Revised: September 2, 2013
Accepted: October 16, 2013
Published online: November 27, 2013
Processing time: 122 Days and 13.4 Hours
The average age of hepatitis C virus (HCV)-infected individuals is becoming increasingly higher in Japan and steps should be taken to treat older individuals infected with HCV. Until an interferon-free regimen becomes available, peginterferon plus ribavirin will play a critical role in the treatment. The perception that older HCV-infected patients may be at higher risk than younger patients for adverse events from peginterferon plus ribavirin treatment but may obtain less clinical benefit from it may be based on the underrepresentation of older patients in clinical trials. A recent genome-wide association study revealed that interleukin-28B (IL28B) genotype closely correlates with the treatment response against HCV. The relationship of IL28B genotype with the treatment response in older HCV-infected patients is also unknown. In this review, we focused on the treatment response in older patients infected with HCV and the effects of IL28B genotype. IL28B major genotype is a useful predictor of sustained virological response in the interferon-including treatment of older patients infected with HCV. It also seems useful for avoiding adverse events, although the mechanisms of the effects of IL28B genotype on the treatment outcome are still poorly understood and are currently under investigation. Further studies will be needed.
Core tip: The exact mechanisms of the effects of interleukin-28B (IL28B) genotype on the treatment response in chronic hepatitis C patients are unclear. However, IL28B genotype is useful for the continued successful treatment of older patients infected with hepatitis C virus (HCV) and avoiding adverse events. Until the eventual availability of interferon-free regimens, it is important to determine IL28B genotype before treating HCV-infected individuals, especially in the screening of older patients.
-
Citation: Kanda T, Nakamoto S, Wu S, Yokosuka O. Role of
IL28B genotype in older hepatitis C virus-infected patients. World J Immunol 2013; 3(3): 54-61 - URL: https://www.wjgnet.com/2219-2824/full/v3/i3/54.htm
- DOI: https://dx.doi.org/10.5411/wji.v3.i3.54
Hepatitis C virus (HCV) infection is a cause of chronic hepatitis, cirrhosis and hepatocellular carcinoma in the United States, Western countries and Japan[1-4]. Most cases of hepatocellular carcinoma are associated with chronic hepatitis B virus or HCV infections[5]. HCV variants are classified into at least 6 genotypes (representing the 6 genetic groups defined by phylogenetic analysis). It is well known that the treatment response differs among the different HCV genotypes[6]. Approximately 50% and 80% achieve a sustained virological response, defined as undetectable HCV RNA at week 24 after stopping the treatment, following the current standard of care combination therapy with peginterferon plus ribavirin for 48 wk in HCV genotype 1 and 24 wk in HCV genotype 2[6].
In Japan, hepatocellular carcinoma is one of the major malignancies and patients with liver cirrhosis are encouraged to undergo examinations using a combination of real-time ultrasonography and alpha-fetoprotein measurement at regular intervals for an early detection of hepatocellular carcinoma[7]. The prognosis of elderly hepatocellular carcinoma patients was found to be similar to that for younger cases[8]. In Japan, as many people live to an advanced age, there are also many elderly hepatocellular carcinoma patients. Therefore, we need to treat elderly patients infected with HCV in order to decrease the mortality and incidence of HCV-related liver diseases[9,10].
The recent recognition that host genetic polymorphisms near interleukin-28B (IL28B) (IL28B genotype), such as a single nucleotide polymorphism (SNP) (rs8099917 or rs12979860), predict the treatment-induced and spontaneous HCV eradications remains an important discovery[11-15]. IL28B encodes interferon lambda 3 (IFNL3), a cytokine distantly related to type I interferons and IL10[16]. IL28B, interleukin-28A and interleukin-29 are three closely related cytokine genes that form a cytokine gene cluster on chromosome 19q13. This review focuses on HCV and host factor IL28B genotype in the treatment response of elderly patients with chronic hepatitis C and describes our experiences in treating such patients with chronic hepatitis C.
Treatment response to standard of care in HCV genotype 1-infected patients is shown in Table 1[17-22]. The reported patients from the United States and European countries were, on average, younger than those from Japan[8,21-23]. This suggests that there might be different time spread and roots of transmission of HCV among these countries. In the United States, HCV genotype 1a-infected individuals appeared around 1960, at least 30 years later than the widespread introduction of HCV genotype 1b in the Japanese population[24]. The spread of HCV genotype 1b in Japan then started to decrease around 1995, whereas HCV genotype 1a in the United States is still growing exponentially[24]. Japan has high mortality rates from hepatocellular carcinoma, counting about 30000 deaths annually[25]. According to hepatitis virus carrier rates among first-time blood donors, the peak frequency of antibody to HCV was demonstrated by the 1931-1935 birth cohort, who are 78-82 years old in 2013[8]. So, to prevent death from hepatocellular carcinoma in Japan, we should treat relatively older patients infected with HCV. We and others previously reported that standard of care was also effective in chronic hepatitis C patients aged > 65 years[21,26].
| Ref. | G | Number of | Naive | Age (yr, mean ± SD)/ | Formula | SVR rates |
| patients | gender (male) | |||||
| McHutchison et al[17] | 1 | 1016 | Yes | 47.5 ± 8.1/59.7% | Low-dose PegIFN alpha-2b plus RBV | 38.00% |
| 1 | 1019 | Yes | 47.5 ± 7.8/60.2% | Standard-dose PegIFN alpha-2b plus RBV | 39.80% | |
| 1 | 1035 | Yes | 47.6 ± 8.2/59.2% | PegIFN alpha-2a plus RBV | 40.90% | |
| Jeffers et al[18] | 1 | 78 (Black) | Yes | 46.3 ± 0.7/72% | PegIFN alpha-2a plus RBV | 26.00% |
| 1 | 28 (White) | Yes | 44.7 ± 1.4/61% | PegIFN alpha-2a plus RBV | 39.00% | |
| Bruno et al[19] | 1 | 163 | Yes | 49.9 ± 11.1/62% | PegIFN alpha-2b plus RBV | 41.10% |
| 1 | 148 | Yes | 49.5 ± 11.1/62% | IFN alpha-2b plus RBV | 29.30% | |
| Conjeevaram et al[20] | 1 | 196 (African-Americans) | Yes | 49.0 ± (45.0, 52.5)/64.8% | PegIFN alpha-2a plus RBV | 28.00% |
| 1 | 205 (Caucasian-Americans) | Yes | 48.0 ± (43.0, 52.0)/65.8% | PegIFN alpha-2a plus RBV | 52.00% | |
| Miyauchi et al[21] | 1 | 383 | - | 55.0 ± 10.9/62% | PegIFN alpha-2b plus RBV | 61.80% |
| Kanda et al[22] | 1 | 127 | Yes | 56.1 ± 10.7/48.8% | PegIFN alpha-2a plus RBV | 56.60% |
| 1 | 69 | No | 59.0 ± 10.1/49.2% | PegIFN alpha-2a plus RBV | 39.10% |
First, we retrospectively analyzed 196 chronic hepatitis C patients aged ≥ 65 years and treated with standard of care in our and affiliated hospitals[21,22,27,28] (Table 2). This work was carried out in accordance with the Declaration of Helsinki of the World Medical Association. The study protocol was approved by the Ethics Committee of Chiba University School of Medicine.
| Total (n= 196) | Male (n= 104) | Female (n= 92) | P value1 | |
| Age (yr) | 67.8 ± 2.5 | 68.1 ± 2.6 | 67.4 ± 2.4 | |
| Gender (male/female) | 104/92 | 104/0 | 0/92 | |
| HCV viral load (high/low/unknown) | 185/7/4 | 6/2/1996 | 1/2/1989 | 0.169 |
| HCV genotypes 1/2 | 164/32 | Nov-93 | 71/21 | 0.033 |
| Treatment-naïve (yes/no/unknown) | 108/87/1 | 55/48/1 | 52/39/1 | 0.704 |
| ALT (IU/L) | 61.3 ± 50.7 | 70.1 ± 57.1 | 51.4 ± 40.3 | 0.010 |
| White blood cells (/μL) | 4940 ± 1490 | 5160 ± 1670 | 4690 ± 1210 | 0.027 |
| Neutrophils (/μL) | 2660 ± 1080 | 2790 ± 1270 | 2530 ± 830 | 0.096 |
| Hemoglobin (g/dL) | 13.7 ± 1.5 | 14.1 ± 1.6 | 13.2 ± 1.3 | 0.000 |
| Platelets (× 104/μL) | 15.1 ± 4.7 | 15.0 ± 4.8 | 15.4 ± 4.6 | 0.553 |
| Peg-IFN α - 2a / Peg-IFN α - 2b | 86/110 | 41/63 | 45/47 | 0.233 |
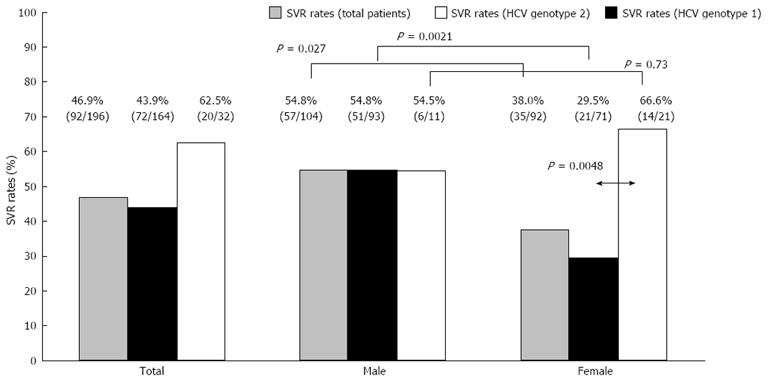
In the 196 patients treated with standard of care, sustained virological response was achieved in 46.9% (92/196). Sustained virological response was achieved in 43.9% (72/164) of HCV genotype 1 compared with 62.5% (20/32) of HCV genotype 2 patients (P = 0.082). In 110 patients treated with peginterferon alpha-2b plus ribavirin, sustained virological response was achieved in 49.4% (44/89) of HCV genotype 1 compared with 66.6% (14/21) of HCV genotype 2 patients. In 86 patients treated with peginterferon alpha-2a plus ribavirin, sustained virological response was achieved in 37.3% (28/75) of HCV genotype 1 compared with 54.5% (6/11) of HCV genotype 2 patients. There were no differences in efficacies or adverse events between the two peginterferons.
In male and female patients aged ≥ 65 years and treated with standard of care, sustained virological response was achieved in 54.8% and 38.0%, respectively (Figure 1). Our results also supported previous studies[8,26]. Gender differences were more remarkable in HCV genotype 1 than in HCV genotype 2 patients (P = 0.0021). In older female patients, the treatment response in HCV genotype 2 was better than that of the others (Figure 1).
Interferon plus ribavirin combination therapy for chronic hepatitis C produces a number of well-described side-effects that are dominated by fatigue, influenza-like symptoms, hematological abnormalities and neuropsychiatric symptoms[29,30]. We defined “severe adverse event” if treatment had to be stopped. In chronic hepatitis C patients aged ≥ 65 years, we identified severe adverse events in 14.7% (29/196) (Table 3). In male and female patients, severe adverse events occurred in 14.4% (15/104) and 15.2% (14/92), respectively. Fried et al[31] reported that severe adverse events were observed in 7% when the mean age of patients treated with standard of care was 42.8 ± 10.1 years. McHutchison et al[17] reported that severe adverse events were observed in 9.6%-13% when the mean age of patients treated with standard of care was 47 years. So, our results suggested that older HCV-infected patients treated with standard of care might be more susceptible to severe adverse events. Among them, occurrence of hepatocellular carcinoma, severe fatigue, severe pulmonary symptoms and severe anemia were observed in 13.5% each, although we did not use growth factors such as erythropoietin[32-34] in Japan. It should be emphasized that clinicians regularly check for the possible development of hepatocellular carcinoma even in chronic hepatitis C patients under treatment[10].
| Severe adverse events | No. of patient |
| discontinuities | |
| Occurrence of malignancies (Hepatocellular carcinoma) | 5 (13.5) |
| Severe fatigue | 5 (13.5) |
| Severe pulmonary symptoms (bloody phlegm, cough, or interstitial pneumonitis) | 5 (13.5) |
| Severe anemia | 5 (13.5) |
| Psychiatric disorders | 3 (8.1) |
| Severe skin lesion | 3 (8.1) |
| Neurological disorders | 3 (8.1) |
| Severe thrombocytopenia or neutropenia | 3 (8.1) |
| Severe denutrition | 2 (5.4) |
| Upper gastrointestinal bleeding | 1 (2.7) |
| Severe infection (tuberculosis) | 1 (2.7) |
| Unknown | 1 (2.7) |
There have been several reports showing a close association between IL28B genotype and treatment response to standard of care[11-14,35-42]. Although the mechanisms for this association are still uncertain, several studies have indicated an association between plasma interferon-gamma-inducible protein-10 (IP-10) levels and IL28B genotypes[41], as well as between the expression of hepatic interferon-stimulated genes and IL28B genotypes[38]. We also reported that hepatic signal transducer and activator of transcription 1 -nuclear translocation and IL28B genotype could predict treatment outcomes in HCV genotype 1-infected patients[36]. A (TA) dinucleotide repeat, rs72258881, located in the promoter region, and the transcriptional activity of the promoter increasing gradually in a (TA)n length-dependent manner, was discovered[43]. A dinucleotide variant, ss469415590 (-G), is a flame-shift variant that creates a novel gene, designated as IFNL4, encoding the interferon-λ4 protein (IFNL4), which is moderately similar to IFNL3[44]. This novel TT/-G polymorphism in the CpG region upstream of IL28B is a better predictor of HCV clearance than rs12979860. Induction of IL28B and interferon-gamma-inducible protein-10 (IP-10) mRNA of peripheral blood mononuclear cells (PBMCs) relies on TT/-G polymorphism[45]. Further studies will be needed to clarify the significance of IL28B-related SNPs. In any case, IL28B genotypes (rs8099917 and rs12979860) are predictive factors of sustained virological response in standard of care for HCV[46,47]. Ge et al[11] reported that the major genotype in rs12979860 leads to 80% sustained virological response with standard of care, but a minor genotype in rs12979860 leads to only 30% sustained virological response. It was reported that the major genotype in rs8099917 leads to 75.3% sustained virological response, but a minor genotype in rs8099917 leads to only 24.7% sustained virological response in HCV genotype 1 patients, and also that major and minor genotypes at rs8099917, respectively, lead to 83.0% and 17.0% sustained virological response in HCV genotype 2 patients[47].
We retrospectively analyzed 41 chronic hepatitis C patients aged ≥ 65 years and treated with standard of care in our hospital (Table 4). This work was carried out in accordance with the Declaration of Helsinki of the World Medical Association. The study protocol was approved by the Ethics Committee of Chiba University School of Medicine. Our results showed that IL28B rs8099917 genotype is predictive of early virological response, which is undetectable HCV RNA at week 12 after the commencement of therapy, in standard of care for HCV-infected older patients. As early virological response is a predictive value for sustained virological response in standard of care treatment, IL28B genotyping could give us useful information for the prediction of sustained virological response with this treatment.
| Total | IL28B TT | IL28B TG | 1P values | |
| (n = 41) | (n = 31) | (n = 10) | ||
| Age (yr) | 67.7 ± 2.6 | 68.0 ± 2.9 | 67.0 ± 1.3 | 0.141 |
| Gender (male/female) | 28/13 | 22/9 | 4/6 | 0.796 |
| HCV viral load, (high/low) | 41/0 | 31/0 | 10/0 | NA |
| HCV genotype (G1/G2) | 41/0 | 25/6 | 8/2 | 0.678 |
| Treatment-naïve (yes/no) | 29/12 | 24/7 | 5/5 | 0.208 |
| ALT (IU/L) | 56.5 ± 40.2 | 54.9 ± 41.2 | 61.1 ± 38.9 | 0.677 |
| γ-GTP (IU/L) | 36.4 ± 33.3 | 33.1 ± 33.1 | 46.6 ± 33.4 | 0.269 |
| White blood cells (/μL) | 4840 ± 1300 | 4730 ± 1160 | 5190 ± 1690 | 0.337 |
| Hemoglobin (g/dL) | 13.5 ± 1.3 | 13.6 ± 1.1 | 13.3 ± 1.8 | 0.528 |
| Platelets (x104/μL) | 14.9 ± 5.4 | 14.4 ± 5.1 | 16.5 ± 6.2 | 0.289 |
| IL28B rs8099917 (TT/TG) | 31/10 | 31/0 | 0/10 | NA |
| EVR rates | 34.1% (14/41) | 45.1% (14/31) | 0% (0/10) | 0.025 |
| SVR rates | 43.9% (18/41) | 48.3% (15/31) | 30.0% (3/10) | 0.514 |
In 2011, telaprevir and boceprevir were approved as direct-acting antivirals against HCV in Japan as well as in the United States and European countries[48]. Triple therapy with peginterferon, ribavirin, plus telaprevir or boceprevir improved sustained virological response rates greatly, up to 70%-80%, in treatment-naïve individuals and previous treatment relapsers[39,49-55], but these triple therapies also brought severe adverse events to the patients[56]. In Japan, only the telaprevir-including regimen was available in clinical practice at this time. We frequently observed dermatological side-effects[57], drug-drug interactions[58], anemia[59] and renal impairment[60] in chronic hepatitis C patients under triple therapy with telaprevir. Akuta et al[61] reported that IL28B genotype could predict the response to peginterferon/ribavirin/telaprevir therapy. The efficacy of triple therapy was high in patients with IL28B rs8099917 genotype TT who accomplished sustained virological response (83.8%), but was low in patients with IL28B rs8099917 genotype TG or GG who accomplished sustained virological response (27.6%). The efficacy of triple therapy was also high in patients with IL28B rs12979860 genotype CC who accomplished sustained virological response (83.8%), but was low in patients with IL28B rs12979860 genotype CT or TT who accomplished sustained virological response (32.3%)[61], although it was reported that IL28B genotype had a limited impact on sustained virological response rates with telaprevir-based therapy in treatment-experienced patients[62].
In our experience, we safely treated a 74 year old female, a previous treatment-relapser who had IL28B rs8099917 TT, with peginterferon/ribavirin/telaprevir and obtained a successful result. Although her dose of telaprevir was 1500 mg/d[63], she did not discontinue the treatment, had no adverse events and finally achieved sustained virological response. Because most Asian individuals are of relatively smaller physical stature compared to American or European individuals, it was reported that 24 wk triple therapy with telaprevir at 1500 mg/d seemed safe and efficacious for elderly patients infected with HCV genotype 1b[63]. In addition, IL28B genotyping seems to be one of the useful screening methods for older patients awaiting treatment with peginterferon/ribavirin/telaprevir[64]. Furusyo et al[64] reported that sustained virological response rates for patients aged > 60 with IL28B rs8099917 TT genotype (89.4%) was significantly higher than for those with IL28B TG/GG genotype (41.2%), and that multivariate analysis extracted IL28BTT and rapid virological response, which was defined as undetectable HCV RNA at week 4 after the commencement of therapy, as independent factors associated with sustained virological response. They observed severe anemia more frequently in patients aged > 60 years than in patients aged ≤ 60 years[64].
In summary, IL28B major genotype is a useful predictor of sustained virological response in the interferon-including treatment of older patients infected with HCV. In addition, it seems to play a useful role for avoiding adverse events. The mechanisms of the effects of IL28B genotype on the treatment outcome are now under investigation and further studies will be needed. Until the appearance of interferon-free regimens, it is important to determine IL28B genotype before treating HCV-infected individuals and this is especially true in the screening of older patients.
P- Reviewers: Rosales C, Velisek J S- Editor: Zhai HH L- Editor: Roemmele A E- Editor: Wang CH
| 1. | Di Bisceglie AM. Hepatitis C and hepatocellular carcinoma. Hepatology. 1997;26:34S-38S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 293] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 2. | Pacholczyk M, Łągiewska B, Lisik W, Tronina O, Wasiak D, Cieciura T, Chmura A. Liver transplantation for HCV cirrhosis; cautious optimism after 10 years of experience. Ann Transplant. 2012;17:5-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 3. | Saito I, Miyamura T, Ohbayashi A, Harada H, Katayama T, Kikuchi S, Watanabe Y, Koi S, Onji M, Ohta Y. Hepatitis C virus infection is associated with the development of hepatocellular carcinoma. Proc Natl Acad Sci USA. 1990;87:6547-6549. [PubMed] |
| 4. | Shiratori Y, Shiina S, Zhang PY, Ohno E, Okudaira T, Payawal DA, Ono-Nita SK, Imamura M, Kato N, Omata M. Does dual infection by hepatitis B and C viruses play an important role in the pathogenesis of hepatocellular carcinoma in Japan? Cancer. 1997;80:2060-2067. [PubMed] |
| 5. | El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology. 2012;142:1264-1273.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2183] [Cited by in RCA: 2498] [Article Influence: 192.2] [Reference Citation Analysis (2)] |
| 6. | Kanda T, Imazeki F, Yokosuka O. New antiviral therapies for chronic hepatitis C. Hepatol Int. 2010;4:548-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 98] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 7. | Nomura F, Ohnishi K, Honda M, Satomura Y, Nakai T, Okuda K. Clinical features of hepatocellular carcinoma in the elderly: a study of 91 patients older than 70 years. Br J Cancer. 1994;70:690-693. [PubMed] |
| 8. | Akita T, Ohisa M, Kimura Y, Fujimoto M, Miyakawa Y, Tanaka J. Validation and limitation of age-period-cohort model in simulating mortality due to hepatocellular carcinoma from 1940 to 2010 in Japan. Hepatol Res. 2013;Jun 3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Di Bisceglie AM, Shiffman ML, Everson GT, Lindsay KL, Everhart JE, Wright EC, Lee WM, Lok AS, Bonkovsky HL, Morgan TR. Prolonged therapy of advanced chronic hepatitis C with low-dose peginterferon. N Engl J Med. 2008;359:2429-2441. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 377] [Cited by in RCA: 339] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 10. | Kanda T, Imazeki F, Mikami S, Kato K, Shimada N, Yonemitsu Y, Miyauchi T, Arai M, Fujiwara K, Tsubota A. Occurrence of hepatocellular carcinoma was not a rare event during and immediately after antiviral treatment in Japanese HCV-positive patients. Oncology. 2011;80:366-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Ge D, Fellay J, Thompson AJ, Simon JS, Shianna KV, Urban TJ, Heinzen EL, Qiu P, Bertelsen AH, Muir AJ. Genetic variation in IL28B predicts hepatitis C treatment-induced viral clearance. Nature. 2009;461:399-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2776] [Cited by in RCA: 2722] [Article Influence: 170.1] [Reference Citation Analysis (0)] |
| 12. | Suppiah V, Moldovan M, Ahlenstiel G, Berg T, Weltman M, Abate ML, Bassendine M, Spengler U, Dore GJ, Powell E. IL28B is associated with response to chronic hepatitis C interferon-alpha and ribavirin therapy. Nat Genet. 2009;41:1100-1104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1505] [Cited by in RCA: 1503] [Article Influence: 93.9] [Reference Citation Analysis (0)] |
| 13. | Tanaka Y, Nishida N, Sugiyama M, Kurosaki M, Matsuura K, Sakamoto N, Nakagawa M, Korenaga M, Hino K, Hige S. Genome-wide association of IL28B with response to pegylated interferon-alpha and ribavirin therapy for chronic hepatitis C. Nat Genet. 2009;41:1105-1109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1779] [Cited by in RCA: 1774] [Article Influence: 110.9] [Reference Citation Analysis (0)] |
| 14. | Rauch A, Kutalik Z, Descombes P, Cai T, Di Iulio J, Mueller T, Bochud M, Battegay M, Bernasconi E, Borovicka J. Genetic variation in IL28B is associated with chronic hepatitis C and treatment failure: a genome-wide association study. Gastroenterology. 2010;138:1338-1345, 1345. e1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 850] [Cited by in RCA: 867] [Article Influence: 57.8] [Reference Citation Analysis (0)] |
| 15. | Booth D, George J. Loss of function of the new interferon IFN-λ4 may confer protection from hepatitis C. Nat Genet. 2013;45:119-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 16. | Kanda T, Jiang X, Nakamoto S, Nakamura M, Miyamura T, Wu S, Yokosuka O. Different effects of three interferons L on Toll-like receptor-related gene expression in HepG2 cells. Cytokine. 2013;64:577-583. [PubMed] |
| 17. | McHutchison JG, Lawitz EJ, Shiffman ML, Muir AJ, Galler GW, McCone J, Nyberg LM, Lee WM, Ghalib RH, Schiff ER, Galati JS, Bacon BR, Davis MN, Mukhopadhyay P, Koury K, Noviello S, Pedicone LD, Brass CA, Albrecht JK, Sulkowski; MSIDEAL Study Team. Peginterferon alfa-2b or alfa-2a with ribavirin for treatment of hepatitis C infection. N Engl J Med. 2009;361:580-593. [PubMed] |
| 18. | Jeffers LJ, Cassidy W, Howell CD, Hu S, Reddy KR. Peginterferon alfa-2a (40 kd) and ribavirin for black American patients with chronic HCV genotype 1. Hepatology. 2004;39:1702-1708. [PubMed] |
| 19. | Bruno S, Cammà C, Di Marco V, Rumi M, Vinci M, Camozzi M, Rebucci C, Di Bona D, Colombo M, Craxì A. Peginterferon alfa-2b plus ribavirin for naïve patients with genotype 1 chronic hepatitis C: a randomized controlled trial. J Hepatol. 2004;41:474-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 80] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 20. | Conjeevaram HS, Fried MW, Jeffers LJ, Terrault NA, Wiley-Lucas TE, Afdhal N, Brown RS, Belle SH, Hoofnagle JH, Kleiner DE, Howell CD; Virahep-C Study Group. Peginterferon and ribavirin treatment in African American and Caucasian American patients with hepatitis C genotype 1. Gastroenterology. 2006;131:470-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 384] [Cited by in RCA: 366] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 21. | Miyauchi T, Kanda T, Imazeki F, Mikata R, Tawada A, Arai M, Fujiwara K, Nakamoto S, Wu S, Tanaka T. Response to peginterferon-alpha 2b and ribavirin in Japanese patients with chronic hepatitis C genotype 1. Hepatol Int. 2013;7:144-152. [RCA] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 22. | Kanda T, Kato K, Tsubota A, Takada N, Nishino T, Mikami S, Miyamura T, Maruoka D, Wu S, Nakamoto S. Platelet count and sustained virological response in hepatitis C treatment. World J Hepatol. 2013;5:182-188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 23. | Sezaki H, Suzuki F, Kawamura Y, Yatsuji H, Hosaka T, Akuta N, Kobayashi M, Suzuki Y, Saitoh S, Arase Y. Poor response to pegylated interferon and ribavirin in older women infected with hepatitis C virus of genotype 1b in high viral loads. Dig Dis Sci. 2009;54:1317-1324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 24. | Tanaka Y, Hanada K, Mizokami M, Yeo AE, Shih JW, Gojobori T, Alter HJ. A comparison of the molecular clock of hepatitis C virus in the United States and Japan predicts that hepatocellular carcinoma incidence in the United States will increase over the next two decades. Proc Natl Acad Sci USA. 2002;99:15584-15589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 229] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 25. | Chen DS, Locarnini S, Wait S, Bae SH, Chen PJ, Fung JY, Kim HS, Lu SN, Sung J, Tanaka J, Wakita T, Ward J, Wallace J; CEVHAP North Asia Workshop on Viral Hepatitis. Report from a Viral Hepatitis Policy Forum on implementing the WHO framework for global action on viral hepatitis in North Asia. J Hepatol. 2013;59:1073-1080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 26. | Kainuma M, Furusyo N, Kajiwara E, Takahashi K, Nomura H, Tanabe Y, Satoh T, Maruyama T, Nakamuta M, Kotoh K, Azuma K, Shimono J, Shimoda S, Hayashi J; Kyushu University Liver Disease Study Group. Pegylated interferon α-2b plus ribavirin for older patients with chronic hepatitis C. World J Gastroenterol. 2010;16:4400-4409. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 39] [Cited by in RCA: 38] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 27. | Kanda T, Imazeki F, Azemoto R, Yonemitsu Y, Mikami S, Kita K, Takashi M, Sunaga M, Wu S, Nakamoto S. Response to peginterferon-alfa 2b and ribavirin in Japanese patients with chronic hepatitis C genotype 2. Dig Dis Sci. 2011;56:3335-3342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 28. | Kanda T, Nakamoto S, Nishino T, Takada N, Tsubota A, Kato K, Miyamura T, Maruoka D, Wu S, Tanaka T. Peginterferon Alfa-2a plus ribavirin in Japanese patients infected with hepatitis C virus genotype 2 who failed previous interferon therapy. Int J Med Sci. 2013;10:43-49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 29. | Fried MW. Side effects of therapy of hepatitis C and their management. Hepatology. 2002;36:S237-S244. [PubMed] |
| 30. | Durante-Mangoni E, Iossa D, Pinto D, De Vincentiis L, Ragone E, Utili R. Safety and efficacy of peginterferon alpha plus ribavirin in patients with chronic hepatitis C and coexisting heart disease. Dig Liver Dis. 2011;43:411-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 31. | Fried MW, Shiffman ML, Reddy KR, Smith C, Marinos G, Gonçales FL, Häussinger D, Diago M, Carosi G, Dhumeaux D. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347:975-982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4847] [Cited by in RCA: 4747] [Article Influence: 206.4] [Reference Citation Analysis (0)] |
| 32. | Pockros PJ, Shiffman ML, Schiff ER, Sulkowski MS, Younossi Z, Dieterich DT, Wright TL, Mody SH, Tang KL, Goon BL, Bowers PJ, Leitz G, Afdhal NH; PROACTIVE Study Group. Epoetin alfa improves quality of life in anemic HCV-infected patients receiving combination therapy. Hepatology. 2004;40:1450-1458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 112] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 33. | Mac Nicholas R, Norris S. Review article: optimizing SVR and management of the haematological side effects of peginterferon/ribavirin antiviral therapy for HCV - the role of epoetin, G-CSF and novel agents. Aliment Pharmacol Ther. 2010;31:929-937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 34. | Bacon BR, Gordon SC, Lawitz E, Marcellin P, Vierling JM, Zeuzem S, Poordad F, Goodman ZD, Sings HL, Boparai N. Boceprevir for previously treated chronic HCV genotype 1 infection. N Engl J Med. 2011;364:1207-1217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1306] [Cited by in RCA: 1308] [Article Influence: 93.4] [Reference Citation Analysis (0)] |
| 35. | Nakamoto S, Kanda T, Imazeki F, Wu S, Arai M, Fujiwara K, Yokosuka O. Simple assay based on restriction fragment length polymorphism associated with IL28B in chronic hepatitis C patients. Scand J Gastroenterol. 2011;46:955-961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 36. | Miyamura T, Kanda T, Nakamoto S, Wu S, Fujiwara K, Imazeki F, Yokosuka O. Hepatic STAT1-nuclear translocation and interleukin 28B polymorphisms predict treatment outcomes in hepatitis C virus genotype 1-infected patients. PLoS One. 2011;6:e28617. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 37. | Miyamura T, Kanda T, Nakamoto S, Wu S, Jiang X, Arai M, Fujiwara K, Imazeki F, Yokosuka O. Roles of ITPA and IL28B genotypes in chronic hepatitis C patients treated with peginterferon plus ribavirin. Viruses. 2012;4:1264-1278. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 38. | Honda M, Sakai A, Yamashita T, Nakamoto Y, Mizukoshi E, Sakai Y, Yamashita T, Nakamura M, Shirasaki T, Horimoto K, Tanaka Y, Tokunaga K, Mizokami M, Kaneko S; Hokuriku Liver Study Group. Hepatic ISG expression is associated with genetic variation in interleukin 28B and the outcome of IFN therapy for chronic hepatitis C. Gastroenterology. 2010;139:499-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 334] [Cited by in RCA: 344] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 39. | Yu ML, Huang CF, Huang JF, Chang NC, Yang JF, Lin ZY, Chen SC, Hsieh MY, Wang LY, Chang WY. Role of interleukin-28B polymorphisms in the treatment of hepatitis C virus genotype 2 infection in Asian patients. Hepatology. 2011;53:7-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 159] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 40. | Yu ML, Liu CH, Huang CF, Tseng TC, Huang JF, Dai CY, Lin ZY, Chen SC, Wang LY, Juo SH. Revisiting the stopping rule for hepatitis C genotype 1 patients treated with peginterferon plus ribavirin. PLoS One. 2012;7:e52048. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 41. | Lagging M, Askarieh G, Negro F, Bibert S, Söderholm J, Westin J, Lindh M, Romero A, Missale G, Ferrari C, Neumann AU, Pawlotsky JM, Haagmans BL, Zeuzem S, Bochud PY, Hellstrand K; DITTO-HCV Study Group. Response prediction in chronic hepatitis C by assessment of IP-10 and IL28B-related single nucleotide polymorphisms. PLoS One. 2011;6:e17232. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 110] [Cited by in RCA: 120] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 42. | Lindh M, Lagging M, Arnholm B, Eilard A, Nilsson S, Norkrans G, Söderholm J, Wahlberg T, Wejstål R, Westin J. IL28B polymorphisms determine early viral kinetics and treatment outcome in patients receiving peginterferon/ribavirin for chronic hepatitis C genotype 1. J Viral Hepat. 2011;18:e325-e331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 43. | Sugiyama M, Tanaka Y, Wakita T, Nakanishi M, Mizokami M. Genetic variation of the IL-28B promoter affecting gene expression. PLoS One. 2011;6:e26620. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 44. | Prokunina-Olsson L, Muchmore B, Tang W, Pfeiffer RM, Park H, Dickensheets H, Hergott D, Porter-Gill P, Mumy A, Kohaar I. A variant upstream of IFNL3 (IL28B) creating a new interferon gene IFNL4 is associated with impaired clearance of hepatitis C virus. Nat Genet. 2013;45:164-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 701] [Cited by in RCA: 759] [Article Influence: 63.3] [Reference Citation Analysis (0)] |
| 45. | Bibert S, Roger T, Calandra T, Bochud M, Cerny A, Semmo N, Duong FH, Gerlach T, Malinverni R, Moradpour D. IL28B expression depends on a novel TT/-G polymorphism which improves HCV clearance prediction. J Exp Med. 2013;210:1109-1116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 168] [Cited by in RCA: 177] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 46. | Ochi H, Maekawa T, Abe H, Hayashida Y, Nakano R, Imamura M, Hiraga N, Kawakami Y, Aimitsu S, Kao JH. IL-28B predicts response to chronic hepatitis C therapy--fine-mapping and replication study in Asian populations. J Gen Virol. 2011;92:1071-1081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 49] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 47. | Kobayashi M, Suzuki F, Akuta N, Sezaki H, Suzuki Y, Hosaka T, Kawamura Y, Kobayashi M, Saitoh S, Arase Y. Association of two polymorphisms of the IL28B gene with viral factors and treatment response in 1,518 patients infected with hepatitis C virus. J Gastroenterol. 2012;47:596-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 48. | Kanda T, Yokosuka O, Omata M. Treatment of hepatitis C virus infection in the future. Clin Transl Med. 2013;2:9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 49. | McHutchison JG, Everson GT, Gordon SC, Jacobson IM, Sulkowski M, Kauffman R, McNair L, Alam J, Muir AJ; PROVE1 Study Team. Telaprevir with peginterferon and ribavirin for chronic HCV genotype 1 infection. N Engl J Med. 2009;360:1827-1838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 851] [Cited by in RCA: 809] [Article Influence: 50.6] [Reference Citation Analysis (0)] |
| 50. | Hézode C, Forestier N, Dusheiko G, Ferenci P, Pol S, Goeser T, Bronowicki JP, Bourlière M, Gharakhanian S, Bengtsson L, McNair L, George S, Kieffer T, Kwong A, Kauffman RS, Alam J, Pawlotsky JM, Zeuzem S; PROVE2 Study Team. Telaprevir and peginterferon with or without ribavirin for chronic HCV infection. N Engl J Med. 2009;360:1839-1850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 849] [Cited by in RCA: 793] [Article Influence: 49.6] [Reference Citation Analysis (0)] |
| 51. | McHutchison JG, Manns MP, Muir AJ, Terrault NA, Jacobson IM, Afdhal NH, Heathcote EJ, Zeuzem S, Reesink HW, Garg J, Bsharat M, George S, Kauffman RS, Adda N, Di Bisceglie AM; PROVE3 Study Team. Telaprevir for previously treated chronic HCV infection. N Engl J Med. 2010;362:1292-1303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 570] [Cited by in RCA: 535] [Article Influence: 35.7] [Reference Citation Analysis (0)] |
| 52. | Jacobson IM, McHutchison JG, Dusheiko G, Di Bisceglie AM, Reddy KR, Bzowej NH, Marcellin P, Muir AJ, Ferenci P, Flisiak R. Telaprevir for previously untreated chronic hepatitis C virus infection. N Engl J Med. 2011;364:2405-2416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1905] [Cited by in RCA: 1861] [Article Influence: 132.9] [Reference Citation Analysis (0)] |
| 53. | Zeuzem S, Andreone P, Pol S, Lawitz E, Diago M, Roberts S, Focaccia R, Younossi Z, Foster GR, Horban A. Telaprevir for retreatment of HCV infection. N Engl J Med. 2011;364:2417-2428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1230] [Cited by in RCA: 1213] [Article Influence: 86.6] [Reference Citation Analysis (0)] |
| 54. | Sherman KE, Flamm SL, Afdhal NH, Nelson DR, Sulkowski MS, Everson GT, Fried MW, Adler M, Reesink HW, Martin M, Sankoh AJ, Adda N, Kauffman RS, George S, Wright CI, Poordad F; ILLUMINATE Study Team. Response-guided telaprevir combination treatment for hepatitis C virus infection. N Engl J Med. 2011;365:1014-1024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 592] [Cited by in RCA: 602] [Article Influence: 43.0] [Reference Citation Analysis (0)] |
| 55. | Poordad F, McCone J, Bacon BR, Bruno S, Manns MP, Sulkowski MS, Jacobson IM, Reddy KR, Goodman ZD, Boparai N. Boceprevir for untreated chronic HCV genotype 1 infection. N Engl J Med. 2011;364:1195-1206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1996] [Cited by in RCA: 1980] [Article Influence: 141.4] [Reference Citation Analysis (0)] |
| 56. | Kumada H, Toyota J, Okanoue T, Chayama K, Tsubouchi H, Hayashi N. Telaprevir with peginterferon and ribavirin for treatment-naive patients chronically infected with HCV of genotype 1 in Japan. J Hepatol. 2012;56:78-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 59] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 57. | Torii H, Sueki H, Kumada H, Sakurai Y, Aoki K, Yamada I, Ohtsuki M. Dermatological side-effects of telaprevir-based triple therapy for chronic hepatitis C in phase III trials in Japan. J Dermatol. 2013;40:587-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 58. | Chauvin B, Drouot S, Barrail-Tran A, Taburet AM. Drug-Drug Interactions Between HMG-CoA Reductase Inhibitors (Statins) and Antiviral Protease Inhibitors. Clin Pharmacokinet. 2013;52:815-831. [PubMed] |
| 59. | Rosa I. Management of side-effects. Clin Res Hepatol Gastroenterol. 2011;35 Suppl 2:S69-S74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 60. | Mauss S, Hueppe D, Alshuth U. Renal impairment is frequent in chronic hepatitis C patients under triple therapy with telaprevir or boceprevir. Hepatology. 2013;Jun 28; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 71] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 61. | Akuta N, Suzuki F, Hirakawa M, Kawamura Y, Yatsuji H, Sezaki H, Suzuki Y, Hosaka T, Kobayashi M, Kobayashi M. Amino acid substitution in hepatitis C virus core region and genetic variation near the interleukin 28B gene predict viral response to telaprevir with peginterferon and ribavirin. Hepatology. 2010;52:421-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 201] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 62. | Pol S, Aerssens J, Zeuzem S, Andreone P, Lawitz EJ, Roberts S, Younossi Z, Foster GR, Focaccia R, Horban A. Limited impact of IL28B genotype on response rates in telaprevir-treated patients with prior treatment failure. J Hepatol. 2013;58:883-889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 63. | Hara T, Akuta N, Suzuki F, Sezaki H, Suzuki Y, Hosaka T, Kobayashi M, Kobayashi M, Saitoh S, Kumada H. A pilot study of triple therapy with telaprevir, peginterferon and ribavirin for elderly patients with genotype 1 chronic hepatitis C. J Med Virol. 2013;85:1746-1753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 64. | Furusyo N, Ogawa E, Nakamuta M, Kajiwara E, Nomura H, Dohmen K, Takahashi K, Satoh T, Azuma K, Kawano A. Telaprevir can be successfully and safely used to treat older patients with genotype 1b chronic hepatitis C. J Hepatol. 2013;59:205-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 63] [Article Influence: 5.3] [Reference Citation Analysis (0)] |