Revised: October 8, 2013
Accepted: November 1, 2013
Published online: November 27, 2013
Processing time: 115 Days and 2.5 Hours
AIM: To investigate the expression of tumor-antigens and major histocompatibility complex (MHC)-machinery components in glioblastoma multiforme cell lines flow cytometry staining methods were applied.
METHODS: Ten GBM cell lines (three commercially available: U-87 MG, U-138-MG and GMS-10 as well as seven newly established cell lines from individual patients in low-passages: HROG02, HROG04, HROG05, HROG06, HROG10, HROG13 and HROG17) were analyzed for expression of (I) general and (II) GBM-related tumor antigens as well as of (III) components of the MHC machinery by flow cytometry.
RESULTS: All cell lines expressed MHC class I with seven out of the ten being HLA-A02 positive. Four of the seven primary cell lines additionally expressed MHC class II in a constitutive manner. Of note, after interferon gamma (IFN-γ) treatment, all seven cell lines expressed MHC class II. The tumor associated antigens (TAA) EGFR and survivin were expressed at high levels in all cell lines; whereas MART-1, RHAMM, WT-1 and IL-13Rα were expressed by at least half of the cell lines and HER2/neu, MAGE-1 and tyrosinase were expressed only by few cell lines. However, all cell lines expressed at least two of the candidate antigens included into this analysis.
CONCLUSION: No obvious differences between commercially available and newly-established cell lines were observed. Thus, the latter in low-passages are interesting for (therapy-) screening and immunotherapeutic strategies.
Core tip: Expression of tumor-antigens and major histocompatibility complex (MHC)-machinery components was analyzed in a series of seven novel low-passage glioblastoma multiforme cell lines by flow cytometry in comparison to three commercially available standard lines. MHC class I was always expressed, MHC class II readily after interferon gamma treatment. All cell lines expressed at least two tumor antigens. No differences between newly-established and commercially available cell lines were observed. Since these novel cell lines are available in low-passages upon request, they are interesting tools for future development of immunotherapeutic strategies, associated screenings and the like.
- Citation: Mullins CS, Walter A, Schmitt M, Classen CF, Linnebacher M. Tumor antigen and MHC expression in glioma cells for immunotherapeutic interventions. World J Immunol 2013; 3(3): 62-67
- URL: https://www.wjgnet.com/2219-2824/full/v3/i3/62.htm
- DOI: https://dx.doi.org/10.5411/wji.v3.i3.62
Glioblastoma multiforme (GBM) is the most common and deadly malignancy of the brain with no current cure. Median survival after standard therapy consisting of resection followed by radio-chemotherapy merely lies at 14.6 mo[1]. Even more devastating is the fact that most patients relapse and die within the following 18 mo[2]. Thus, more potent treatment strategies are inevitable.
In the last decade, immunotherapy emerged as a very promising strategy applied in many different varieties. For chimeric antigen receptors and bi-specific T cell engagers, most dendritic cell vaccines, and T cell transfers, the target structures should be known. In general, these can be categorized as tumor associated antigens (TAA), which are overexpressed on tumor tissue (but may also to a lower extent be present on healthy tissue), or tumor specific antigens (TSA), which are exclusively expressed on the malignant tissue[3]. Further, the distinction between shared or general tumor antigens (e.g., CEA, EGFR, Her2/neu, survivin, mutated TP53) and (entity) specific antigens (e.g., MART and MAGE for melanoma or PSA for prostate cancer) is common[4].
The most widely used models for (drug and therapy) screenings are cell lines, enabling a multitude of analyses concerning the tumor’s genetic and epigenetic composition, biology, phenotype and more. Among these, primary or low passage cultures are most favorable[5-7], since especially patient individual models have been described to serve most accurately for response and resistance prediction[8-11]. These patient individual tumor models may be established as in vitro (primary cell culture) or in vivo (xenograft) models[12].
In this study, we analyzed the expression of various tumor antigens and in addition that of major histocompatibility complex (MHC) machinery components in a series of patient-derived low-passage glioblastoma cell lines in parallel to commercially available ones. To mimic the in vivo situations of absent or weak versus strong inflammation in the tumor bed, expression analyses were performed in the absence and in the presence of interferon gamma (IFN-γ).
Human GBM cell lines U-87 MG (from cell line service, Eppelheim, Germany), U-138-MG and GMS-10 (both obtained at the DSMZ; Braunschweig, Germany) as well as the newly established cell lines (HROG02, HROG04, HROG05, HROG06, HROG10, HROG13 and HROG17) from our lab[13] were cultured in DMEM/Ham’s F12 supplemented with 10% fetal calf serum and 2 mmol/L L-glutamine. All analyses with HROG cells were performed at least three times in different but always low passages. Exactly, the following passage numbers apply: HROG02 P23-P29, HROG04 P26-P35, HROG05 P21-P26, HROG06 P16-P25, HROG10 P17-P26, HROG13 P15-P25 and HROG17 P22-P25. The cells were incubated at 37 °C and 5% CO2. Media and supplements were purchased from PAA (Cölbe, Germany) and all plastic materials were from Greiner bio one (Frickenhausen, Germany).
For IFN-γ treatment cells were harvested by trypsin treatment and 1.5 × 106 cells were seeded in a T75 culture flask. Cells were allowed to attach overnight and clinical grade IFN-γ (Boeringer Ingelheim, Germany) was added at a final concentration of 200 IU/mL for 48 h. Cells treated in the same manner without IFN-γ addition served as controls.
Cells were harvested by incubation with trypsin; the enzymatic reaction was stopped by adding cell culture media. Cells were washed with PBS, counted, and 5 × 105 cells were stained with 1 μg of the respective antibody or isotype control (Table 1) for surface staining. Cells were washed with PBS and resuspended in final volume of 200 μL PBS. In case of unlabeled primary antibodies, excess antibody was washed out with PBS and respective secondary antibodies were added and final wash step was performed as above.
| Host | Antigen | Clone | Label | Company |
| Mouse | HLA-ABC | W6/32 | FITC | Immunotools |
| Mouse | HLA-DR, -DP | HL-38 | FITC | Immunotools |
| Mouse | HLA-E | 3D12 | PE | Bio Legend (London, United Kiongdom) |
| Mouse | HLA-G | 87G | APC | BioLegend |
| Mouse | β2-microglobulin | 2M2 | FITC | BioLegend |
| Mouse | MIC A | 2C10 | FITC | Santa Cruz (Santa Cruz, United States) |
| Mouse | MIC B | Jan-47 | None | Santa Cruz |
| Humanized | EGFR | Cetuximab | None | Bristol-Myers Squibb (New York, United States) |
| Humanized | HER2/neu | Trastuzumab | None | Roche (Basel, Switzerland) |
| Mouse | ICAM-1 | 1H4 | APC | Immunotools |
| Rabbit | IL-13Rα | Polyclonal | None | AssaybioTech (Sunnyvale, United States) |
| Mouse | MAGE-1 | MA454 | None | Thermo Scientific (Waltham, United States) |
| Mouse | MART-1 | M2-7C10 | None | Thermo Scientific |
| Mouse | RHAMM | 2D6 | None | Leica (Wetzlar, Germany) |
| Mouse | survivin | 3F342 | None | Santa Cruz |
| Mouse | tyrosinase | T311 | None | Thermo Scientific |
| Mouse | WT-1 | 6F-H2 | None | Chemicon international (Temecula, United States) |
Similarly, 5 × 105 cells were fixed with 2% Formafix and then treated with buffer P for 10 min to permeabilize the cell membrane for an intra-cellular staining. Cells were incubated with the antibody and washed with buffer P. After a second 10 min incubation period, the respective secondary antibody was added in buffer P. Cells were washed and resuspended in 2% Formafix at a final volume of 200 μL.
For the staining with unlabeled primary antibodies, cells handled the same way with no primary antibody served as negative controls. All incubations were performed on ice for 30 min.
All isotype controls were obtained from immunotools (Friesoythe, Germany). The secondary antibodies were purchased from Dako (Glostrup, Denmark) in case of the anti-mouse antibody and from Bethyl (Montgomery, United States) in case of the anti-human antibody.
Typing of the HLA-A locus was performed in the department for transfusion medicine at the University medicine in Rostock, Germany.
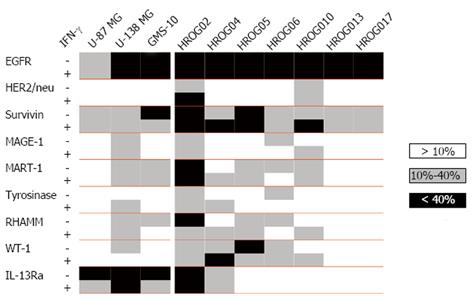
Knowledge about the expression of TAA or even better TSA is of interest for a wide range of therapeutic interventions; especially for targeted therapies and immunotherapeutic approaches. Thus, the expression of a variety of antigens was assessed in seven newly established GBM cell lines in comparison to commercially available GBM cell lines (U-87, U-138 and GMS-10). This analysis included TAA shared between different tumor entities (EGFR, HER2/neu and survivin), classical melanoma (MAGE, MART and tyrosinase) and leukemia-associated antigens (RHAMM and WT-1), as well as the glioma specific IL-13Rα.
The more general antigens EGFR and survivin were found to be expressed-highly in most cases-in all cell lines (Figure 1). MART-1 and RHAMM were expressed in seven, WT-1 in six cell lines. The glioma specific antigen IL-13Rα was expressed in half of the cell lines; however, if positive, than with a high percentage. MAGE-1 and tyrosinase were only expressed in four cell lines and to a low degree. HER2/neu was expressed rarely (HROG02 and HROG10). In summary, each cell line expressed at least two antigens (EGFR and survivin) and half even seven or more. Of note, the cell line HROG02 expressed all of the assessed antigens.
In addition, we analyzed the influence of IFN-γ on the intensity of antigen expression in these cell lines. Here, the degree of antigen expression was rarely and if only barely affected by IFN-γ treatment (Figure 1).
Expression of MHC class I and II
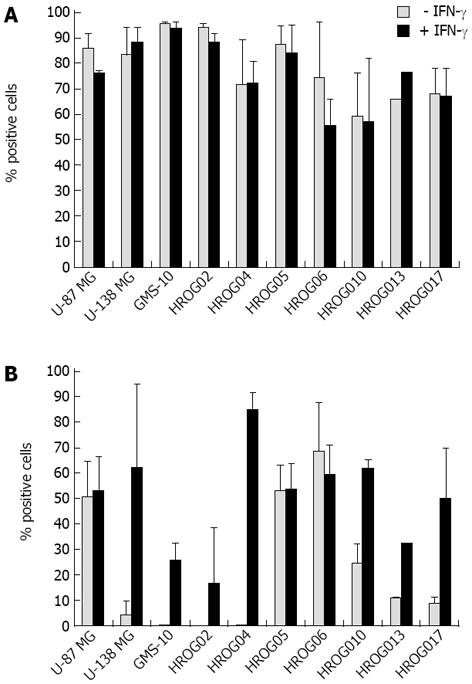
Next, the expression of MHC class I and II molecules of the GBM cell lines was analyzed. All cell lines expressed high levels (> 50% expression) of the HLA-ABC antigens; though no further up-regulation could be achieved by IFN-γ treatment (Figure 2A). In contrast, only three cell lines (HROG05, HROG06 and U-87 MG) highly expressed HLA-DR, -DP, three (HROG10, HROG13 and HROG17) had intermediate levels and four (HROG02, HROG04, U-138-MG and GMS-10) did not express MHC class II at all. Here, treatment of the cells with IFN-γ resulted in a clear induction of expression in the non-expressing cell lines and an up-regulation in the intermediate ones (Figure 2B).
With regard to unraveling novel T cell epitopes from candidate tumor antigens, the HLA-A02 sub-type is most frequently used as it is the most common one in Caucasian and Asian population. Thus, HLA-A02 expression was assessed by flow cytometric staining. Results were subsequently confirmed by a molecular genetic HLA-A typing (Table 2). Five out of the seven cell lines stained positive for HLA-A02 and were at least heterozygous for *A2. Of the three commercially available cell lines, GMS-10 and U-87 MG were additionally found to express HLA-A02 as judged by flow cytometric staining analysis (data not shown).
| HROG02 | HROG04 | HROG05 | HROG06 | HROG10 | HROG13 | HROG17 | ||||||||
| HLA-A typing | *01 | *02 | *01 | *02 | *02 | - | *01 | *03 | *02 | *23 | *02 | - | *11 | *66 |
| Flow cytometry (HLA-A02) | 88% ( ± 10%) | 95% ( ± 1%) | 83% ( ± 10%) | 1% ( ± 1%) | 89% ( ± 4%) | 92% ( ± 3%) | 0% ( ± 0%) | |||||||
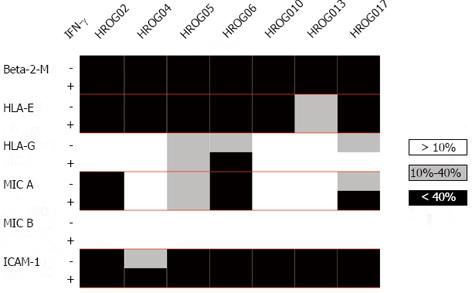
The cell lines were further characterized with regard to a broader range of MHC machinery components such as HLA-E and -G, MIC A and B as well as β2-microglobulin (Figure 3). In line with the results of the HLA-ABC expression analysis, β2-microglobulin was highly expressed in all cell lines. Further, all cell lines expressed HLA-E; with the exception of HROG13 all cell lines even very high levels (> 75% expression). HLA-G was expressed by three cell lines (HROG05, HROG06 and HROG17). MIC A was highly expressed by two cell lines (HROG02 and HROG06), two cell lines (HROG05 and HROG17) had intermediate and three (HROG04, HROG10 and HROG13) no MIC A expression. None of the cell lines stained positive for MIC B. In addition, the immune-recognition-enhancing molecule ICAM-1 was analyzed (Figure 3). This was highly expressed by all cell lines.
Cell lines have served in unraveling cellular- and tumor-related mechanisms as well as in therapy development for many decades. Since no severe differences were observed between the standard cultures and our patient-derived low-passage cell lines with regard to TAA expression, the latter are preferable for such analyses. By using (ultra) low-passage cell lines, culture artifacts can (nearly) be excluded. Long term cultures are reported to acquire a multitude of culture related changes such as increase in doubling time with the number of passages[14,15], a more methylated MGMT promoter phenotype[16] and the acquisition of novel mutations and chromosomal aberrations[17].
Individual patients’ tumor models are especially interesting for testing of drug responses and resistance development[8,9] but are also ideal for establishing and testing of immune-based treatment strategies if autologous or tumor infiltrating lymphocytes (TIL) are available[18,19].Together with expert pathological assessment of tumor characteristics and lymphocytic infiltration, low-passage cell lines offer an outstanding possibility to develop and optimize immunotherapeutic approaches. Such approaches seem to at least not be hindered by inflammation or IFN-γ secreted, e.g., by T cells, since no adverse effects of IFN-γ on TAA expression were observed. However, we did not analyze changes in the presentation of TAA-derived peptides in MHC class I and II molecules.
In case of MHC class II, an induction or up-regulation of the molecules by IFN-γ was frequently observed. This phenomenon is described in the literature[20,21].
However, the biological background of the high variation in MHC class II expression is not known at present. We suspect that because of the role of MHC class II in T cell activation and regulation this to be a potential novel mechanism of immune evasion. Respective experiments are ongoing in our laboratory.
Immune evasion is one of the major mechanisms employed by tumors to avoid destruction by the patient’s immune system. Beside low expression of MHC class I and secretion of immunosuppressive factors like transforming growth factor-β, prostaglandin E2 or Interleukin-6 particularly the upregulation of molecules like Fas and programmed cell death 1 ligand 1 (PD-L1) allow tumor cells (and especially glioblastoma cells) to directly induce T cell anergy or even apoptosis and, as a consequence, to from an immunosuppressive environment[22]. Especially glioma stem cells are capable of most if not all of these mechansism[22].
To sum up our findings, all cell lines expressed multiple TAA; this enables targeting more than one antigen in order to prevent immune escape and resistance development[23,24]. Additionally, antigen spreading could readily be analyzed.
Finally, the models allow testing the immunological potential of the (selected) antigen (combinations) through in vitro T cell stimulation and functional readouts (for example ELISpot or kill assays). As we have recently shown that the generation of GBM cell lines is feasible and successful to a high percentage even from vitally frozen material[12], accompanying research on immunotherapies becomes possible.
A decade ago, Liu and colleagues assessed the expression of the antigens HER-2, gp100 and MAGE-1 in human glioblastoma. They additionally described a correlation between the cytolytic potential of antigen-specific T cells and the level of antigen expression[25]. A few years later, Zhang et al[26] performed an antigenic profiling of 20 commercially available GBM cell lines with potential regard to allogenic immunotherapies. In their study, all cell lines were found to express the antigens HER2/neu, IL-13Rα, MAGE-1 and survivin and two thirds were positive for tyrosinase.
Recently peptide-pulsed dendritic cells entered a phase I clinical study for patients with recurrent high grade gliomas. The peptides included in the study were derived from the antigens gp100, HER-2, MAGE and WT-1[27]. With this approach, one out of nine patients had stable disease after vaccination; thus hinting toward efficacy of the strategy but likewise demonstrating the necessity of improvement. The antigens survivin[28] and RHAMM[29] are further being exploited for their potential to cure cancer in vaccination strategies for head and neck cancer as well as chronic B cell lymphoma.
For development of therapeutic strategies, cell lines are an inevitable tool. With the rise of immunotherapy, precise knowledge on the expression of relevant molecules in the cell models used is appreciated by basic and translational researchers. For the most part, these molecules include components of the major histocompatibility complex (MHC) machinery on the one hand and tumor antigens on the other.
Development of novel immunotherapeutic approaches is an exploding field of research. Examples here for are chimeric antigen receptors, bi specific T cell engagers, cellular (mostly dendritic cell) vaccines and T cell transfers.
Similar phenotypic characterizations of cell lines from numerous different tumor entities have been performed many times. However, the authors group’s focus for several years is development and characterization of low-passage, patient-individual cell lines. These have several major advantages when compared to standard cell lines. Lower passages mean less change in the cell’s genome and epigenome. Lymphocytes and sera biobanked together with the cell models allow research to be performed in a complete autologous setting.
These novel cell lines are interesting tools for drug screening, therapy optimization and even basic and further translational research applications. With the data presented here, they additionally recommend themselves for future development of immunotherapeutic strategies, associated screenings and the like. The cell lines are available in low-passages upon request and thus it is very likely that subsequent studies will broaden the data presented here.
Patient-derived, low-passage cell lines: a cell line which has been established only recently from a tumor of a patient and underwent a very limited number of cell divisions.
The authors have conducted an investigation of the expression of tumor-antigens and MHC-machinery components in glioblastoma multiforme cell lines. The conception and relevance of this work could be enormous; indeed the authors have analyzed patient specific cell cultures. This is of extreme relevance and actuality due to the more and more recognized limitations of the immortalized cell line model.
P- Reviewers: CardinaleV, Berardinis P S- Editor: Song XX L- Editor: A E- Editor: Liu XM
| 1. | Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987-996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14033] [Cited by in RCA: 15799] [Article Influence: 790.0] [Reference Citation Analysis (0)] |
| 2. | De Vleeschouwer S, Fieuws S, Rutkowski S, Van Calenbergh F, Van Loon J, Goffin J, Sciot R, Wilms G, Demaerel P, Warmuth-Metz M. Postoperative adjuvant dendritic cell-based immunotherapy in patients with relapsed glioblastoma multiforme. Clin Cancer Res. 2008;14:3098-3104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 194] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 3. | Mullins CS, Linnebacher M. Endogenous retrovirus sequences as a novel class of tumor-specific antigens: an example of HERV-H env encoding strong CTL epitopes. Cancer Immunol Immunother. 2012;61:1093-1100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 51] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 4. | Yang F, Yang XF. New concepts in tumor antigens: their significance in future immunotherapies for tumors. Cell Mol Immunol. 2005;2:331-341. [PubMed] |
| 5. | Barretina J, Caponigro G, Stransky N, Venkatesan K, Margolin AA, Kim S, Wilson CJ, Lehár J, Kryukov GV, Sonkin D. The Cancer Cell Line Encyclopedia enables predictive modelling of anticancer drug sensitivity. Nature. 2012;483:603-607. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5947] [Cited by in RCA: 5808] [Article Influence: 446.8] [Reference Citation Analysis (0)] |
| 6. | Reyes G, Villanueva A, García C, Sancho FJ, Piulats J, Lluís F, Capellá G. Orthotopic xenografts of human pancreatic carcinomas acquire genetic aberrations during dissemination in nude mice. Cancer Res. 1996;56:5713-5719. [PubMed] |
| 7. | Vogel CL, Reddy JC, Reyno LM. Efficacy of trastuzumab. Cancer Res. 2005;65:2044. [PubMed] |
| 8. | Voskoglou-Nomikos T, Pater JL, Seymour L. Clinical predictive value of the in vitro cell line, human xenograft, and mouse allograft preclinical cancer models. Clin Cancer Res. 2003;9:4227-4239. [PubMed] |
| 9. | Fiebig HH, Maier A, Burger AM. Clonogenic assay with established human tumour xenografts: correlation of in vitro to in vivo activity as a basis for anticancer drug discovery. Eur J Cancer. 2004;40:802-820. [PubMed] |
| 10. | Francia G, Cruz-Munoz W, Man S, Xu P, Kerbel RS. Mouse models of advanced spontaneous metastasis for experimental therapeutics. Nat Rev Cancer. 2011;11:135-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 284] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 11. | Kerbel RS. Human tumor xenografts as predictive preclinical models for anticancer drug activity in humans: better than commonly perceived-but they can be improved. Cancer Biol Ther. 2003;2:S134-S139. [PubMed] |
| 12. | Mullins CS, Schneider B, Stockhammer F, Krohn M, Classen CF, Linnebacher M. Establishment and characterization of primary glioblastoma cell lines from fresh and frozen material: a detailed comparison. PLoS One. 2013;8:e71070. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 13. | Mullins CS, Schubert J, Schneider B, Linnebacher M, Classen CF. Cilengitide response in ultra-low passage glioblastoma cell lines: relation to molecular markers. J Cancer Res Clin Oncol. 2013;139:1425-1431. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 14. | Park YB, Kim YY, Oh SK, Chung SG, Ku SY, Kim SH, Choi YM, Moon SY. Alterations of proliferative and differentiation potentials of human embryonic stem cells during long-term culture. Exp Mol Med. 2008;40:98-108. [PubMed] |
| 15. | Chang-Liu CM, Woloschak GE. Effect of passage number on cellular response to DNA-damaging agents: cell survival and gene expression. Cancer Lett. 1997;113:77-86. [PubMed] |
| 16. | Danam RP, Howell SR, Remack JS, Brent TP. Heterogeneous methylation of the O (6)-methylguanine-DNA methyltransferase promoter in immortalized IMR90 cell lines. Int J Oncol. 2001;18:1187-1193. [PubMed] |
| 17. | Huszthy PC, Daphu I, Niclou SP, Stieber D, Nigro JM, Sakariassen PØ, Miletic H, Thorsen F, Bjerkvig R. In vivo models of primary brain tumors: pitfalls and perspectives. Neuro Oncol. 2012;14:979-993. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 162] [Cited by in RCA: 190] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 18. | Klier U, Maletzki C, Kreikemeyer B, Klar E, Linnebacher M. Combining bacterial-immunotherapy with therapeutic antibodies: a novel therapeutic concept. Vaccine. 2012;30:2786-2794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 19. | Dudley ME, Gross CA, Somerville RP, Hong Y, Schaub NP, Rosati SF, White DE, Nathan D, Restifo NP, Steinberg SM, Wunderlich JR, Kammula US, Sherry RM, Yang JC, Phan GQ, Hughes MS, Laurencot CM, Rosenberg SA. Randomized selection design trial evaluating CD8+-enriched versus unselected tumor-infiltrating lymphocytes for adoptive cell therapy for patients with melanoma. J Clin Oncol. 2013;31:2152-2159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 177] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 20. | Michel S, Linnebacher M, Alcaniz J, Voss M, Wagner R, Dippold W, Becker C, von Knebel Doeberitz M, Ferrone S, Kloor M. Lack of HLA class II antigen expression in microsatellite unstable colorectal carcinomas is caused by mutations in HLA class II regulatory genes. Int J Cancer. 2010;127:889-898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 21. | Meissner M, Whiteside TL, Kaufmann R, Seliger B. CIITA versus IFN-gamma induced MHC class II expression in head and neck cancer cells. Arch Dermatol Res. 2009;301:189-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 22. | Toda M. Glioma stem cells and immunotherapy for the treatment of malignant gliomas. ISRN Oncol. 2013;2013:673793. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 23. | Zhou G, Levitsky H. Towards curative cancer immunotherapy: overcoming posttherapy tumor escape. Clin Dev Immunol. 2012;2012:124187. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 24. | Harandi A. Immunoplacental therapy, a potential multi-epitope cancer vaccine. Med Hypotheses. 2006;66:1182-1187. [PubMed] |
| 25. | Liu G, Ying H, Zeng G, Wheeler CJ, Black KL, Yu JS. HER-2, gp100, and MAGE-1 are expressed in human glioblastoma and recognized by cytotoxic T cells. Cancer Res. 2004;64:4980-4986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 150] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 26. | Zhang JG, Eguchi J, Kruse CA, Gomez GG, Fakhrai H, Schroter S, Ma W, Hoa N, Minev B, Delgado C. Antigenic profiling of glioma cells to generate allogeneic vaccines or dendritic cell-based therapeutics. Clin Cancer Res. 2007;13:566-575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 113] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 27. | Akiyama Y, Oshita C, Kume A, Iizuka A, Miyata H, Komiyama M, Ashizawa T, Yagoto M, Abe Y, Mitsuya K. α-type-1 polarized dendritic cell-based vaccination in recurrent high-grade glioma: a phase I clinical trial. BMC Cancer. 2012;12:623. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 69] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 28. | Turksma AW, Bontkes HJ, Ruizendaal JJ, Scholten KB, Akershoek J, Rampersad S, Moesbergen LM, Cillessen SA, Santegoets SJ, de Gruijl TD. Exploring dendritic cell based vaccines targeting survivin for the treatment of head and neck cancer patients. J Transl Med. 2013;11:152. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 29. | Giannopoulos K, Własiuk P, Dmoszyńska A, Roliński J, Schmitt M. Peptide vaccination induces profound changes in the immune system in patients with B-cell chronic lymphocytic leukemia. Folia Histochem Cytobiol. 2011;49:161-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |