Revised: December 5, 2013
Accepted: February 16, 2014
Published online: March 27, 2014
Processing time: 155 Days and 16.7 Hours
Protein energy malnutrition is the main cause of immunodeficiency and, secondarily, of several infections. However, immune cell activation is involved in several pathophysiological processes that play a crucial role in the appearance of cardiovascular disease (CVD) or cancer. The aim of this review is to update the knowledge of the modulation of immune cell activation by different dietary patterns and its components focusing on CVD or cancer. While a westernized high-saturated fat high-carbohydrate diet is positively associated with low-grade inflammation, vegetable- and fruit-based diets rich in monounsaturated fatty acids, polyunsaturated fatty acids and polyphenols, key nutrients of Mediterranean diet, decrease the levels of cellular and circulating inflammatory biomarkers thereby reducing the risk of related chronic diseases.
Core tip: Immune cell activation is involved in several pathophysiological processes that play a crucial role in the appearance of cardiovascular disease or cancer. The aim of this review is to update the knowledge of the modulation of immune cell activation by different dietary patterns. A westernized high-saturated fat high-carbohydrate diet is positively associated with low-grade inflammation, but a Mediterranean diet, rich in vegetables and fruits decrease the levels of cellular and circulating inflammatory biomarkers thereby reducing the risk of related chronic diseases.
- Citation: Chiva-Blanch G, Estruch R. Circulating immune cell activation and diet: A review on human trials. World J Immunol 2014; 4(1): 12-19
- URL: https://www.wjgnet.com/2219-2824/full/v4/i1/12.htm
- DOI: https://dx.doi.org/10.5411/wji.v4.i1.12
Although some food compounds can originate several immune reactions such as allergies or celiac disease[1], most of nutrients in the diet are essential for maintaining the function of immune cells. Thus, protein energy malnutrition is the main cause of immunodeficiency worldwide[2] and deficits of nutrients commonly included in the diet such as vitamin E, vitamin C, β-carotene, selenium, copper, iron and zinc modify different immune functions related to infections caused by bacteria, viruses or parasites. A deficiency in vitamin E diminishes the ability of the immune system to respond to infectious microorganisms and under some conditions, a pharmacological level of vitamin E is needed to achieve an optimal immune response suggesting that the recommended dietary allowances for vitamin E might not be adequate for immunological vigor and health[3]. Therefore, the knowledge of the effects of nutrition on immune function now extends beyond clinical nutrient deficiency. A growing body of literature demonstrates the immune benefits of increasing the intake of some specific nutrients. Compared with human infants fed formula without nucleotides, infants fed breast milk or formula supplemented with nucleotides had higher natural killer cell activity and interleukin (IL)-2 production[4] and consequently, improved immune function. On the other hand, in an elderly population, zinc supplementation eliminated the effect of seasonal variations on the incidence of infections and also decreased their mean incidence compared to a placebo group (common cold, cold sores, and the flu)[5].
In addition to protection against infections, immune cell activation is involved in several pathophysiological processes. Cell activation is a complex process, implying several plasma membrane-associated events in which chemokines and adhesion molecules play a pivotal role. These processes ultimately result in proliferation, target cell lysis, increased production of cytokines and the expression of immune cell activation markers.
Many chronic diseases, such as atherosclerosis, cancer, neurodegenerative disorders, rheumatoid arthritis, and even aging, are due to chronically increased pro-inflammatory cytokines and oxidative stress, and in consequence, due to immune cell activation. Therefore, dietetic strategies to decrease low-grade inflammation and immune cell activation may be useful tools for preventing or decreasing the progression of many chronic disorders.
Many studies have focused on the mechanisms by which one single nutrient or compound alters immune cell activation, but these studies have the limitation that the interactions between the different compounds of food are not considered. There is an increasing interest to consider a whole dietary pattern in addition to single compounds in order to have a holistic approach of the effects of diet on cell activation.
The aim of this review is to update the knowledge of the modulation of immune cell activation by diet and its components from a chronic disease point of view through human interventional studies, those which provide the greatest scientific evidence.
Chronic inflammatory diseases are defined by long-term inflammatory processes directed at a particular endogenous or exogenous antigen and considered as an underlying pathophysiological mechanism linking behavioral factors and obesity to risk of chronic disease. Inflammation is characterized by a complex biological cascade of molecular and cellular signals that alter physiological responses. At the site of the injury, cells release molecular signals such as cytokines that cause a number of changes in the affected area, such as dilation of blood vessels, increased blood flow, increased vascular permeability, exudation of fluids containing antibodies and invasion by monocytes and macrophages and, to a lesser extent, lymphocytes, through the expression of integrins and other adhesion molecules. In addition, lesion progression is associated with the predominance of the proinflammatory M1 over the antiinflammatory M2 macrophage phenotype, which can be switched to M1 by several transcription factors, chemokines and lipid accumulation in macrophages[6,7].
Elevated levels of inflammatory biomarkers such as C-reactive protein (CRP), tumor necrosis factor-α (TNF-α), IL-6 and 18, fibrinogen and adhesion molecules [E-selectin, intercellular adhesion molecule 1 (ICAM-1) and vascular cell adhesion protein 1 (VCAM-1)] have been shown to predict type 2 diabetes, cardiovascular disease (CVD) and cancer[8-13]. In this setting, the activation of the inflammasome has been linked to the pathogenesis of obesity, type 2 diabetes and atherosclerosis[14-19]. Several studies have suggested that the development of tolerance and control of inflammation are strongly correlated with specific immune mechanisms that may be altered by an inadequate supply of either macronutrients or micronutrients. Therefore, the intake of some nutrients or specific dietary patterns may influence the concentrations of inflammatory biomarkers and therefore, the risk and/or progression of inflammatory diseases.
A westernized high-fat high-carbohydrate diet is positively associated with low-grade inflammation, and therefore, contributes to disease development and progression. Likewise, these types of diets can have direct adverse effects on human physiology[20] resulting in chronic immune and inflammatory imbalances. Overall, the intake of a high-fat, high-carbohydrate westernized diet has potent direct and indirect effects on local as well as systemic inflammation. This has led to a dramatic upswing in the incidence of inflammatory diseases such as rheumatoid arthritis, inflammatory bowel disease, diabetes, and non-alcoholic fatty liver disease, among others[21]. On the other hand, vegetable- and fruit-based diet and a priori healthy dietary patterns appeared to be inversely related to inflammatory biomarkers; this fact is particularly well supported by intervention studies investigating the effects of Mediterranean diet (MedDiet) on health.
CVD is the main cause of mortality worldwide and is principally caused by the appearance and progression of atherosclerotic lesions. Although atherosclerosis has been historically considered an oxidative disease, nowadays it is considered a systemic disease characterized by low-grade arterial inflammation, in which the cell and endothelial expression of adhesion molecules and chemokines participate in the recruitment of circulating leukocytes to the vascular endothelium and further migration into subendothelial spaces. In addition, the metabolic syndrome is a risk factor CVD, which also has an immunological and inflammatory component. Immune cell infiltration of adipose tissue giving rise to chronic low-grade inflammation is, in part, responsible for the pathogenesis of insulin resistance in obesity[22] and lastly, CVD.
As explained before, the western diet enriched in total fat (and an imbalanced ratio of n-6:n-3[23]), animal protein, n-6 polyunsaturated fatty acids (PUFA) and refined sugars, leads to an increased proinflammatory status[24] and is, therefore, considered as a risk factor for the development of CVD[25]. On the other hand, several studies have highlighted that a Mediterranean-like diet decreases cardiovascular risk[26-29], by up to 30% in a high cardiovascular risk population[30]. Several mechanisms have been proposed for the effects observed, and some of them, which will be discussed below, are related to immune cell activation.
While n-6 PUFA have been shown to exert an inflammatory effect[31], it has been demonstrated that n-3 PUFAs have beneficial effects on cardiovascular and inflammatory diseases[32,33], probably linked to the nuclear factor (NF)-κB pathway[34] and the inhibition of the inflammasome activation[35]. Meta-analyses of randomized controlled trials studying the n-6 PUFA-specific effect on CVDs[36] showed a direct effect of n-6 fatty acids on the risk of non fatal and fatal heart failure, although linoleic acid could not be linked to an increase in systemic inflammatory biomarkers[37]. Hypercholesterolemic subjects receiveing 2 diets low in saturated fat and cholesterol, and high in PUFA varying in α-linolenic acid (10.5% linoleic acid; 6.5% α-linolenic acid) and linoleic acid (12.6% linoleic acid; 3.6% α-linolenic acid) were compared with other who followed an average American diet (7.7% linoleic acid; 0.8% α-linolenic acid). The α-linolenic acid diet decreased circulating CRP, VCAM-1 and E-selectin plasma concentrations, and the 2 high-PUFA diets similarly decreased ICAM-1[38], although other studies in hypercholesterolemic subjects observed no such results[39]. In healthy women, a 2-wk intervention of a n-3 PUFA-enriched juice or a plain tomato juice decreased VCAM-1 levels but only the n-3 PUFA-enriched juice decreased ICAM-1 plasma concentrations[40]. In addition, a high monounsaturated fatty acid (MUFA) intake has also been shown to exert anti-inflammatory effects. A crossover feeding trial observed that a breakfast rich in butter [saturated fatty acids (SFA)] increased leukocyte mRNA expression of TNF-α compared to an olive oil (rich in MUFA, and concretely oleic acid) or walnut breakfast (rich in PUFA n-6)[41]. On the other hand, in overweight men, a low-fat and a very-low-carbohydrate diet resulted in significant decreases of TNF-α, IL-6, CRP and sICAM-1 but not P-Selectin[42], although in another study with overweight or obese women with metabolic syndrome, the substitution of carbohydrates by PUFA resulted in no changes in CRP, TNF-α, IL-6, sICAM-1 and sVCAM-1 serum concentrations[43]. It can be summarized that the overall quantity of fat intake, the sources and type of dietary fat, with special emphasis on α-linolenic acid and oleic acid, and the ratio of n-6:n-3 fatty acids in the diet, collectively play a crucial role in modulating inflammation.
Other dietetic compounds influencing immune cell activation are polyphenols. These products are antioxidant phytochemicals that have been found in vegetables, fruits and derivatives such as cocoa, red wine or tea, shown to decrease TNF-α and CRP levels[44]. In healthy volunteers cocoa consumption reduced NF-κB activation in peripheral blood mononuclear cells[45], and in men at high CVD risk, cocoa consumption decreased monocyte expression of very late antigen (VLA)-4, CD40 and CD36 and serum concentrations of P-selectin and ICAM-1[46]. Grape polyphenols and specially resveratrol are among the polyphenols most frequently studied. In hemodialysis patients, red grape juice supplementation for 3-wk significantly reduced plasma monocyte chemoattractant protein 1 (MCP-1)[47], and in overweight or obese subjects with metabolic syndrome, grapefruit supplementation for 6-wk decreased F2-isoprostane concentrations in those subjects with high baseline F2-isoprostane concentrations, but no changes in CRP and VCAM-1 were observed[48]. These results suggest different responses to polyphenol intake depending on the pathophysiological conditions of the study subjects and probably the type of polyphenols administered in the intervention group. These differential effects were also observed after moderate red wine consumption, where in healthy male volunteers red wine consumption significantly reduced plasma concentrations of VCAM-1, ICAM-1 and IL-1α and VLA-4 lymphocyte expression and lymphocyte function-associated antigen (LFA)-1, Mac-1, VLA-4 and MCP-1 monocyte expression[49]. On the other hand, in high cardiovascular risk subjects, moderate red wine consumption and dealcoholized red wine consumption (therefore, the non alcoholic fraction of red wine, mainly polyphenols) decreased serum concentrations of CD40 antigen, CD40 Ligand, ICAM-1, E-Selectin, IL-16 and IL-6, MCP-1 and VCAM-1 and inhibited the expression of LFA-1 in T-lymphocytes and Mac-1, SLex and C-C chemokine receptor type 2 expression in monocytes[50].
In the recent years, the effects of a MedDiet as a dietary pattern and not a sum of nutrients have been considered from a multidisciplinary point of view. The MedDiet is characterized by a high intake of cereals, fruit and vegetable products (and therefore, polyphenols), a moderate consumption of fish, olive oil, nuts and wine, and a low intake of meat and dairy and industrial bakery products[51]. According to scientific evidence, the MedDiet is currently considered the more anti-inflammatory dietary pattern, and this is translated to a decreased risk in cardiovascular mortality[30]. In patients with metabolic syndrome, a 2-year follow-up MedDiet reduced serum concentrations of CRP, IL-6, IL-7 and IL-18, accompanied with decreased insulin resistance and an improved endothelial function score[52]. In older subjects with diabetes or ≥ 3 CVD risk factors randomly allocated to a 3-mo MedDiet with supplemented with extra-virgin olive oil, a MedDiet supplemented with nuts or a low-fat diet, after both MedDiets CRP, IL-6, ICAM-1 and VCAM-1 plasma concentrations decreased as did CD40 and CD49d monocyte expression, whereas IL-6, ICAM-1 and VCAM-1 increased after the low-fat diet[26]. In addition, after 1 year both MedDiet groups showed lower plasma concentrations of IL-6, tumor necrosis factor receptor 60 (TNFR60), and TNFR80, whereas ICAM-1, TNFR60, and TNFR80 concentrations increased in the low-fat diet group[27]. The MedDiet has also shown anti-inflammatory effects in healthy subjects. Four weeks of a MedDiet compared to an ordinary Swedish diet decreased the number of platelets and leukocytes and serum concentrations of vascular endothelial growth factor (VEGF), although it did not change the CRP and IL-6 concentrations[28], perhaps because of their low baseline concentration. Interestingly, in a middle-aged twin population, adherence to a MedDiet was highly associated with lower levels of IL-6 but not CRP[29]. Overall, the MedDiet has an antiinflammatory and an inhibitory immune cell activation effect decreasing the onset and progression of CVD, while a low-fat diet or a westernized diet has the opposite effect.
Cancer is the second cause of mortality worldwide and is mediated by both the innate (nonspecific) and acquired (specific) immune systems[53,54]. The molecular mechanisms by which chronic inflammation drives cancer initiation and promotion include increased production of pro-inflammatory mediators, such as cytokines, chemokines, reactive oxygen intermediates, increased expression of oncogenes, cyclooxygenases, lipoxygenases and matrix metalloproteinases, and pro-inflammatory transcription factors such as NF-κB, that mediate tumor cell proliferation, transformation, metastasis, survival, invasion, angiogenesis, chemoresistance and radioresistance[55]. Taking into account that adherence to ideal cardiovascular health, as proposed by the American Heart Association, is associated with a lower incidence of cancer[56], one may suspect that dietary benefits on CVD may reduce the risk of cancer.
Although epidemiological studies have pinpointed that diet may influence more than one-third of human malignancies, probably through the high consumption of pesticides[57], heavy metals[58], heterocyclic amines from over-cooked meats and sex steroid hormones[59], few interventional trials have focused on the modulation of angiogenesis and carcinogenesis through dietary patterns. A recent review[60] of epidemiological studies concluded that there is no significant effect of n-3 PUFA on cancer risk. However these studies only accounted for absolute as opposed to relative levels of n-3 and n-6 PUFA. In fact, n-6 PUFA metabolites promote tumor angiogenesis through a variety of signaling pathways, encouraging epithelial cell proliferation and migration, and decreasing tumor apoptosis, while n-3 PUFA and their metabolites can reverse the pro-angiogenic consequences of high n-6 fatty acids. On the other hand, a MedDiet supplemented with nuts and walnuts (rich in n-6 and polyphenols) also associated with a high intake of vegetables, fruit and fish, decreased the risk of cancer mortality[61].
Intake of total catechin, epicatechin, kaempferol, and myricetin and consumption of black tea were associated with a decreased risk of stage III/IV or stage IV prostate cancer in the Netherlands Cohort study[62], probably because of the anti-inflammatory and antiproliferative effects of flavonoids observed in vitro[63]. In prostate cancer men, 30 d of low-fat diet decreased 19 cytokines and angiogenic factors including proangiogenic factors (stromal-cell derived-1α) and myeloid factors [granulocyte-colony-stimulating factor, macrophage colony-stimulating factor (-M-CSF-)] and VEGF, probably through the NF-κB pathway[64]. Regarding breast cancer, diets high in n-6 PUFA have a clear stimulating influence on breast cancer development, whereas diets rich in extra virgin olive oil mainly have a negative modulatory effect[65]. A recent meta-analysis[66], showed a significant inverse association with the highest fiber intakes and the risk of esophageal cancer, probably through weight control and therefore, inflammation status control. The isothiocyanate sulforaphane [SF; 1-isothiocyanato-4(R)-methylsulfinylbutane] is abundant in broccoli sprouts in the form of its glucosinolate precursor (glucoraphanin). SF is powerful bactericidal against Helicobacter pylori (H. pylori) infections, which are strongly associated with the worldwide pandemic of gastric cancer. H. pylori-infected patients were randomly assigned to feeding of broccoli sprouts (70 g/d; containing 420 micromol of SF precursor) for 8 wk or to consumption of an equal weight of alfalfa sprouts (not containing SF) as placebo. Intervention with broccoli sprouts, but not with alfalfa, decreased the levels of urease measured by the urea breath test and H. pylori stool antigen (both biomarkers of H. pylori colonization) and serum pepsinogens I and II (biomarkers of gastric inflammation). Therefore, daily intake of SF-rich broccoli sprouts for 2 mo enhanced the chemoprotection of the gastric mucosa against H. pylori[67]. In colorectal cancer (CRC) patients, drinking a slurry of black raspberry powder 3 times-a-day for 9 wk increased granulocyte -M-CSF- and decreased IL-8 plasma concentrations and CD105 colorectal tissue expression[68], while in another large CRC cohort, red and processed meat intake before CRC diagnosis was associated with higher risks of death due to all causes and from CVD but not CRC. Although red and processed meat consumption after CRC diagnosis was not associated with mortality, survivors with consistently high (median or higher) intakes before and after diagnosis had a higher risk of CRC-specific mortality compared with those with consistently low intakes[69]. Nonetheless, it should be taken into account that, in addition to the diet, colon cancer risk is influenced by the balance between microbial production of health-promoting metabolites and potentially carcinogenic metabolites[70]. In summary, few interventional studies have been performed investigating the link between diet, immune cell activation and cancer, but it can be postulated that a MedDiet brings together all the dietary protective nutrients related to cancer and specially cancers of the digestive system decreasing its risk of appearance[71], although there are still not enough data to develop guidelines regarding specific foods and cancer risk.
Dietary intake in relation to low-grade inflammation has been investigated in a number of studies exploring nutrients, foods or dietary patterns. Although there is increasing evidence that dietary patterns modulate immune cell activation and low-grade systemic inflammation, there is still a long way to understand the interactions between dietary compounds, dietary patterns, microbiota metabolites and individual polymorphisms and how these affect the body response to the intake of a determined food compound. The integration of dietary behaviors is warranted, given the fact that nutrients or foods are rarely eaten alone, and dietary patterns consider synergistic or antagonistic biochemical interactions among nutrients as well as different food sources of the same nutrient.
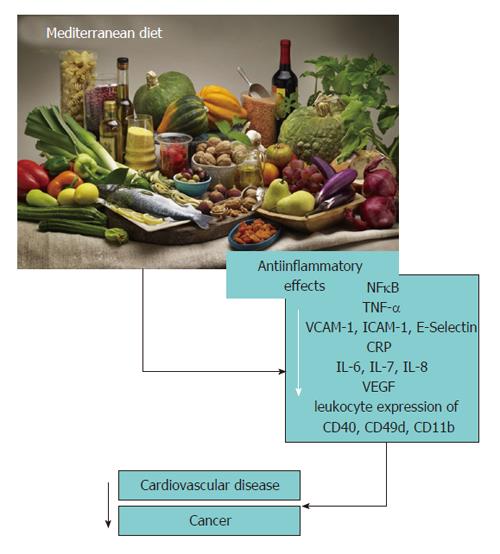
As summarized in Figure 1, there is compelling scientific evidence that a MedDiet rich in MUFA, PUFA (with an adequate ratio of n-3:n-6), polyphenols and with mild-to-low carbohydrate, animal protein and SFA content is the most effective pattern to prevent immune cell activation and inflammation related to chronic diseases by decreasing the expression of leukocyte adhesion molecules and circulating inflammatory biomarkers.
CIBERobn is an initiative of Instituto de Salud Carlos III, Spain.
P- Reviewers: Kucuker A, Kindy MS, Movahed A S- Editor: Song XX L- Editor: A E- Editor: Liu SQ
| 1. | Volta U, Caio G, Tovoli F, De Giorgio R. Non-celiac gluten sensitivity: questions still to be answered despite increasing awareness. Cell Mol Immunol. 2013;10:383-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 85] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 2. | Delafuente JC. Nutrients and immune responses. Rheum Dis Clin North Am. 1991;17:203-212. [PubMed] |
| 3. | Beharka A, Redican S, Leka L, Meydani SN. Vitamin E status and immune function. Methods Enzymol. 1997;282:247-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 53] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 4. | Carver JD. Dietary nucleotides: cellular immune, intestinal and hepatic system effects. J Nutr. 1994;124:144S-148S. [PubMed] |
| 5. | Prasad AS, Beck FW, Bao B, Fitzgerald JT, Snell DC, Steinberg JD, Cardozo LJ. Zinc supplementation decreases incidence of infections in the elderly: effect of zinc on generation of cytokines and oxidative stress. Am J Clin Nutr. 2007;85:837-844. [PubMed] |
| 6. | Chinetti-Gbaguidi G, Staels B. Macrophage polarization in metabolic disorders: functions and regulation. Curr Opin Lipidol. 2011;22:365-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 153] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 7. | Harford KA, Reynolds CM, McGillicuddy FC, Roche HM. Fats, inflammation and insulin resistance: insights to the role of macrophage and T-cell accumulation in adipose tissue. Proc Nutr Soc. 2011;70:408-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 198] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 8. | Panagiotakos DB, Pitsavos C, Yannakoulia M, Chrysohoou C, Stefanadis C. The implication of obesity and central fat on markers of chronic inflammation: The ATTICA study. Atherosclerosis. 2005;183:308-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 258] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 9. | Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA. 2001;286:327-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2974] [Cited by in RCA: 3028] [Article Influence: 126.2] [Reference Citation Analysis (1)] |
| 10. | Thorand B, Kolb H, Baumert J, Koenig W, Chambless L, Meisinger C, Illig T, Martin S, Herder C. Elevated levels of interleukin-18 predict the development of type 2 diabetes: results from the MONICA/KORA Augsburg Study, 1984-2002. Diabetes. 2005;54:2932-2938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 171] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 11. | Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO, Criqui M, Fadl YY, Fortmann SP, Hong Y, Myers GL. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107:499-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4411] [Cited by in RCA: 4648] [Article Influence: 211.3] [Reference Citation Analysis (0)] |
| 12. | II'yasova D, Colbert LH, Harris TB, Newman AB, Bauer DC, Satterfield S, Kritchevsky SB. Circulating levels of inflammatory markers and cancer risk in the health aging and body composition cohort. Cancer Epidemiol Biomarkers Prev. 2005;14:2413-2418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 344] [Cited by in RCA: 355] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 13. | Lindahl B, Toss H, Siegbahn A, Venge P, Wallentin L. Markers of myocardial damage and inflammation in relation to long-term mortality in unstable coronary artery disease. FRISC Study Group. Fragmin during Instability in Coronary Artery Disease. N Engl J Med. 2000;343:1139-1147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 846] [Cited by in RCA: 813] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 14. | Wen H, Ting JP, O’Neill LA. A role for the NLRP3 inflammasome in metabolic diseases--did Warburg miss inflammation? Nat Immunol. 2012;13:352-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 369] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 15. | Henao-Mejia J, Elinav E, Jin C, Hao L, Mehal WZ, Strowig T, Thaiss CA, Kau AL, Eisenbarth SC, Jurczak MJ. Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nature. 2012;482:179-185. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1620] [Cited by in RCA: 1878] [Article Influence: 144.5] [Reference Citation Analysis (0)] |
| 16. | Strowig T, Henao-Mejia J, Elinav E, Flavell R. Inflammasomes in health and disease. Nature. 2012;481:278-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1530] [Cited by in RCA: 1783] [Article Influence: 137.2] [Reference Citation Analysis (1)] |
| 17. | Stienstra R, van Diepen JA, Tack CJ, Zaki MH, van de Veerdonk FL, Perera D, Neale GA, Hooiveld GJ, Hijmans A, Vroegrijk I. Inflammasome is a central player in the induction of obesity and insulin resistance. Proc Natl Acad Sci USA. 2011;108:15324-15329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 485] [Cited by in RCA: 580] [Article Influence: 41.4] [Reference Citation Analysis (0)] |
| 18. | Freigang S, Ampenberger F, Spohn G, Heer S, Shamshiev AT, Kisielow J, Hersberger M, Yamamoto M, Bachmann MF, Kopf M. Nrf2 is essential for cholesterol crystal-induced inflammasome activation and exacerbation of atherosclerosis. Eur J Immunol. 2011;41:2040-2051. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 254] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 19. | Duewell P, Kono H, Rayner KJ, Sirois CM, Vladimer G, Bauernfeind FG, Abela GS, Franchi L, Nuñez G, Schnurr M. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010;464:1357-1361. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3165] [Cited by in RCA: 3016] [Article Influence: 201.1] [Reference Citation Analysis (0)] |
| 20. | Barbaresko J, Koch M, Schulze MB, Nöthlings U. Dietary pattern analysis and biomarkers of low-grade inflammation: a systematic literature review. Nutr Rev. 2013;71:511-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 338] [Cited by in RCA: 443] [Article Influence: 36.9] [Reference Citation Analysis (0)] |
| 21. | Huang EY, Devkota S, Moscoso D, Chang EB, Leone VA. The role of diet in triggering human inflammatory disorders in the modern age. Microbes Infect. 2013;15:765-774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 22. | Kalupahana NS, Moustaid-Moussa N, Claycombe KJ. Immunity as a link between obesity and insulin resistance. Mol Aspects Med. 2012;33:26-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 185] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 23. | Patterson E, Wall R, Fitzgerald GF, Ross RP, Stanton C. Health implications of high dietary omega-6 polyunsaturated Fatty acids. J Nutr Metab. 2012;2012:539426. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 530] [Cited by in RCA: 542] [Article Influence: 41.7] [Reference Citation Analysis (0)] |
| 24. | Giugliano D, Ceriello A, Esposito K. The effects of diet on inflammation: emphasis on the metabolic syndrome. J Am Coll Cardiol. 2006;48:677-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 483] [Cited by in RCA: 602] [Article Influence: 31.7] [Reference Citation Analysis (0)] |
| 25. | Menotti A, Kromhout D, Blackburn H, Fidanza F, Buzina R, Nissinen A. Food intake patterns and 25-year mortality from coronary heart disease: cross-cultural correlations in the Seven Countries Study. The Seven Countries Study Research Group. Eur J Epidemiol. 1999;15:507-515. [PubMed] |
| 26. | Mena MP, Sacanella E, Vazquez-Agell M, Morales M, Fitó M, Escoda R, Serrano-Martínez M, Salas-Salvadó J, Benages N, Casas R. Inhibition of circulating immune cell activation: a molecular antiinflammatory effect of the Mediterranean diet. Am J Clin Nutr. 2009;89:248-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 184] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 27. | Urpi-Sarda M, Casas R, Chiva-Blanch G, Romero-Mamani ES, Valderas-Martínez P, Salas-Salvadó J, Covas MI, Toledo E, Andres-Lacueva C, Llorach R. The Mediterranean diet pattern and its main components are associated with lower plasma concentrations of tumor necrosis factor receptor 60 in patients at high risk for cardiovascular disease. J Nutr. 2012;142:1019-1025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 73] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 28. | Ambring A, Johansson M, Axelsen M, Gan L, Strandvik B, Friberg P. Mediterranean-inspired diet lowers the ratio of serum phospholipid n-6 to n-3 fatty acids, the number of leukocytes and platelets, and vascular endothelial growth factor in healthy subjects. Am J Clin Nutr. 2006;83:575-581. [PubMed] |
| 29. | Dai J, Miller AH, Bremner JD, Goldberg J, Jones L, Shallenberger L, Buckham R, Murrah NV, Veledar E, Wilson PW. Adherence to the mediterranean diet is inversely associated with circulating interleukin-6 among middle-aged men: a twin study. Circulation. 2008;117:169-175. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 115] [Cited by in RCA: 109] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 30. | Estruch R, Ros E, Salas-Salvadó J, Covas MI, Corella D, Arós F, Gómez-Gracia E, Ruiz-Gutiérrez V, Fiol M, Lapetra J. Primary prevention of cardiovascular disease with a Mediterranean diet. N Engl J Med. 2013;368:1279-1290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3419] [Cited by in RCA: 2835] [Article Influence: 236.3] [Reference Citation Analysis (0)] |
| 31. | Ramsden CE, Ringel A, Feldstein AE, Taha AY, MacIntosh BA, Hibbeln JR, Majchrzak-Hong SF, Faurot KR, Rapoport SI, Cheon Y. Lowering dietary linoleic acid reduces bioactive oxidized linoleic acid metabolites in humans. Prostaglandins Leukot Essent Fatty Acids. 2012;87:135-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 146] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 32. | Wall R, Ross RP, Fitzgerald GF, Stanton C. Fatty acids from fish: the anti-inflammatory potential of long-chain omega-3 fatty acids. Nutr Rev. 2010;68:280-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 716] [Cited by in RCA: 723] [Article Influence: 48.2] [Reference Citation Analysis (0)] |
| 33. | Marchioli R, Barzi F, Bomba E, Chieffo C, Di Gregorio D, Di Mascio R, Franzosi MG, Geraci E, Levantesi G, Maggioni AP. Early protection against sudden death by n-3 polyunsaturated fatty acids after myocardial infarction: time-course analysis of the results of the Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto Miocardico (GISSI)-Prevenzione. Circulation. 2002;105:1897-1903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 953] [Cited by in RCA: 857] [Article Influence: 37.3] [Reference Citation Analysis (0)] |
| 34. | Perkins ND. Integrating cell-signalling pathways with NF-kappaB and IKK function. Nat Rev Mol Cell Biol. 2007;8:49-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1708] [Cited by in RCA: 1858] [Article Influence: 103.2] [Reference Citation Analysis (0)] |
| 35. | Yan Y, Jiang W, Spinetti T, Tardivel A, Castillo R, Bourquin C, Guarda G, Tian Z, Tschopp J, Zhou R. Omega-3 fatty acids prevent inflammation and metabolic disorder through inhibition of NLRP3 inflammasome activation. Immunity. 2013;38:1154-1163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 459] [Cited by in RCA: 580] [Article Influence: 48.3] [Reference Citation Analysis (0)] |
| 36. | Ramsden CE, Hibbeln JR, Majchrzak SF, Davis JM. n-6 fatty acid-specific and mixed polyunsaturate dietary interventions have different effects on CHD risk: a meta-analysis of randomised controlled trials. Br J Nutr. 2010;104:1586-1600. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 226] [Cited by in RCA: 208] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 37. | Johnson GH, Fritsche K. Effect of dietary linoleic acid on markers of inflammation in healthy persons: a systematic review of randomized controlled trials. J Acad Nutr Diet. 2012;112:1029-1041, 1041.e1-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 180] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 38. | Zhao G, Etherton TD, Martin KR, West SG, Gillies PJ, Kris-Etherton PM. Dietary alpha-linolenic acid reduces inflammatory and lipid cardiovascular risk factors in hypercholesterolemic men and women. J Nutr. 2004;134:2991-2997. [PubMed] |
| 39. | Bemelmans WJ, Lefrandt JD, Feskens EJ, van Haelst PL, Broer J, Meyboom-de Jong B, May JF, Tervaert JW, Smit AJ. Increased alpha-linolenic acid intake lowers C-reactive protein, but has no effect on markers of atherosclerosis. Eur J Clin Nutr. 2004;58:1083-1089. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 92] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 40. | García-Alonso FJ, Jorge-Vidal V, Ros G, Periago MJ. Effect of consumption of tomato juice enriched with n-3 polyunsaturated fatty acids on the lipid profile, antioxidant biomarker status, and cardiovascular disease risk in healthy women. Eur J Nutr. 2012;51:415-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 41. | Jiménez-Gómez Y, López-Miranda J, Blanco-Colio LM, Marín C, Pérez-Martínez P, Ruano J, Paniagua JA, Rodríguez F, Egido J, Pérez-Jiménez F. Olive oil and walnut breakfasts reduce the postprandial inflammatory response in mononuclear cells compared with a butter breakfast in healthy men. Atherosclerosis. 2009;204:e70-e76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 116] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 42. | Sharman MJ, Volek JS. Weight loss leads to reductions in inflammatory biomarkers after a very-low-carbohydrate diet and a low-fat diet in overweight men. Clin Sci (Lond). 2004;107:365-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 93] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 43. | Rajaie S, Azadbakht L, Saneei P, Khazaei M, Esmaillzadeh A. Comparative effects of carbohydrate versus fat restriction on serum levels of adipocytokines, markers of inflammation, and endothelial function among women with the metabolic syndrome: a randomized cross-over clinical trial. Ann Nutr Metab. 2013;63:159-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 44. | Bogdanski P, Suliburska J, Szulinska M, Stepien M, Pupek-Musialik D, Jablecka A. Green tea extract reduces blood pressure, inflammatory biomarkers, and oxidative stress and improves parameters associated with insulin resistance in obese, hypertensive patients. Nutr Res. 2012;32:421-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 224] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 45. | Vázquez-Agell M, Urpi-Sarda M, Sacanella E, Camino-López S, Chiva-Blanch G, Llorente-Cortés V, Tobias E, Roura E, Andres-Lacueva C, Lamuela-Raventós RM. Cocoa consumption reduces NF-κB activation in peripheral blood mononuclear cells in humans. Nutr Metab Cardiovasc Dis. 2013;23:257-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 46. | Monagas M, Khan N, Andres-Lacueva C, Casas R, Urpí-Sardà M, Llorach R, Lamuela-Raventós RM, Estruch R. Effect of cocoa powder on the modulation of inflammatory biomarkers in patients at high risk of cardiovascular disease. Am J Clin Nutr. 2009;90:1144-1150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 135] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 47. | Castilla P, Echarri R, Dávalos A, Cerrato F, Ortega H, Teruel JL, Lucas MF, Gómez-Coronado D, Ortuño J, Lasunción MA. Concentrated red grape juice exerts antioxidant, hypolipidemic, and antiinflammatory effects in both hemodialysis patients and healthy subjects. Am J Clin Nutr. 2006;84:252-262. [PubMed] |
| 48. | Dow CA, Wertheim BC, Patil BS, Thomson CA. Daily consumption of grapefruit for 6 weeks reduces urine F2-isoprostanes in overweight adults with high baseline values but has no effect on plasma high-sensitivity C-reactive protein or soluble vascular cellular adhesion molecule 1. J Nutr. 2013;143:1586-1592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 49. | Estruch R, Sacanella E, Badia E, Antúnez E, Nicolás JM, Fernández-Solá J, Rotilio D, de Gaetano G, Rubin E, Urbano-Márquez A. Different effects of red wine and gin consumption on inflammatory biomarkers of atherosclerosis: a prospective randomized crossover trial. Effects of wine on inflammatory markers. Atherosclerosis. 2004;175:117-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 176] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 50. | Chiva-Blanch G, Urpi-Sarda M, Llorach R, Rotches-Ribalta M, Guillén M, Casas R, Arranz S, Valderas-Martinez P, Portoles O, Corella D. Differential effects of polyphenols and alcohol of red wine on the expression of adhesion molecules and inflammatory cytokines related to atherosclerosis: a randomized clinical trial. Am J Clin Nutr. 2012;95:326-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 122] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 51. | Trichopoulou A, Lagiou P. Healthy traditional Mediterranean diet: an expression of culture, history, and lifestyle. Nutr Rev. 1997;55:383-389. [PubMed] |
| 52. | Esposito K, Marfella R, Ciotola M, Di Palo C, Giugliano F, Giugliano G, D’Armiento M, D’Andrea F, Giugliano D. Effect of a mediterranean-style diet on endothelial dysfunction and markers of vascular inflammation in the metabolic syndrome: a randomized trial. JAMA. 2004;292:1440-1446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1446] [Cited by in RCA: 1385] [Article Influence: 66.0] [Reference Citation Analysis (0)] |
| 53. | Whiteside TL, Herberman RB. The role of natural killer cells in immune surveillance of cancer. Curr Opin Immunol. 1995;7:704-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 198] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 54. | Robins RA. T-cell responses at the host: tumour interface. Biochim Biophys Acta. 1986;865:289-305. [PubMed] |
| 55. | Sethi G, Shanmugam MK, Ramachandran L, Kumar AP, Tergaonkar V. Multifaceted link between cancer and inflammation. Biosci Rep. 2012;32:1-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 254] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 56. | Rasmussen-Torvik LJ, Shay CM, Abramson JG, Friedrich CA, Nettleton JA, Prizment AE, Folsom AR. Ideal cardiovascular health is inversely associated with incident cancer: the Atherosclerosis Risk In Communities study. Circulation. 2013;127:1270-1275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 251] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 57. | ACOG Committee Opinion No 575. Exposure to toxic environmental agents. Fertil Steril. 2013;100:931-934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 58. | Mannello F, Ligi D, Canale M. Aluminium, carbonyls and cytokines in human nipple aspirate fluids: Possible relationship between inflammation, oxidative stress and breast cancer microenvironment. J Inorg Biochem. 2013;128:250-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 59. | Lee HJ, Wu K, Cox DG, Hunter D, Hankinson SE, Willett WC, Sinha R, Cho E. Polymorphisms in xenobiotic metabolizing genes, intakes of heterocyclic amines and red meat, and postmenopausal breast cancer. Nutr Cancer. 2013;65:1122-1131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 60. | Kang JX, Liu A. The role of the tissue omega-6/omega-3 fatty acid ratio in regulating tumor angiogenesis. Cancer Metastasis Rev. 2013;32:201-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 61. | Guasch-Ferré M, Bulló M, Martínez-González MÁ, Ros E, Corella D, Estruch R, Fitó M, Arós F, Wärnberg J, Fiol M. Frequency of nut consumption and mortality risk in the PREDIMED nutrition intervention trial. BMC Med. 2013;11:164. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 134] [Cited by in RCA: 112] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 62. | Geybels MS, Verhage BA, Arts IC, van Schooten FJ, Goldbohm RA, van den Brandt PA. Dietary flavonoid intake, black tea consumption, and risk of overall and advanced stage prostate cancer. Am J Epidemiol. 2013;177:1388-1398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 65] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 63. | García-Lafuente A, Guillamón E, Villares A, Rostagno MA, Martínez JA. Flavonoids as anti-inflammatory agents: implications in cancer and cardiovascular disease. Inflamm Res. 2009;58:537-552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 633] [Cited by in RCA: 614] [Article Influence: 38.4] [Reference Citation Analysis (0)] |
| 64. | Heymach JV, Shackleford TJ, Tran HT, Yoo SY, Do KA, Wergin M, Saintigny P, Vollmer RT, Polascik TJ, Snyder DC. Effect of low-fat diets on plasma levels of NF-κB-regulated inflammatory cytokines and angiogenic factors in men with prostate cancer. Cancer Prev Res (Phila). 2011;4:1590-1598. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 65. | Escrich E, Solanas M, Moral R. Olive oil and other dietary lipids in breast cancer. Cancer Treat Res. 2014;159:289-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 66. | Coleman HG, Murray LJ, Hicks B, Bhat SK, Kubo A, Corley DA, Cardwell CR, Cantwell MM. Dietary fiber and the risk of precancerous lesions and cancer of the esophagus: a systematic review and meta-analysis. Nutr Rev. 2013;71:474-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 67. | Yanaka A, Fahey JW, Fukumoto A, Nakayama M, Inoue S, Zhang S, Tauchi M, Suzuki H, Hyodo I, Yamamoto M. Dietary sulforaphane-rich broccoli sprouts reduce colonization and attenuate gastritis in Helicobacter pylori-infected mice and humans. Cancer Prev Res (Phila). 2009;2:353-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 99] [Reference Citation Analysis (0)] |
| 68. | Mentor-Marcel RA, Bobe G, Sardo C, Wang LS, Kuo CT, Stoner G, Colburn NH. Plasma cytokines as potential response indicators to dietary freeze-dried black raspberries in colorectal cancer patients. Nutr Cancer. 2012;64:820-825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 69. | McCullough ML, Gapstur SM, Shah R, Jacobs EJ, Campbell PT. Association between red and processed meat intake and mortality among colorectal cancer survivors. J Clin Oncol. 2013;31:2773-2782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 77] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 70. | Ou J, Carbonero F, Zoetendal EG, DeLany JP, Wang M, Newton K, Gaskins HR, O’Keefe SJ. Diet, microbiota, and microbial metabolites in colon cancer risk in rural Africans and African Americans. Am J Clin Nutr. 2013;98:111-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 426] [Cited by in RCA: 462] [Article Influence: 38.5] [Reference Citation Analysis (0)] |
| 71. | Bamia C, Lagiou P, Buckland G, Grioni S, Agnoli C, Taylor AJ, Dahm CC, Overvad K, Olsen A, Tjønneland A. Mediterranean diet and colorectal cancer risk: results from a European cohort. Eur J Epidemiol. 2013;28:317-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 131] [Article Influence: 10.9] [Reference Citation Analysis (0)] |