Published online Mar 24, 2016. doi: 10.5410/wjcu.v5.i1.45
Peer-review started: September 8, 2015
First decision: October 16, 2015
Revised: November 24, 2015
Accepted: January 8, 2016
Article in press: January 11, 2016
Published online: March 24, 2016
Processing time: 198 Days and 14 Hours
AIM: To evaluate the long-term outcomes of patients receiving adjuvant and salvage radiotherapy following prostatectomy with adverse pathologic features and an undetectable prostate specific antigen (PSA).
METHODS: A retrospective review was performed of patients who received post-prostatectomy radiation at Loyola University Medical Center between 1992 and 2013. Adverse pathologic features (Gleason score ≥ 8, seminal vesicle invasion, extracapsular extension, pathologic T4 disease, and/or positive surgical margins) and an undetectable PSA following prostatectomy were required for inclusion. Adjuvant patients received therapy with an undetectable PSA, salvage patients following biochemical recurrence (BCR). Post-radiation BCR, overall survival, bone metastases, and initiation of hormonal therapy were assessed. Kaplan-Meier time-to-event analyses and stepwise Cox proportional hazards regression (HR) were performed.
RESULTS: Post-prostatectomy patients (n = 134) received either adjuvant (n = 47) or salvage (n = 87) radiation. Median age at radiotherapy (RT) was 63 years, and median follow-up was 53 mo. Five-year post-radiation BCR-free survival was 78% for adjuvant vs 50% salvage radiotherapy (SRT) (Logrank P = 0.001). Patients with radiation administered following a detectable PSA had an increased risk of BCR compared to undetectable: PSA > 0.0-0.2: HR = 4.1 (95%CI: 1.5-11.2; P = 0.005); PSA > 0.2-1.0: HR = 4.4 (95%CI: 1.6-11.9; P = 0.003); and PSA > 1.0: HR = 52 (95%CI: 12.9-210; P < 0.001). There was no demonstrable difference in rates of overall survival, bone metastases or utilization of hormonal therapy between adjuvant and SRT patients.
CONCLUSION: Adjuvant RT improves BCR-free survival compared to SRT in patients with adverse pathologic features and an undetectable post-prostatectomy PSA.
Core tip: We evaluated the outcomes of patients who received post-prostatectomy radiotherapy (RT) who had adverse features on the pathologic specimen and an immediately undetectable prostate specific antigen (PSA) postoperatively. In this cohort of patients, those who received RT in the adjuvant therapy (e.g., while PSA remains undetectable) had an improved 5-year biochemical recurrence (BCR)-free survival of 78%, compared to 50% for patients receiving RT in the salvage setting (e.g., after the postoperative PSA has again become detectable). As such, adjuvant RT improves BCR free survival in post-prostatectomy patients with adverse pathologic features and an undetectable PSA compared to salvage RT.
- Citation: Blackwell RH, Gange W, Kandabarow AM, Harkenrider MM, Gupta GN, Quek ML, Flanigan RC. Adjuvant radiotherapy for pathologically advanced prostate cancer improves biochemical recurrence free survival compared to salvage radiotherapy. World J Clin Urol 2016; 5(1): 45-52
- URL: https://www.wjgnet.com/2219-2816/full/v5/i1/45.htm
- DOI: https://dx.doi.org/10.5410/wjcu.v5.i1.45
An estimated 233000 men in the United States will be diagnosed with prostate cancer (PCa) in 2014[1]. While radical prostatectomy (RP) is a curative treatment for many patients, approximately one-third of patients will experience recurrence of disease within 10 years of surgery[2-4]. Pathological features such as positive surgical margins (PSM), seminal vesicle invasion (SVI), extracapsular extension (ECE), Gleason score ≥ 8, and/or pathologic adjacent organ invasion are associated with a higher risk of biochemical recurrence (BCR)[5-8]. In these high risk patients adjuvant radiotherapy (ART) can be offered, however this leads to overtreatment of approximately 55% of patients who may never experience a BCR[4,9]. Patients who defer initial adjuvant therapy are closely monitored and offered salvage radiotherapy (SRT) if and when they experience BCR.
Three randomized controlled trials (SWOG 8794, EORTC 22911, and ARO 96-02) have been conducted comparing ART with observation following RP in patients with adverse pathologic features and an undetectable prostate specific antigen (PSA). These have demonstrated improved BCR-free survival with ART compared with observation (patients may or may not have received SRT)[5,6,10,11]. Despite these convincing data, only approximately 11.7% of patients with pT3-4N0 disease undergo ART according to an analysis of the Surveillance Epidemiology and End Results database[12]. Investigation into SRT in this same patient population (adverse pathologic features with undetectable PSA) has not been as purposefully studied in randomized controlled trials, although several retrospective studies[9,13-18], including two matched-control analyses[17,18], have been performed in this area.
In this study, we present our experience with the outcomes of ART and SRT in post-RP patients at a high risk for recurrence, with adverse pathologic features and an initial post-RP undetectable PSA.
Following institutional review board approval a retrospective chart review was performed. All patients who were counseled for RT for PCa between 1992 and 2013 were identified. Of the 886 patients who subsequently received RT at our institution, 248 had a history of prior RP.
The patient demographic and pathologic PCa information listed in Table 1 was abstracted from via a comprehensive review of physician notes and laboratory reports (bloodwork, pathology reports, etc.).
| Received adjuvant therapy | Adjuvant candidate and received salvage radiotherapy | P value | ||
| Age at RT (median, IQR), mo | 60 (54-65) | 63 (59-68) | 0.2 | |
| Follow-up (mo) | 53 (19-83) | 50 (22-854) | 0.1 | |
| Time from RP to RT, mo | 0-12 | 43 (93%) | 12 (14%) | < 0.001 |
| > 12-24 | 3 (7%) | 18 (21%) | ||
| > 24-48 | 0 (0%) | 29 (33%) | ||
| > 48 | 0 (0%) | 28 (32%) | ||
| Pre-RT PSA | Undetectable | 46 (100%) | 0 (0%) | < 0.001 |
| > 0-0.2 | 0 (0%) | 39 (46%) | ||
| > 0.2-1.0 | 0 (0%) | 38 (45%) | ||
| > 1.0 | 0 (0%) | 7 (8%) | ||
| Received | No | 37 (79%) | 67 (77%) | 0.8 |
| Peri-RT ADT | Yes | 10 (21%) | 20 (23%) | |
| Coronary artery disease | No | 36 (86%) | 64 (84%) | 0.8 |
| Yes | 6 (14%) | 12 (16%) | ||
| Diabetes mellitus, type II | No | 37 (88%) | 58 (76%) | 0.1 |
| Yes | 5 (12%) | 18 (24%) | ||
| Hypertension | No | 19 (45%) | 37 (49%) | 0.6 |
| Yes | 23 (55%) | 39 (51%) | ||
| Obesity | No | 31 (74%) | 51 (67%) | 0.4 |
| Yes | 11 (26%) | 25 (33%) | ||
| Peripheral vascular disease | No | 39 (93%) | 73 (96%) | 0.4 |
| Yes | 3 (7%) | 3 (4%) | ||
| Smoking history | No | 37 (88%) | 70 (92%) | 0.5 |
| Yes | 5 (12%) | 6 (8%) | ||
| Pathologic | 2-6 | 9 (20%) | 18 (21%) | 0.4 |
| Gleason score | 7 | 21 (48%) | 48 (57%) | |
| 8-10 | 14 (32%) | 18 (21%) | ||
| Pathologic | T1 | 0 (0%) | 0 (0%) | 0.08 |
| Tumor stage | T2 | 11 (25%) | 35 (41%) | |
| T3/T4 | 33 (75%) | 51 (59%) | ||
| Positive surgical margin | Absent | 12 (25%) | 28 (32%) | 0.4 |
| Present | 35 (75%) | 59 (68%) | ||
| Extracapsular extension | Absent | 20 (43%) | 41 (47%) | 0.6 |
| Present | 27 (57%) | 46 (53%) | ||
| Seminal vesicle invasion | Absent | 37 (79%) | 80 (92%) | 0.03 |
| Present | 10 (21%) | 7 (8%) |
Post-prostatectomy RT patients were grouped according to pathologic characteristics, postoperative PSA nadir level, and the timing of administration of post-prostatectomy RT (before/after BCR). Adjuvant therapy candidates were defined as patients with one or more adverse pathologic features (total Gleason score ≥ 8, SVI, PSM, ECE, and/or adjacent organ invasion) and an undetectable post-RP nadir PSA level. For this study, an undetectable PSA was defined as a PSA with a value of < 0.05 ng/mL. Patients with a detectable post-RP PSA (n = 54, 21.8%), the absence of adverse pathologic features (n = 50, 20.2%), or both of the aforementioned criteria (n = 10, 4.0%) were not considered to be adjuvant therapy candidates and excluded from analysis. Adjuvant therapy candidates who received RT with an undetectable PSA were classified as having received ART. Salvage therapy candidates were defined as those who following an undetectable postoperative PSA level, who later developed a detectable PSA level. Phoenix criteria of post-RP BCR were utilized (a PSA of ≥ 0.2 ng/mL, with a second consecutive test at or above this level) to define BCR following RT[19].
Standard post-prostatectomy RT was provided to patients as either adjuvant or SRT (as above) and administered at 66.6 Gy fractionated over approximately 37 doses to the prostatic fossa and seminal vesicle remnants, if present.
The outcomes of interest that were evaluated include time to BCR, overall survival (OS), bone metastasis (BMet), and hormonal therapy (HT). BCR was considered to take place on the date of the first of two or more successive PSA values ≥ 0.2 ng/mL after RT. OS was defined as death from any cause. BMet was defined as any radiologic, pathologic, or clinical evidence of bony metastasis. HT was defined the initiation of androgen deprivation therapy following post-RT BCR.
Kaplan-Meier method was utilized to analyze BCR-, OS-. BMet, and HT-free survival functions. The time span between the event of interest and the final day of RT was analyzed. Patients entered the model at the date of completion of RT. If an event did not occur, the patient was considered to be right-censored for that event with the time between the day of the last follow-up and the final day of RT. A stepwise Cox proportion hazard regression was modelled to evaluate the independent effect of the categorical variables and treatment modalities in Table 1. Variables were selected in a forward fashion, with P = 0.05 meeting the standard for inclusion into the model. Variables with P ≥ 0.10 were deemed insignificant and removed from the model.
SPSS® version 20 (SPSS, Chicago, IL), was utilized, with all comparisons 2-sided and a P-value < 0.05 was considered statistically significant.
Between 1992 and 2013 our institution treated 886 patients with RT for PCa, of whom 248 received post-prostatectomy RT. Patients with adverse pathologic features, an undetectable nadir PSA, and who received post-RP RT accounted for 134 patients. Of these, 47 (35%) received ART and 87 (65%) received SRT. The median follow-up after RT was 53 (22-96) mo, and median age at RT was 63 (58-68) years old (Table 1).
For patients receiving ART vs SRT, pre-RT patient characteristics differed only in time from RP to RT (93% ART patients received therapy within 12 mo, compared with 14% SRT, P < 0.001), pre-RT PSA level (undetectable in 100% ART and 0% SRT, P < 0.001), and a higher rate of SVI in the ART cohort (12% vs 8%, P = 0.028). Medical comorbidities were comparable between the groups. There were no statistical differences in total Gleason score or frequency of PSM, ECE, or pathologic T4 disease between the two treatment groups.
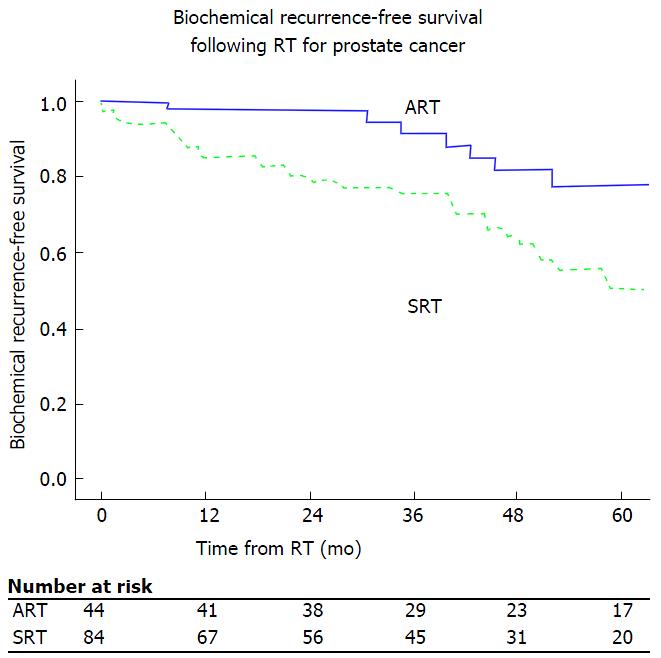
Kaplan-Meier 5-year BCR-free survival were 78% and 50% for ART and SRT, respectively (Logrank, P = 0.001) (Figure 1). On univariate analysis, receipt of RT at an undetectable level, and pathologic Gleason score < 8 were associated with improved BCR-free survival. On multivariate analysis, the predominant factor associated with BCR was PSA level at time of RT. Compared with RT administered with an undetectable PSA (ART), BCR was more likely when RT was administered as SRT with detectable pre-RT PSA levels as follows: > 0.0 to 0.2 ng/mL (HR = 4.1; P = 0.005), > 0.2-1.0 ng/mL (HR = 5.5; P = 0.003), and ≥ 1.0 ng/mL (HR = 52, P < 0.001) (Table 2). A sensitivity analysis was performed with pre-RT cutoff of PSA ≤ 0.5 ng/mL compared to undetectable, which demonstrated a similar improved BCR-free survival with adjuvant therapy (data not shown). Pathologic Gleason score of ≥ 8 also increases risk of BCR in the multivariate model (HR = 3.1; P = 0.02).
| Biochemical recurrence | Overall survival | Bone metastases | Hormonal therapy | ||||||
| HR (95%CI) | P value | HR (95%CI) | P value | HR (95%CI) | P value | HR (95%CI) | P value | ||
| Pathologic gleason score | 2-6 | Referent | 0.01 | Referent | 0.4 | Referent | 0.3 | Referent | 0.005 |
| 7 | 1.2 (0.4-3.1) | 0.8 | 0.7 | 0.9 | 1.2 (0.3-4.6) | 0.8 | |||
| 8-10 | 3.1 (1.2-8.1) | 0.02 | 0.2 | 0.1 | 4.9 (1.4-16.7) | 0.01 | |||
| Pre-RT PSA | Undetectable | Referent | < 0.001 | Referent | 0.8 | Referent | 0.045 | Referent | < 0.001 |
| > 0.0-0.2 | 4.1 (1.5 -11.2) | 0.005 | 0.8 | 3.3 (0.2-54.2) | 0.4 | 2.6 (0.8-9.1) | 0.1 | ||
| > 0.2-1.0 | 4.4 (1.6-11.9) | 0.003 | 0.6 | 0.6 (0.04-8.6) | 0.7 | 1.7 (0.5-5.8) | 0.4 | ||
| > 1.0 | 52 (12.9-210) | < 0.001 | 0.7 | 39.8 (1.8-868) | 0.02 | 67.8 (13.7-336) | < 0.001 | ||
| Received peri-RT ADT | 0.7 | 0.2 | 0.1 | 0.6 | |||||
| Seminal vesicle invasion | 2.2 (0.99-4.8) | 0.053 | 0.9 | 0.2 | 2.7 (1.1-6.6) | 0.036 | |||
| Positive surgical margin | 0.056 | 0.08 | 0.08 | 0.09 | |||||
| Extracapsular extension | 0.3 | 0.1 | 0.6 | 0.6 | |||||
| Pathologic stage | T2 | Referent | 0.9 | Referent | 0.1 | Referent | 0.9 | Referent | 0.8 |
| T3/4 | |||||||||
Kaplan-Meier estimates of 5-year OS were 97% for both ART and SRT patients. A total of 3 (6%) ART and 8 (9%) SRT patients have died since RT (Logrank, P = 0.5). No variables contributed to OS on multivariate analysis (Table 2).
Five-year actuarial risks of bone metastasis were 0% and 6% for ART and SRT, respectively (Logrank, P = 0.9). Three ART patients (6%) and five SRT patients (6%) developed metastatic disease to the bone over the course of follow-up. On univariate analysis, patients who received ART had improved bone metastasis-free survival (Logrank P = 0.004). On multivariate analysis, patients who received SRT with a PSA ≥ 1.0 had an increased risk of bone metastases (HR = 39.806; P = 0.02) compared to patients who received ART (undetectable PSA) (Table 2).
There was trend toward decreased utilization of hormonal therapy at 5 years post-RT in ART (6%) compared with SRT (21%) patients (Logrank, P = 0.08). Median time from RT to additional treatment was 218 mo for ART and 142 mo for SRT. Based on pre-RT PSA level, there was a worse HT-free survival in patients receiving RT with a PSA > 1.0, which remained true on multivariate analysis (HR = 67.841; P < 0.001) (Table 2). A sensitivity analysis run with pre-RT PSA ≤ 0.5 ng/mL compared to undetectable demonstrated a similar risk of progression to HT. With a PSA >0.5 ng/mL, there was an increased risk of receipt of HT (data not shown). Further, a pathologic Gleason score of 8-10 was associated with an increased risk for receipt of HT on univariate and multivariate analyses (Table 2).
Our results demonstrate that patients with adverse pathologic features (Gleason 8-10, SVI, ECE, PSM, and/or pathologic T4 disease) and an undetectable PSA have improved oncologic results with ART compared to SRT. When patients were observed until PSA became detectable and then received SRT there was an increased risk of post-RT BCR, even in the early RT (PSA < 0.2) setting. When SRT was administered with a pre-RT PSA level > 1.0, the risk of BCR, BMet and HT increased dramatically. While pathologic Gleason score 8-10 was also associated with BCR, and both pathologic Gleason 8-10 and SVI were associated with progression to HT, the receipt of RT prior to detectable PSA was shown to be the only modifiable risk factor available to the treating clinician to impact BCR-free survival.
Three randomized controlled trials (SWOG 8794, EORTC22911, and ARO 96-02) have definitely demonstrated that ART in this high-risk patient population results in improved BCR-free survival compared to RP and observation alone[5,6,10,11]. Two of these studies (SWOG 8794 and EORTC 22911) have demonstrated reduced need for salvage therapy for RT failure when patients were administered ART compared to RP and observation[5,10]. The benefit of an observational approach would be to spare men exposure to RT until they experience a BCR, which would never occur for an as of yet unspecified population. The question of whether there is a benefit to administration of ART compared with SRT at the time of BCR, as assessed in this study, has yet to be reported in a randomized controlled trial. Three trials which will address this question are currently enrolling patients in Australia/New Zealand (RAVES)[20], France (GETUG-17)[21], and in the United Kingdom and Canada (RADICALS)[22], although results are pending.
Until these trials meet accrual and have sufficient follow-up to produce meaningful conclusion, the literature remains sparse. A recent review and meta-analysis has been performed on the available, retrospective data, demonstrating an improved BCR-free survival in ART-treated patients compared to those treated with SRT[23]. While this analysis is in agreement with our findings, caution is necessary when interpreting a review of this topic. The current literature has markedly variability of the definitions of both ART and SRT, which confound generalizability and interpretation. Our study included strict inclusion criteria for analysis, including only patients with adverse pathologic features following RP and an undetectable post-RP PSA. Of the 18 studies included in the review above, eight did not require an undetectable post-RP PSA for SRT patients and four allowed ART patients to have a detectable post-RP PSA. While SRT may be administered in a different settings (e.g., detectable PSA immediately post-RP, rising PSA from undetectable post-RP), ART should be administered within 6-12 mo post-operatively with an undetectable PSA. It is important to strictly define these criteria prior to analysis in order to compare treatment effects on comparable baseline patient cohorts.
Of the retrospective studies available, three deserve special mention and represent the best evidence to date regarding ART vs SRT in post-RP patients with adverse pathologic features and an undetectable PSA. Trabulsi et al[18] reported on 449 patients received postoperative RT for adverse pathologic features with an undetectable postoperative nadir PSA. After propensity score matching, 96 patients remained in each treatment group (ART and SRT). With a median follow-up of 73 mo from RT, there was improvement in five-year BCR-free survival in the ART group (73% vs 50%; HR = 2.3; P = 0.007). Comparable to the present study, pathologic Gleason score 8-10 was found to be associated with BCR (HR = 2.5; P = 0.005).
Ost et al[17] and coworkers reported a comparable match-controlled analysis of 178 patients, with 89 in each group. Three-year BCR-free survival was improved for ART vs SRT (90% and 65%, P < 0.05) in this analysis as well. Further, patients with Gleason score ≥ 4 + 3, preoperative PSA > 10 ng/mL, and omission of concomitant androgen deprivation therapy had an increase in risk for BCR.
Briganti et al[9] performed a multi-institutional retrospective review of 390 patients who received ART. These patients were matched in a one-to-one fashion based on pathologic Gleason score, pathologic stage and surgical margin status, with patients who underwent initial observation and SRT as needed for BCR. Kaplan-Meier analysis indicated comparable BCR-free survival between the ART and observation/SRT matched cohorts. While this analysis does examine optimal patient management with post-RP SRT prior to PSA 0.5 ng/mL, exclusion of patients who may have presented with a recurrence PSA of ≥ 0.5 ng/mL may omit more aggressive cases, and artificially improve BCR-free survival rates in the observation/SRT cohort. Further, Briganti’s study assesses time to BCR following RP, while Ost, Trabulsi, and the present study assess time to BCR following RT, limiting the ability to make comparisons between the studies.
Taken together with the present studies, it appears that when patients are compared following the receipt of RT, there is improvement in BCR-free survival with ART compared to SRT. The randomized, controlled trials above will hopefully provide definitive evidence regarding the timing of RT following RP, as well as define the patients who are adjuvant therapy candidates (adverse pathologic features with an undetectable post-RP PSA) who will or will not ultimately experience a BCR necessitating RT.
The primary limitation of our study is selection bias, specifically how patients arrived at the decision to pursue ART vs SRT. This decision is not solely dependent on pathologic and laboratory values, and patients may have been counseled to either of these treatment strategies based on personal preference, physician preference, their recovery from surgery, and convenience of therapy availability. Further, the potential side-effects of RT (including urethral stricture disease, hematuria, proctitis, cystitis, secondary malignancy, etc.) are well documented[24-30], and play an integral role in the decision making process for both the patient and provider. These subjective choices are not reflected in our analysis. This analysis also does not have the denominator for how many patients elected for observational follow-up and did not recur. Avoiding overtreatment of patients with RT is a commendable goal, however until prospective trials are completed it is difficult to characterize which patients will or will not experience BCR. Finally, there was greater SVI in the ART compared to the SRT group. While this difference between treatment groups does exist, it should not influence the reported results as the greater SVI should have negatively impacted outcomes in the ART cohort, which was not seen.
Radiotherapy (RT) for prostate cancer (PCa) following RT is a treatment option available for patients with adverse pathologic features (positive surgical margins, seminal vesicle invasion, extracapsular extension, a Gleason score ≥ 8, and/or pathologic adjacent organ invasion. While prior prospective, randomized trials have shown improved biochemical recurrence (BCR) free survival following adjuvant radiotherapy (ART) (immediately following recovery from prostatectomy) compared to observation, the comparison of adjuvant compared to salvage radiotherapy (SRT) [after postoperative prostate specific antigen (PSA) has risen from an undetectable level] has yet to be as rigorously studied.
While adjuvant post-prostatectomy RT is known to improve BCR free survival, the optimal timing of administration RT is yet to be determined. Given the additional morbidity of RT and potential overtreatment of patients who may never recur with adjuvant radiation, the results of this study contribute to the understanding of outcomes between early (adjuvant) RT compared to delayed (salvage) RT in the post-prostatectomy population.
In this study, patients who received either adjuvant or SRT following radical prostatectomy with the presence of adverse pathologic features and an undetectable PSA were identified. This was a well-matched group when comparing baseline and pathologic characteristics. It is clear that patients who received ART had an improved BCR free survival at 5 years (78%) compared to those who received SRT (50%).
This study suggests that the receipt of ART for post-prostatectomy adverse pathologic features improved BCR free survival compared to patients who receive salvage radiation following a rise in PSA from undetectable.
PSA: Prostate specific antigen, a serum marker produced only by prostate and PCa cells. Adverse pathologic features: Poor prognostic findings on the prostate specimen including positive surgical margins, seminal vesicle invasion, extracapsular extension, a Gleason score ≥ 8, and/or pathologic adjacent organ invasion. ART: The administration of radiation to the prostatectomy surgical bed following recovery of surgery, while the patient has an undetectable PSA. SRT: The administration of radiation to the prostatectomy surgical bed following recovery of surgery, following an increase in PSA from undetectable to a detectable value.
This is an interesting retrospective study comparing the effects of adjuvant vs SRT on BCR free survival of high risk PCa pts with initially undetectable post-op PSA. This is well-written work and both the results and limitations of the study are adequately documented.
P- Reviewer: Gofrit ON, Vlachostergios PJ S- Editor: Qiu S L- Editor: A E- Editor: Li D
| 1. | American Cancer Society: Prostate cancer key statistics 2014. [accessed 2015 Dec 14]. Available from: http//www.cancer.org/cancer/prostatecancer/detailedguide/prostate-cancer-key-statistics. |
| 2. | Amling CL, Blute ML, Bergstralh EJ, Seay TM, Slezak J, Zincke H. Long-term hazard of progression after radical prostatectomy for clinically localized prostate cancer: continued risk of biochemical failure after 5 years. J Urol. 2000;164:101-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 228] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 3. | Roehl KA, Han M, Ramos CG, Antenor JA, Catalona WJ. Cancer progression and survival rates following anatomical radical retropubic prostatectomy in 3,478 consecutive patients: long-term results. J Urol. 2004;172:910-914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 606] [Cited by in RCA: 640] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 4. | Thompson IM, Valicenti RK, Albertsen P, Davis BJ, Goldenberg SL, Hahn C, Klein E, Michalski J, Roach M, Sartor O. Adjuvant and salvage radiotherapy after prostatectomy: AUA/ASTRO Guideline. J Urol. 2013;190:441-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 293] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 5. | Bolla M, van Poppel H, Collette L, van Cangh P, Vekemans K, Da Pozzo L, de Reijke TM, Verbaeys A, Bosset JF, van Velthoven R. Postoperative radiotherapy after radical prostatectomy: a randomised controlled trial (EORTC trial 22911). Lancet. 2005;366:572-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 931] [Cited by in RCA: 877] [Article Influence: 43.9] [Reference Citation Analysis (0)] |
| 6. | Bolla M, van Poppel H, Tombal B, Vekemans K, Da Pozzo L, de Reijke TM, Verbaeys A, Bosset JF, van Velthoven R, Colombel M. Postoperative radiotherapy after radical prostatectomy for high-risk prostate cancer: long-term results of a randomised controlled trial (EORTC trial 22911). Lancet. 2012;380:2018-2027. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 654] [Cited by in RCA: 676] [Article Influence: 52.0] [Reference Citation Analysis (0)] |
| 7. | Stephenson AJ, Scardino PT, Kattan MW, Pisansky TM, Slawin KM, Klein EA, Anscher MS, Michalski JM, Sandler HM, Lin DW. Predicting the outcome of salvage radiation therapy for recurrent prostate cancer after radical prostatectomy. J Clin Oncol. 2007;25:2035-2041. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 743] [Cited by in RCA: 692] [Article Influence: 38.4] [Reference Citation Analysis (0)] |
| 8. | Venyo A, Imran S, Shah S, Gordon R, Barnes D. Serum PSA, Gleason Score and Clinical Staging in Predicting Biochemical (PSA) Failure After Radical Prostatectomy for Carcinoma of Prostate. Webmed Central Urology. 2012;3:WMC003222. [DOI] [Full Text] |
| 9. | Briganti A, Wiegel T, Joniau S, Cozzarini C, Bianchi M, Sun M, Tombal B, Haustermans K, Budiharto T, Hinkelbein W. Early salvage radiation therapy does not compromise cancer control in patients with pT3N0 prostate cancer after radical prostatectomy: results of a match-controlled multi-institutional analysis. Eur Urol. 2012;62:472-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 132] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 10. | Thompson IM, Tangen CM, Paradelo J, Lucia MS, Miller G, Troyer D, Messing E, Forman J, Chin J, Swanson G. Adjuvant radiotherapy for pathologically advanced prostate cancer: a randomized clinical trial. JAMA. 2006;296:2329-2335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 750] [Cited by in RCA: 714] [Article Influence: 37.6] [Reference Citation Analysis (0)] |
| 11. | Wiegel T, Bottke D, Steiner U, Siegmann A, Golz R, Störkel S, Willich N, Semjonow A, Souchon R, Stöckle M. Phase III postoperative adjuvant radiotherapy after radical prostatectomy compared with radical prostatectomy alone in pT3 prostate cancer with postoperative undetectable prostate-specific antigen: ARO 96-02/AUO AP 09/95. J Clin Oncol. 2009;27:2924-2930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 657] [Cited by in RCA: 640] [Article Influence: 40.0] [Reference Citation Analysis (0)] |
| 12. | Gandaglia G, Karakiewicz PI, Briganti A, Trudeau V, Trinh QD, Kim SP, Montorsi F, Nguyen PL, Abdollah F, Sun M. Early radiotherapy after radical prostatectomy improves cancer-specific survival only in patients with highly aggressive prostate cancer: validation of recently released criteria. Int J Urol. 2015;22:89-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 13. | Wadasaki K, Kaneyasu Y, Kenjo M, Matsuura K, Murakami Y, Hashimoto Y, Ito K, Kiriu H, Ito A. Treatment results of adjuvant radiotherapy and salvage radiotherapy after radical prostatectomy for prostate cancer. Int J Clin Oncol. 2007;12:37-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 14. | Taylor N, Kelly JF, Kuban DA, Babaian RJ, Pisters LL, Pollack A. Adjuvant and salvage radiotherapy after radical prostatectomy for prostate cancer. Int J Radiat Oncol Biol Phys. 2003;56:755-763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 77] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 15. | Catton C, Gospodarowicz M, Warde P, Panzarella T, Catton P, McLean M, Milosevic M. Adjuvant and salvage radiation therapy after radical prostatectomy for adenocarcinoma of the prostate. Radiother Oncol. 2001;59:51-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 109] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 16. | Morris MM, Dallow KC, Zietman AL, Park J, Althausen A, Heney NM, Shipley WU. Adjuvant and salvage irradiation following radical prostatectomy for prostate cancer. Int J Radiat Oncol Biol Phys. 1997;38:731-736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 123] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 17. | Ost P, De Troyer B, Fonteyne V, Oosterlinck W, De Meerleer G. A matched control analysis of adjuvant and salvage high-dose postoperative intensity-modulated radiotherapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2011;80:1316-1322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 66] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 18. | Trabulsi EJ, Valicenti RK, Hanlon AL, Pisansky TM, Sandler HM, Kuban DA, Catton CN, Michalski JM, Zelefsky MJ, Kupelian PA. A multi-institutional matched-control analysis of adjuvant and salvage postoperative radiation therapy for pT3-4N0 prostate cancer. Urology. 2008;72:1298-1302; discussion 1302-1304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 78] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 19. | Roach M, Hanks G, Thames H, Schellhammer P, Shipley WU, Sokol GH, Sandler H. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int J Radiat Oncol Biol Phys. 2006;65:965-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1888] [Cited by in RCA: 2133] [Article Influence: 112.3] [Reference Citation Analysis (0)] |
| 20. | Pearse M, Fraser-Browne C, Davis ID, Duchesne GM, Fisher R, Frydenberg M, Haworth A, Jose C, Joseph DJ, Lim TS. A Phase III trial to investigate the timing of radiotherapy for prostate cancer with high-risk features: background and rationale of the Radiotherapy -- Adjuvant Versus Early Salvage (RAVES) trial. BJU Int. 2014;113 Suppl 2:7-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 97] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 21. | Richaud P, Sargos P, Henriques de Figueiredo B, Latorzeff I, Mongiat-Artus P, Houédé N, Salomon L, Wallerand H. [Postoperative radiotherapy of prostate cancer]. Cancer Radiother. 2010;14:500-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 44] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 22. | Parker C, Sydes MR, Catton C, Kynaston H, Logue J, Murphy C, Morgan RC, Mellon K, Morash C, Parulekar W. Radiotherapy and androgen deprivation in combination after local surgery (RADICALS): a new Medical Research Council/National Cancer Institute of Canada phase III trial of adjuvant treatment after radical prostatectomy. BJU Int. 2007;99:1376-1379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 95] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 23. | Chen C, Lin T, Zhou Y, Li D, Xu K, Li Z, Fan X, Zhong G, He W, Chen X. Adjuvant and salvage radiotherapy after prostatectomy: a systematic review and meta-analysis. PLoS One. 2014;9:e104918. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 24. | Blackwell RH, Kandabarow AM, Gupta GN, Harkenrider MM, Quek ML, Flanigan RC. Long-term incidence of hematuria, urethral stricture and bladder cancer after radiation therapy for prostate cancer. Urology Practice. 2015;2:349-358. [RCA] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 25. | Albert M, Tempany CM, Schultz D, Chen MH, Cormack RA, Kumar S, Hurwitz MD, Beard C, Tuncali K, O’Leary M. Late genitourinary and gastrointestinal toxicity after magnetic resonance image-guided prostate brachytherapy with or without neoadjuvant external beam radiation therapy. Cancer. 2003;98:949-954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 72] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 26. | Crook J, Esche B, Futter N. Effect of pelvic radiotherapy for prostate cancer on bowel, bladder, and sexual function: the patient’s perspective. Urology. 1996;47:387-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 119] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 27. | Nguyen LN, Pollack A, Zagars GK. Late effects after radiotherapy for prostate cancer in a randomized dose-response study: results of a self-assessment questionnaire. Urology. 1998;51:991-997. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 84] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 28. | Hunter GK, Reddy CA, Klein EA, Kupelian P, Angermeier K, Ulchaker J, Chehade N, Altman A, Ciezki JP. Long-term (10-year) gastrointestinal and genitourinary toxicity after treatment with external beam radiotherapy, radical prostatectomy, or brachytherapy for prostate cancer. Prostate Cancer. 2012;2012:853487. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 29. | Feng M, Hanlon AL, Pisansky TM, Kuban D, Catton CN, Michalski JM, Zelefsky MJ, Kupelian PA, Pollack A, Kestin LL. Predictive factors for late genitourinary and gastrointestinal toxicity in patients with prostate cancer treated with adjuvant or salvage radiotherapy. Int J Radiat Oncol Biol Phys. 2007;68:1417-1423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 91] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 30. | Elliott SP, Meng MV, Elkin EP, McAninch JW, Duchane J, Carroll PR. Incidence of urethral stricture after primary treatment for prostate cancer: data From CaPSURE. J Urol. 2007;178:529-534; discussion 534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 190] [Article Influence: 10.6] [Reference Citation Analysis (0)] |