Published online Nov 24, 2015. doi: 10.5410/wjcu.v4.i3.100
Peer-review started: January 24, 2015
First decision: March 6, 2015
Revised: March 26, 2015
Accepted: October 12, 2015
Article in press: October 13, 2015
Published online: November 24, 2015
Processing time: 310 Days and 15 Hours
Recent interests have focused on the exploration of the mechanical properties (elasticity, stiffness and deformity) of parenchymatous organs using tissue strain imaging techniques and elastosonography. Measures of the mechanical properties of the kidneys have given conflicting results. There are various conditions that affect the variability of renal parenchymal measures, the main target of the investigations. They can be classified as intrinsic (depending upon the patient), extrinsic (depending upon the operator) and mixed (both intrinsic and extrinsic). Indeed, the mechanical properties of the kidney depend on various conditions that alter its histology, mainly the amount of fibrosis in the renal parenchymal interstitium. Anatomical factors play an important role because the kidney is a highly anisotropic organ with important differences when considering the cortex and the medulla. Physical factors include the frequency of the probe, compression and distance from source to target. Many factors can affect measurements and it is necessary to find an accurate technique in order to avoid mistakes and to obtain reproducible data. Indeed, it is imperative to define a standardized examination technique in order to get comparable results. Therefore, the utility of acoustic radiation force imaging technique to predict only renal fibrosis or progression of chronic kidney disease is of dubious value because several variables - blood perfusion and urinary pressure - can contribute to a given measure, even with a standardized method able to minimize intra- and inter-operator variability.
Core tip: Recent interests have focused on the exploration of the mechanical properties (elasticity, stiffness and deformity) of parenchymatous organs using tissue strain imaging techniques and elastosonography. Many factors can affect measurements and an accurate technique is necessary in order to avoid mistakes and to obtain reproducible data. The use of tissue strain imaging techniques to predict only renal fibrosis or progression of chronic kidney disease is of dubious value because other variables - blood perfusion and urinary pressure - can contribute to a given measure, even with a standardized method able to minimize intra- and inter-operator variability.
- Citation: Zaffanello M, Bruno C. Clinical perspective on renal elasticity quantification by acoustic radiation force impulse: Where we are and where we are going. World J Clin Urol 2015; 4(3): 100-103
- URL: https://www.wjgnet.com/2219-2816/full/v4/i3/100.htm
- DOI: https://dx.doi.org/10.5410/wjcu.v4.i3.100
Recent interests have focused on the exploration of the mechanical properties (elasticity, stiffness and deformity) of parenchymatous organs using tissue strain imaging techniques and elastosonography. They basically work in the same way: A force induces a modification in a tissue that is closely related to its elasticity[1].
Tissue strain imaging can be divided into two categories: Quasi-static and dynamic methods[2]. The dynamic methods are based on detection and tracking of shear waves resulting from mechanical vibration of tissue[3]. Two main categories can be identified (external and internal methods) based on the applied stimulus[4]. Internal methods [i.e., acoustic radiation force imaging technique (ARFI) and supersonic shear waves (SSW) elastography] involve selecting a region of interest (ROI) on a gray-scale map, after which an acoustic push pulse is aimed directly at it, inducing the generation of SSW[5].
ARFI imaging has been demonstrated in many clinical settings, using various transducers and beam sequences depending upon the application, with promising results. These include: Abdominal imaging, cardiac imaging, vascular imaging, breast imaging, nerve imaging, prostate imaging, and monitoring thermal ablation procedures[6]. To date, the liver is the organ most frequently studied with the ARFI technique, especially to obtain a measure of fibrosis and cirrhosis[7]. In these sceneries, no particular debates have been reported.
Measures of the mechanical properties of the kidneys have given conflicting results. For example, the levels of renal shear wave velocity (SWV) reported in an Italian paper[8] are twice the levels obtained by a Turkish team[9] and a Chinese team[10] that used the same device (Acuson S2000). Moreover, Göya et al[9] reported that the more serious the renal damage, the slower the propagation of shear waves[11], in contrast to the results reported by Bruno et al[8] for older children. The explanation comes from understanding the factors which can influence ARFI measurements.
There are various conditions that affect the variability of renal parenchymal measures, the main target of the investigations. They can be classified as intrinsic (depending on the patient), extrinsic (depending on the operator) and mixed (both intrinsic and extrinsic). These variables indicate some problems related to the technical approach and to the failure of standardized procedures. They make it difficult to have comparable results among different studies.
The first intrinsic factor is the age of the patients. Normal kidneys in healthy subjects showed variations of SWV values according to age[10]. Recently, kidney shear wave speed values assessed by ARFI elastography in “normal” patients were reportedly influenced mainly by age and gender, with lower values being obtained in older patients and in men[12].
Moreover, as well as other parenchymatous organs, the mechanical properties of the kidney depend on various conditions that alter its histology, mainly the amount of fibrosis in the renal parenchymal interstitium. Theoretically, the more fibrosis and inflammation present in the parenchyma, the greater the ARFI measures should be.
Various degrees of fibrosis depending on the cause of damage, the time elapsed since damage, the degree of anisotropy, and the level of vascular and urinary pressure may have an impact on SWV of the renal cortex[11]. Changes in intrarenal vascular structure and renal blood flow may have a greater influence on SWV values. Asano et al[13] showed that a decrease in blood flow affects SWV values in the kidneys more than the progression of tissue fibrosis. Gennisson et al[14], in pig models, showed that the intrarenal elasticity values varied with tissue anisotropy and with vascular and urinary pressure levels. In particular, parenchymal elasticity increased linearly with elevation of urinary pressure. Nephro-urological pathology is another condition that may influence results since it is responsible for changes in renal blood perfusion and urinary pressure[8]. Studies have shown that variability of measurements in the renal parenchyma is a real issue, whichever elastography technique is used[15,16].
Other variables, in addition to tissue conditions, such as measure setting and target of the measures (subcapsular, cortex and medulla) can affect results. Anatomical factors play an important role[17] as well because the kidney is a highly anisotropic organ with important differences when considering the cortex and the medulla[18]. When observing each renal segment, the collecting ducts and vasa recta are disposed perpendicularly to the capsule and constitute a pathway along which shear waves travel at different speeds.
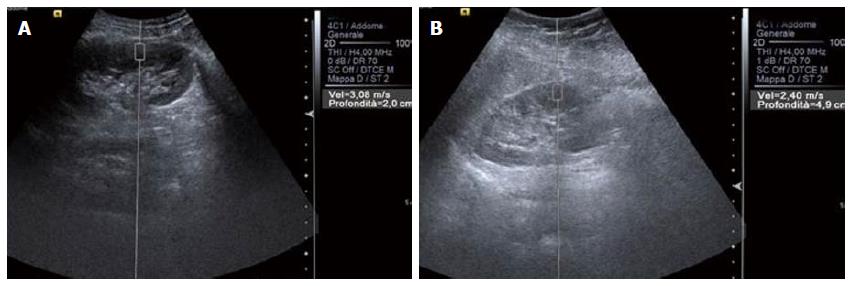
Physical factors include frequency of the probe, compression and distance from source to target. A lower frequency is related to lower frequency of the radiation force pulses and thus to a higher acoustic pressure with higher SWV[19]. However, greater compression gives higher SWV values because it gives a higher elastic modulus with greater SWV[20]. The distance from source to target is also important because for each frequency an optimal distance can be found where variability is minimum. Bota et al[12] showed that measurement depth negatively influences kidney shear wave speed values: the lower the depth the higher the SWV.
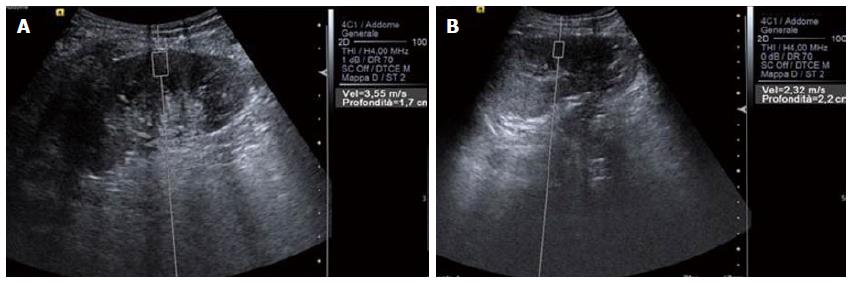
Generally speaking, SWV is related to the strength of compression[20], the source to target distance (Figure 1), the orientation of the ROI[21] and, most important of all, anisotropy, which has great influence when dealing with kidneys. The kidney is a highly anisotropic organ[18], and the particular decubitus when evaluating patients (for instance lying prone or supine or in lateral decubitus) is able to alter results (Figure 2).
Again, many factors can affect measurements and it is imperative to find an accurate technique in order to avoid mistakes and to obtain reproducible data. So the issue is to define a protocol for ARFI evaluation (as was done for the liver) able to minimize the differences between operators, and which includes standardization of the source to target distance, positioning of the ROI (avoiding the medulla or the dilated pyelo-caliceal system) and, of course, the patient’s decubitus. Moreover, in order to get comparable results, it is mandatory to have a standardized examination technique that clearly states that the operator should always exert the same compression on the kidney, with an ROI completely included in the cortex. The main axis of the US beam must be placed parallel to the pyramids in the segment of interest, always with the probe at the same angle, in order to minimize any effects of anisotropy[14].
Based on the findings cited above, the utility of ARFI analysis to predict only renal fibrosis or the progression of chronic kidney disease is of dubious value because other variables - blood perfusion and urinary pressure - can contribute to a given measure, even with a standardized method that can minimize intra- and inter-operator variability (patient position, probe frequency, ROI, compression, etc.). Accordingly, the clinical significance of the measures obtained remains to be explored.
P- Reviewer: Ohashi N, Schemmer P, Watanabe T S- Editor: Gong XM L- Editor: A E- Editor: Wu HL
| 1. | Varghese T. Quasi-Static Ultrasound Elastography. Ultrasound Clin. 2009;4:323-338. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 136] [Cited by in RCA: 100] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 2. | Zaffanello M, Piacentini G, Bruno C, Brugnara M, Fanos V. Renal elasticity quantification by acoustic radiation force impulse applied to the evaluation of kidney diseases: a review. J Investig Med. 2015;63:605-612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 3. | Ophir J, Garra B, Kallel F, Konofagou E, Krouskop T, Righetti R, Varghese T. Elastographic imaging. Ultrasound Med Biol. 2000;26 Suppl 1:S23-S29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 108] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 4. | Bharat S, Varghese T. Radiofrequency electrode vibration-induced shear wave imaging for tissue modulus estimation: a simulation study. J Acoust Soc Am. 2010;128:1582-1585. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 5. | Nightingale KR, Palmeri ML, Nightingale RW, Trahey GE. On the feasibility of remote palpation using acoustic radiation force. J Acoust Soc Am. 2001;110:625-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 553] [Cited by in RCA: 409] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 6. | Nightingale K. Acoustic Radiation Force Impulse (ARFI) Imaging: a Review. Curr Med Imaging Rev. 2011;7:328-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 250] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 7. | Sporea I, Gilja OH, Bota S, Şirli R, Popescu A. Liver elastography - an update. Med Ultrason. 2013;15:304-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 8. | Bruno C, Caliari G, Zaffanello M, Brugnara M, Zuffante M, Cecchetto M, Minniti S, Pedot A, Talamini G, Pozzi-Mucelli R. Acoustic radiation force impulse (ARFI) in the evaluation of the renal parenchymal stiffness in paediatric patients with vesicoureteral reflux: preliminary results. Eur Radiol. 2013;23:3477-3484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 9. | Göya C, Hamidi C, Ece A, Okur MH, Taşdemir B, Çetinçakmak MG, Hattapoğlu S, Teke M, Şahin C. Acoustic radiation force impulse (ARFI) elastography for detection of renal damage in children. Pediatr Radiol. 2015;45:55-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 10. | Lee MJ, Kim MJ, Han KH, Yoon CS. Age-related changes in liver, kidney, and spleen stiffness in healthy children measured with acoustic radiation force impulse imaging. Eur J Radiol. 2013;82:e290-e294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 85] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 11. | Olsen ØE. Shear wave velocities in damaged kidneys: fast and slow. Pediatr Radiol. 2015;45:2-3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 12. | Bota S, Bob F, Sporea I, Şirli R, Popescu A. Factors that influence kidney shear wave speed assessed by acoustic radiation force impulse elastography in patients without kidney pathology. Ultrasound Med Biol. 2015;41:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 13. | Asano K, Ogata A, Tanaka K, Ide Y, Sankoda A, Kawakita C, Nishikawa M, Ohmori K, Kinomura M, Shimada N. Acoustic radiation force impulse elastography of the kidneys: is shear wave velocity affected by tissue fibrosis or renal blood flow? J Ultrasound Med. 2014;33:793-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 106] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 14. | Gennisson JL, Grenier N, Combe C, Tanter M. Supersonic shear wave elastography of in vivo pig kidney: influence of blood pressure, urinary pressure and tissue anisotropy. Ultrasound Med Biol. 2012;38:1559-1567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 184] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 15. | Grenier N, Gennisson JL, Cornelis F, Le Bras Y, Couzi L. Renal ultrasound elastography. Diagn Interv Imaging. 2013;94:545-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 100] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 16. | Ozkan F, Menzilcioglu MS, Duymus M, Yildiz S, Avcu S. Acoustic radiation force impulse elastography for evaluating renal parenchymal stiffness in children. Pediatr Radiol. 2015;45:461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 17. | Goertz RS, Amann K, Heide R, Bernatik T, Neurath MF, Strobel D. An abdominal and thyroid status with Acoustic Radiation Force Impulse Elastometry--a feasibility study: Acoustic Radiation Force Impulse Elastometry of human organs. Eur J Radiol. 2011;80:e226-e230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 82] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 18. | Ries M, Jones RA, Basseau F, Moonen CT, Grenier N. Diffusion tensor MRI of the human kidney. J Magn Reson Imaging. 2001;14:42-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 181] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 19. | Chang S, Kim MJ, Kim J, Lee MJ. Variability of shear wave velocity using different frequencies in acoustic radiation force impulse (ARFI) elastography: a phantom and normal liver study. Ultraschall Med. 2013;34:260-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 20. | Syversveen T, Midtvedt K, Berstad AE, Brabrand K, Strøm EH, Abildgaard A. Tissue elasticity estimated by acoustic radiation force impulse quantification depends on the applied transducer force: an experimental study in kidney transplant patients. Eur Radiol. 2012;22:2130-2137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 84] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 21. | Fahey BJ, Nightingale KR, Nelson RC, Palmeri ML, Trahey GE. Acoustic radiation force impulse imaging of the abdomen: demonstration of feasibility and utility. Ultrasound Med Biol. 2005;31:1185-1198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 108] [Article Influence: 5.4] [Reference Citation Analysis (0)] |