Revised: January 8, 2013
Accepted: January 31, 2013
Published online: March 27, 2013
To better understand the pathogenesis of Sézary cells, distinguish them from reactive skin-infiltrating T-cells and improve disease treatment, efforts have been made to identify molecular targets deregulated by the malignant process. From immunophenotypic analysis and subtractive differential expression experiments to pan-genomic studies, many approaches have been used to identify markers of the disease. During the last decade several natural killer (NK) cell markers have been found aberrantly expressed at the surface of Sézary cells. In particular, KIR3DL2/CD158k, expressed by less than 2% of healthy individuals CD4+ T-cells, is an excellent marker to identify and follow the tumor burden in the blood of Sézary syndrome patients. It may also represent a valuable target for specific immunotherapy. Other products of the NK cluster on chromosome 19q13 have been detected on Sézary cells, raising the hypothesis of an NK reprogramming process associated with the malignant transformation that may induce survival functions.
- Citation: Schmitt C, Marie-Cardine A, Bagot M, Bensussan A. Natural killer reprogramming in cutaneous T-cell lymphomas: Facts and hypotheses. World J Immunol 2013; 3(1): 1-6
- URL: https://www.wjgnet.com/2219-2824/full/v3/i1/1.htm
- DOI: https://dx.doi.org/10.5411/wji.v3.i1.1
Cutaneous T-cell lymphomas (CTCL) are a heterogeneous group of lymphoproliferative disorders involving primarily the skin. The most common subtypes of CTCL are mycosis fungoides (MF) and Sézary syndrome (SS). MF is characterized by a slowly progressing skin invasion by clonally derived mature CD4+ T-lymphocytes, these malignant cells residing primarily in the infiltrating skin lesion. SS is a more aggressive leukemic and erythrodermic form of CTCL involving malignant CD4+CD45RO+ T-cells. Despite the fact that MF and SS are classified as distinct disease entities, their clinical relationship is still a matter of debate as they share common features and similarities suggesting that they might be variants of the same disease spectrum[1-3]. Patients with transformed MF can have blood findings characteristic of SS and can sometimes develop typical SS[4]. On the other hand, the majority of patients diagnosed with early-stage MF will never progress to advanced-stage disease. The finding that MF and SS arise from two distinct functional T-cell subsets, central memory for SS (CCR7+L-selectin+ CD27+CCR4+CLA+/-) vs effector memory T cells for MF (CCR7-L-selectin- CD27-CCR4+CLA+), if confirmed, favors the notion that they should be considered as separate lymphomas[5].
The prognosis of MF and SS depends on the type and extent of skin lesions and extracutaneous disease. This is reflected in the TNMB classification of MF/SS defined by the International Society for Cutaneous Lymphomas, involving evaluation of the skin (T), lymph nodes (N), visceral organs (M) and blood (B)[6]. SS is thus defined as meeting T4 plus B2 criteria, where T4 refers to a confluence of erythema covering at least 80% of the body surface area and B2 a high blood tumor burden[6]. Peripheral blood studies are important for establishing the diagnosis and staging for SS. While determining the tumor mass by histological examination of blood smears, with Sézary cells defined by a cerebriform nuclear morphology, is widely used and valuable, flow cytometry analysis of T-cell blood subsets provides a more objective and reproducible means to quantify and track blood involvement in patients with MF/SS. For example, a CD4:CD8 ratio higher than 10 is observed in about 80% of patients with SS, whereas loss of CD7 (CD4+CD7-≥ 30%) or CD26 (CD4+CD26-≥ 40%) are found in about half of the SS patients[7-9]. However, although it is possible to show using Vß-specific TCR antibodies that clonally expanded cells in SS may have these immunophenotypes, loss of CD7 or CD26 among CD4+ T-cells can also be found in benign inflammatory erythroderma or even healthy blood. SS is considered as a clonal expansion of a T-cell subset and the analysis of T-cell clonality by PCR amplification of TCR-γ or -ß chain genes can allow the detection of a dominant T-cell clone in the peripheral blood in most SS patients. However, a T-cell clonality can also be detected in 34% of cases with benign inflammatory erythroderma[10]. Therefore the identification of a predominant T-cell clone might reflect a reactive rather than a neoplastic T-cell clone. The evaluation of other potential Sézary cell markers is consequently important for the diagnosis, prognosis and follow-up of SS. Among the proposed potential markers, several belong to the natural killer (NK) cell lineage, raising the question of a hypothetical NK-cell reprogramming mechanism occurring in the transformation of some CTCL. This editorial will focus on that provocative question.
Malignant T cells in MF and SS produce and respond to various cytokines in their microenvironment. Among them, interleukin (IL)-7 is sufficient to enhance the proliferation of healthy skin resident T-cells and is necessary to sustain an in vitro proliferation of malignant T-cells from SS or MF[11-13]. The observation that IL7-trangenic mice develop cutaneous lymphomas at high frequency further illustrates the role of this cytokine in inducing the proliferation of skin infiltrating lymphocytes[14]. This allowed us to develop T-cell lines derived from circulating Sézary cells as attested by their expression of TCR-Vß and TCRß-VDJ sequences identical to the in vivo tumor cells[15,16]. Such long-term cultured cell lines have been valuable tools to study Sézary cells and were used to initially describe their expression of KIR3DL2/CD158k[17].
The cell surface receptor KIR3DL2/CD158k belongs to the killer immunoglobulin-like receptor (KIR) family and is normally expressed by minor subsets of circulating NK cells and cytotoxic CD8+ T-lymphocytes. The KIRs display a clonally distributed expression in human NK cells and KIR3DL2/CD158k is only expressed on a few percentage of circulating blood lymphocytes[18]. The KIR nomenclature is based on the biochemical structure of the receptors. Thus, they may have 2 (2D) or 3 (3D) extracellular immunoglobulin domains associated with a long (L) or short (S) cytoplasmic tail, responsible for an inhibiting or activating signaling activity respectively. KIRs recognize mainly determinants shared by a group of HLA class-I allotypes. The KIR3DL2/CD158k is an inhibitory receptor with specificity for HLA-A3 and -A11[18] and has been reported recently to also recognize CpG oligodeoxynucleotides[19].
Our group has identified KIR3DL2/CD158k as a new phenotypic marker for circulating Sézary cells[17,20,21]. Despite the lack of commercially available anti-CD158k antibodies other groups have confirmed these observations[8,22]. The proportion and absolute count of CD158k+ lymphocytes strongly correlate with the percentage and absolute count of atypical cells determined by cytomorphology[20]. Interestingly, CD158k+ cells can be detected even in SS patients with low tumor burden[20,22]. The CD4+CD158k+ cells found in the blood were shown to correspond to the malignant clonal cell population as assessed by the immunoscope technique [21]. In the skin, KIR3DL2/CD158k transcripts were found to be significantly overexpressed in SS compared to erythrodermic inflammatory diseases[23]. The only occasional expression of KIR3DL2/CD158k on rare CD4+ T-cells from healthy individuals makes it a valuable positive marker to identify malignant Sézary cells, even when present at low levels, and to monitor the tumor cell load during therapy. In some cases however, CD158k expression may not identify all the neoplastic T-cells, due to clonal evolution during tumoral progression[24]. This raises the question of whether the appearance of CD158k is a relatively late event in the SS pathogenesis, occuring when genetic deregulation increases, or if it parallels the early oncogenic events. No definitive answer can be given but one may note that in normal T-cells KIR expression occurs after T-cell activation, and that in MF no CD158k+ T-cells are detected in the skin at the patch-plaque stage but can be found in patients at the transformed stage, favoring the acquisition of KIR3DL2/CD158k expression as a late event[25]. It remains to understand what can be the consequences of this expression on the tumor cell biology in terms of proliferation or survival. CD158k/KIR3DL2 is an inhibitory receptor that upon engagement mediates an inhibitory signaling cascade through the ITIM domains located within its cytoplasmic tail. One can speculate that it may down regulate TCR-mediated signaling, in line with the reported hyporesponsiveness of Sézary cells to an anti-CD3 mAb stimulation[12]. This may be seen as an advantage for tumor cells to resist to antigen receptor-mediated cell death associated to chronic antigenic stimulation, as observed on normal T-cells. However, as what was recently observed, KIR may act differently on Sézary cells, and behave as co-activating receptors through a JNK-dependent pathway[26]. Clearly the exact function of KIR3DL2/CD158k in T-cells from SS patients has still to be defined. In a lower proportion of patients KIR3DL2 is not the only KIR expressed by Sézary cells. In particular, a significant expression of CD158a/KIR2DL1 and CD158b/KIR2DL2/3 can be observed in less than 10% of patients[22,26].
An abnormal expression of other NK receptors has been observed at the surface of Sézary cells. The CD85j/Ig-like transcript 2 (ILT2) receptor belongs to a family of receptors homologous to the KIR, encompassing both inhibitory forms recruiting SHP-1 phosphatase and short-tailed activating forms[27-29]. ILT2 is an inhibitory receptor specific for an a3-domain epitope shared by many MHC class Ia and Ib molecules and the class I-like protein UL18 of human cytomegalovirus[30]. It is expressed by myeloid cells, B lymphocytes and some NK and CD8+ T-cells with memory phenotype[31]. Most circulating CD4+ lymphocytes fail to express ILT2 at their cell surface, whereas the molecule is in fact present in the cytoplasm of all T-cells[32]. As for KIR, its action on circulating CD8+ T-cells is to reduce antigen driven activation-induced cell death without affecting proliferation and survival induced by cytokines and particularly IL-7[31]. In SS, circulating malignant Sézary cells may be distinguished from non malignant reactive CD4+ autologous T-cells through the detection of ILT2 at the cell surface[33]. In addition these receptors are functional as they can inhibit an anti-CD3 mAb-induced signaling and therefore perpetuate the survival of SS malignant cells by protecting them from CD3/TCR engagement induced apoptosis. Of note, in the skin, MF cells lack expression of ILT2[33].
Another essential NK cell marker reported at the surface of Sézary cells is NKp46/NCR1 that is not detected on normal circulating CD4+ T-cells[34]. NKp46/NCR1, together with NKp44/NCR2 and NKp30/NCR3, forms the family of activating natural cytotoxicity receptors(NCR)[35]. These receptors are normally confined to NK cells, and their engagement induces strong activation of NK-mediated cytolysis. However, umbilical cord blood CD8+ T-cells, when stimulated for a long period of time with IL-15, expressed NKp30 and NKp44, although only NKp30 was functional to induce cytotoxicity[36]. Whereas NKp30 and NKp46 expression are constitutive, NKp44 is acquired upon activation of NK cells. NKp46 mediates signal transduction through its association with the ITAM-bearing molecules CD3ξ or FceRIγ, that become tyrosine phosphorylated upon receptor cross-linking. NKp46 was detected at the surface of malignant Sézary cells in the absence of external stimulus. This expression, that parallels the one of KIR3DL2/CD158k, is specific to Sézary cells as it is not detected on cells isolated from MF or inflammatory erythroderma patients[34]. In NK cells, NKp46 acts as a full receptor and its engagement triggers their natural cytotoxicity against target cells. In Sézary cells however, its triggering does not induce CD3ξ tyrosine phosphorylation and fails to initiate the activating events leading to cell proliferation. In fact, when brought to close proximity to the CD3/TCR, NKp46 prevents the phosphorylation of CD3 chain, resulting in an overall inhibition of the TCR-mediated activation pathway. As mentioned above, SS cells are usually hyporesponsive to CD3/TCR-mediated triggering, which can be seen as a way to escape to antigen receptor-mediated cell death associated to chronic antigenic stimulation of T-cells. One can speculate that whereas KIR can mediate proliferation through JNK activation pathway, NKp46 may downregulate TCR signaling to promote survival. Could such a behavior reflect a perversion of normal functions when placed in an ectopic environment?
Cell transformation and tumoral progression is generally associated with a reprogramming of the cell differentiation program. Celiac disease (CD) is a chronic inflammation of the small intestine secondary to gluten intolerance. This leads to a chronic activation of the intraepithelial lymphocytes (IEL), that are tissue specialized CD8+ cytotoxic T-lymphocytes, and to the alteration of the intestinal mucosa and the progression towards enteropathy-associated T-cell lymphoma[37]. IL-15 production, which is greatly increased in the mucosa of patients with CD, has an important role in the disease process[38]. Subjects expressing HLA-DQ2/DQ8, that form stable complexes with gluten peptides, elicit exacerbated response of DQ2/DQ8-restricted CD4+ T-cells leading to villous atrophy and malabsorption. However, despite the expansion of IEL in the mucosa, gluten-specific IEL are rare or absent[39]. In fact, in CD patients, a massive expansion of few IEL cytotoxic T-cell clones that have undergone a genetic reprogramming under the IL-15 stimulation occurs, that essentially convert them into NK-like cells capable of cytolysis independently of a CD3/TCR signaling[40]. This reprogramming consists in the aberrant expression on these IEL of a panoply of normally restricted cytolytic NK lineage receptors, such as NKG2C, NKp44, NKp46, or KIR. Such reprogramming has also been reported in the cytotoxic T-cells from cytomegalovirus-seropositve patients[41]. This raises the question whether NK reprogramming may underlie the transformation of chronically stimulated T-cells.
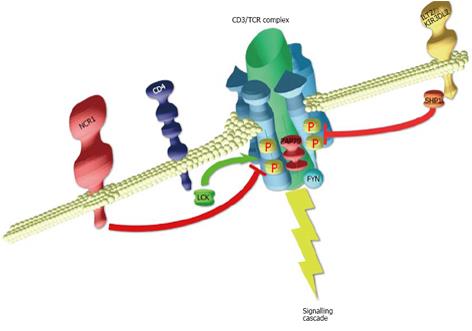
In SS, the aberrant expression of NK-cell lineage receptors such as KIR, ILT2 or NKp46 has been observed, that are all encoded in the NK cluster region on chromosome 19q13. Although the Sézary cells do not acquire cytotoxic capacity, signaling capacity through these receptors were observed in the malignant SS cells, suggesting an NK-like differentiation process. Chronic stimulation through antigen or allergen has been proposed to play a role in SS and MF[42]. With this perspective, Figure 1 illustrates how NK receptors may interfere with T-cell stimulation in Sézary cells, tuning the signalling threshold of the CD3/TCR, preventing the tumoral cells from activation-induced cell apoptosis. Of note a high level of T-cell stimulating cytokines is present in the skin, such as IL7. Elevated levels of IL-15, an important cytokine for NK reprogramming in CD, have been reported in SS[43,44]. Could there be a concerted aberrant expression of NK markers at the surface of Sézary cells, playing an important role in the pathobiology and tumoral progression of SS? Future work will tell us the truth, but that track is worth to be followed.
The authors would like to thanks the Inserm, Société de Recherches Dermatologiques (SRD; C.S), and Société Française de Dermatologie (SFD; A.M-C) for their support as well as the European Union through the Euro-Trans-Bio grant (M.B and A.B).
P- Reviewer Maghazachi A S- Editor Cheng JX L- Editor A E- Editor Lu YJ
| 1. | Laharanne E, Oumouhou N, Bonnet F, Carlotti M, Gentil C, Chevret E, Jouary T, Longy M, Vergier B, Beylot-Barry M. Genome-wide analysis of cutaneous T-cell lymphomas identifies three clinically relevant classes. J Invest Dermatol. 2010;130:1707-1718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 83] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 2. | Mao X, Lillington DM, Czepulkowski B, Russell-Jones R, Young BD, Whittaker S. Molecular cytogenetic characterization of Sézary syndrome. Genes Chromosomes Cancer. 2003;36:250-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 82] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 3. | van Doorn R, van Kester MS, Dijkman R, Vermeer MH, Mulder AA, Szuhai K, Knijnenburg J, Boer JM, Willemze R, Tensen CP. Oncogenomic analysis of mycosis fungoides reveals major differences with Sezary syndrome. Blood. 2009;113:127-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 149] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 4. | Kari L, Loboda A, Nebozhyn M, Rook AH, Vonderheid EC, Nichols C, Virok D, Chang C, Horng WH, Johnston J. Classification and prediction of survival in patients with the leukemic phase of cutaneous T cell lymphoma. J Exp Med. 2003;197:1477-1488. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 159] [Cited by in RCA: 137] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 5. | Campbell JJ, Clark RA, Watanabe R, Kupper TS. Sezary syndrome and mycosis fungoides arise from distinct T-cell subsets: a biologic rationale for their distinct clinical behaviors. Blood. 2010;116:767-771. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 435] [Cited by in RCA: 371] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 6. | Olsen E, Vonderheid E, Pimpinelli N, Willemze R, Kim Y, Knobler R, Zackheim H, Duvic M, Estrach T, Lamberg S. Revisions to the staging and classification of mycosis fungoides and Sezary syndrome: a proposal of the International Society for Cutaneous Lymphomas (ISCL) and the cutaneous lymphoma task force of the European Organization of Research and Treatment of Cancer (EORTC). Blood. 2007;110:1713-1722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 976] [Cited by in RCA: 996] [Article Influence: 55.3] [Reference Citation Analysis (0)] |
| 7. | Kelemen K, Guitart J, Kuzel TM, Goolsby CL, Peterson LC. The usefulness of CD26 in flow cytometric analysis of peripheral blood in Sézary syndrome. Am J Clin Pathol. 2008;129:146-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 52] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 8. | Klemke CD, Brade J, Weckesser S, Sachse MM, Booken N, Neumaier M, Goerdt S, Nebe TC. The diagnosis of Sézary syndrome on peripheral blood by flow cytometry requires the use of multiple markers. Br J Dermatol. 2008;159:871-880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 53] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 9. | Nagler AR, Samimi S, Schaffer A, Vittorio CC, Kim EJ, Rook AH. Peripheral blood findings in erythrodermic patients: importance for the differential diagnosis of Sézary syndrome. J Am Acad Dermatol. 2012;66:503-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 10. | Delfau-Larue MH, Laroche L, Wechsler J, Lepage E, Lahet C, Asso-Bonnet M, Bagot M, Farcet JP. Diagnostic value of dominant T-cell clones in peripheral blood in 363 patients presenting consecutively with a clinical suspicion of cutaneous lymphoma. Blood. 2000;96:2987-2992. [PubMed] |
| 11. | Bagot M, Charue D, Boulland ML, Gaulard P, Revuz J, Schmitt C, Wechsler J. Interleukin-7 receptor expression in cutaneous T-cell lymphomas. Br J Dermatol. 1996;135:572-575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 12. | Dalloul A, Laroche L, Bagot M, Mossalayi MD, Fourcade C, Thacker DJ, Hogge DE, Merle-Béral H, Debré P, Schmitt C. Interleukin-7 is a growth factor for Sézary lymphoma cells. J Clin Invest. 1992;90:1054-1060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 108] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 13. | Yamanaka K, Clark R, Rich B, Dowgiert R, Hirahara K, Hurwitz D, Shibata M, Mirchandani N, Jones DA, Goddard DS. Skin-derived interleukin-7 contributes to the proliferation of lymphocytes in cutaneous T-cell lymphoma. Blood. 2006;107:2440-2445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 56] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 14. | Rich BE, Campos-Torres J, Tepper RI, Moreadith RW, Leder P. Cutaneous lymphoproliferation and lymphomas in interleukin 7 transgenic mice. J Exp Med. 1993;177:305-316. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 186] [Cited by in RCA: 181] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 15. | Bagot M, Echchakir H, Mami-Chouaib F, Delfau-Larue MH, Charue D, Bernheim A, Chouaib S, Boumsell L, Bensussan A. Isolation of tumor-specific cytotoxic CD4+ and CD4+CD8dim+ T-cell clones infiltrating a cutaneous T-cell lymphoma. Blood. 1998;91:4331-4341. [PubMed] |
| 16. | Poszepczynska E, Bagot M, Echchakir H, Martinvalet D, Ramez M, Charue D, Boumsell L, Bensussan A. Functional characterization of an IL-7-dependent CD4(+)CD8alphaalpha(+) Th3-type malignant cell line derived from a patient with a cutaneous T-cell lymphoma. Blood. 2000;96:1056-1063. [PubMed] |
| 17. | Bagot M, Moretta A, Sivori S, Biassoni R, Cantoni C, Bottino C, Boumsell L, Bensussan A. CD4(+) cutaneous T-cell lymphoma cells express the p140-killer cell immunoglobulin-like receptor. Blood. 2001;97:1388-1391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 91] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 18. | Moretta L, Moretta A. Killer immunoglobulin-like receptors. Curr Opin Immunol. 2004;16:626-633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 259] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 19. | Sivori S, Falco M, Carlomagno S, Romeo E, Soldani C, Bensussan A, Viola A, Moretta L, Moretta A. A novel KIR-associated function: evidence that CpG DNA uptake and shuttling to early endosomes is mediated by KIR3DL2. Blood. 2010;116:1637-1647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 76] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 20. | Bouaziz JD, Remtoula N, Bensussan A, Marie-Cardine A, Bagot M. Absolute CD3+ CD158k+ lymphocyte count is reliable and more sensitive than cytomorphology to evaluate blood tumour burden in Sézary syndrome. Br J Dermatol. 2010;162:123-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 33] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 21. | Poszepczynska-Guigné E, Schiavon V, D’Incan M, Echchakir H, Musette P, Ortonne N, Boumsell L, Moretta A, Bensussan A, Bagot M. CD158k/KIR3DL2 is a new phenotypic marker of Sezary cells: relevance for the diagnosis and follow-up of Sezary syndrome. J Invest Dermatol. 2004;122:820-823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 105] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 22. | Bahler DW, Hartung L, Hill S, Bowen GM, Vonderheid EC. CD158k/KIR3DL2 is a useful marker for identifying neoplastic T-cells in Sézary syndrome by flow cytometry. Cytometry B Clin Cytom. 2008;74:156-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 53] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 23. | Ortonne N, Le Gouvello S, Mansour H, Poillet C, Martin N, Delfau-Larue MH, Leroy K, Farcet JP, Bagot M, Bensussan A. CD158K/KIR3DL2 transcript detection in lesional skin of patients with erythroderma is a tool for the diagnosis of Sézary syndrome. J Invest Dermatol. 2008;128:465-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 37] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 24. | Ortonne N, Huet D, Gaudez C, Marie-Cardine A, Schiavon V, Bagot M, Musette P, Bensussan A. Significance of circulating T-cell clones in Sezary syndrome. Blood. 2006;107:4030-4038. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 45] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 25. | Wechsler J, Bagot M, Nikolova M, Parolini S, Martin-Garcia N, Boumsell L, Moretta A, Bensussan A. Killer cell immunoglobulin-like receptor expression delineates in situ Sézary syndrome lymphocytes. J Pathol. 2003;199:77-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 34] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 26. | Marie-Cardine A, Huet D, Ortonne N, Remtoula N, Le Gouvello S, Bagot M, Bensussan A. Killer cell Ig-like receptors CD158a and CD158b display a coactivatory function, involving the c-Jun NH2-terminal protein kinase signaling pathway, when expressed on malignant CD4+ T cells from a patient with Sezary syndrome. Blood. 2007;109:5064-5065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 27. | Allan DS, McMichael AJ, Braud VM. The ILT family of leukocyte receptors. Immunobiology. 2000;202:34-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 28. | Samaridis J, Colonna M. Cloning of novel immunoglobulin superfamily receptors expressed on human myeloid and lymphoid cells: structural evidence for new stimulatory and inhibitory pathways. Eur J Immunol. 1997;27:660-665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 228] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 29. | Trowsdale J. Genetic and functional relationships between MHC and NK receptor genes. Immunity. 2001;15:363-374. [PubMed] [DOI] [Full Text] |
| 30. | Chapman TL, Heikeman AP, Bjorkman PJ. The inhibitory receptor LIR-1 uses a common binding interaction to recognize class I MHC molecules and the viral homolog UL18. Immunity. 1999;11:603-613. [PubMed] [DOI] [Full Text] |
| 31. | Young NT, Uhrberg M, Phillips JH, Lanier LL, Parham P. Differential expression of leukocyte receptor complex-encoded Ig-like receptors correlates with the transition from effector to memory CTL. J Immunol. 2001;166:3933-3941. [PubMed] |
| 32. | Saverino D, Fabbi M, Ghiotto F, Merlo A, Bruno S, Zarcone D, Tenca C, Tiso M, Santoro G, Anastasi G. The CD85/LIR-1/ILT2 inhibitory receptor is expressed by all human T lymphocytes and down-regulates their functions. J Immunol. 2000;165:3742-3755. [PubMed] |
| 33. | Nikolova M, Musette P, Bagot M, Boumsell L, Bensussan A. Engagement of ILT2/CD85j in Sézary syndrome cells inhibits their CD3/TCR signaling. Blood. 2002;100:1019-1025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 34. | Bensussan A, Remtoula N, Sivori S, Bagot M, Moretta A, Marie-Cardine A. Expression and function of the natural cytotoxicity receptor NKp46 on circulating malignant CD4+ T lymphocytes of Sézary syndrome patients. J Invest Dermatol. 2011;131:969-976. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 35. | Biassoni R, Cantoni C, Pende D, Sivori S, Parolini S, Vitale M, Bottino C, Moretta A. Human natural killer cell receptors and co-receptors. Immunol Rev. 2001;181:203-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 226] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 36. | Tang Q, Grzywacz B, Wang H, Kataria N, Cao Q, Wagner JE, Blazar BR, Miller JS, Verneris MR. Umbilical cord blood T cells express multiple natural cytotoxicity receptors after IL-15 stimulation, but only NKp30 is functional. J Immunol. 2008;181:4507-4515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 37. | Chandesris MO, Malamut G, Verkarre V, Meresse B, Macintyre E, Delarue R, Rubio MT, Suarez F, Deau-Fischer B, Cerf-Bensussan N. Enteropathy-associated T-cell lymphoma: a review on clinical presentation, diagnosis, therapeutic strategies and perspectives. Gastroenterol Clin Biol. 2010;34:590-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 38. | Meresse B, Chen Z, Ciszewski C, Tretiakova M, Bhagat G, Krausz TN, Raulet DH, Lanier LL, Groh V, Spies T. Coordinated induction by IL15 of a TCR-independent NKG2D signaling pathway converts CTL into lymphokine-activated killer cells in celiac disease. Immunity. 2004;21:357-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 583] [Cited by in RCA: 618] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 39. | Green PH, Jabri B. Coeliac disease. Lancet. 2003;362:383-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 614] [Cited by in RCA: 593] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 40. | Meresse B, Curran SA, Ciszewski C, Orbelyan G, Setty M, Bhagat G, Lee L, Tretiakova M, Semrad C, Kistner E. Reprogramming of CTLs into natural killer-like cells in celiac disease. J Exp Med. 2006;203:1343-1355. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 209] [Cited by in RCA: 230] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 41. | Gumá M, Angulo A, Vilches C, Gómez-Lozano N, Malats N, López-Botet M. Imprint of human cytomegalovirus infection on the NK cell receptor repertoire. Blood. 2004;104:3664-3671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 642] [Cited by in RCA: 695] [Article Influence: 33.1] [Reference Citation Analysis (0)] |
| 42. | Hwang ST, Janik JE, Jaffe ES, Wilson WH. Mycosis fungoides and Sézary syndrome. Lancet. 2008;371:945-957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 182] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 43. | Asadullah K, Haeussler-Quade A, Gellrich S, Hanneken S, Hansen-Hagge TE, Döcke WD, Volk HD, Sterry W. IL-15 and IL-16 overexpression in cutaneous T-cell lymphomas: stage-dependent increase in mycosis fungoides progression. Exp Dermatol. 2000;9:248-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 1.5] [Reference Citation Analysis (0)] |