M, Timm J, Kuntz M, Blum HE, Pircher H, Thimme R. Coexpression of PD-1, 2B4, CD160 and KLRG1 on exhausted HCV-specific CD8+ T cells is linked to antigen recognition and T cell differentiation. PLoS Pathog. 2010;6:e1000947. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 273] [Cited by in RCA: 305] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 45. | Rutebemberwa A, Ray SC, Astemborski J, Levine J, Liu L, Dowd KA, Clute S, Wang C, Korman A, Sette A. High-programmed death-1 levels on hepatitis C virus-specific T cells during acute infection are associated with viral persistence and require preservation of cognate antigen during chronic infection. J Immunol. 2008;181:8215-8225. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 95] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 46. | Thimme R, Opitz OG. Interleukin-10 and viral clearance: translation to viral hepatitis. Gastroenterology. 2007;132:2611-2613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 47. | Boettler T, Spangenberg HC, Neumann-Haefelin C, Panther E, Urbani S, Ferrari C, Blum HE, von Weizsäcker F, Thimme R. T cells with a CD4+CD25+ regulatory phenotype suppress in vitro proliferation of virus-specific CD8+ T cells during chronic hepatitis C virus infection. J Virol. 2005;79:7860-7867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 327] [Cited by in RCA: 329] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 48. | Cabrera R, Tu Z, Xu Y, Firpi RJ, Rosen HR, Liu C, Nelson DR. An immunomodulatory role for CD4(+)CD25(+) regulatory T lymphocytes in hepatitis C virus infection. Hepatology. 2004;40:1062-1071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 426] [Cited by in RCA: 430] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 49. | Amoroso A, D’Amico F, Consolo M, Skarmoutsou E, Neri S, Dianzani U, Spandidos DA, Mazzarino MC. Evaluation of circulating CD4+CD25+ and liver-infiltrating Foxp3+ cells in HCV-associated liver disease. Int J Mol Med. 2012;29:983-988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 50. | Sugimoto K, Ikeda F, Stadanlick J, Nunes FA, Alter HJ, Chang KM. Suppression of HCV-specific T cells without differential hierarchy demonstrated ex vivo in persistent HCV infection. Hepatology. 2003;38:1437-1448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 126] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 51. | Ward SM, Fox BC, Brown PJ, Worthington J, Fox SB, Chapman RW, Fleming KA, Banham AH, Klenerman P. Quantification and localisation of FOXP3+ T lymphocytes and relation to hepatic inflammation during chronic HCV infection. J Hepatol. 2007;47:316-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 122] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 52. | Bowen DG, Walker CM. Adaptive immune responses in acute and chronic hepatitis C virus infection. Nature. 2005;436:946-952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 570] [Cited by in RCA: 578] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 53. | Keynan Y, Card CM, McLaren PJ, Dawood MR, Kasper K, Fowke KR. The role of regulatory T cells in chronic and acute viral infections. Clin Infect Dis. 2008;46:1046-1052. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 61] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 54. | Hartling HJ, Gaardbo JC, Ronit A, Knudsen LS, Ullum H, Vainer B, Clausen MR, Skogstrand K, Gerstoft J, Nielsen SD. CD4⁺ and CD8⁺ regulatory T cells (Tregs) are elevated and display an active phenotype in patients with chronic HCV mono-infection and HIV/HCV co-infection. Scand J Immunol. 2012;76:294-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 55. | Bolacchi F, Sinistro A, Ciaprini C, Demin F, Capozzi M, Carducci FC, Drapeau CM, Rocchi G, Bergamini A. Increased hepatitis C virus (HCV)-specific CD4+CD25+ regulatory T lymphocytes and reduced HCV-specific CD4+ T cell response in HCV-infected patients with normal versus abnormal alanine aminotransferase levels. Clin Exp Immunol. 2006;144:188-196. [PubMed] |
| 56. | Sturm N, Thélu MA, Camous X, Dimitrov G, Ramzan M, Dufeu-Duchesne T, Bonorino P, Guillermet C, Brambilla E, Arvers P. Characterization and role of intra-hepatic regulatory T cells in chronic hepatitis C pathogenesis. J Hepatol. 2010;53:25-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 57. | Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133:775-787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3445] [Cited by in RCA: 3906] [Article Influence: 229.8] [Reference Citation Analysis (0)] |
| 58. | Magg T, Mannert J, Ellwart JW, Schmid I, Albert MH. Subcellular localization of FOXP3 in human regulatory and nonregulatory T cells. Eur J Immunol. 2012;42:1627-1638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 69] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 59. | Rushbrook SM, Ward SM, Unitt E, Vowler SL, Lucas M, Klenerman P, Alexander GJ. Regulatory T cells suppress in vitro proliferation of virus-specific CD8+ T cells during persistent hepatitis C virus infection. J Virol. 2005;79:7852-7859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 227] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 60. | Itose I, Kanto T, Kakita N, Takebe S, Inoue M, Higashitani K, Miyazaki M, Miyatake H, Sakakibara M, Hiramatsu N. Enhanced ability of regulatory T cells in chronic hepatitis C patients with persistently normal alanine aminotransferase levels than those with active hepatitis. J Viral Hepat. 2009;16:844-852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 61. | Brandtzaeg P. Mucosal immunity: induction, dissemination, and effector functions. Scand J Immunol. 2009;70:505-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 302] [Cited by in RCA: 316] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 62. | Brandtzaeg P. History of oral tolerance and mucosal immunity. Ann N Y Acad Sci. 1996;778:1-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 101] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 63. | Brandtzaeg P. ‘ABC’ of mucosal immunology. Nestle Nutr Workshop Ser Pediatr Program. 2009;64:23-38; discussion 38-43, 251-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 64. | Artis D. Epithelial-cell recognition of commensal bacteria and maintenance of immune homeostasis in the gut. Nat Rev Immunol. 2008;8:411-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 733] [Cited by in RCA: 784] [Article Influence: 46.1] [Reference Citation Analysis (0)] |
| 65. | Husby S, Jensenius JC, Svehag SE. Passage of undegraded dietary antigen into the blood of healthy adults. Quantification, estimation of size distribution, and relation of uptake to levels of specific antibodies. Scand J Immunol. 1985;22:83-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 133] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 66. | Limmer A, Ohl J, Wingender G, Berg M, Jüngerkes F, Schumak B, Djandji D, Scholz K, Klevenz A, Hegenbarth S. Cross-presentation of oral antigens by liver sinusoidal endothelial cells leads to CD8 T cell tolerance. Eur J Immunol. 2005;35:2970-2981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 134] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 67. | Adams DH, Eksteen B, Curbishley SM. Immunology of the gut and liver: a love/hate relationship. Gut. 2008;57:838-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 57] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 68. | Yang R, Liu Q, Grosfeld JL, Pescovitz MD. Intestinal venous drainage through the liver is a prerequisite for oral tolerance induction. J Pediatr Surg. 1994;29:1145-1148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 86] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 69. | Holmgren J, Czerkinsky C. Mucosal immunity and vaccines. Nat Med. 2005;11:S45-S53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1078] [Cited by in RCA: 1155] [Article Influence: 57.8] [Reference Citation Analysis (0)] |
| 70. | Agace WW. Tissue-tropic effector T cells: generation and targeting opportunities. Nat Rev Immunol. 2006;6:682-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 129] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 71. | Shata MT, Abdel-Hameed EA, Hetta HF, Sherman KE. Immune activation in HIV/HCV-infected patients is associated with low-level expression of liver expressed antimicrobial peptide-2 (LEAP-2). J Clin Pathol. 2013;66:967-975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 72. | Yuan Q, Walker WA. Innate immunity of the gut: mucosal defense in health and disease. J Pediatr Gastroenterol Nutr. 2004;38:463-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 62] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 73. | Heremans JF, Bazin H. Antibodies induced by local antigenic stimulation of mucosal surfaces. Ann N Y Acad Sci. 1971;190:268-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 74. | Doe WF. The intestinal immune system. Gut. 1989;30:1679-1685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 41] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 75. | Ogra PL, Karzon DT. Poliovirus antibody response in serum and nasal secretions following intranasal inoculation with inactivated poliovaccine. J Immunol. 1969;102:15-23. [PubMed] |
| 76. | Lycke N, Eriksen L, Holmgren J. Protection against cholera toxin after oral immunization is thymus-dependent and associated with intestinal production of neutralizing IgA antitoxin. Scand J Immunol. 1987;25:413-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 65] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 77. | Goodrich ME, McGee DW. Regulation of mucosal B cell immunoglobulin secretion by intestinal epithelial cell-derived cytokines. Cytokine. 1998;10:948-955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 78. | Asano T, Kaneko H, Terada T, Kasahara Y, Fukao T, Kasahara K, Kondo N. Molecular analysis of B-cell differentiation in selective or partial IgA deficiency. Clin Exp Immunol. 2004;136:284-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 79. | Franco MA, Greenberg HB. Role of B cells and cytotoxic T lymphocytes in clearance of and immunity to rotavirus infection in mice. J Virol. 1995;69:7800-7806. [PubMed] |
| 80. | Buzoni-Gatel D, Lepage AC, Dimier-Poisson IH, Bout DT, Kasper LH. Adoptive transfer of gut intraepithelial lymphocytes protects against murine infection with Toxoplasma gondii. J Immunol. 1997;158:5883-5889. [PubMed] |
| 81. | Iwasaki A, Kelsall BL. Freshly isolated Peyer’s patch, but not spleen, dendritic cells produce interleukin 10 and induce the differentiation of T helper type 2 cells. J Exp Med. 1999;190:229-239. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 498] [Cited by in RCA: 487] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 82. | Rossi M, Bot A. The Th17 cell population and the immune homeostasis of the gastrointestinal tract. Int Rev Immunol. 2013;32:471-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 83. | Bilsborough J, Viney JL. Gastrointestinal dendritic cells play a role in immunity, tolerance, and disease. Gastroenterology. 2004;127:300-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 83] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 84. | Mowat AM. Anatomical basis of tolerance and immunity to intestinal antigens. Nat Rev Immunol. 2003;3:331-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 936] [Cited by in RCA: 911] [Article Influence: 41.4] [Reference Citation Analysis (0)] |
| 85. | Faria AM, Weiner HL. Oral tolerance. Immunol Rev. 2005;206:232-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 523] [Cited by in RCA: 531] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 86. | Whitacre CC, Gienapp IE, Orosz CG, Bitar DM. Oral tolerance in experimental autoimmune encephalomyelitis. III. Evidence for clonal anergy. J Immunol. 1991;147:2155-2163. [PubMed] |
| 87. | Chen Y, Inobe J, Marks R, Gonnella P, Kuchroo VK, Weiner HL. Peripheral deletion of antigen-reactive T cells in oral tolerance. Nature. 1995;376:177-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 595] [Cited by in RCA: 586] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 88. | Groux H, O’Garra A, Bigler M, Rouleau M, Antonenko S, de Vries JE, Roncarolo MG. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 1997;389:737-742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2752] [Cited by in RCA: 2709] [Article Influence: 96.8] [Reference Citation Analysis (2)] |
| 89. | Chen Y, Kuchroo VK, Inobe J, Hafler DA, Weiner HL. Regulatory T cell clones induced by oral tolerance: suppression of autoimmune encephalomyelitis. Science. 1994;265:1237-1240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1456] [Cited by in RCA: 1428] [Article Influence: 46.1] [Reference Citation Analysis (0)] |
| 90. | Thornton AM, Shevach EM. CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J Exp Med. 1998;188:287-296. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1907] [Cited by in RCA: 1955] [Article Influence: 72.4] [Reference Citation Analysis (0)] |
| 91. | Stassen M, Fondel S, Bopp T, Richter C, Müller C, Kubach J, Becker C, Knop J, Enk AH, Schmitt S. Human CD25+ regulatory T cells: two subsets defined by the integrins alpha 4 beta 7 or alpha 4 beta 1 confer distinct suppressive properties upon CD4+ T helper cells. Eur J Immunol. 2004;34:1303-1311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 125] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 92. | McMenamin C, Pimm C, McKersey M, Holt PG. Regulation of IgE responses to inhaled antigen in mice by antigen-specific gamma delta T cells. Science. 1994;265:1869-1871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 300] [Cited by in RCA: 304] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 93. | Knolle PA, Limmer A. Neighborhood politics: the immunoregulatory function of organ-resident liver endothelial cells. Trends Immunol. 2001;22:432-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 112] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 94. | Crispe IN. Hepatic T cells and liver tolerance. Nat Rev Immunol. 2003;3:51-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 506] [Cited by in RCA: 514] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 95. | Bowen DG, McCaughan GW, Bertolino P. Intrahepatic immunity: a tale of two sites? Trends Immunol. 2005;26:512-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 75] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 96. | Gualdi R, Bossard P, Zheng M, Hamada Y, Coleman JR, Zaret KS. Hepatic specification of the gut endoderm in vitro: cell signaling and transcriptional control. Genes Dev. 1996;10:1670-1682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 453] [Cited by in RCA: 443] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 97. | Yoshida H, Kawamoto H, Santee SM, Hashi H, Honda K, Nishikawa S, Ware CF, Katsura Y, Nishikawa SI. Expression of alpha(4)beta(7) integrin defines a distinct pathway of lymphoid progenitors committed to T cells, fetal intestinal lymphotoxin producer, NK, and dendritic cells. J Immunol. 2001;167:2511-2521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 113] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 98. | Ward SM, Jonsson JR, Sierro S, Clouston AD, Lucas M, Vargas AL, Powell EE, Klenerman P. Virus-specific CD8+ T lymphocytes within the normal human liver. Eur J Immunol. 2004;34:1526-1531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 99. | Grant AJ, Lalor PF, Hübscher SG, Briskin M, Adams DH. MAdCAM-1 expressed in chronic inflammatory liver disease supports mucosal lymphocyte adhesion to hepatic endothelium (MAdCAM-1 in chronic inflammatory liver disease). Hepatology. 2001;33:1065-1072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 207] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 100. | Eksteen B, Grant AJ, Miles A, Curbishley SM, Lalor PF, Hübscher SG, Briskin M, Salmon M, Adams DH. Hepatic endothelial CCL25 mediates the recruitment of CCR9+ gut-homing lymphocytes to the liver in primary sclerosing cholangitis. J Exp Med. 2004;200:1511-1517. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 233] [Cited by in RCA: 239] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 101. | Napoli J, Bishop GA, McGuinness PH, Painter DM, McCaughan GW. Progressive liver injury in chronic hepatitis C infection correlates with increased intrahepatic expression of Th1-associated cytokines. Hepatology. 1996;24:759-765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 278] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 102. | Quiroga JA, Martín J, Navas S, Carreño V. Induction of interleukin-12 production in chronic hepatitis C virus infection correlates with the hepatocellular damage. J Infect Dis. 1998;178:247-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 103. | Abrignani S. Immune responses throughout hepatitis C virus (HCV) infection: HCV from the immune system point of view. Springer Semin Immunopathol. 1997;19:47-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 104. | Fried MW, Shiffman ML, Reddy KR, Smith C, Marinos G, Gonçales FL, Häussinger D, Diago M, Carosi G, Dhumeaux D. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347:975-982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4847] [Cited by in RCA: 4748] [Article Influence: 206.4] [Reference Citation Analysis (0)] |
| 105. | Manns MP, McHutchison JG, Gordon SC, Rustgi VK, Shiffman M, Reindollar R, Goodman ZD, Koury K, Ling M, Albrecht JK. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001;358:958-965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4736] [Cited by in RCA: 4558] [Article Influence: 189.9] [Reference Citation Analysis (0)] |
| 106. | Hadziyannis SJ, Sette H, Morgan TR, Balan V, Diago M, Marcellin P, Ramadori G, Bodenheimer H, Bernstein D, Rizzetto M. Peginterferon-alpha2a and ribavirin combination therapy in chronic hepatitis C: a randomized study of treatment duration and ribavirin dose. Ann Intern Med. 2004;140:346-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2216] [Cited by in RCA: 2109] [Article Influence: 100.4] [Reference Citation Analysis (0)] |
| 107. | Ghany MG, Nelson DR, Strader DB, Thomas DL, Seeff LB. An update on treatment of genotype 1 chronic hepatitis C virus infection: 2011 practice guideline by the American Association for the Study of Liver Diseases. Hepatology. 2011;54:1433-1444. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 803] [Cited by in RCA: 844] [Article Influence: 60.3] [Reference Citation Analysis (0)] |
| 108. | Chae HB, Park SM, Youn SJ. Direct-acting antivirals for the treatment of chronic hepatitis C: open issues and future perspectives. ScientificWorldJournal. 2013;2013:704912. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 109. | Koff RS. Review article: the efficacy and safety of sofosbuvir, a novel, oral nucleotide NS5B polymerase inhibitor, in the treatment of chronic hepatitis C virus infection. Aliment Pharmacol Ther. 2014;39:478-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 90] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 110. | Ge D, Fellay J, Thompson AJ, Simon JS, Shianna KV, Urban TJ, Heinzen EL, Qiu P, Bertelsen AH, Muir AJ. Genetic variation in IL28B predicts hepatitis C treatment-induced viral clearance. Nature. 2009;461:399-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2776] [Cited by in RCA: 2723] [Article Influence: 170.2] [Reference Citation Analysis (0)] |
| 111. | Pearlman BL. The IL-28 genotype: how it will affect the care of patients with hepatitis C virus infection. Curr Gastroenterol Rep. 2011;13:78-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 112. | Thompson AJ, Muir AJ, Sulkowski MS, Ge D, Fellay J, Shianna KV, Urban T, Afdhal NH, Jacobson IM, Esteban R. Interleukin-28B polymorphism improves viral kinetics and is the strongest pretreatment predictor of sustained virologic response in genotype 1 hepatitis C virus. Gastroenterology. 2010;139:120-129.e18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 513] [Cited by in RCA: 535] [Article Influence: 35.7] [Reference Citation Analysis (0)] |
| 113. | Ank N, Iversen MB, Bartholdy C, Staeheli P, Hartmann R, Jensen UB, Dagnaes-Hansen F, Thomsen AR, Chen Z, Haugen H. An important role for type III interferon (IFN-lambda/IL-28) in TLR-induced antiviral activity. J Immunol. 2008;180:2474-2485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 330] [Cited by in RCA: 352] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 114. | Sheppard P, Kindsvogel W, Xu W, Henderson K, Schlutsmeyer S, Whitmore TE, Kuestner R, Garrigues U, Birks C, Roraback J. IL-28, IL-29 and their class II cytokine receptor IL-28R. Nat Immunol. 2003;4:63-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1221] [Cited by in RCA: 1217] [Article Influence: 55.3] [Reference Citation Analysis (0)] |
| 115. | Stark GR, Kerr IM, Williams BR, Silverman RH, Schreiber RD. How cells respond to interferons. Annu Rev Biochem. 1998;67:227-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3048] [Cited by in RCA: 3080] [Article Influence: 114.1] [Reference Citation Analysis (0)] |
| 116. | Samuel CE. Antiviral actions of interferons. Clin Microbiol Rev. 2001;14:778-809, table of contents. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2029] [Cited by in RCA: 2003] [Article Influence: 83.5] [Reference Citation Analysis (0)] |
| 117. | Dolganiuc A, Kodys K, Marshall C, Saha B, Zhang S, Bala S, Szabo G. Type III interferons, IL-28 and IL-29, are increased in chronic HCV infection and induce myeloid dendritic cell-mediated FoxP3+ regulatory T cells. PLoS One. 2012;7:e44915. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 54] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 118. | Perrella A, Vitiello L, Atripaldi L, Conti P, Sbreglia C, Altamura S, Patarino T, Vela R, Morelli G, Bellopede P. Elevated CD4+/CD25+ T cell frequency and function during acute hepatitis C presage chronic evolution. Gut. 2006;55:1370-1371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 119. | Mangia A, Thompson AJ, Santoro R, Piazzolla V, Tillmann HL, Patel K, Shianna KV, Mottola L, Petruzzellis D, Bacca D. An IL28B polymorphism determines treatment response of hepatitis C virus genotype 2 or 3 patients who do not achieve a rapid virologic response. Gastroenterology. 2010;139:821-827, 827.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 235] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 120. | Moghaddam A, Melum E, Reinton N, Ring-Larsen H, Verbaan H, Bjøro K, Dalgard O. IL28B genetic variation and treatment response in patients with hepatitis C virus genotype 3 infection. Hepatology. 2011;53:746-754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 103] [Article Influence: 7.4] [Reference Citation Analysis (0)] |