Published online Nov 24, 2014. doi: 10.5410/wjcu.v3.i3.325
Revised: August 7, 2014
Accepted: September 4, 2014
Published online: November 24, 2014
Processing time: 173 Days and 20.9 Hours
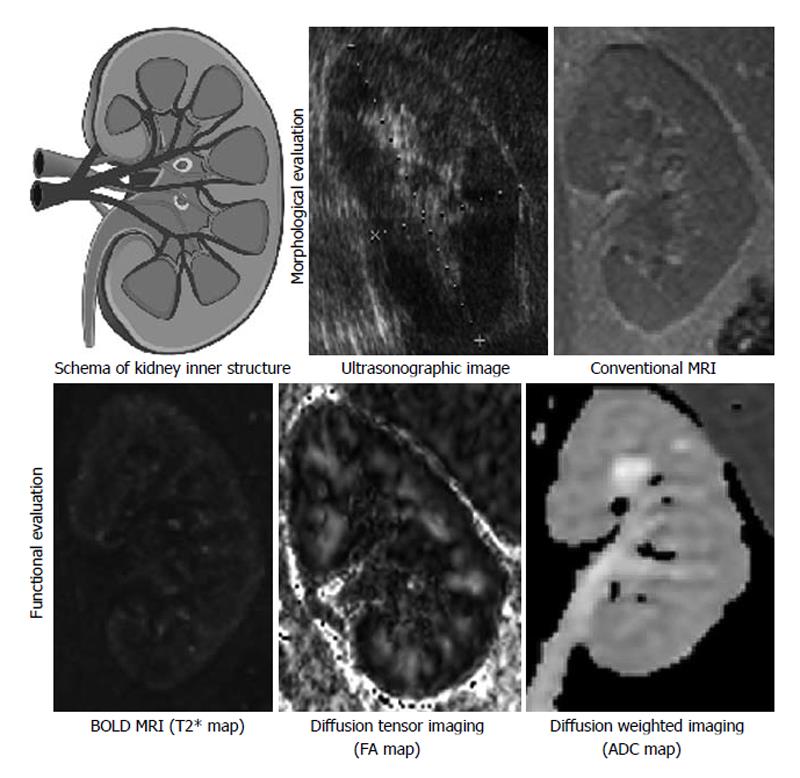
X-ray computed tomography (CT), ultrasonography (US) and radionuclide scanning are important clinical methods for evaluating morphology of the kidney. These modalities are also applicable for estimating kidney function with time lapse analysis using proper contrast-media as may be necessary. In the case of US, it can estimate kidney function based on the measurement of blood flow using the Doppler effect. Formerly, magnetic resonance imaging (MRI) was an inappropriate diagnostic imaging technique for abdominal organs because of their respiratory displacements. However, MRI is now actively used for kidney as well as liver or other parenchymal organs, in tandem with the technological advances. Unlike unenhanced X-ray CT, “conventional” MRI can distinguish the border between cortex and medulla in T1 or T2 weighted images. It was known that the border blurred with decreasing kidney function. Moreover, several other particular imaging methods were introduced in recent years, and these could be called “functional” MRI. In this review, the following are discussed: functional MRI for chronic kidney disease, which include blood oxygenation level-dependent MRI for evaluation of hypoxia, diffusion-weighted imaging for evaluation of fibrosis, diffusion tensor imaging for evaluation of microstructure, and arterial spin labeling to evaluate the amount of organ perfusion, accompanied with several related articles. The ultimate goal of functional MRI is to provide useful in vivo information repeatedly for daily medical treatment non-invasively.
Core tip: Recent advances in diagnostic imaging technology allow us to evaluate the function of parenchymal organs such as kidney and liver. For example in the kidney, magnetic resonance imaging technique can visualize changes in the ratio of oxy-/deoxy-hemoglobin, the accumulation of extra cellular matrix, the alteration of microstructure of tubules and blood capillaries in the interstitium, and the amount of organ perfusion without using any contrast medium. Thus, with imaging technique, the nephrologist can evaluate a kidney from multiple points of view. This is an emerging modality, and it’s hoped-for mission is providing significant information for daily clinical decisions. Accumulation of further knowledge is keenly anticipated.
- Citation: Inoue T, Kozawa E, Okada H, Suzuki H. Morphological and functional evaluation of chronic kidney disease using magnetic resonance imaging. World J Clin Urol 2014; 3(3): 325-329
- URL: https://www.wjgnet.com/2219-2816/full/v3/i3/325.htm
- DOI: https://dx.doi.org/10.5410/wjcu.v3.i3.325
Magnetic resonance imaging (MRI) until now has had an important role as a diagnostic imaging method for urology and nephrology. Gadolinium contrast medium was used frequently for examinations in the past. However, since nephrogenic systemic fibrosis was identified, the frequency in use of the contrast media in renal disease seems to have decreased. However, significance of the role of MRI is increasing. MRI, which is superior in contrast resolution of soft tissues to X-ray computed tomography (CT) and ultrasonography, provides important information for internal architecture of the kidney and adrenal gland, or qualitative diagnosis of tumor lesions (e.g., presence of fat composition) particularly in cases of kidney dysfunction for which it is difficult to use iodine-based contrast medium. Furthermore, magnetic resonance angiography is useful for evaluation of renal artery lesions similar to ultrasonographic examinations. Even for similar tomography, MRI, unlike X-ray computed tomography, enables the evaluation of the inner structure such as the corticomedullary border in the kidney without using contrast media. Changes related to renal functions have been known to occur, such as the disappearance of the corticomedullary border as renal dysfunction worsens. Moreover, what is frequently used for clinical MRI examinations presently is nuclear magnetic resonance of the hydrogen (proton) atomic nuclei abundantly contained in human bodies as moisture, which enables various evaluations by devising imaging methods as one of the features of MRI.
Development of origin of functional MRI (fMRI) originally started as a noninvasive imaging method for brain functions. The phenomenon that is frequently targeted in fMRI is blood oxygenation level-dependent (BOLD) effects, indicating that difference in susceptibility of oxidized hemoglobin and reduced hemoglobin exerts a particular influence on the T2* relaxation time. In an activating site of the brain, the blood flow that surpasses it increases with an increase of the oxygen consumption, the reduced hemoglobin which is paramagnetic material decreases, and prolongation of the T2* relaxation time occurs. In other words, it is a method to grasp an activating site of a brain not by electrical activity of cerebral nerve cells but by changes in accompanying hemodynamics[1]. Brain MRI is captured continually and an activating site is mapped in comparison with the resting state, while having the subject repeat specific tasks such as visual/auditory stimulation and finger motion.
Renal functions are evaluated in tests related to the renal blood flow such as the glomerular filtration rate and renal plasma flow (RPF), which is still a gold standard. However, in imaging studies, in addition to X-ray CT with contrast medium and MRI, quantitative blood flow evaluation is realized by using carbon dioxide contrast medium and ultrasonography. It has already been shown that the renal blood flow measured by these imaging studies correlate well with estimated glomerular filtration rate (eGFR) with serum creatinine, and it can be regarded as functional image evaluation of kidney. The decrease of renal blood flow in chronic kidney disease (CKD) occurs not only in large or medium vascular diseases such as renal-artery stenosis but also accompanies degeneration of the kidney as in glomerulosclerosis and tubular atrophy/interstitial fibrosis. A number of past studies have revealed that a decrease in the renal blood flow causes hypoxia, which causes further inflammation and fibrosis and lowers the renal blood flow, with ensuing CKD[2]. From the above, it was decided that MRI for evaluating the clinical condition of CKD including blood flow and partial pressure of oxygen, as well as fibrosis of the kidney is called fMRI for kidney. It is distinguished from the conventional MRI, which focuses on forms/morphology. The renal fMRI, which is expected to be applied to future clinical practice, is described below (Figure 1).
The first report was from Prasad et al[3] in 1996. His group evaluated kidneys based on T2* weighted images and revealed that when energy consumption of the renal tubule is reduced with administration of furosemide and a large quantity of drinking water, T2* relaxation rate: R2* (= 1/T2*) in the medulla decreases. They concluded that the change was reflected by a decrease in reduced hemoglobin, capturing improved oxygenation in the site. Further, partial pressure of oxygen was higher in the cortex than in the medulla even before the drug administration and drinking water load and its change was still poor thereafter. Based on the past results, it was expected with more blood flow per unit weight in the cortex than in the medulla and the high partial pressure of oxygen in the tissues that much energy is consumed by the medulla for urine concentration. Then the difference in the partial pressure of oxygen in renal parenchyma and the physiological function of each tubular segment was reconfirmed by fMRI, which is a totally different method. In 2005, the relationship between the microelectrode method, which is a direct oxygen partial pressure evaluation method, and R2* values of BOLD MRI was studied using porcine kidneys. Changing inspired oxygen pressure using a respirator continually, the authors measured pO2 by the microelectrode method and R2* values of the MRI for both medulla and cortex of kidney and a significant correlation between them was found[4]. As described above, the utility of MRI has been recognized since it is an experimental method that can examine an acute and transient oxygen pressure change in a noninvasive manner before and after medication in place of the microelectrode method. In 2006, Thoeny et al[5] studied 15 cases of renal-transplant recipients and healthy subjects after drinking sufficient water. They reported a significant correlation of the serum creatinine level and R2* value of MRI, with the apparent diffusion coefficient (ADC) of diffusion weighted images described below[5]. It was reported for the first time that R2* values enable evaluation not only of the relative change in transient oxygenation but also oxygenation (or hypoxia) when renal function and hemodynamics are stable. It was also an important report in indicating the possibility of MRI as an evaluation method for chronic hypoxia in CKD.
The diffusion weighted image (DWI) is one that visualizes the difference in random movements of water molecules in tissues (Brownian motion). It is thought that perfusion in capillaries and diffusion in the target tissues are more purely evaluated for small b values (s/mm2) and great b values, respectively. Further, ADC values calculated with plural diffusion weighted images from different b values can be quantitatively evaluated without being affected by T2-weighted images. Namimoto et al[6] measured ADC values of cortex and medulla of 16 kidneys from 8 CKD cases in which renal function had deteriorated, and 8 kidneys from 16 cases of subjects with normal renal function. They showed ADC was significantly decreased in both cortex and medulla in CKD[6]. For b values, Thoeny et al[5] studied ADC for b = 0.50, 100 s/mm2 (low); that for b = 500, 750, 1000 s/mm2 (high); and separately for the average (avg) ADC. They found all ADC (low), ADC (high) and ADC (avg) decreased in both cortex and medulla of CKD patients as compared with those of normal kidneys from healthy volunteers although there were only 18 healthy volunteer cases and 15 CKD cases[7]. In healthy kidneys, both ADC (high) and ADC (avg) were always lower in the medulla than in the cortex whereas a difference was not seen in ADC (low) between cortex and medulla. Possibly this is because the regularly-structured renal tubules and blood capillaries, which are a characteristic of medulla, are generated by the diffusion limit of water molecules. In 2007, for 110 kidneys from 55 cases, the relation of ADC values (b = 0 and 500 s/mm2) was studied with 99mTc-diethylenetriaminepenta-acetic acid renal dynamic scintigrams and their positive correlations were found[8]. It has been known for a long time that the remaining renal function of CKD correlates well with degrees of tubular degeneration and fibrosis of interstitial tissue. It has been inferred that ADC values are an index of fibrosis from studies on imaging principle and cirrhosis; in 2010, Togao et al[9] reported that ADC values decreased renal parenchyma on the ligation side and it is the change corresponding to fibrosis that was histologically demonstrated, using the unilateral ureteric ligation model, which is a model of postrenal failure[9].
Originally, diffusion of water molecules is random three-dimensionally, and does not have specific directionality though diffusion that has “directionality” when there is structure disturbing the diffusion occurs. This is called “anisotropic diffusion” and diffusion tensor imaging (DTI) is the method to image this anisotropic diffusion as well as evaluating it quantitatively. The myelin sheath of nerve fibers is an obstacle to the diffusion of water molecules in the brain and spinal cord and therefore courses of the nerve fibers can be visualized in DTI. Fractional anisotropy (FA) is an index that reflects diffusive anisotropy. This is an index that shows how the diffusion deviates from isotropic diffusion (fully random diffusion without diffusion restrictions), and it gets close to 0 in the case of isotropic diffusion and to 1 when the anisotropic diffusion is large. Moreover, in tractography the direction of anisotropy is tracked linearly and displayed three-dimensionally, and in particular an image of a brain seems to imitate the course of nerve fibers[10]. Since DWI has begun to be applied to kidneys, anisotropic diffusion is likely to occur in the renal structure, which has regularity in renal tubules and vascular courses. As a result of studying FA values of kidneys for 10 healthy subject cases, FA values of medullas that had regular radial structures were higher than those of cortex, indicating that DTI enables evaluation of renal microstructure. In a study on kidney transplantation, a significant positive correlation was recognized in FA values and eGFR, and it is presumed that change in the inner structure accompanied by a decrease of the renal function was captured[11]. The past studies reported a significant correlation between the renal function and FA values of medulla, indicating mainly that they reflect the degrees of disorder of the tubular structure[12].
The preceding material described methods of renal fMRI that have been most widely reported. Hypoxia and fibrosis, two major factors that are related to the development of CKD, can be evaluated. If it can be applied to clinical practice as in a periodic examination, clinical conditions of CKD at the time of the primary diagnosis can be understood and can capture the changes in the kidneys before alterations are detected in the serum creatinine levels and proteinuria. In addition, it is useful for measurement of effect after treatment intervention, and can contribute to efficient innovative drug development according to the authors. Indeed, reports on its usability as a laboratory study have been increasing. The author measured T2* values of the BOLD MRI and ADC values of DWI for CKD of 43 diabetic nephropathy cases and 76 nondiabetic nephropathy cases, (approximately 120 cases in total) and examined their correlations with eGFR and renal major axes[13]. The results revealed that T2* values and ADC values had significant correlation with eGFR and renal major axes in nondiabetic nephropathy and that major axes of kidneys with decreasing GFR were shortened (= atrophy), and fibrosis developed in such kidneys and were hypoxic. Since renal biopsy was performed for 37 cases, deposition of collagen fibers was measured by Masson trichrome stain as evaluation of interstitial fibrosis. In the kidneys in which interstitial fibrosis had developed, the ADC value significantly decreased, which was the result expected by imaging principle and animal experiment. The degrees of atrophy of the kidneys and ADC values were correlated even for diabetic nephropathy and “fibrosis kidney = kidney atrophic” though hypoxic degrees were independent from fibrosis and eGFR. The cases indicating high hypoxia without noteworthy fibrosis nor decrease of eGFR were remarkable, because future aggravation of renal function is expected. In addition, the hypoxia is likely occurring by a reversible cause and therefore it is amenable for stricter intervention by nephrologists. Recently, results of tracking recipients from 9 renal transplant cases for 3 years have been reported[14]. ADC values and the R2* values of the transplanted kidney by cortex and medulla were compared soon after the transplantation (7 ± 3 mo) and approximately three years later (32 ± 2 mo). In the eight cases in which renal function was stable for three years, other measured values except the R2* values of cortex were almost similar and no difference was noted. On the other hand, in the one case in which renal function decreased, increase in R2* values and decrease in ADC values were recognized, suggesting aggravation of hypoxia and development of fibrosis. These results showed reproducibility that can be evaluated longitudinally for at least DWI and BOLD MRI. In addition, the interesting point is that change in measured values accompanied by the degenerating renal function was captured in the same case. Further, even when renal function is stable on the blood test, significant change in the R2* values suggest progress of hypoxia in cortex is recognized, and it is particularly worth noting that it reminds us of its relation with the immunosuppressive drug used frequently after transplantation, particularly calcineurin inhibitor.
There is an imaging method called arterial spin labeling (ASL), which is a method to evaluate brain perfusion. This is a method by which the spin in blood flowing into the target organ is labeled by magnetizing with radio frequency to evaluate the amount of organ perfusion with the blood itself as an endogenous tracer. A perfusion image is obtained from the difference of the images before and after the labeling. If however, the signal noise ratio (S/N) is low, it is necessary to image repeatedly and integrate them and therefore the imaging time is increased. Instruments having a magnetostatic field of 3 teslas have been introduced for clinical use. The improvement of images and shortening of imaging time are being attempted and therefore their clinical application is approaching feasibility. There still are a few reports that examined the amount of kidney perfusion by similar methods. The relationship of kidney perfusion with clearance of para-aminohippuric acid that is RPF was studied in 2010, and a significant correlation was shown with amounts of perfusion per unit weight measured by ASL[15]. In addition, reports on kidney transplantation and reports that clarified a decrease in amount of perfusion in a CKD case in comparison with healthy subjects have come to be recognized[16]. MRI images not only protons but also other atoms. In evaluation of kidneys by Sodium-23 (23Na) MRI, the behavior of Na concentration increasing progressively toward the depths of the medulla from a cortex surface layer can be obtained as an image. MRI also observed that a Na concentration gradient gradually disappears when renal ischemia is caused experimentally. It is probably the change that reflects tubular impairment due to ischemia[17].
Most of the research reports have been published within the past ten years and renal fMRI is now in the dawn of its emergence. Correlation with eGFR and RPF, which are classic indices of the renal function, is discussed in most papers. However, in kidneys for which positional information is not as important as in the brain (though there is difference between right and left), it is meaningless to introduce a large-scale device and re-evaluate the indices that are not needed because serum creatinine is sufficient for evaluation. The ultimate goal of the fMRI study was to provide useful information for daily medical treatment such as, for example, a renal biopsy diagnosis by evaluating kidneys non-invasively over time. Accumulation of further knowledge is eagerly anticipated.
P- Reviewer: Tian YC S- Editor: Ji FF L- Editor: A E- Editor: Liu SQ
| 1. | Ogawa S. Finding the BOLD effect in brain images. Neuroimage. 2012;62:608-609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 2. | Mimura I, Nangaku M. The suffocating kidney: tubulointerstitial hypoxia in end-stage renal disease. Nat Rev Nephrol. 2010;6:667-678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 239] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 3. | Prasad PV, Edelman RR, Epstein FH. Noninvasive evaluation of intrarenal oxygenation with BOLD MRI. Circulation. 1996;94:3271-3275. [PubMed] |
| 4. | Pedersen M, Dissing TH, Mørkenborg J, Stødkilde-Jørgensen H, Hansen LH, Pedersen LB, Grenier N, Frøkiaer J. Validation of quantitative BOLD MRI measurements in kidney: application to unilateral ureteral obstruction. Kidney Int. 2005;67:2305-2312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 135] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 5. | Thoeny HC, Zumstein D, Simon-Zoula S, Eisenberger U, De Keyzer F, Hofmann L, Vock P, Boesch C, Frey FJ, Vermathen P. Functional evaluation of transplanted kidneys with diffusion-weighted and BOLD MR imaging: initial experience. Radiology. 2006;241:812-821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 212] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 6. | Namimoto T, Yamashita Y, Mitsuzaki K, Nakayama Y, Tang Y, Takahashi M. Measurement of the apparent diffusion coefficient in diffuse renal disease by diffusion-weighted echo-planar MR imaging. J Magn Reson Imaging. 1999;9:832-837. [PubMed] |
| 7. | Thoeny HC, De Keyzer F, Oyen RH, Peeters RR. Diffusion-weighted MR imaging of kidneys in healthy volunteers and patients with parenchymal diseases: initial experience. Radiology. 2005;235:911-917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 321] [Cited by in RCA: 308] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 8. | Xu Y, Wang X, Jiang X. Relationship between the renal apparent diffusion coefficient and glomerular filtration rate: preliminary experience. J Magn Reson Imaging. 2007;26:678-681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 81] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 9. | Togao O, Doi S, Kuro-o M, Masaki T, Yorioka N, Takahashi M. Assessment of renal fibrosis with diffusion-weighted MR imaging: study with murine model of unilateral ureteral obstruction. Radiology. 2010;255:772-780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 140] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 10. | Mori S, Zhang J. Principles of diffusion tensor imaging and its applications to basic neuroscience research. Neuron. 2006;51:527-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1186] [Cited by in RCA: 1193] [Article Influence: 62.8] [Reference Citation Analysis (0)] |
| 11. | Hueper K, Gutberlet M, Rodt T, Gwinner W, Lehner F, Wacker F, Galanski M, Hartung D. Diffusion tensor imaging and tractography for assessment of renal allograft dysfunction-initial results. Eur Radiol. 2011;21:2427-2433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 78] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 12. | Gaudiano C, Clementi V, Busato F, Corcioni B, Orrei MG, Ferramosca E, Fabbri E, Berardi P, Santoro A, Golfieri R. Diffusion tensor imaging and tractography of the kidneys: assessment of chronic parenchymal diseases. Eur Radiol. 2013;23:1678-1685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 75] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 13. | Inoue T, Kozawa E, Okada H, Inukai K, Watanabe S, Kikuta T, Watanabe Y, Takenaka T, Katayama S, Tanaka J. Noninvasive evaluation of kidney hypoxia and fibrosis using magnetic resonance imaging. J Am Soc Nephrol. 2011;22:1429-1434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 293] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 14. | Vermathen P, Binser T, Boesch C, Eisenberger U, Thoeny HC. Three-year follow-up of human transplanted kidneys by diffusion-weighted MRI and blood oxygenation level-dependent imaging. J Magn Reson Imaging. 2012;35:1133-1138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 15. | Ritt M, Janka R, Schneider MP, Martirosian P, Hornegger J, Bautz W, Uder M, Schmieder RE. Measurement of kidney perfusion by magnetic resonance imaging: comparison of MRI with arterial spin labeling to para-aminohippuric acid plasma clearance in male subjects with metabolic syndrome. Nephrol Dial Transplant. 2010;25:1126-1133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 66] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 16. | Artz NS, Sadowski EA, Wentland AL, Grist TM, Seo S, Djamali A, Fain SB. Arterial spin labeling MRI for assessment of perfusion in native and transplanted kidneys. Magn Reson Imaging. 2011;29:74-82. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 72] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 17. | Atthe BK, Babsky AM, Hopewell PN, Phillips CL, Molitoris BA, Bansal N. Early monitoring of acute tubular necrosis in the rat kidney by 23Na-MRI. Am J Physiol Renal Physiol. 2009;297:F1288-F1298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 1.5] [Reference Citation Analysis (0)] |