INTRODUCTION
Stress urinary incontinence (SUI) is a common disorder that affects a large number of women and their quality of life. According to the National Center for Health Statistics 16% of adult United States women suffer from moderate to severe urinary incontinence, which has a significant socioeconomic impact with an estimated treatment cost of up to $16 billion annually in the United States[1-4].
The pathophysiology of SUI is multifactorial and poorly understood. It appears to be due to the dysfunction of the sphincteric mechanism of the urethra, changes in connective tissues, poor blood perfusion in the periurethral and submucosal areas, and neuronal dysfunction[5,6].
The aim of SUI therapy is to restore the existing urethral function via physical therapy, biofeedback, pelvic floor rehabilitation, pharmacological therapy, bulking agents and surgical approaches. Currently, the gold standard treatment for the management of SUI is the tension free vaginal sling, which provides structural support to the female urethra through a minimally invasive surgical procedure. However, even minimally invasive surgical procedure carries risks for the patients, lost efficacy over the time and has long-term complications such as voiding dysfunction and “de novo urgency”. For this reason, new therapeutic modalities are needed[7-9].
Cell therapy has been emerged as an alternative to be used on the treatment of different diseases. Despite being described for more than 40 years in hematopoietic system regeneration, their therapeutic potential has been started only in 1999, when the cover of Science, one of the most respected scientific journal, announced “Breakthrough of the year: stem cells show their potential”[10].
The use of stem cells as a therapeutic option for SUI is an attractive alternative because, theoretically, injected cells could restore functional muscle cells and improve the urethral sphincter activity in women with sphincter-associated incontinence. Cell types considered for this therapeutic purpose include bone marrow stem cells (BMSC), adult mesenchymal stem cells (MSC), human cord blood stem cells, human amniotic stem cells (HASC), induced pluripotent stem cells (iPSC)[11]. This study aims to review the current literature regarding evidences for using stem cell therapy on stress urinary incontinence in women.
STEM CELLS AND ITS POTENTIAL FOR DIFFERENTIATION
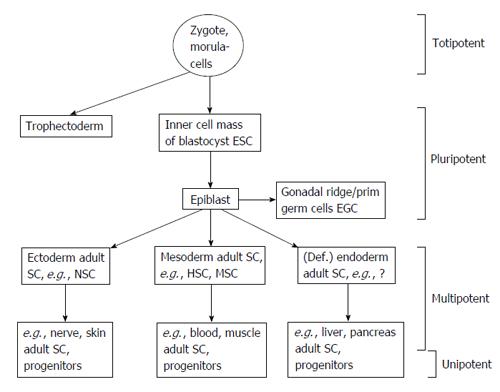
Stem cells are undifferentiated cells that are defined by their abilities of self-renewal and differentiation capacity[12,13]. There is a lack of defined morphologic and molecular characteristics of stem cells. Therefore, they are classified according to their potency. The hierarchic order for stem cells ranges from totipotency to pluri and multipotency to unipotency[14]. Totipotent cells are capable of forming cells of the ectoderm, mesoderm, endoderm, and trophoblast. Pluripotent cells can give rise to cells of the three germ layers, but not extra-embryonic tissues. Multipotent cells can be isolated from the developing germ layers or descended adult organs and are capable of self-renewal and differentiate into multiple cell types. Ultimately, the unipotent cells can differentiate into only one cell type[15-18] (Figure 1).
Figure 1 Stem cells and its potential for differentiation.
Adapted from Ref. [12]. ESC: Embryonic stem cells; EGC: Embryonic germ cells; SC: Stem cells; HSC: Hematopoietic stem cells; MSC: Mesenchymal stem cells.
EXPERIMENTAL ANIMAL MODEL: AN ESSENTIAL STEP TO DEMONSTRATE THE EFFECTIVENESS OF CELL-BASED THERAPY FOR STRESS URINARY INCONTINENCE
The development of a reliable experimental animal model is the first step to demonstrate the effectiveness of cell-based therapy for stress urinary incontinence. However, once the physiopathology of SUI has not been well established a reliable animal model for urinary incontinence remains a big challenge[4,11].
Mice, rats, rabbits, pigs, and more recently monkeys have been used in experimental studies. There are different ways to induce urinary incontinence in animals such as vaginal balloon dilation, urethrolysis, urethrolysis and cardiotoxin injection, electrocauterization of pudendal branches, and bilateral pudendal nerve transection. Despite some studies have shown promising outcomes, the main question that should be addressed is: Any of these current methods reproduce the pathophysiology stress urinary incontinence in women[19-25]?
Badra et al[4] published the first study performed in monkeys demonstrating a long-term animal model of stable urethral deficiency. They emphasize that nonhuman primates have a natural menarche, a 28-d menstrual cycle, natural menopause as well as age associated health risks that closely mirror those of women.
The objectives of creating a reliable animal model are to avoid methodological bias and outcomes misinterpretation. In the literature, most of the studies, which demonstrate promising outcomes have a short follow up and were performed in small young animals. Therefore, the translation of this therapy for humans needs further and more detailed investigation[9,11].
STEM CELLS AND STRESS URINARY INCONTINENCE
There are two different mechanisms of actions for cell therapy. First, the cells can differentiate, integrate into the site of injury and then, replace the damaged tissue. Second, cells can release cytokines, chemokines, and growth factors, which act in paracrine or endocrine manner. Ideally, autologous cells are always recommended to decrease the risk of immune response and rejection[26].
Embryonic stem cells
Embryonic stem cells (ESC) are pluripotent cells derived from blastocysts. These cells propagate readily and remain undifferentiated when cultured with leukemia inhibitory factor (LIF). When LIF is withdrawn, ESCs form aggregates called embryoid bodies that generate a variety of specialized cell types. However, the extraction of these cells involves the destruction of embryos, therefore their use is associated with controversial ethical dilemmas. Despite the self-renewing potential and the capability of differentiation into tissues derived from the three germ layers, embryonic stem cells are associated with uncontrolled growth and teratoma formation. For this reason, embryonic stem cells have never been tested for stress urinary incontinence[27].
Adult stem cells
The regenerative capacity of a tissue is determined in part by whether it contains endogenous stem cells. These stem cells remain quiescent in the niche for long periods until they are activated by the requirement of new cells to maintain the tissue or because of the tissue damage. Despite the limited potential of differentiation and self-renewal, adult stem cells are an attractive source of cells for urinary sphincter regeneration. Most of the studies regarding cell-based therapy and SUI were performed using adult stem cells derived from adipose tissue, skeletal muscle, bone marrow, human cord blood, and amniotic fluid[27].
Bone marrow-derived stem cells: The bone marrow contains two major populations of stem cells: hematopoietic stem cells (HSCs) and MSC, which provide stromal support for HSCs. It has been demonstrated that bone marrow derived stem cells have in vitro myogenic differentiation potential, and when placed in culture they expressed myosin heavy chain and desmin[8,9,12].
There is a lack of studies correlating SUI and BMSC. Kinebuchi et al[28] transplanted BMSC into rat’s injured urethral sphincter. After 13 wk, cell-treated rats did not have improvements on leak point pressure whether compared to controls. On the other hand, Kim et al[29] demonstrated that the periurethral injection of BMSC increased the leak point pressure and urethral closure pressure at one-month follow up.
The outcomes regarding bone marrow-derived stem cells and SUI are controversial. Some studies have shown histological and functional recovery, whereas other studies have shown no improvements. These conflicting results may be related to different methodologies, animal models, route of administration, and amount of injected cells. The current literature does not have strong evidences that support the use of BMSC for SUI.
Adipose derived stem cells: The adipose tissue is derived from mesoderm, is easily accessible and overwhelmingly abundant. It has been demonstrated that under specific conditions the adipose tissue has stem cells, which can differentiate into myogenic, adipogenic, osteogenic, chondrogenic, and neurogenic tissue[30].
Shi et al[31] injected a tissue engineering bulking agent composed of adipose derived stem cells (ADSC) and silk fibrin microspheres in 4 different points around the urethra of rats with urinary incontinence. In this study, urinary incontinence was established by bilateral pudendal nerve transection and confirmed by decreasing leak-point pressure (LPP). Injection of silk fibroin microspheres without cells could recover LPP and lumen area at 4 wk but its efficacy disappears at 8 and 12 wk follow up. The injection of microspheres with ADSC brought long-term efficacy at 8 and 12 wk post-injection and improved the urethral sphincter regeneration[31].
Li et al[32] developed a model of postpartum urinary incontinence in rats through the placement of vaginal balloon dilator for four hours followed by bilateral ovariectomy. They labeled isolated ADSC’s from the peri-ovarian fat with thymidine analog 5-ethynyl-2-deoxyuridine. After four weeks leak-point pressure and bladder capacity were significantly higher in ADSC-treated rats compared to the balloon-injured ovariectomized rats. Histological analysis of ADSCs treated group demonstrated a higher density of peri-urethral blood vessel and the preservation of the urethral fibromuscular structure[32].
Jack et al[30] isolated pluripotent adipose derived stem cells from human lipoaspirate. These cells were stained with Vybrant Red and injected around the urethra of athymic rats. Eight weeks later, injected cells remained viable and randomly distributed in the local submucosa and lamina propria[30].
Muscle derived stem cells
There are different cell populations that can be isolated form skeletal muscle biopsies. These cells include myoblasts, satellite cells, muscle progenitor cells, and muscle derived stem cells (MDSC). As well as adipose derived stem cells, it has been demonstrated that adult stem cells isolated from skeletal muscle also have multilineage differentiation capacity[33].
Xu et al[34] isolated MDSCs from the hind gastrocnemius of 4-wk-old Wistar rats and infected them with lentivirus encoding green fluorescent protein. In this study, urinary incontinence was established by bilateral pudendal nerve transection and confirmed by LPP decrease. MDSCs were injected at proximal urethra and the animals were sacrificed at four weeks. MDSC-treated rats had higher leak-point pressure and sphincter muscle thickness than controls[34].
Badra et al[4] developed a model of urinary sphincter deficiency in adult premenopausal female cynomolgus monkeys cauterizing and then bilaterally transecting the pudendal nerve. Autologous muscle progenitor cells were injected at four locations (12, 3, 6 and 9 o’clock positions) around the sphincter area. At 12 mo follow up, cell-treated monkeys had an improvement in resting, somatic and adrenergic nerve stimulated maximal urethral closure pressure, and a greater percentage of sphincter area occupied by muscle. The GFP labeled precursors cells were found in the skeletal muscle layer and expressed desmin and connexin-43. According to the authors it seems reasonable to assume that their outcomes have a high translational values[4].
Cord blood stem cells
Cord blood is a rich source of stem cells, including hematopoietic and mesenchymal stem cells, which have been used in the treatment of many diseases, including leukemia, lymphoma and anemia[35].
There are few experimental studies that use stem cells derived from cord blood for SUI therapy[35-37]. Lim et al[35] reported the short-term effects of human umbilical cord blood mononuclear transplantation in incontinent rats. Urinary incontinence was created through the electro-cauterization of periurethral soft tissue and confirmed by leak point pressure measurement. At four weeks, cell-treated rats had significant higher leak-point pressure than controls; however, the injected cells were not found in the urethral tissue[35].
Human amniotic fluid stem cells
Amniotic fluid, due to its contact with the fetus, has been considered an interesting source for undifferentiated or partially differentiated cells. HASC express surface markers and transcription factors distinctive of embryonic stem cells. These include octamer-binding transcription factor 4 and stage specific embryonic antigen-4. HASCs have high replicative self-renewal potential and multilineage differentiation capacity[38-41].
Chun et al[42] injected three different lineages of early differentiation human amniotic fluid-derived cell to restore urinary sphincter function in mice with urinary incontinence. In this study, urinary incontinence was created through bilateral pudendal nerve transection and the follow up was two and four weeks. HASC’s were early differentiated into muscle, neuron, and endothelial progenitor cells, and then injected into the urethral sphincter as a single, double, or triple combination. Urodynamic study showed that mice treated with the three cell populations had better outcomes than the other ones. These functional results were confirmed by histological and immunohistochemical analysis, as evidenced by the formation of new striated muscle fibers and neuromuscular junctions at the cell injection site[42].
iPSC
iPSC were first described by Takahashi et al[43] in 2006 when they reprogrammed human fibroblasts to become pluripotent stem cells by the addition of four different genes: Oct3/4, Sox2, c-Myc, and Klf4. Despite being a good source of cells, not all adult stem cells can be reprogrammed using the same method, which means that each cell type may have critical factors. Unlike embryonic stem cells, iPSC have no ethical issues and no immune rejection. On the other hand, these cells are reprogrammed through the addition of oncogenes, which increase the risk of uncontrolled growth[44]. These cells have never been tested to treat stress urinary incontinence.
REGENERATIVE MEDICINE OF THE CONNECTIVE TISSUES-PELVIC PROLAPSE AND LIGAMENT REINFORCEMENT
The current surgical approaches for pelvic organ prolapse treatment are associated with high rates of long-term complications such as erosions, infections, pain, and vagina shrinkage. There is a lack of studies in the literature with regards to cell-therapy and pelvic organ prolapse[45].
Boennelycke et al[46] used fresh muscle fiber fragments seeded on synthetic biodegradable methoxy polyethylene glycol-polylactide-co-glycolic acid scaffolds implanted subcutaneously on the abdomen of rats. After 8 wk, new striated muscle was created and the scaffolds had disappeared[46]. Petros et al[47] implanted mersilene tapes between rectus abdominis and midurerhra in 13 canines for periods of 6-12 wk. They observed a linear deposition of collagen around all implanted tapes and some interstitial macrophages. In this study, the main goal was to reinforce damaged pubourethral ligaments using an autogenic collagenous ligament[47].
Regarding prolapse and regenerative medicine approaches, the current literature does not have sufficient data to support cell-based therapy. Further studies using a reliable animal model, different cell sources and scaffolds should be addressed before clinical use.
STEM CELL THERAPY AND STRESS URINARY INCONTINENCE-CLINICAL OUTCOMES
The translation of basic science to humans is the main goal of scientists and researchers. When a new therapy reaches acceptable outcomes in animal models and has the potential to be applied in humans, some issues should be considered before moving forward. Despite expectations inherent to new breakthroughs, a carefully analysis of pros and cons regarding the procedure should be considered to avoid potential avoidable mistakes.
Aref-Adib et al[11] published a systematic review addressing the use of stem cell therapy for stress urinary incontinence in humans. They assessed different outcomes such as adverse effects, incontinence, quality of life, urodynamic, transurethral ultrasound and urethral EMG. Out of 89 studies, only eight met the inclusion criteria, which was a quality score tool designed for use in systematic reviews. According to the authors, the outcomes from the studies published to date are promising. Stem cell therapy may improve patients’ quality of life as well as objective measures of urinary incontinence. Furthermore, the procedure is safe with minimal adverse effects. However, despite promising results, there is no long-term follow up data and many patients lost to follow up without any explanation and withdrew from the studies[11].
Shirvan et al[48] described the safety assessment of urethra injections of autologous nucleated cells harvested from peripheral blood along with platelets in 9 women with stress urinary incontinence. At 6 mo follow up all the patients considered themselves completely cures with 8 women completely continent and one marked improvement. The idea is that peripheral blood has multipotential cell, which can improve the tissue regeneration[48].
FUTURE PERSPECTIVES AND DIRECTIONS
The stress urinary incontinence is a benign condition that has other effective, simple, cheap and safe treatment alternatives. I believe that stem cell therapy may be an alternative to treat those patients who has urethral sphincter deficiency and did not respond to well-established procedures. Historically, any new therapeutic modality has an initial enthusiasm; however, as well demonstrated in this paper, there are still few evidences, which support cell-based therapy for SUI. The enthusiasm surrounding the new therapeutic possibility should be replaced by moderation, reflection, and careful analysis of the available data associated with consistent experimental studies. The indiscriminate use without sufficient basic evidence could lead to discredit and retrocession.
P- Reviewer: De Petro G, Niyibizi C, Sacco E S- Editor: Ji FF L- Editor: A E- Editor: Liu SQ