Published online Nov 24, 2014. doi: 10.5410/wjcu.v3.i3.351
Revised: July 10, 2014
Accepted: August 27, 2014
Published online: November 24, 2014
Processing time: 251 Days and 22.5 Hours
AIM: To investigate the expression of programmed cell death 4 (Pdcd4) tumor suppressor gene in tissue specimen of renal cell carcinoma (RCC), testicular germ cell cancer and penile cancer.
METHODS: Pdcd4 expression was studied using immunohistochemistry in 188 cases of RCC and 28 controls (including 9 oncocytoma); in 74 cases of penile carcinoma (including 17 metastatic tissue samples) and 26 controls; in 11 cases of seminoma, in 14 cases of non-seminoma and 5 controls.
RESULTS: Control tissues exhibited strong core and cytoplasmatic Pdcd4 staining. In contrast, core and cytoplasmatic Pdcd4 levels were significantly decreased in cancer tissues.
CONCLUSION: Our data support a role for Pdcd4 (down-) regulation in urologic tumors. Interestingly, Pdcd4 expression seem to be a potential diagnostic marker for renal or penile tumors.
Core tip: Programmed cell death 4 has increasingly become the focus of investigative tumor research in the last years. It has shown to be involved in many tumorous entities, some of which we present for the first time in this paper. Its involvment in apoptosis, invasion and metastasis has been proved in numerous works and showed to be a target for diagnostic and therapeutic measures. We investigate its role and cellular expression patterns in urologic tumors, especially some, that haven’t been investigated to this extent or at all to this date.
- Citation: Fischer N, Göke F, Kahl P, Splittstößer V, Lankat-Buttgereit B, Müller SC, Ellinger J. Programmed cell death protein 4 expression in renal cell carcinoma, penile carcinoma and testicular germ cell cancer. World J Clin Urol 2014; 3(3): 351-357
- URL: https://www.wjgnet.com/2219-2816/full/v3/i3/351.htm
- DOI: https://dx.doi.org/10.5410/wjcu.v3.i3.351
Programmed cell death 4 (Pdcd4) expression is known to be suppressed in many tumors, for example urothelial- or colorectal cancer[1,2]. As shown in a previous study, Pdcd4 expression levels may have a tumor specific expression pattern and a potential as a diagnostic marker[1]. It inhibits RNA binding of the initiations factors eIF4A and eIF4G[3,4]. The Pdcd4 gene is located on chromosome 10q24 and encodes a 469 aminoacids long protein. A major regulator of Pdcd4 expression, microRNA 21 (miR-21) is induced by the transforming growth factor-β pathway. High miR-21 expression leads to a suppression of Pdcd4 expression and an induction of metastasis, invasion and intravasation in cell culture[5]. In Pdcd4 over-expressing cells, the subsequent carbonic anhydrase II down-regulation shows its influence on the translational level[6]. At the transcriptional level Pdcd4 influences the uPAR gene promoter through phosphorylation of the Sp transcription factors in colorectal cells[7]. Interestingly, this pathway is not confirmed for breast cancer: the lack of suppression of uPAR transcription by Pdcd4 overexpression therefore shows a possible tissue specific role of Pdcd4 and its involvement in carcinogenesis[8].
Renal cell carcinoma (RCC) stands for 2%-3% of all human cancers with an incidence of 5.8 and a mortality of 1.4 per 100000. Mostly men in the age of 60 to 70 are affected. Important risk factors are smoking, obesity and hypertension as well as having a first-degree relative with RCC. The different subtypes of RCC are clear cell RCC (ccRCC), papillary RCC (pRCC), chromophobe RCC (chRCC) and sarcomatoid RCC (sRCC).
This entity represents between 1%-1.5% of male cancers and about 5% of all urological tumors, with an incidence of 3-10 per 100000 males/year Peak incidence is in the third decade of life for non-seminoma and in the fourth decade for seminoma. Risk factors are cryptorchidism, undescended testes, Klinefelter’s syndrome, positive familial history, a contralateral tumour or a precancerous lesion and infertility. The two subtypes include seminoma and non-seminomatous germ cell tumor (NSGCT).
Penile cancer has an incidence of < 1/100000 males in western countries. It is related to race and ethnicity, most frequently affecting white hispanics (1/100000). Risk factors are social, cultural, hygienic and/or religious practices.
The role of Pdcd4 in penile- and testicular carcinoma has not been reported or investigated to this date. Recent studies to RCC stipulate a role for Pdcd4 in tumor progress and survival[9]. We thus investigated its expression pattern in human renal-, testicular-and penile tissue in the largest cohorts published to this date.
The expression of Pdcd4 in tumorous and normal tissue was studied using a tissue microarray as described in a previous study[10]. The microarray included 188 RCC, 74 penile squamous cell carcinoma and 25 testicular cancer (11 seminoma, 14 NSGCT) samples with their complementary controls; see Supplementary tables for clinicopathological parameters. The study was approved by our institutional ethics committee (ethic vote 199/10). The clinical pathological parameters are listed in Tables 1-4.
| Variables | Carcinoma (n = 11) | Control (n = 5) |
| Age in years mean (range) | 40.58 (33-53) | 30.75 (24-42) |
| Pathological stage | ||
| pT1 | 7 (63.6) | NA |
| pT2 | 2 (18.2) | NA |
| pT3 | 2 (18.2) | NA |
| Lymphnode metastasis | ||
| cNx | 1 (9.1) | NA |
| cN0 | 7 (63.6) | NA |
| cN1 | 1 (9.1) | NA |
| cN2 | 2 (18.2) | NA |
| M-stage | ||
| cMx | 1 (9.1) | NA |
| cM0 | 10 (90.9) | NA |
| Tumor marker | ||
| HCG increased | 5 (45.5) | NA |
| LDH increased | 3 (45.5) | NA |
| AFP increased | 0 (0) | NA |
| Clinical tumor stage | ||
| CSIA | 5 (45.5) | NA |
| CSIS | 3 (27.3) | NA |
| CSIIB | 1 (9.1) | NA |
| CSIIC | 2 (18.2) | NA |
| Variables | Carcinoma (n = 14) | Control (n = 5) |
| Age in years mean (range) | 32.58 (16) | 30.75 (18) |
| Pathological stage | ||
| pT1 | 5 (26.3) | NA |
| pT2 | 5 (26.3) | NA |
| pT3 | 3 (15.8) | NA |
| pTx | 1 (18.2) | NA |
| Lymphnode metastasis | ||
| Nx | 7 (36.8) | NA |
| N0 | 4 (21.1) | NA |
| N3 | 3 (15.8) | NA |
| M-stage | ||
| Mx | 4 (21.1) | NA |
| M0 | 9 (47.7) | NA |
| M1 | 1 (5.3) | NA |
| Tumor markers | ||
| HCG increased | 13 (68.8) | NA |
| LDH increased | 7 (36.8) | NA |
| AFP increased | 12 (85.7) | NA |
| Clinical stage | ||
| IS | 8 (42.1) | NA |
| IIA | 2 (10.5) | NA |
| IIC | 3 (15.8) | NA |
| NA | 1 (5.3) | NA |
| Variables | RCC (n = 188) | Normal/oncocytoma (n = 28) |
| Age in years mean (range) | 60.4 (0-85) | 56.86 (26-75) |
| Sex | ||
| Male | 128 (68.1) | 12 (42.9) |
| Female | 60 (31.9) | 16 (57.1) |
| Pathological stage | ||
| pT1 | 81 (37.5) | NA |
| pT2 | 40 (18.5) | NA |
| pT3 | 57 (26.4) | NA |
| pT4 | 3 (1.4) | NA |
| Missing | 7 (16.2) | NA |
| Lymphnode metastasis | ||
| pN0 | 96 (44.4) | NA |
| pN1 | 6 (2.8) | NA |
| pN2 | 9 (4.2) | NA |
| pNx | 72 (33.3) | NA |
| Missing | 5 (15.3) | NA |
| Metastasis | ||
| M0 | 114 (52.8) | NA |
| M1 | 24 (11.1) | NA |
| M2 | 1 (0.5) | NA |
| Mx | 42 (19.4) | NA |
| Missing | 7 (16.2) | NA |
| Grade | ||
| G1 | 56 (25.9) | NA |
| G2 | 111 (51.4) | NA |
| G3 | 12 (5.6) | NA |
| G4 | 2 (0.9) | NA |
| Missing | 7 (16.2) | NA |
| Variables | Carcinoma (n = 57) | Metastasis (n = 17) | Control (n = 26) |
| Age in years mean (range) | 63.32 (35-93) | 62.96 (11-87) | 64.15 (37-97) |
| Pathological stage | |||
| Cis | 4 (7) | NA | |
| pT1 | 26 (45.6) | NA | |
| pT2 | 15 (26.3) | NA | |
| pT3 | 11 (19.3) | NA | |
| pT4 | 1 (1.8) | NA | |
| Lymph node metastasis | |||
| cN0 | 50 (87.7) | NA | |
| pN+ | 7 (12.3) | NA | |
| M-stage | |||
| cM0 | 57 (100) | NA | |
| Grading | |||
| G1 | 9 (17) | NA | |
| G2 | 36 (67.9) | NA | |
| G3 | 8 (15.1) | NA | |
| Missing | 4 (7) | NA | |
| Surgical margins | |||
| R0 | 55 (79.7) | 10 (58.8) | |
| R1 | 2 (2.9) | 0 (0) | |
| R2 | 0 (0) | 2 (11.8) |
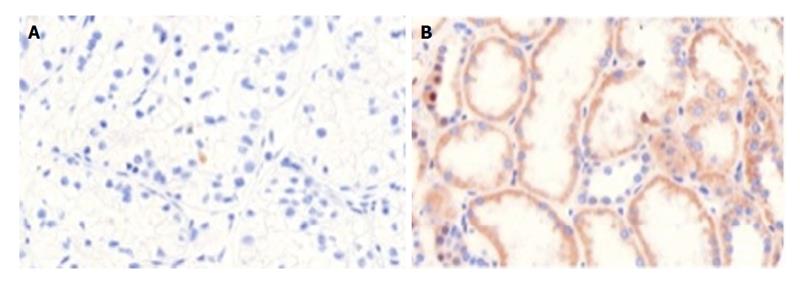
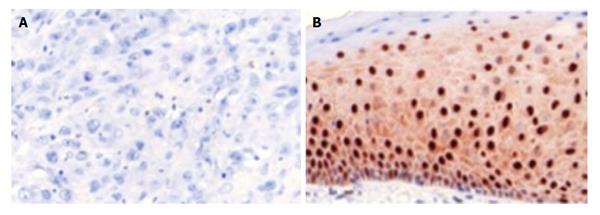
The construction of the tissue microarray was reported earlier[1]. Immunohistochemical staining was performed automatically (DAKO TechMate™ 500, Danmark) for Pdcd4 [1:400, Anti-Pdcd4 (rabbit) antibody, United States] following the manufacturer′s instructions as described in a previous work[1]. Negative and positive controls were run using rabbit igG-isotype in a concurrent manner. Inflammatory, stromal and normal cells expressed Pdcd4 and therefore served as internal positive control. The stained microarrays were archived with a pathology scanner (Pannoramic MIDI Scanner, 3D-HISTECH, Hungary) for subsequent analyses (Figures 1 and 2).
Pdcd4 expression was scored by one pathologist who was unaware of the patients’ clinical history. According to Mudduluru et al[11], we evaluated the core and cytoplasmatic immunostainings. Core Pdcd4 staining was classified in four groups according to the amount (in percent) of stain-positive nuclei (core quantity: score): none: 0; ≤ 30%: 1; 30%-70%: 2 and ≥ 70%: 3. Cytoplasmic and core stainings were matched by the intensity of the staining results (none, weak, intermediate or strong). The sum of these scores was assesed as well.
Clinicopathological parameters were correlated with Pdcd4 expression using the χ2-test. Statistical analyses were performed with SPSS, version 20 (IBM Corporation, United States). Statistical significance was concluded at P < 0.05.
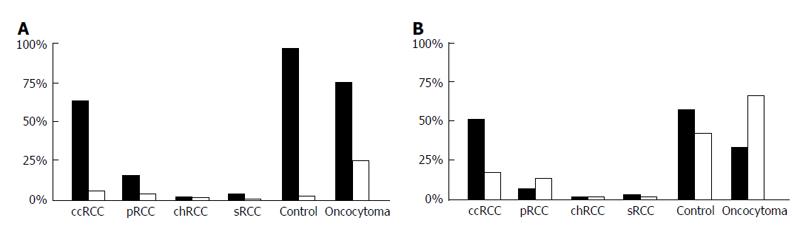
Cytoplasmic and core Pdcd4 levels were increased in normal renal tissue compared to RCC (P < 0.001). Pdcd4 expression was reduced in patients with locally advanced RCC. Furthermore, statistical analysis showed high significance levels in staining patterns between the renal tumor types (sRCC, pRCC, ccRCC and chRCC). Especially cytoplasmatic staining showed the strongest correlation levels (P < 0.0001). Further significant results showed in T-stage dependent stainings (P = 0.001) and tumor size (P = 0.011). Figure 3 presents tumor type dependent staining results.
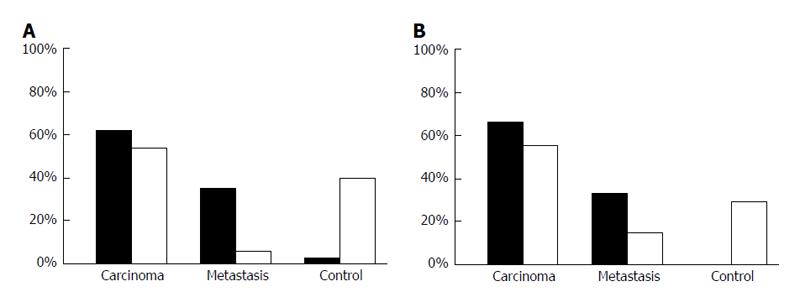
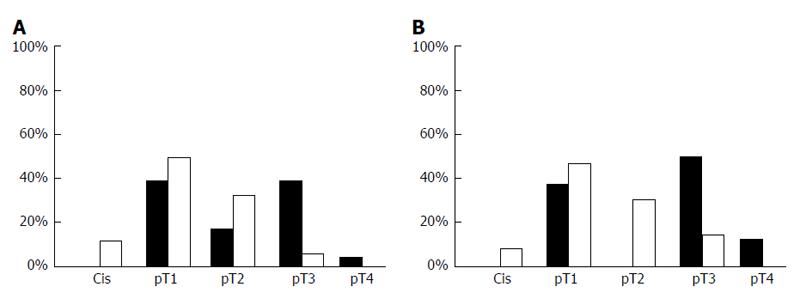
Both, core (P < 0.001) and cytoplasmic (P = 0.047) Pdcd4 levels were decreased in penile carcinoma tissue when compared to non-malignant penile skin (Figure 4). Furthermore, we observed lower Pdcd4 levels in locally advanced and less-differentiated penile carcinomas. A strong correlation in differentiating tumorous from healthy tissue was found. Especially core stainings showed the strongest significance (P < 0.0001) level in this group. T-Stage dependent stainings also correlate with core and cytoplasmatic Pdcd4 expression, insinuating a stage dependent expression and regulation of Pdcd4 (Figure 5). Other significant results were calculated when immunohistochemistry (IHC) results were compared to grading and tumor recurrence. There is no correlation with nodal status.
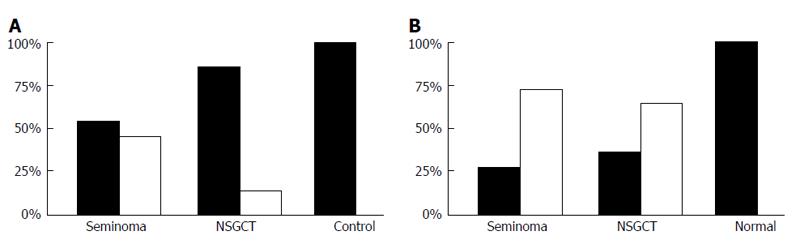
In seminoma and NSGCT, only weak correlation levels to clinicopathological parameters were calculated. Thus, Pdcd4 staining results correlate in differentiating healthy from malignant tissue in both entities (P = 0.007). Another notable significance is cytoplasmatic stainings to alpha-fetoprotein (AFP)-levels (P = 0.032) (Figure 6). This insinuates a potential for Pdcd4 as a diagnostic marker in this tumorous entity.
Our results show a decreasing Pdcd4 expression in urologic tumors (i.e., RCC and penile carcinoma). As shown in a previous study, Pdcd4 expression levels may have a tumor specific expression pattern[1].
Shiota et al[12] showed that Pdcd4 interacts with the DNA binding domain of Twist1, through inhibition of the DNA binding ability and Y-box binding protein-1 (YB-1) expression, reducing cell growth. The immunohistochemical stainings showed an inverse correlation between core Pdcd4 and YB-1 expression.
Shi et al[13] could show an effect of miR-21-expression on docetaxel resistance, insinuating a more complex role of Pdcd4 in tumor aggressivity and chemoresistance. Pdcd4 overexpression in the study of Shiota et al[12] showed a high sensitivity to chemotherapeutics (cisplatin and paclitaxel). Thus, the data displayed there (clinicopathological parameters and follow-up) and the data of our study is not extensive enough to correlate Pdcd4 expression levels and tumor chemosensitivity. A COX regression analysis could not confirm this hypothesis of Pdcd4 as a prognostic marker in survival or progress. The weak correlation levels are presumably due to the small cohort restraining the arithmetic power of this analysis. Because of these results and the cohort size, a multivariate analysis was redundant. Another possible explanation for these results are the weak responses of metastatic disease to further therapy in advanced tumor stages.
A recent study by Li et al[9] showed a T-stage dependent expression of Pdcd4. Thus, the significant correlation between Pdcd4 expression and survival could not be confirmed in our study. A possible explanation for this is our differentiation of RCC subtypes in our study design. Our analysis shows a clear difference in Pdcd4 expression patterns within the subgroups, this being probably due to their distinct tumor biology. IHC Pdcd4 expression was positive in the core and cytoplasmatic compartment in RCC samples: The levels changed according to tumor progression (T-stage dependent). This supports the hypothesis of tumor-specific expression patterns of Pdcd4 and a possible shuttle function between the cellular compartments. Thus, Li et al[9] described a solely core expression; the use of a different antibody for IHC could explain these diverging results. However, this differentiation is important regarding the good discriminative potential of our IHC for differing malignant RCC tissue from the benign renal tissue and oncocytoma. Prognostic relevance such as described by Li et al[9] could not be verified in our study.
A high sensitivity and specificity could be confirmed for penile carcinoma as well. These data are similar to results in bladder carcinoma samples obtained in a previous study and suggerate a similar regulation in these tumorous entities[1]. To assess these similarities and to evaluate the role of a shuttle function of the gene, we calculated different IHC-expression variables (core and cytoplasmatic intensity and quantity). A statistical evaluation (χ2-test) of these IHC expression patterns with established variables (T-stage, resection status and tumor grade) support the similarities between these epithelial tumors. Thus, a validating COX regression analysis could again not confirm this hypothesis of Pdcd4 as a prognostic marker in survival or progress.
In testicular samples, expression levels correlated significantly in the malignant/healthy group, in T-stage-dependent staining results and to AFP-levels. This insinuates a potential for Pdcd4 as a diagnostic marker. However, our cohort did not have a sufficient size to evaluate clinical outcome. Accordingly, a ROC analysis revealed only weak sensitivity and specificity and a survival analysis (COX) was not possible. Another problem in elucidating the role of Pdcd4 in NSGCT is the cellular inhomogeneity of the tumor cells within each probe (chorion-, embryonal-, cystic-carcinoma and yolk sack tumor).
Our study supports the important role for Pdcd4 downregulation in renal-, testicular- and penile carcinoma. Interestingly, Pdcd4 expression seem to be a potential diagnostic marker for renal or penile tumors.
Programmed cell death 4 (Pdcd4) is involved in the process of apoptosis. It was first described in 1995 by Shibahara et al. Its upregulation has an effect on transcription, translation and many signal transduction pathways. In many tumorous entities its expression levels are suppressed, so that it was stipulated that it has a tumor-suppressive function through the initiation of cellular apoptosis. Especially translation and transcription pathways seem to be affected by the active gene. This could not be verified in all tumorous entities, so that the authors assume a cancer-specific expression pattern. Wei et al (2009), Matsuhashi et al (2007) and Mudduluru et al (2009) compared expression levels in healthy and tumorous tissues of ovarian-, squamous- and colorectal-carcinoma. In these probes there was a “shift” from the nuclear-in healthy probes to the cytoplasmatic compartment in malignant tissue. These results could not be verified in all tumorous entities. Studies with urologic tumors have shown diverging results concerning the expression levels or were not published at all. In a previous work, the authors could show a very significant correlation between the tumor stage and expression patterns in transitional cell carcinoma.
Shi et al could show an effect of microRNA 21 (miR-21)-expression on docetaxel resistance, insinuating a more complex role of Pdcd4 in tumor aggressivity and chemoresistance. In this study, there was a negative correlation between low miR-21 expression levels with high docetaxel chemosensitivity and high Pdcd4 expression. The expression levels of Pdcd4 could be used to elaborate an individual therapeutic strategy and a prognostic evidence for each patient.
The studies of Woodard et al (2008) and Jansen et al (2004) demonstrated high Pdcd4 expression in clear cell renal cell carcinoma cell lines after having treated these with Fluvostatin and a higher geldamycin sensitivity in cells with high Pdcd4 expression levels. Fluvostatin being an inhibitory agent for pAkt and S6K1, inhibitors of Pdcd4. A recent study by Li et al (2012) showed a T-stage dependent expression of Pdcd4. This analysis shows a clear difference in Pdcd4 expression patterns within the subgroups, this being probably due to their distinct tumor biology. Immunohistochemistry (IHC) Pdcd4 expression was positive in the core and cytoplasmatic compartment in RCC samples: The levels changed according to tumor progression (T-stage dependent). This supports the hypothesis of tumor-specific expression patterns of Pdcd4 and a possible shuttle function between the cellular compartments.
This study supports the important role for Pdcd4 downregulation in renal-, testicular- and penile-carcinoma. Therapeutic and prognostic possibilities are discussed for other tumourous entities (i.e., squamous carcinoma, Dou et al 2014). Especially chemosensitivity and therapeutic options can be evaluated for patients showing high IHC Pdcd4 expression levels.
Pdcd4: Programmed cell death 4; NSGCT: Non-seminomatous germ cell tumor.
The manuscript by Fisher et al is to investigate the Pdcd4 expression in renal cell carcinoma, penile carcinoma, and testicular germ cell cancer. The authors found that the Pdcd4 is strongly expressed in core and cytoplasm, but core and cytoplasmic Pdcd4 levels is significantly decreased in the cancer tissues. These findings are important and may reveal a novel biomarker for renal and prostate cancers.
P- Reviewer: Brisinda G, Yang HS S- Editor: Song XX L- Editor: A E- Editor: Liu SQ
| 1. | Fischer N, Göke F, Splittstösser V, Lankat-Buttgereit B, Müller SC, Ellinger J. Expression of programmed cell death protein 4 (PDCD4) and miR-21 in urothelial carcinoma. Biochem Biophys Res Commun. 2012;417:29-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 2. | Ma G, Zhang H, Dong M, Zheng X, Ozaki I, Matsuhashi S, Guo K. Downregulation of programmed cell death 4 (PDCD4) in tumorigenesis and progression of human digestive tract cancers. Tumour Biol. 2013;34:3879-3885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 3. | Yang HS, Jansen AP, Komar AA, Zheng X, Merrick WC, Costes S, Lockett SJ, Sonenberg N, Colburn NH. The transformation suppressor Pdcd4 is a novel eukaryotic translation initiation factor 4A binding protein that inhibits translation. Mol Cell Biol. 2003;23:26-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 401] [Cited by in RCA: 412] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 4. | Yang HS, Jansen AP, Nair R, Shibahara K, Verma AK, Cmarik JL, Colburn NH. A novel transformation suppressor, Pdcd4, inhibits AP-1 transactivation but not NF-kappaB or ODC transactivation. Oncogene. 2001;20:669-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 157] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 5. | Asangani IA, Rasheed SA, Nikolova DA, Leupold JH, Colburn NH, Post S, Allgayer H. MicroRNA-21 (miR-21) post-transcriptionally downregulates tumor suppressor Pdcd4 and stimulates invasion, intravasation and metastasis in colorectal cancer. Oncogene. 2008;27:2128-2136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 6. | Lankat-Buttgereit B, Gregel C, Knolle A, Hasilik A, Arnold R, Göke R. Pdcd4 inhibits growth of tumor cells by suppression of carbonic anhydrase type II. Mol Cell Endocrinol. 2004;214:149-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 62] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 7. | Leupold JH, Yang HS, Colburn NH, Asangani I, Post S, Allgayer H. Tumor suppressor Pdcd4 inhibits invasion/intravasation and regulates urokinase receptor (u-PAR) gene expression via Sp-transcription factors. Oncogene. 2007;26:4550-4562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 132] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 8. | Nieves-Alicea R, Colburn NH, Simeone AM, Tari AM. Programmed cell death 4 inhibits breast cancer cell invasion by increasing tissue inhibitor of metalloproteinases-2 expression. Breast Cancer Res Treat. 2009;114:203-209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 65] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 9. | Li X, Xin S, Yang D, Li X, He Z, Che X, Wang J, Chen F, Wang X, Song X. Down-regulation of PDCD4 expression is an independent predictor of poor prognosis in human renal cell carcinoma patients. J Cancer Res Clin Oncol. 2012;138:529-535. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 10. | Schneider AC, Heukamp LC, Rogenhofer S, Fechner G, Bastian PJ, von Ruecker A, Müller SC, Ellinger J. Global histone H4K20 trimethylation predicts cancer-specific survival in patients with muscle-invasive bladder cancer. BJU Int. 2011;108:E290-E296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 58] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 11. | Mudduluru G, Medved F, Grobholz R, Jost C, Gruber A, Leupold JH, Post S, Jansen A, Colburn NH, Allgayer H. Loss of programmed cell death 4 expression marks adenoma-carcinoma transition, correlates inversely with phosphorylated protein kinase B, and is an independent prognostic factor in resected colorectal cancer. Cancer. 2007;110:1697-1707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 167] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 12. | Shiota M, Izumi H, Tanimoto A, Takahashi M, Miyamoto N, Kashiwagi E, Kidani A, Hirano G, Masubuchi D, Fukunaka Y. Programmed cell death protein 4 down-regulates Y-box binding protein-1 expression via a direct interaction with Twist1 to suppress cancer cell growth. Cancer Res. 2009;69:3148-3156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 108] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 13. | Shi GH, Ye DW, Yao XD, Zhang SL, Dai B, Zhang HL, Shen YJ, Zhu Y, Zhu YP, Xiao WJ. Involvement of microRNA-21 in mediating chemo-resistance to docetaxel in androgen-independent prostate cancer PC3 cells. Acta Pharmacol Sin. 2010;31:867-873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 122] [Article Influence: 8.1] [Reference Citation Analysis (0)] |