Peer-review started: July 2, 2021
First decision: July 30, 2021
Revised: August 13, 2021
Accepted: July 24, 2022
Article in press: July 24, 2022
Published online: August 24, 2022
Processing time: 416 Days and 6.4 Hours
To control the pandemic, efficient vaccines must be applied to the population, including patients with autoimmune diseases. Therefore, one can expect that coronavirus disease 2019 (COVID-19) vaccines may influence the underlying autoimmune processes in these patients. Additionally, it is essential to understand whether COVID-19 vaccines would be effective, safe, and provide long-lasting immunological protection and memory. However, the currently available and approved COVID-19 vaccines turned out to be safe, effective, and reliable in patients with autoimmune inflammatory and rheumatic diseases. Furthermore, most patients said they felt safer after getting vaccinations for COVID-19 and reported enhanced overall quality of life and psychological wellbeing. In general, the COVID-19 vaccines have been highly tolerated by autoimmune patients. Such findings might comfort patients who are reluctant to use COVID-19 vaccines and assist doctors in guiding their patients into receiving vaccinations more easily and quickly.
Core Tip: Coronavirus disease 2019 (COVID-19) vaccines have created concerns about their efficacy and safety, notably in autoimmune patients. Which vaccine adverse events are related to the underlying autoimmunity is unclear. Additional data is needed to evaluate the immunological impact of COVID-19 vaccines in terms of effectiveness and immune-driven adverse effects that might provoke a disease flare in individuals with a history of autoimmune-related symptoms. However, the risk of autoimmune disease flare after vaccination was considered low, while the immune responses after vaccination showed great immunogenicity for these patients. In addition, vaccination will considerably decrease related morbidity and mortality from COVID-19 in autoimmune patients.
- Citation: Velikova T. Vaccines and autoimmunity during the COVID-19 pandemic. World J Immunol 2022; 12(2): 9-14
- URL: https://www.wjgnet.com/2219-2824/full/v12/i2/9.htm
- DOI: https://dx.doi.org/10.5411/wji.v12.i2.9
We still do not know all the mechanisms involved in the immune system - severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) interaction during coronavirus disease 2019 (COVID-19) infection. However, it was demonstrated that the virus possesses a tremendous ability to inhibit the immune mechanisms, both innate and adaptive[1]. Nevertheless, there are still controversial data on which immunity is better - naturally acquired or vaccine[3].
There are concerns regarding people living with autoimmune diseases as well. In patients with autoimmune diseases, the body’s immune system is overactive and destroys its own cells through various mechanisms, including autoantibodies and immune cells[4,5]; therefore, one can expect that COVID-19 vaccines may influence the autoimmune processes in these patients. Additionally, it is essential to understand whether COVID-19 vaccines would be effective and safe in patients with autoimmune diseases and whether vaccines will provide long-lasting immunological protection and memory[6]. However, in order to take control of the pandemic, the medical community has stressed that efficient vaccines must be applied to the population. This approach includes vaccinating patients with autoimmune diseases.
Data showed that the immune hyperactivation and cytokine-excessive release in patients with COVID-19 resulted in multi-organ failure and death[7]. In line with this, patients with already activated immune system could be more prone to severe SARS-CoV-2; however, this was not proven for patients with autoimmune diseases. The main concerns are severe outcomes for patients on immunosuppressive therapy or developing severe clinical complications[8]. Indeed, it was shown that SARS-CoV-2 could induce a robust immune response in immunocompromised patients[9,10].
On the other hand, COVID-19 vaccines have also created concerns about their efficacy and safety, notably in autoimmune patients. We recently published a paper addressing the known pros and cons of vaccinating patients with autoimmune disorders, stressing the absence of data on the advantages and disadvantages of newly discovered COVID-19 in patients with autoinflammatory and rheumatic diseases[11]. Various pathways that contribute to the increase in acute autoimmune responses have been suggested[12]. For example, molecular mimicry, i.e., antibodies against SARS-CoV-2 spike glycoproteins, has the theoretical potential to trigger autoimmunity, as Vojdani and Kharrazian[13] recently demonstrated. Talotta[14] further suggested that an injectable nucleic acid vaccination might put young women in danger of undesired, unexpected immunological side effects, especially those already susceptible to autoimmune or auto-inflammatory disease. However, even in the autoimmune population, serious adverse events are rare[15,16].
Akinosoglou et al[7] further hypothesize that immunization with COVID-19 is not the cause for de novo immune-mediated adverse events. In contrast, the immunological reaction might lead to dysregulation of the pre-existing underlying pathways. This might result from the polyclonal expansion of the B cells leading to the development of immunological features of autoimmunity. It should be noted that autoimmune disorders can be provoked in genetically sensitive individuals through various autoimmune mechanisms, including epitope spreading and bystander activation[17]. Which vaccine adverse events are related to the underlying autoimmunity is unclear. An unsolved issue remains whether to provide a second dosage after such reactions in patients with rheumatic diseases. Additional data is needed to evaluate the immunological impact of COVID-19 vaccines in terms of effectiveness and immune-driven adverse effects that might provoke a disease flare in individuals with a history of autoimmune-related symptoms[7].
As demonstrated previously, elderly populations with chronic disorders such as diabetes, asthma, and cardiovascular disease are especially susceptible to severe SARS-CoV-2[18]. The same concerns were raised regarding patients with autoimmune inflammatory rheumatic diseases (AIIRDs)[19,20]. However, recent studies demonstrated that patients receiving immunosuppressive therapy for AIIRDs produced sufficient and protective immune response after SARS-CoV-2 mRNA vaccination without experiencing severe side effects or flares[21].
Since AIIRD patients are usually not included in phase III clinical trials of vaccines, immunological response to COVID-19 vaccination in AIIRD patients under the immunosuppression treatment remains unknown. Although the COVID-19 vaccine efficacy was demonstrated between 60%-95% with acceptable safety, uncertainty in AIIRD patients for the COVID-19 vaccines, especially the novel RNA and viral vector vaccines, led to hesitancy in both physicians and patients[22,23]. However, the currently available and approved COVID-19 vaccines turned out to be safe, effective, and reliable in patients with AIIRD. Furthermore, unless contraindicated for medical conditions, such as previous allergy/anaphylaxis to the COVID-19 vaccine or its ingredients, any patient with AIIRD should receive one of the available COVID-19 vaccines.
Patients have to continue immunosuppressive therapy for their underlying AIIRD, which may include biological and selective synthetic disease-modifying anti-rheumatic medications. Korean College of Rheumatology issued guidelines recommending limiting corticosteroids to the lowest possible dosage without exacerbating AIIRD. Methotrexate may be deferred for 1-2 wk following each injection to increase vaccine response. The duration of rituximab and abatacept infusions may also be adjusted[21]. The overall vaccine benefits exceed possible vaccine dangers, as the study showed. Additionally, the risk of disease flare of AIIRD after vaccination is low. However, the currently accepted surrogate markers for the immune response after vaccination (i.e., antibodies against SARS-CoV-2 and activated T cells) showed great immunogenicity of the vaccines in these patients[21].
Another concern that must be discussed is assessing vaccine effectiveness in the IV phase, a.k.a. the real-world studies. Clinical studies investigating high-risk for infection people, i.e., healthcare workers, showed that the absolute risk of testing positive for SARS-CoV-2 after vaccination with mRNA vaccine in a cohort of healthcare workers was 0.97%-1.19%[24]. One must consider that the healthcare staff was younger and more susceptible to SARS-CoV-2 than the clinical trial participants. As stated above, hesitancy in autoimmune patients may have arisen because these individuals were mainly omitted from vaccination studies of COVID-19. Boekel et al[18] have already shown that more than one-third of autoimmune patients are reluctant to get vaccinations against COVID-19. The primary concerns are the anticipated side effects and the lack of long-term studies.
Additionally, there are currently very little data on the safety of COVID-19 vaccines in patients with autoimmune disorders, and no research available can compare the impact of different types of vaccinations between patients and healthy controls. For example, worldwide vaccination recommendations for COVID-19 for autoimmune illness patients is based on experts’ opinion[25]. In their previous study, Boekel et al[26] presented the results from a survey that evaluated the adverse events following COVID-19 vaccinations in systemic AIIRD patients and healthy control (Netherlands Trial Register, trial ID NL8513 and NCT04498286). Of all participants, 1780 patients and 660 controls filled out the questionnaire, whereas 46% and 41% of patients received ChAdOx1 nCoV-19 (AstraZeneca) and BNT162b2 (Pfizer/BioNTech), respectively. Thirteen percent of patients were vaccinated with Moderna. Half of the patients and controls reported at least one mild adverse event, and about 20% of all participants had moderate adverse events. Severe adverse events remained below 1%, with no serious adverse events. Complaints of joints and bones were stated more frequently by patients with AIIRD than controls (10% vs 1%, respectively). Fortunately, only 2% of patients reported flare or deterioration of the disease up to 2 mo after COVID-19 vaccination[26].
The results from the survey show that, regardless of the kind of vaccine, adverse effects of immunization with COVID-19 in patients with autoimmune disorders are equivalent to controls. The adverse effects included also predicted local or systemic hyperreactivity responses, which were largely self-limiting. The incidence of individuals who reported adverse events in the clinical trials was lower than the number stated[15], similar to the national study of COVID-19 adverse events in the United Kingdom general population[27]. In conclusion, the survey demonstrated that the vaccines against COVID-19 do not tend to induce autoimmunity flares, as shown in previous limited studies that evaluated mRNA vaccines’ impact on patients with autoimmune diseases[20,28].
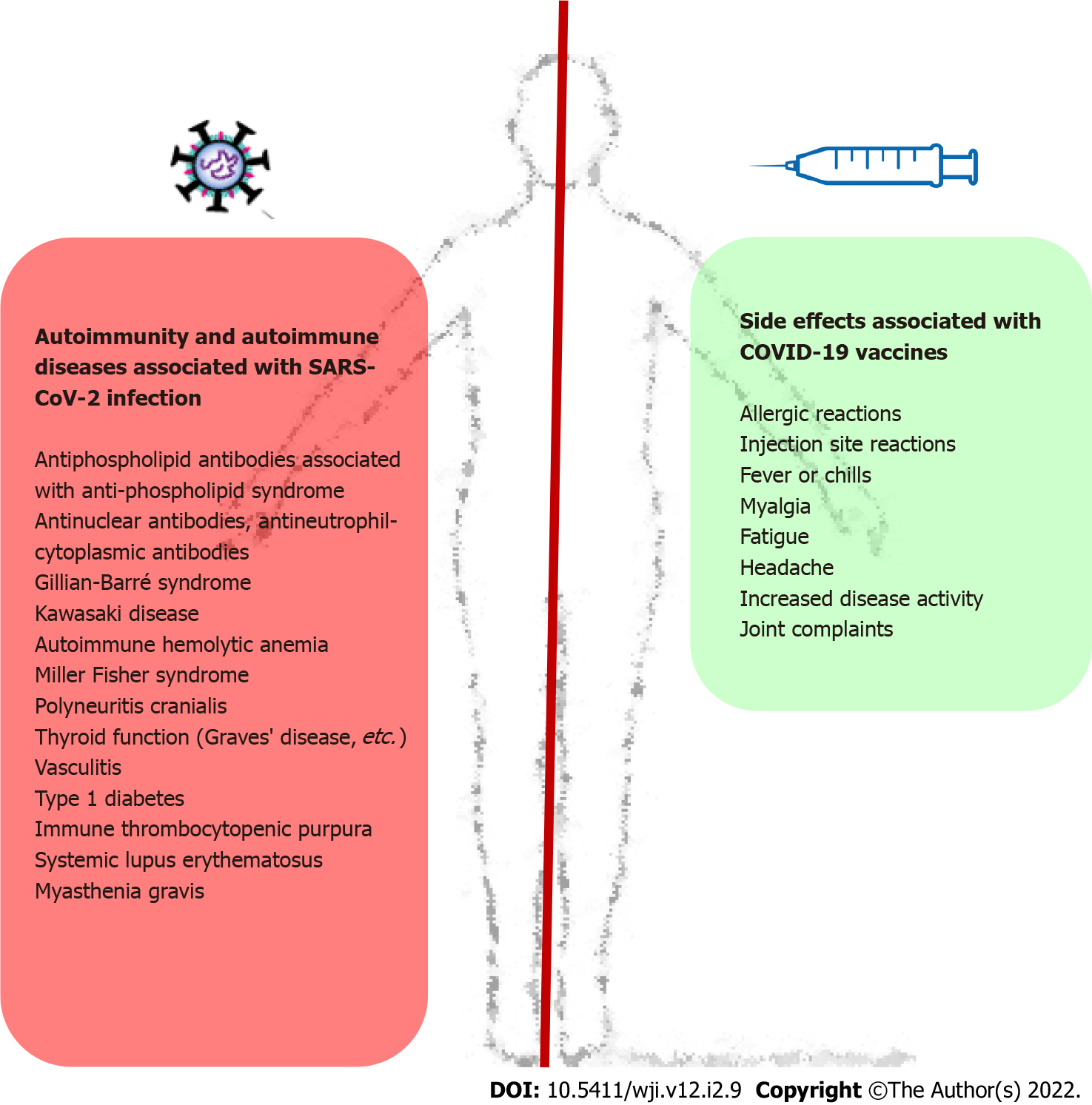
Known pathophysiological effects mRNA may be both immunostimulatory and immunosuppressive to the innate immune system as COVID-19 vaccines are the first to be widely applied, and prospective, monitored studies of the long-run effects of COVID-19 vaccines on their activities require robust conclusions[11]. Nevertheless, most participants said they felt safer after getting vaccinations for COVID-19, and 20% of individuals with autoimmune disorders reported enhanced overall quality of life and psychological wellbeing[26]. If we compare these adverse effects associated with the application of COVID-19 vaccines with autoimmune complications during SARS-CoV-2 infection[29], the benefits of vaccines significantly outweigh the side effects of vaccination. This comparison is presented in Figure 1.
In general, the COVID-19 vaccines have been highly tolerated by autoimmune patients. Such findings might comfort patients who are reluctant to use COVID-19 vaccines and assist doctors in guiding their patients in vaccination timely. Therefore, the therapy and management of COVID-19 should be given priority to reduce the catastrophic effect of COVID-19 in autoimmune patients, and SARS-CoV-2 immunization is one of the most effective protection against infection. Additionally, significant research with the acquisition of new data is required to assess the safety and efficiency of COVID-19 vaccines in immunocompromised patients. In addition, medical practitioners should counsel their immunocompromised patients to support SARS-CoV-2 vaccinations, as this might considerably decrease related morbidity and mortality from COVID-19.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Immunology
Country/Territory of origin: Bulgaria
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Wang CY, Taiwan S-Editor: Wang JJ L-Editor: Filipodia P-Editor: Wang JJ
| 1. | Molaei S, Dadkhah M, Asghariazar V, Karami C, Safarzadeh E. The immune response and immune evasion characteristics in SARS-CoV, MERS-CoV, and SARS-CoV-2: Vaccine design strategies. Int Immunopharmacol. 2021;92:107051. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 2. | Velikova TV, Kotsev SV, Georgiev DS, Batselova HM. Immunological aspects of COVID-19: What do we know? World J Biol Chem. 2020;11:14-29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 29] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (1)] |
| 3. | Velikova T. Infection-acquired versus vaccine-induced immunity against COVID-19. Cent Asian J Med Hypotheses Ethics. 2021;2:29-35. [RCA] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 4. | Cooper GS, Bynum ML, Somers EC. Recent insights in the epidemiology of autoimmune diseases: improved prevalence estimates and understanding of clustering of diseases. J Autoimmun. 2009;33:197-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 444] [Cited by in RCA: 521] [Article Influence: 32.6] [Reference Citation Analysis (0)] |
| 5. | Theofilopoulos AN, Kono DH, Baccala R. The multiple pathways to autoimmunity. Nat Immunol. 2017;18:716-724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 395] [Article Influence: 49.4] [Reference Citation Analysis (0)] |
| 6. | Tang F, Quan Y, Xin ZT, Wrammert J, Ma MJ, Lv H, Wang TB, Yang H, Richardus JH, Liu W, Cao WC. Lack of peripheral memory B cell responses in recovered patients with severe acute respiratory syndrome: a six-year follow-up study. J Immunol. 2011;186:7264-7268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 390] [Cited by in RCA: 381] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 7. | Akinosoglou K, Tzivaki I, Marangos M. Covid-19 vaccine and autoimmunity: Awakening the sleeping dragon. Clin Immunol. 2021;226:108721. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 40] [Article Influence: 10.0] [Reference Citation Analysis (1)] |
| 8. | Robinson PC; Senior staff specialist2, Bursle EC; Infectious diseases physician3; Clinical microbiologist34. Management of autoimmune disease during the COVID-19 pandemic. Aust Prescr. 2020;43:146-147. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 9. | Ali Z, Sarwar M, Ansar S, Awan UA, Ahmed H, Aftab N, Afzal MS. COVID-19 vaccination hesitancy in patients with autoimmune diseases: A mystery that needs an immediate solution! J Med Virol. 2021;93:5216-5218. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 10. | Liu Y, Sawalha AH, Lu Q. COVID-19 and autoimmune diseases. Curr Opin Rheumatol. 2021;33:155-162. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 290] [Cited by in RCA: 325] [Article Influence: 81.3] [Reference Citation Analysis (0)] |
| 11. | Velikova T, Georgiev T. SARS-CoV-2 vaccines and autoimmune diseases amidst the COVID-19 crisis. Rheumatol Int. 2021;41:509-518. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 124] [Cited by in RCA: 113] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 12. | Poland GA, Ovsyannikova IG, Kennedy RB. SARS-CoV-2 immunity: review and applications to phase 3 vaccine candidates. Lancet. 2020;396:1595-1606. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 472] [Cited by in RCA: 437] [Article Influence: 87.4] [Reference Citation Analysis (0)] |
| 13. | Vojdani A, Kharrazian D. Potential antigenic cross-reactivity between SARS-CoV-2 and human tissue with a possible link to an increase in autoimmune diseases. Clin Immunol. 2020;217:108480. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 228] [Cited by in RCA: 421] [Article Influence: 84.2] [Reference Citation Analysis (0)] |
| 14. | Talotta R. Do COVID-19 RNA-based vaccines put at risk of immune-mediated diseases? Clin Immunol. 2021;224:108665. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 158] [Article Influence: 39.5] [Reference Citation Analysis (0)] |
| 15. | Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Pérez Marc G, Moreira ED, Zerbini C, Bailey R, Swanson KA, Roychoudhury S, Koury K, Li P, Kalina WV, Cooper D, Frenck RW Jr, Hammitt LL, Türeci Ö, Nell H, Schaefer A, Ünal S, Tresnan DB, Mather S, Dormitzer PR, Şahin U, Jansen KU, Gruber WC; C4591001 Clinical Trial Group. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med. 2020;383:2603-2615. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10556] [Cited by in RCA: 10655] [Article Influence: 2131.0] [Reference Citation Analysis (1)] |
| 16. | Ehrenfeld M, Tincani A, Andreoli L, Cattalini M, Greenbaum A, Kanduc D, Alijotas-Reig J, Zinserling V, Semenova N, Amital H, Shoenfeld Y. Covid-19 and autoimmunity. Autoimmun Rev. 2020;19:102597. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 416] [Cited by in RCA: 363] [Article Influence: 72.6] [Reference Citation Analysis (0)] |
| 17. | Caso F, Costa L, Ruscitti P, Navarini L, Del Puente A, Giacomelli R, Scarpa R. Could Sars-coronavirus-2 trigger autoimmune and/or autoinflammatory mechanisms in genetically predisposed subjects? Autoimmun Rev. 2020;19:102524. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 152] [Cited by in RCA: 225] [Article Influence: 45.0] [Reference Citation Analysis (0)] |
| 18. | Boekel L, Hooijberg F, van Kempen ZLE, Vogelzang EH, Tas SW, Killestein J, Nurmohamed MT, Boers M, Kuijpers TW, van Ham SM, Eftimov F, Wieske L, Rispens T, Wolbink GJ. Perspective of patients with autoimmune diseases on COVID-19 vaccination. Lancet Rheumatol. 2021;3:e241-e243. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 67] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 19. | Felten R, Dubois M, Ugarte-Gil MF, Chaudier A, Kawka L, Bergier H, Costecalde C, Pijnenburg L, Fort J, Chatelus E, Sordet C, Javier RM, Gottenberg JE, Sibilia J, Fuentes-Silva Y, Arnaud L. Vaccination against COVID-19: Expectations and concerns of patients with autoimmune and rheumatic diseases. Lancet Rheumatol. 2021;3:e243-e245. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 91] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 20. | Geisen UM, Berner DK, Tran F, Sümbül M, Vullriede L, Ciripoi M, Reid HM, Schaffarzyk A, Longardt AC, Franzenburg J, Hoff P, Schirmer JH, Zeuner R, Friedrichs A, Steinbach A, Knies C, Markewitz RD, Morrison PJ, Gerdes S, Schreiber S, Hoyer BF. Immunogenicity and safety of anti-SARS-CoV-2 mRNA vaccines in patients with chronic inflammatory conditions and immunosuppressive therapy in a monocentric cohort. Ann Rheum Dis. 2021;80:1306-1311. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 302] [Cited by in RCA: 270] [Article Influence: 67.5] [Reference Citation Analysis (0)] |
| 21. | Park JK, Lee EB, Shin K, Sung YK, Kim TH, Kwon SR, Lee MS, Hong SJ, Choi BY, Lee SS, Back HJ; Korean College of Rheumatology Task Force for COVID-19 Vaccine Guidance for Patients with Autoimmune Inflammatory Rheumatic Diseases. COVID-19 Vaccination in Patients with Autoimmune Inflammatory Rheumatic Diseases: Clinical Guidance of the Korean College of Rheumatology. J Korean Med Sci. 2021;36:e95. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 37] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 22. | Rosenbaum L. Escaping Catch-22 - Overcoming Covid Vaccine Hesitancy. N Engl J Med. 2021;384:1367-1371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 79] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 23. | Schwarzinger M, Watson V, Arwidson P, Alla F, Luchini S. COVID-19 vaccine hesitancy in a representative working-age population in France: a survey experiment based on vaccine characteristics. Lancet Public Health. 2021;6:e210-e221. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 347] [Cited by in RCA: 492] [Article Influence: 123.0] [Reference Citation Analysis (0)] |
| 24. | Keehner J, Horton LE, Pfeffer MA, Longhurst CA, Schooley RT, Currier JS, Abeles SR, Torriani FJ. SARS-CoV-2 Infection after Vaccination in Health Care Workers in California. N Engl J Med. 2021;384:1774-1775. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 208] [Cited by in RCA: 185] [Article Influence: 46.3] [Reference Citation Analysis (0)] |
| 25. | American College of Rheumatology. American College of Rheumatology COVID-19 vaccine clinical guidance summary for patients with rheumatic and musculoskeletal diseases. [cited 13 May 2021]. Available from: https://www.rheumatology.org/Portals/0/Files/COVID-19-Vaccine-Clinical-Guidance-Rheumatic-Diseases-Summary.pdf. |
| 26. | Boekel L, Kummer LY, van Dam KPJ, Hooijberg F, van Kempen Z, Vogelzang EH, Wieske L, Eftimov F, van Vollenhoven R, Kuijpers TW, van Ham SM, Tas SW, Killestein J, Boers M, Nurmohamed MT, Rispens T, Wolbink G. Adverse events after first COVID-19 vaccination in patients with autoimmune diseases. Lancet Rheumatol. 2021;3:e542-e545. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 50] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 27. | Menni C, Klaser K, May A, Polidori L, Capdevila J, Louca P, Sudre CH, Nguyen LH, Drew DA, Merino J, Hu C, Selvachandran S, Antonelli M, Murray B, Canas LS, Molteni E, Graham MS, Modat M, Joshi AD, Mangino M, Hammers A, Goodman AL, Chan AT, Wolf J, Steves CJ, Valdes AM, Ourselin S, Spector TD. Vaccine side-effects and SARS-CoV-2 infection after vaccination in users of the COVID Symptom Study app in the UK: a prospective observational study. Lancet Infect Dis. 2021;21:939-949. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 766] [Cited by in RCA: 657] [Article Influence: 164.3] [Reference Citation Analysis (0)] |
| 28. | Connolly CM, Ruddy JA, Boyarsky BJ, Avery RK, Werbel WA, Segev DL, Garonzik-Wang J, Paik JJ. Safety of the first dose of mRNA SARS-CoV-2 vaccines in patients with rheumatic and musculoskeletal diseases. Ann Rheum Dis. 2021;80:1100-1101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 38] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 29. | Dotan A, Muller S, Kanduc D, David P, Halpert G, Shoenfeld Y. The SARS-CoV-2 as an instrumental trigger of autoimmunity. Autoimmun Rev. 2021;20:102792. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 390] [Cited by in RCA: 324] [Article Influence: 81.0] [Reference Citation Analysis (0)] |