Published online Jul 27, 2014. doi: 10.5411/wji.v4.i2.116
Revised: May 10, 2014
Accepted: June 27, 2014
Published online: July 27, 2014
Processing time: 121 Days and 17 Hours
Cardiovascular diseases, especially atherosclerosis, found to be the dreadful diseases worldwide. There are diverse pathways associated with the progression of atherosclerosis. One of the important signaling pathways to target atherosclerotic plaque rupture is toll-like receptor 4 (TLR4) Pathway. Several studies are available for illustrating the role of TLR4 in health and diseases. Different types of immune cell are activated in atherosclerosis but primary cells that are activated by the TLR4 signaling are macrophages and endothelial cells. Mechanisms by which macrophages uptake lipids are diverse and it is very important to target signaling pathway responsible for controlling foam cell formation. The process of macrophages transformed foam cell formation is the critical event in progression of atherosclerotic lesion and TLR4 found to have actively participate in the event through mitogen activated protein kinases (MAPKs) activation. The activation of MAPKs signaling pathway leads to the accumulation of cholesterol in the macrophages and also contribute to the dissociation of IκB and the nuclear translocation of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) p65 subunit, thereby activating key inflammatory cascade activation by MAPKs/NF-κB signaling pathway to induce toxicity by activating different inflammatory parameters. Hence, the review focussed on exploring the role of TLR4/MAPKs signaling pathway for the therapeutic inhibition of atherosclerosis.
Core tip: The inhibition of atherosclerosis is one of primary target for the therapeutics of cardiovascular diseases, which is the eminent health problem worldwide. The important function of toll-like receptor 4 (TLR4) in the activation and progression of atherosclerosis is justified here. The TLR4 in turn activates the mitogen activated protein kinases (MAPKs) and nuclear factor kappa-light-chain-enhancer of activated B cells which are responsible for most of the inflammatory events. Hence, therapeutic inhibition of TLR4/MAPKs signaling pathway is one of the best method of inhibiting atherosclerosis.
- Citation: Ayyappan JP, Abraham A. Targeting TLR4/MAPKs signaling pathway: A better option for therapeutic inhibition of atherosclerosis. World J Immunol 2014; 4(2): 116-121
- URL: https://www.wjgnet.com/2219-2824/full/v4/i2/116.htm
- DOI: https://dx.doi.org/10.5411/wji.v4.i2.116
Cardiovascular disease, especially atherosclerosis is a main health problem worldwide and it is a disease characterised by the deposition of lipid in the blood vessels. There are several studies undertaken to know the proximal role of immune system in atherosclerosis[1]. Macrophages are the primary cells which are present in atherosclerotic lesions and they uptake lipids and get transformed to foam cells. These foam cells are risky and contribute to the development of atherosclerotic plaque rupture.
It was known that inflammatory process and its further cascade by activating immune system may contribute to the development of inflammation related atherosclerosis[2]. Usually the luminal side of the blood vessel walls are prone to atherosclerotic injury[3]. The presence of human histocompatibility leukocyte antigen is widely upregulated as the result of inflammatory processes[4]. There are studies reporting the role of variety of Infectious organisms and HSP60 as trigger of atherosclerosis[5].
It is very important to know the mechanism by which macrophages uptakes lipid and transformed get into foam cells. Targeting of macrophages transformed to foam cells are very important therapeutic strategies[6]. The studies on mechanism by which macrophages accumulate OxLDL and its further activation cascades are very important. It usually activates further cascades by activating components like polyoxygenated cholesteryl ester hydroperoxides and in turn activates toll-like receptor 4 (TLR4)[6].
It was suggested that TLR4 act as a link between inflammation and atherosclerosis[7]. TLR4 found to have an active participation in the progression of atherosclerotic diseases. It can also interferes with the cholesterol metabolic machinery in macrophages[8]. The research in TLR4 shown that, silencing of TLR4 gene seems to have reduced the size of atherosclerotic lesion, lipid content and macrophage polarisation in mice fed a high cholesterol diet for continuous six months[9].
TLR4 found to have act as an important receptor for arterial remodelling[10]. The activation of TLR4 receptor leads to the further activation of MYD88 protein and through protein cascade further activates mitogen activated protein kinases (MAPKs). The activation of MAPKs are essential for the secretion of chemoattract protein to direct monocytes to the atherosclerotic site[11]. The study on inhibition of tyrosine phosphatases like MAPKs found to have demolished the atherosclerotic lesion size in mice[11].
The phosphorylation of MAPKs triggers the activation of several downstream proteins and further activates the nuclear factor translocation (NF-κB) which ultimately leading to the progression and rupture of atherosclerotic plaque [12]. Hence, the review focussed on exploring the role of TLR4/MAPKs signaling pathway in therapeutic inhibition of atherosclerosis.
The immune system is considered to be the guardian of host and its activation as a result to solve the denudation of endothelium. If the immune system unable to control this activation, then it will result in the chronic immune reaction and can result in the development of atherosclerotic plaque formation[13]. The regions of atherosclerotic lesions are usually crowded with macrophages and T cells which usually plays an adequate role in innate and acquired immune reactions. It was known in atherosclerotic disease condition there is an clonal expansion of differentiated T cells, which are common in all adaptive immune reactions[14].
Toll-like receptor-4 (TLR4) pattern-recognition receptors are found to have an important role in the immune function. TLRs resides in the family of type I transmembrane receptor which consists of intracellular domain and an extracellular leucine repeat domain[15-17]. It was known that human TLR4 was the first characterised form of mammalian toll[15]. TLR4 is expressed in different types of cells, among them most abundant cell type is macrophages and dendric cells[15]. Usually, it is an membrane receptor which act as a signal transducing agent in different inflammatory insult condition like LPS induced[18-21].
The extensive research in the field of TLRs resulted in knowing mechanism of immune response induced by TLR4, it is by recognition the pathogen associated molecular pattern. The recent studies using mouse knock out genes demonstrated the active role of TLR4 in triggering and development of atherosclerotic plaque[15].
Among the toll like receptors, the best characterised form is the TLR4, which has found to have prominent role in the atherosclerosis[22]. The tissue slice from aorta of atherosclerotic plaque area showed an prominent expression of TLR4 by immunohistochemical analysis[23].
The research studies on cardiovascular diseases shown that infection associated with C pneumonia found to have role in the progression of atherosclerotic diseases[24]. It usually triggers the diseases by activating TLR4 receptor to induce the migration and proliferation of smooth muscle cells[25]. The patient with up regulated expression of human TLR4 results in the elevation of IL-12 expression on the downstream activation of TLR4[22].
Lipopolysaccharide are released upon microbial infection and might triggers the plaque cells to promote the production of different cytokines which initiates the progression of plaque and its rupture which results in severe complications[26]. The up regulated expression of hTLR4 in patients results in the enhanced expression of MYD88 protein level[27]. Extensive genetic study on TLR4 gene showed that any polymorphism in TLR4 gene found to have slow down the progress of atherosclerosis. It is due to the mutation on TLR4 (Asp 299 Gly and Thr 399ile) residues. The analysis on TLR4 polymorphism in different patient showed that the patient with acute coronary syndrome showed less polymorphism were as healthy old people showed least polymorphism[28].
Macrophages and the endothelial cells are the main two types of cells which primary respond to the microbial infection. TLR4 expression in macrophages triggers the local differentiation of these cells to antigen presenting one[29,30]. Finally it act as the bridge between innate and adaptive immune response to local antigen such as heat shock proteins and OxLDL[31].
TLR4 has active role in cholesterol metabolism in macrophages[8], which elucidates the process by which TLR4 affect the disease pathology. It has been found that deficiency in TLR4 gene was associated with reduction in the atherosclerotic lesion in cholesterol fed mice for six months[9]. The gene polymorphism in TLR4 results in the 25% reduction in plaque of double mutant mice. The levels of plasma cholesterol didn’t affect significantly on TLR4 deficiency. Over all the genetic polymorphism in TLR4 results in the reduction in levels of cholesterol, conforming the active role of TLR4 in atherosclerosis.
The innate immune system can be activated by variety of pathogen by TLR4 signaling pathways[16,18]. Lipopolysacharide can specifically activates TLR4 ligand[31], which is the major component of gram negative bacteria. Cholesterol induced toxicity causes tissue injury and which releases cellular fibronectin and HSP60 which triggers the activation of TLR4 receptor and results in the atherosclerotic progression[32,33].
The activation of TLR4 leads to the accumulation of different cells in the atherosclerotic walls like endothelial cells[20,30], macrophages[7,20,30], adventitial fibroblast[20,34] and dendric cells[20,35,36]. TLRs have two important domains like extracellular leucine rich (LRR) domain and intracellular domain (TIR). When the TLR4 receptor stimulates, the TIR domain bind to TIR domain adaptor protein MYD88, then to adaptor protein (AD) to form TIRAP complex which is known as MYD88-MAIL and TIR domain consist of adaptor inducing IFN-β (TRIF), the TRIF-related adaptor molecule (TRAM) resulting in two distinct signaling mechanism. MyD88-dependent and the MyD88-independent/TRIF-dependent pathways[37].
TLR4 is widely expressed in atherosclerotic plaques and results in the activation of macrophages and endothelial cells. There comes a link between TLR4/MAPKs/NF-κB pathway in inducing inflammatory stress and ultimately resulting in atherosclerotic plaque rupture[30]. Upon activation TLR4 receptor leads to the activation of IRAK associated protein TRAF6 which induces activation of TAK1 and MKK6 via JNK/p38 to activates NF-κB and resulting in the activation of downstream signaling to promote the progression of the disease[38,39].
TLR4 found to have an eminent role in the innate immune system. When it comes in with microbial product TLR4 activates intracellular signaling pathway. The execution of the mechanism is through NF-κB signaling pathway. It is known that TLR4 induced NF-κB activation is an critical component in ancient host defence system, which is phylogenetically conserved in most of insects and mammals[40].
The alterations in the mechanisms regulating the activation of MAPKs and NF-κB are responsible for the most of inflammatory events[12]. In normal cells the NF-κB resides in the cytoplasm and usually associated with Iκ-B, a family of inhibitory proteins, which usually binds to NF-κB and inhibits the nuclear translocation[41]. NF-κB usually regulates the cell survival and inflammatory stress on the active κB binding sites called the promoter gene[12]. Active NF-κB complexes are dimers of combinations of Rel family polypeptides (p50, p52 and p65) that respond to a wide variety of stimuli. The NF-κB subunit determines the biological effect by nuclear translocation and further binding to κB-regulatory elements[42,43].
Research study on MAPKs pathway suggests the active participation of MAPKs in the translocation of NF-κB subunits. Upon inflammatory stress the cells elicts inflammatory responses via MAPKs signaling pathway. It regulates various cellular activities like gene expression, mitosis, programmed cell death, etc. The phosphorylation of MAPKs act as switch for tuning the activation of target protein on/off[44,45].
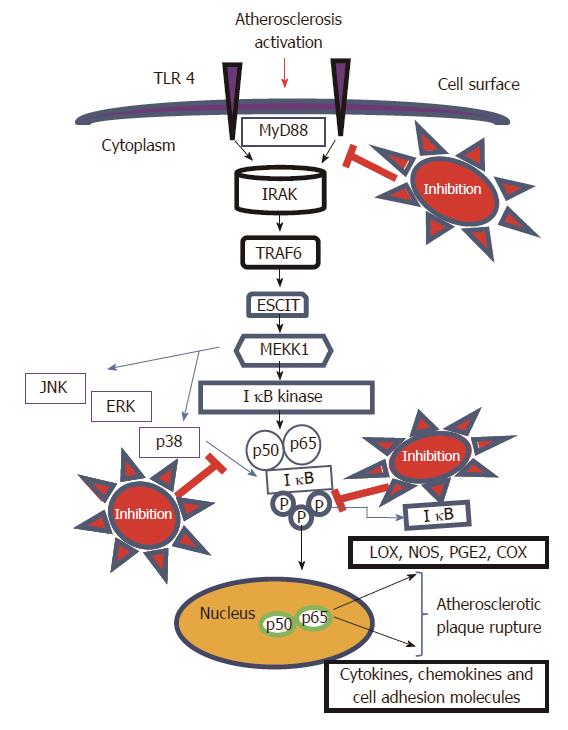
Natural products have long been recognized as an important source of therapeutically effective medicines. It is recognized that natural-product structures have great chemical diversity, biochemical specificity and other molecular properties that make them favourable lead structures[46]. There are several plant compounds which can be used to target this pathway. We have recently published our research paper on Robinin a bioflavonoid from Vigna unguiculata leaf[47,48] which selectively modulates TLR/NF-κB signaling pathway in oxidized LDL induced human peripheral blood mononuclear cells[49]. Targeting of TLR4/MAPKs signaling pathway (Figure 1) is very essential for the therapeutic inhibition of atherosclerosis. The activation of TLR4 in turn activates cascades of proteins and IKK dependent phosphorylation of IκB. There is also an activation of MAPKs which contribute to the dissociation of IκB and the nuclear translocation of NF-κB p65 subunit (Figure 1) resulting in the activation of key inflammatory cascade through MAPKs/NF-κB signaling pathway. Hence we can target the TLR4/MAPKs signaling pathway at different places in the signaling pathway as indicated in the proposed mechanism in Figure 1. Hence, Identification of naturally occurring phytocompounds that can suppress or downregulate TLR4/MAPKs signaling pathway would be an efficient strategy for inhibition of atherosclerosis
The inhibition of atherosclerosis is one of primary target for the therapeutics of atherosclerosis, the leading cause of death worldwide. The important role of TLR4 in the activation and progression of atherosclerosis is justified here. The TLR4 in turn activates the MAPKs and NF-κB which are responsible for most of inflammatory events. Hence, therapeutic inhibition of TLR4/MAPKs signaling pathway is one of the best method for inhibiting atherosclerosis.
P- Reviewer: Amiya E, Lin GM, Shi GY S- Editor: Ji FF L- Editor: A E- Editor: Wang CH
| 1. | Hansson GK, Hermansson A. The immune system in atherosclerosis. Nat Immunol. 2011;12:204-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1445] [Cited by in RCA: 1681] [Article Influence: 120.1] [Reference Citation Analysis (0)] |
| 2. | Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med. 1999;340:115-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15621] [Cited by in RCA: 15527] [Article Influence: 597.2] [Reference Citation Analysis (0)] |
| 3. | Laine P, Kaartinen M, Penttilä A, Panula P, Paavonen T, Kovanen PT. Association between myocardial infarction and the mast cells in the adventitia of the infarct-related coronary artery. Circulation. 1999;99:361-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 223] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 4. | Ramshaw AL, Parums DV. Immunohistochemical characterization of inflammatory cells associated with advanced atherosclerosis. Histopathology. 1990;17:543-552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 61] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 5. | Xu Q, Schett G, Li C, Hu Y, Wick G. Mechanical stress-induced heat shock protein 70 expression in vascular smooth muscle cells is regulated by Rac and Ras small G proteins but not mitogen-activated protein kinases. Circ Res. 2000;86:1122-1128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 332] [Cited by in RCA: 301] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 6. | Miller YI, Choi SH, Fang L, Harkewicz R. Toll-like receptor-4 and lipoprotein accumulation in macrophages. Trends Cardiovasc Med. 2009;19:227-232. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 53] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 7. | Xu XH, Shah PK, Faure E, Equils O, Thomas L, Fishbein MC, Luthringer D, Xu XP, Rajavashisth TB, Yano J. Toll-like receptor-4 is expressed by macrophages in murine and human lipid-rich atherosclerotic plaques and upregulated by oxidized LDL. Circulation. 2001;104:3103-3108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 432] [Cited by in RCA: 455] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 8. | Castrillo A, Joseph SB, Vaidya SA, Haberland M, Fogelman AM, Cheng G, Tontonoz P. Crosstalk between LXR and toll-like receptor signaling mediates bacterial and viral antagonism of cholesterol metabolism. Mol Cell. 2003;12:805-816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 361] [Cited by in RCA: 381] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 9. | Michelsen KS, Wong MH, Shah PK, Zhang W, Yano J, Doherty TM, Akira S, Rajavashisth TB, Arditi M. Lack of Toll-like receptor 4 or myeloid differentiation factor 88 reduces atherosclerosis and alters plaque phenotype in mice deficient in apolipoprotein E. Proc Natl Acad Sci USA. 2004;101:10679-10684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 771] [Cited by in RCA: 804] [Article Influence: 38.3] [Reference Citation Analysis (0)] |
| 10. | Hollestelle SC, De Vries MR, Van Keulen JK, Schoneveld AH, Vink A, Strijder CF, Van Middelaar BJ, Pasterkamp G, Quax PH, De Kleijn DP. Toll-like receptor 4 is involved in outward arterial remodeling. Circulation. 2004;109:393-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 150] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 11. | Imaizumi S, Grijalva V, Priceman S, Wu L, Su F, Farias-Eisner R, Hama S, Navab M, Fogelman AM, Reddy ST. Mitogen-activated protein kinase phosphatase-1 deficiency decreases atherosclerosis in apolipoprotein E null mice by reducing monocyte chemoattractant protein-1 levels. Mol Genet Metab. 2010;101:66-75. [PubMed] |
| 12. | Garcia-Garcia FJ, Mullol J, Perez-Gonzalez M, Pujols L, Alobid I, Roca-Ferrer J, Picado C. Signal transduction pathways (MAPKs, NF-κB, and C/EBP) regulating COX-2 expression in nasal fibroblasts from asthma patients with aspirin intolerance. PLoS One. 2012;7:e51281. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 13. | Barbic J, Leef MF, Burns DL, Shahin RD. Role of gamma interferon in natural clearance of Bordetella pertussis infection. Infect Immun. 1997;65:4904-4908. [PubMed] |
| 14. | Hansson GK, Libby P, Schönbeck U, Yan ZQ. Innate and adaptive immunity in the pathogenesis of atherosclerosis. Circ Res. 2002;91:281-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 727] [Cited by in RCA: 714] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 15. | Medzhitov R, Preston-Hurlburt P, Janeway CA. A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388:394-397. [PubMed] |
| 16. | Hashimoto C, Hudson KL, Anderson KV. The Toll gene of Drosophila, required for dorsal-ventral embryonic polarity, appears to encode a transmembrane protein. Cell. 1988;52:269-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 682] [Cited by in RCA: 692] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 17. | Rock FL, Hardiman G, Timans JC, Kastelein RA, Bazan JF. A family of human receptors structurally related to Drosophila Toll. Proc Natl Acad Sci USA. 1998;95:588-593. [PubMed] |
| 18. | Poltorak A, He X, Smirnova I, Liu MY, Van Huffel C, Du X, Birdwell D, Alejos E, Silva M, Galanos C. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085-2088. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 19. | Qureshi ST, Larivière L, Leveque G, Clermont S, Moore KJ, Gros P, Malo D. Endotoxin-tolerant mice have mutations in Toll-like receptor 4 (Tlr4). J Exp Med. 1999;189:615-625. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1210] [Cited by in RCA: 1180] [Article Influence: 45.4] [Reference Citation Analysis (0)] |
| 20. | Pasterkamp G, Van Keulen JK, De Kleijn DP. Role of Toll-like receptor 4 in the initiation and progression of atherosclerotic disease. Eur J Clin Invest. 2004;34:328-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 111] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 21. | Hoshino K, Tsutsui H, Kawai T, Takeda K, Nakanishi K, Takeda Y, Akira S. Cutting edge: generation of IL-18 receptor-deficient mice: evidence for IL-1 receptor-related protein as an essential IL-18 binding receptor. J Immunol. 1999;162:5041-5044. [PubMed] |
| 22. | Andrea D, Tamara A, Edo D, Snjezana D, Jerko B. 5 Toll-like receptors and atherosclerosis. Med Glas. 2009;6:23–31. |
| 23. | Yamamoto M, Sato S, Hemmi H, Sanjo H, Uematsu S, Kaisho T, Hoshino K, Takeuchi O, Kobayashi M, Fujita T. Essential role for TIRAP in activation of the signalling cascade shared by TLR2 and TLR4. Nature. 2002;420:324-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 727] [Cited by in RCA: 726] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 24. | Saikku P. Epidemiologic association of Chlamydia pneumoniae and atherosclerosis: the initial serologic observation and more. J Infect Dis. 2000;181 Suppl 3:S411-S413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 20] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 25. | Sasu S, LaVerda D, Qureshi N, Golenbock DT, Beasley D. Chlamydia pneumoniae and chlamydial heat shock protein 60 stimulate proliferation of human vascular smooth muscle cells via toll-like receptor 4 and p44/p42 mitogen-activated protein kinase activation. Circ Res. 2001;89:244-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 202] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 26. | Libby P, Egan D, Skarlatos S. Roles of infectious agents in atherosclerosis and restenosis: an assessment of the evidence and need for future research. Circulation. 1997;96:4095-4103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 314] [Cited by in RCA: 310] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 27. | Björkbacka H, Kunjathoor VV, Moore KJ, Koehn S, Ordija CM, Lee MA, Means T, Halmen K, Luster AD, Golenbock DT. Reduced atherosclerosis in MyD88-null mice links elevated serum cholesterol levels to activation of innate immunity signaling pathways. Nat Med. 2004;10:416-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 488] [Cited by in RCA: 485] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 28. | Balistreri CR, Candore G, Colonna-Romano G, Lio D, Caruso M, Hoffmann E, Franceschi C, Caruso C. Role of Toll-like receptor 4 in acute myocardial infarction and longevity. JAMA. 2004;292:2339-2340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 58] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 29. | Hertz CJ, Kiertscher SM, Godowski PJ, Bouis DA, Norgard MV, Roth MD, Modlin RL. Microbial lipopeptides stimulate dendritic cell maturation via Toll-like receptor 2. J Immunol. 2001;166:2444-2450. [PubMed] |
| 30. | Edfeldt K, Swedenborg J, Hansson GK, Yan ZQ. Expression of toll-like receptors in human atherosclerotic lesions: a possible pathway for plaque activation. Circulation. 2002;105:1158-1161. [PubMed] |
| 31. | Kobe B, Deisenhofer J. Proteins with leucine-rich repeats. Curr Opin Struct Biol. 1995;5:409-416. [PubMed] |
| 32. | Aravind L, Dixit VM, Koonin EV. Apoptotic molecular machinery: vastly increased complexity in vertebrates revealed by genome comparisons. Science. 2001;291:1279-1284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 243] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 33. | Muzio M, Ni J, Feng P, Dixit VM. IRAK (Pelle) family member IRAK-2 and MyD88 as proximal mediators of IL-1 signaling. Science. 1997;278:1612-1615. [PubMed] |
| 34. | Vink A, Schoneveld AH, van der Meer JJ, van Middelaar BJ, Sluijter JP, Smeets MB, Quax PH, Lim SK, Borst C, Pasterkamp G. In vivo evidence for a role of toll-like receptor 4 in the development of intimal lesions. Circulation. 2002;106:1985-1990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 35. | Bobryshev YV, Ikezawa T, Watanabe T. Formation of Birbeck granule-like structures in vascular dendritic cells in human atherosclerotic aorta. Lag-antibody to epidermal Langerhans cells recognizes cells in the aortic wall. Atherosclerosis. 1997;133:193-202. [PubMed] |
| 36. | Hoshino K, Kaisho T, Iwabe T, Takeuchi O, Akira S. Differential involvement of IFN-beta in Toll-like receptor-stimulated dendritic cell activation. Int Immunol. 2002;14:1225-1231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 216] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 37. | Falck-Hansen M, Kassiteridi C, Monaco C. Toll-like receptors in atherosclerosis. Int J Mol Sci. 2013;14:14008-14023. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 95] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 38. | Cao Z, Henzel WJ, Gao X. IRAK: a kinase associated with the interleukin-1 receptor. Science. 1996;271:1128-1131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 685] [Cited by in RCA: 685] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 39. | Wang C, Deng L, Hong M, Akkaraju GR, Inoue J, Chen ZJ. TAK1 is a ubiquitin-dependent kinase of MKK and IKK. Nature. 2001;412:346-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1558] [Cited by in RCA: 1634] [Article Influence: 68.1] [Reference Citation Analysis (0)] |
| 40. | Zhang G, Ghosh S. Toll-like receptor-mediated NF-kappaB activation: a phylogenetically conserved paradigm in innate immunity. J Clin Invest. 2001;107:13-19. [PubMed] |
| 41. | Baldwin AS. The NF-kappa B and I kappa B proteins: new discoveries and insights. Annu Rev Immunol. 1996;14:649-683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4738] [Cited by in RCA: 4818] [Article Influence: 166.1] [Reference Citation Analysis (0)] |
| 42. | Chun KS, Surh YJ. Signal transduction pathways regulating cyclooxygenase-2 expression: potential molecular targets for chemoprevention. Biochem Pharmacol. 2004;68:1089-1100. [PubMed] |
| 43. | Syeda F, Grosjean J, Houliston RA, Keogh RJ, Carter TD, Paleolog E, Wheeler-Jones CP. Cyclooxygenase-2 induction and prostacyclin release by protease-activated receptors in endothelial cells require cooperation between mitogen-activated protein kinase and NF-kappaB pathways. J Biol Chem. 2006;281:11792-11804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 78] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 44. | Dong C, Davis RJ, Flavell RA. MAP kinases in the immune response. Annu Rev Immunol. 2002;20:55-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1313] [Cited by in RCA: 1331] [Article Influence: 55.5] [Reference Citation Analysis (0)] |
| 45. | Raman M, Chen W, Cobb MH. Differential regulation and properties of MAPKs. Oncogene. 2007;26:3100-3112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1022] [Cited by in RCA: 1126] [Article Influence: 62.6] [Reference Citation Analysis (0)] |
| 46. | Souto AL, Tavares JF, da Silva MS, Diniz Mde F, de Athayde-Filho PF, Barbosa Filho JM. Anti-inflammatory activity of alkaloids: an update from 2000 to 2010. Molecules. 2011;16:8515-8534. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 108] [Cited by in RCA: 100] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 47. | Janeesh PA, Abraham A. Amelioration of cholesterol induced atherosclerosis by normalizing gene expression, cholesterol profile and antioxidant enzymes by Vigna unguiculata. Plant Foods Hum Nutr. 2013;68:118-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 48. | Janeesh PA, Abraham A. Vigna unguiculata modulates cholesterol induced cardiac markers, genotoxicity and gene expressions profile in an experimental rabbit model. Food Funct. 2013;4:568-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 49. | Janeesh PA, Sasikala V, Dhanya CR, Abraham A. Robinin modulates TLR/NF-κB signaling pathway in oxidized LDL induced human peripheral blood mononuclear cells. Int Immunopharmacol. 2014;18:191-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |