Published online Nov 24, 2014. doi: 10.5410/wjcu.v3.i3.272
Revised: May 3, 2014
Accepted: October 1, 2014
Published online: November 24, 2014
Processing time: 232 Days and 1.1 Hours
Erectile dysfunction (ED) is an important health problem that has commonly been clinically treated using phosphodiesterase type 5 inhibitors (PDE5Is). However, PDE5Is are less effective when the structure of the cavernous body has been severely injured, and thus regeneration is required. Stem cell therapy has been investigated as a possible means for regenerating the injured cavernous body. Stem cells are classified into embryonic stem cells and adult stem cells (ASCs), and the intracavernous injection of ASCs has been explored as a therapy in animal ED models. Bone marrow-derived mesenchymal stem cells and adipose tissue-derived stem cells are major sources of ASCs used for the treatment of ED, and accumulated evidence now suggests that ASCs are useful in the restoration of erectile function and the regeneration of the cavernous body. However, the mechanisms by which ASCs recover erectile function remain controversial. Some studies indicated that ASCs were differentiated into the vascular endothelial cells, vascular smooth muscle cells, and nerve cells that originally resided in the cavernous body, whereas other studies have suggested that ASCs improved erectile function via the secretion of anti-apoptotic and/or proangiogenic cytokines rather than differentiation into other cell types. In this paper, we reviewed the characteristics of stem cells used for the treatment of ED, and the possible mechanisms by which these cells exert their effects. We also discussed the problems to be solved before implementation in the clinical setting.
Core tip: Adult stem cells (ASCs) have been used for the treatment of erectile dysfunction. Although previous studies reported that ASCs differentiated into cells that originally resided in the cavernous body, recent studies indicate that the major, if not all, effects of ASCs on erectile function are achieved through the secretion of paracrine factors rather than their direct differentiation into the cells in the cavernous body. Among various cytokines that ASCs produce, we have recently identified adrenomedullin as a candidate peptide that is implicated in the restoration of erectile function. We introduced these data in this review.
- Citation: Suzuki E, Nishimatsu H, Homma Y. Stem cell therapy for erectile dysfunction. World J Clin Urol 2014; 3(3): 272-282
- URL: https://www.wjgnet.com/2219-2816/full/v3/i3/272.htm
- DOI: https://dx.doi.org/10.5410/wjcu.v3.i3.272
Erectile dysfunction (ED) is a worldwide health problem. Although psychogenic factors are a major cause of ED, other factors such as age, diabetes, total prostatectomy and radiation in the pelvis also contribute to the occurrence of ED. These factors cause structural changes as well as functional abnormalities in the cavernous body, and therefore selective phosphodiesterase type 5 inhibitors (PDE5Is) are not so effective in the treatment of these diseases. For the recovery of erectile function in these patients, the regeneration of the cavernous body is necessary. In this regard, much attention has recently been placed on gene therapy and stem cell therapy.
Stem cells are defined as being capable of self-renewal and of differentiation into a variety of phenotypes[1]. There are two categories of stem cells: embryonic stem cells (ESCs) and adult stem cells (ASCs). ESCs were originally isolated from the inner cell mass of blastocysts[2]. ESCs are pluripotent stem cells that can give rise to the three germ layers. However, harvesting ESCs requires the destruction of human embryos and has therefore raised ethical concerns. To overcome this limitation, induced pluripotent stem (iPS) cells have been produced. Adult fibroblasts were reprogrammed by introducing four factors, Oct3/4, Sox2, c-Myc and Klf4 under ESCs culture conditions[3,4]. iPS cells are pluripotent stem cells with very similar characteristics to ESCs. Furthermore, many groups have now succeeded in reprograming somatic cells to create iPS cells by overexpression of variable sets of several transcription factors in cells without employing viruses or vectors[5-7]. Therefore, iPS cells are a promising option for regenerative medicine in the near future. A further option for avoiding the ethical problems of ESCs is the use of ASCs, which are basically multipotent stem cells that reside in various tissues, including the brain, skeletal muscle, bone marrow, adipose tissue, and dental pulp[8-11]. Besides having the potential to differentiate into various cell types, ASCs produce a broad range of cytokines that exert their effects in a paracrine and/or autocrine manner. Among ASCs, bone marrow-derived mesenchymal stem cells (BMMSCs) are the most commonly studied. BMMSCs reportedly have a potential to differentiate into various cell types including bone, cartilage, cardiac muscle, skeletal muscle, vascular endothelial cells (VECs) and vascular smooth muscle cells (VSMCs)[12,13]. Recently, adipose tissue-derived stem cells (ADSCs) have gained much attention because of the simplicity of harvesting hundreds of grams of subcutaneous adipose tissue without using invasive procedures, whereas painful bone marrow aspiration is required to collect just grams of bone marrow. Similar to BMMSCs, ADSCs have the potential to differentiate into various cell types[14]. ADSCs strongly resemble BMMSCs in that they share similar expression patterns of cell surface markers and similar gene expression profiles[15,16].
Many studies have demonstrated the efficacy of BMMSCs in the treatment of ED, and their efficacy seems to be reliable[17-27]. However, the mechanism by which BMMSCs restore erectile function remains controversial. BMMSCs were originally believed to home in on damaged tissues efficiently and differentiate into various cells at the target site. Genetically manipulated BMMSCs were also used expecting that these cells remain in the tissues and express a specific gene over a long period. Bivalacqua et al[17] infected BMMSCs with an adenovirus expressing endothelial nitric oxide synthase (eNOS) and injected these cells into the penis of aged rats. The eNOS-modified BMMSCs restored erectile function, with the recovery being associated with increased eNOS protein expression, NOS activity, and cyclic guanosine monophosphate levels. Furthermore, the authors demonstrated that the injected BMMSCs survived for at least 21 d in the cavernous body and expressed markers for VECs and VSMCs. Song et al[18] used immortalized human BMMSCs and injected them into rat penises, where the cells expressed markers for VECs and VSMCs in the cavernous body, although the authors did not examine whether erectile function was restored or not. Qiu et al[21] injected BMMSCs into the penis of streptozotocin (STZ)-induced diabetic rats and found that the BMMSCs injection restored erectile function. The authors also demonstrated that injected cells remained in the cavernous body for at least 4 wk and some expressed markers for VECs and VSMCs. Although the injection of BMMSCs restored erectile function in these studies, there are several problems. First, these studies did not clearly calculate the percentage of injected BMMSCs that remained in the cavernous body and obtained markers for VECs and VSMCs, or the percentage mentioned in these studies was not sufficient to explain the recovery of erectile function. Second, there was a possibility that the injected BMMSCs fused with the residing cells in the cavernous body and acquired the phenotype of the residing cells. Terada et al[28] used BMMSCs obtained from female transgenic mice expressing green fluorescent protein (GFP) and puromycin resistance gene. The BMMSCs were cocultured with a male embryonic stem cell line and then puromycin was added to remove the embryonic stem cells. The remaining cells were GFP positive and puromycin resistant, and morphologically similar to embryonic stem cells. These cells could be induced to differentiate into cardiac myocytes and neuronal cells, suggesting that embryonic stem cell-like pluripotent stem cells were established from BMMSCs. However, following DNA ploidy (the number of DNA copies) analysis using fluorescence-activated cell sorting, the cells were found to be tetraploid (4n) or hexaploid (6n), suggesting that they had developed from spontaneous fusion between the BMMSCs and the embryonic stem cells. The possibility of cell fusion in vivo was also reported. Alvarez-Dolado et al[29] used transgenic mice that contain the lacZ reporter gene downstream of a stop codon flanked by loxP sites (floxed). Therefore, the lacZ reporter gene was only expressed when the loxP-flanked stop codon was excised by Cre recombinase. The authors lethally irradiated these mice and intraperitoneally injected BMMSCs from mice that ubiquitously express Cre recombinase and GFP. If cells from the donor and recipient fused, the Cre enzyme would excise the Lox P-flanked stop codon, thereby allowing expression of the lacZ gene. Results revealed that β-gal+ (fused) and GFP+ cells were found in the brain, heart, and liver of recipients, 2 and 4 mo post-transplantation[29]. Thus, BMMSCs potentially fuse with other cell types in vivo and it appears that BMMSCs are differentiated into other cell types because of this phenomenon. In contrast, the paracrine effects of BMMSCs have been reported. Kendirci et al[20] isolated BMMSCs positive for p75 low-affinity nerve growth factor receptor using magnetic-activated cell sorting, and injected these cells into the penis of rats following bilateral cavernous nerve crush injury. The injection of these cells restored erectile function[20]. The engraftment of these cells in the cavernous tissue occurred very rarely, and the engrafted cells appeared fibroblastic. Furthermore, these cells secreted fibroblast growth factor 2 (FGF2), which suggested that FGF2 might protect the cavernous nerve after crush injury. More direct evidence of the paracrine effects of BMMSCs was reported by Yeghiazarians et al[30] who injected BMMSCs extracts into infarcted hearts and found that the procedure effectively improved cardiac function, suggesting that BMMSCs per se were not required for their tissue protective effects. Although this scenario is attractive, no cytokines that are implicated in the recovery of erectile function have been specifically identified.
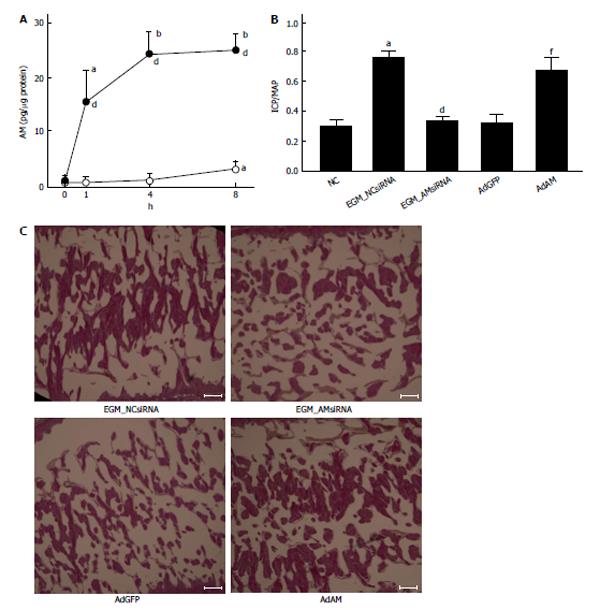
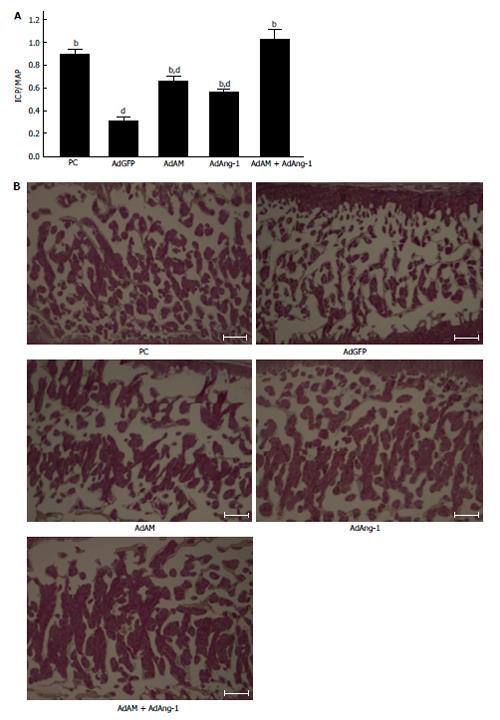
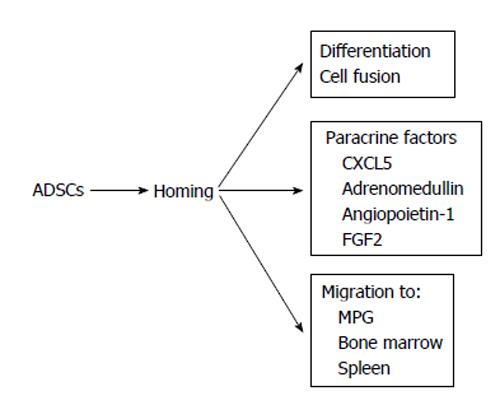
The efficacy of ADSCs in the treatment of ED seems to be reliable from the results of many previous reports[31-45]. However, the mechanisms by which ADSCs restore erectile function remain controversial. Ning et al[46] reported that ADSCs could differentiate into VECs. ADSCs injected into the penis obtained a marker for VECs. FGF2 appeared to be necessary for the differentiation of ADSCs into VECs in vitro, although the functional role of FGF2 in the differentiation of ADSCs into VECs in vivo was not studied[46]. Ryu et al[40] demonstrated that the injection of ADSCs into the penis of STZ-induced diabetic mice restored erectile function. They also found that some injected ADSCs became CD31 positive, suggesting that these cells differentiated into VECs[40]. However, ADSCs injected into the penis disappeared within 14 d. Kim et al[43] applied human ADSCs and nerve growth factor-incorporated hyaluronic acid-based hydrogel to the cavernous nerve of rats following bilateral cavernous nerve crush injury. The authors demonstrated that this treatment restored erectile function. They also showed that some ADSCs were engrafted into the cavernous nerve 4 wk after treatment, suggesting that ADSCs could differentiate into nerve tissue[43]. Although these studies showed that ADSCs have the ability to differentiate into the cells located in the cavernous body, the possibility of cell-cell fusion was not excluded in these studies. Alternatively, paracrine effects of ADSCs have been suggested. Several reports suggested the possibility of paracrine effects of ADSCs because ADSCs did not remain in the cavernous body for a long period[32,33]. Albersen et al[31] injected both ADSCs and the lysate of ADSCs into the penis of rats that were subjected to bilateral cavernous nerve crush injury. They demonstrated that the injection of ADSCs lysate significantly restored erectile function[31]. These results suggested that most, if not all, of the effects of ADSCs were mediated through their production of cytokines and/or immune modulators, although the authors did not identify any molecules that are functionally implicated in the restoration of erectile function. Zhang et al[47] reported that ADSCs produced chemokine (C-X-C motif) ligand 5 (CXCL5) and that CXCL5 was implicated in the neurotrophic effects of ADSCs in vitro, although they did not confirm this finding in vivo. We recently reported that adrenomedullin (AM) is implicated in ADSCs-induced restoration of erectile function in diabetic rats[36]. AM was originally isolated from human pheochromocytoma tissue, and has potent vasorelaxant and diuretic effects[48]. In addition, AM is also produced by VECs, VSMCs and macrophages[49-51] and has the ability to stimulate angiogenesis[52-54]. When rat ADSCs were cultured in a medium containing growth factors for VECs, ADSCs produced significant amounts of AM (Figure 1A). The injection of ADSCs into the penis of diabetic rats restored erectile function (Figure1B), the morphology of the cavernous body (Figure 1C), and the expression of vascular endothelial (VE)-cadherin, a marker for VECs. However, when the expression of AM was knocked down using a small interfering RNA for AM, the favorable effects of ADSCs disappeared (Figure 1B and C). Furthermore, when AM was overexpressed in the penis using an adenovirus expressing AM, erectile function and the morphology of the cavernous body were restored in diabetic rats (Figure 1B and C). We also demonstrated that ADSCs produce angiopoietin-1 (Ang-1) and Ang-1 secreted from ADSCs are implicated in ADSCs-induced suppression of neointimal formation and stimulation of reendothelialization in a wire injury model of the rat femoral artery[55]. Furthermore, we reported that overexpression of both AM and Ang-1 using adenoviruses expressing those proteins restored erectile function in diabetic rats to the same level as that observed in age-matched positive control rats (Figure 2)[44]. Therefore, it seems obvious that ADSCs produce various cytokines that potentially restore erectile function. The limitation is that ADSCs do not remain in the cavernous body for a long period, usually disappearing within a month[32,33,36]. Where do they go? Do they die or migrate to other tissues? Several interesting papers have been published regarding these points. Lin et al[56] injected rat ADSCs and traced their locations after 2 and 7 d. ADSCs remained not only in the penis but also in the major pelvic ganglia (MPG), spleen and bone marrow. ADSCs preferentially remained in the bone marrow and the number of ADSCs remaining in the bone marrow was much larger than that remaining in the penis. Because ADSCs secrete various cytokines, this result suggests that ADSCs remaining outside the penis may affect the restoration of erectile function, although the survival of ADSCs for long periods was not examined in the study. Several studies have reported that ADSCs injected into the penis or placed around the prostate gland migrated to the MPG[35,39,45]. Fandel et al[35] demonstrated that ADSCs injected into the penis migrated to the MPG, although ADSCs were not engrafted in the nerve tissue. Interestingly, the expression of stromal cell derived factor-1 (SDF-1) increased in MPG, suggesting that ADSCs preferentially migrated to the site of SDF-1 production. Qiu et al[39] also reported that intracavernously injected ADSCs migrated to the MPG, where they remained 17 wk after injection, although the number of ADSCs remaining in the MPG was quite low. You et al[45] showed that periprostatic implantation of ADSCs, but not their intracavernous injection, resulted in the migration of ADSCs to MPG. In summary, some ADSCs injected into the penis migrated to the tissues such as the bone marrow and MPG, and these cells may be implicated in the restoration of erectile function. The possible mechanisms by which ADSCs restore erectile function are summarized in Figure 3.
Endothelial progenitor cells (EPCs) were originally isolated from human peripheral blood by Asahara et al[57]. They isolated CD34-positive mononuclear blood cells and demonstrated that these cells obtained the characteristics of VECs when cultured on fibronectin-coated dishes. They also demonstrated that these cells were incorporated in ischemic tissues in vivo and expressed markers for VECs such as CD31 when introduced into the circulation using a hindlimb ischemia model. Furthermore, the authors showed that Flk-1-positive mononuclear blood cells were also integrated in the capillaries and small arteries when the hindlimb ischemia model was used. These cells were designated as EPCs. EPCs are progenitor cells whose differentiation potential is restricted to one lineage (VECs); therefore, they are not multipotent stem cells. Results of subsequent studies revealed that EPCs express three cell surface markers; CD133 (termed originally AC133), CD34, and Flk-1[58-60]. Premature EPCs either in the bone marrow or immediately after entering into the systemic circulation are positive for CD133/CD34/Flk-1. However, when EPCs become more mature, they lose the expression of CD133 and begin to express CD31 and VE-cadherin. An attempt to use EPCs for the treatment of ED has been reported by Gou et al[61]. They transfected EPCs with the vascular endothelial growth factor gene and injected the transfected cells into the penis of diabetic rats. The authors found that this treatment restored erectile function and the injected cells were integrated into the sites of neovascularization in the cavernous body[61]. However, most researchers in this field regard EPCs as a marker for ED rather than a therapeutic tool for ED. Factors affecting the number of circulating EPCs or their functions have been reported. The number of circulating EPCs and their migratory activity are reportedly reduced in patients with coronary risk factors[62,63]. EPCs isolated from type 2 diabetes patients have a decreased capacity for proliferation and the formation of capillary tubes in vitro[64]. In contrast, the number of circulating EPCs rapidly increases after limb ischemia and acute myocardial infarction[65,66]. Because atherosclerosis and ED share a common feature, i.e., endothelial dysfunction, it has been speculated that the dynamics of EPCs might change in ED. Indeed, several studies have reported that the number of circulating EPCs decreased in ED patients[67-69], suggesting that the decrease in the number of circulating EPCs can predict the presence of ED as well as cardiovascular diseases. Interestingly, the number of circulating EPCs increased when patients were administered statins or PDE5Is[70-73] probably via the mobilization of EPCs from the bone marrow. Therefore, these drugs may improve erectile function via the mobilization of EPCs to the cavernous body.
Muscle-derived stem cells (MDSCs) are ASCs that exist in skeletal muscle. MDSCs can be obtained from autologous muscle biopsies and have been used for the treatment of ED in several studies. Nolazco et al[74] injected MDSCs into the penis of aged rats and found that MDSCs differentiated into VSMCs in the cavernous body, resulting in the recovery of erectile function. Woo et al[75] used a bilateral cavernous nerve injury model in rats and examined the effects of the injection of MDSCs into the penis on erectile function. They demonstrated that MDSCs remained in the cavernous body 4 wk after injection, and erectile function was significantly restored. Kovanecz et al[76] used a bilateral cavernosal nerve resection model of rats and, following the injection of MDSCs into the cavernous body, found that erectile function was restored and α-smooth muscle actin expression was increased. The injection of MDSCs also increased the expression of neural nitric oxide synthase and brain-derived neurotrophic factor[76]. In summary, MDSCs seem to be useful for the treatment of ED. However, because harvesting MDSCs from the skeletal muscle is relatively more invasive than the collection of ADSCs, the beneficial characteristics of MDSCs compared with ADSCs should be clarified before introducing them to clinical application.
Umbilical cord blood stem cells (UCBSCs) are an attractive type of stem cells in that they are youngest stem cells among a variety of ASCs. Because they are young, they have less possibility to have DNA damage than other ASCs[77,78]. Bahk et al[79] used human UCBSCs to treat diabetic patients with ED, and demonstrated that erectile function was restored and that blood glucose levels decreased in these patients, although the mechanisms remain unknown.
Brain-derived stem cells (BDSCs) reportedly have capacity to differentiate into VSMCs[80]. Song et al[81] isolated fetal BDSCs from embryonal 12-d rats and injected them into the penis. They demonstrated that injected BDSCs obtained characteristics of VSMCs in vivo 6 wk after injection[81], although they did not examine their effect on erectile function. Considering the source of BDSCs, it will be difficult to use them in clinical settings.
Neural crest stem cells (NCSCs) are the progenitor cells of several cell types that constitute the peripheral nervous system, including neurons, Schwann cells, adrenal chromaffin cells and smooth muscle cells. Transplantation of NCSCs could reportedly induce the regeneration of connective tissues, VSMCs, skeletal muscle and VECs[82,83]. Song et al[84] injected NCSCs into the penis of rats and demonstrated that they obtained markers for VECs and VSMCs 2 wk after injection, although their effects on erectile function was not analyzed[84]. Clinical application of NCSCs may also be difficult considering the source of these cells.
Because iPS cells have been created, clinical application of pluripotent stem cells will be intensively explored in the future. To our knowledge, no studies have been published in which iPS cells or iPS cells-derived cells were used to treat ED. Bochinski et al[85] used ESCs that had differentiated into the neural cell line, and injected them into the MPG or cavernous body using a bilateral cavernous nerve injury model. They found that the injection into the both MPG and cavernous body restored erectile function. They also found that neurofilament staining was recovered in the ESCs-injected group[85]. Therefore, ESCs and iPS cells may be useful for treatment of ED. However, these cells may not efficiently home and survive for a long period under persistent inflammation. For example, in diabetic conditions hyperglycemia and adipocytokines induce persistent inflammation in the tissues. Implanted cells may not home and survive under these conditions unless such an inflammation is sufficiently controlled.
As mentioned above, stem cell therapy for ED appears to be a promising strategy. However, several problems should be solved before moving to clinical application.
It is well known that ESCs and iPS cells easily form tumors, because these cells are pluripotent. Although ASCs seem to be less prone to forming tumors, ASCs can form malignant tumors when transplanted in vivo[86]. Jeong et al[86] injected BMMSCs into the peri-infarct area of myocardial infaction (MI) model of mice and hindlimb muscle of mice with diabetic neuropathy. They found sarcoma formation in 30% of hearts in the MI model and in 46% of hindlimbs in the diabetic neuropathy model[86]. Therefore, it will be necessary to sufficiently investigate the malignant potential of stem cells prior to their clinical use and establish methodology to select “healthy” stem cells that will not form tumors.
As described above, some (most) of stem cells injected into the penis do not remain in the penis and migrate to other tissues such as the bone marrow and spleen. Little is known about the fate of these cells that have migrated to non-diseased organs. Detailed examinations will be necessary to detect the fate of these cells before moving to clinical applications.
Most studies suggested that injected stem cells disappeared from the penis in one month. It is crucially important to explore methods to improve homing and survival of stem cells. It was reported that expression of SDF-1 was increased in the MPG and SDF-1 might stimulate migration of ADSCs to the MPG[35]. Therefore, SDF-1 is a candidate that stimulates migration and homing of stem cells into injured sites. Intensive studies will be necessary to identify molecules that are implicated in migration, homing and survival of stem cells.
It is suggested from EPC study that statins or PDE5Is can stimulate mobilization of EPCs from the bone marrow[70-73]. Therefore, it may be possible to activate and/or mobilize tissue-residual endogenous stem cells by some drugs. If endogenous stem cells residing in the penis can be efficiently activated, it will help to regenerate the cavernous body. This possibility should be examined in the future.
ASCs produce a variety of paracrine factors that potentially regenerate the cavernous body. However, paracrine factors that are implicated in the regeneration of the cavernous body have not been sufficiently identified. Furthermore, it remains unknown what combinations of these paracrine factors are most suitable to stimulate the regeneration of the cavernous body. If these problems are solved, administration of cytokines cocktail may be more effective to treat ED than ASCs injection.
Stem cells especially ASCs have been used in the treatment of ED, and stem cell therapy seems to be effective at least in animal modes. Major, if not all, effects of ASCs on erectile function appear to be achieved by secretion of paracrine factors rather than their direct differentiation into cells residing in the cavernous body. Before moving to clinical application, malignant potential of stem cells should be carefully considered. It is also necessary to explore methods to improve homing and survival of stem cells.
P- Reviewer: Abdel-Salam OME, Carcas-Sansuan AJ, El-Assmy A, Saeki K S- Editor: Ji FF L- Editor: A E- Editor: Liu SQ
| 1. | Morrison SJ, Shah NM, Anderson DJ. Regulatory mechanisms in stem cell biology. Cell. 1997;88:287-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 767] [Cited by in RCA: 707] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 2. | Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292:154-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5956] [Cited by in RCA: 5434] [Article Influence: 123.5] [Reference Citation Analysis (0)] |
| 3. | Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17989] [Cited by in RCA: 18198] [Article Influence: 957.8] [Reference Citation Analysis (0)] |
| 4. | Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861-872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14327] [Cited by in RCA: 14319] [Article Influence: 842.3] [Reference Citation Analysis (0)] |
| 5. | Stadtfeld M, Nagaya M, Utikal J, Weir G, Hochedlinger K. Induced pluripotent stem cells generated without viral integration. Science. 2008;322:945-949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1270] [Cited by in RCA: 1123] [Article Influence: 66.1] [Reference Citation Analysis (0)] |
| 6. | Yu J, Hu K, Smuga-Otto K, Tian S, Stewart R, Slukvin II, Thomson JA. Human induced pluripotent stem cells free of vector and transgene sequences. Science. 2009;324:797-801. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1896] [Cited by in RCA: 1692] [Article Influence: 105.8] [Reference Citation Analysis (0)] |
| 7. | Zhou H, Wu S, Joo JY, Zhu S, Han DW, Lin T, Trauger S, Bien G, Yao S, Zhu Y. Generation of induced pluripotent stem cells using recombinant proteins. Cell Stem Cell. 2009;4:381-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1309] [Cited by in RCA: 1229] [Article Influence: 76.8] [Reference Citation Analysis (0)] |
| 8. | Jiang Y, Vaessen B, Lenvik T, Blackstad M, Reyes M, Verfaillie CM. Multipotent progenitor cells can be isolated from postnatal murine bone marrow, muscle, and brain. Exp Hematol. 2002;30:896-904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 634] [Cited by in RCA: 576] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 9. | Presnell SC, Petersen B, Heidaran M. Stem cells in adult tissues. Semin Cell Dev Biol. 2002;13:369-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 43] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 10. | Schäffler A, Büchler C. Concise review: adipose tissue-derived stromal cells--basic and clinical implications for novel cell-based therapies. Stem Cells. 2007;25:818-827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 767] [Cited by in RCA: 768] [Article Influence: 42.7] [Reference Citation Analysis (0)] |
| 11. | Hipp J, Atala A. Sources of stem cells for regenerative medicine. Stem Cell Rev. 2008;4:3-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 122] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 12. | Herzog EL, Chai L, Krause DS. Plasticity of marrow-derived stem cells. Blood. 2003;102:3483-3493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 561] [Cited by in RCA: 532] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 13. | Grove JE, Bruscia E, Krause DS. Plasticity of bone marrow-derived stem cells. Stem Cells. 2004;22:487-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 274] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 14. | Gimble JM, Katz AJ, Bunnell BA. Adipose-derived stem cells for regenerative medicine. Circ Res. 2007;100:1249-1260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1660] [Cited by in RCA: 1921] [Article Influence: 106.7] [Reference Citation Analysis (0)] |
| 15. | Peroni D, Scambi I, Pasini A, Lisi V, Bifari F, Krampera M, Rigotti G, Sbarbati A, Galiè M. Stem molecular signature of adipose-derived stromal cells. Exp Cell Res. 2008;314:603-615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 88] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 16. | Torensma R, Prins HJ, Schrama E, Verwiel ET, Martens AC, Roelofs H, Jansen BJ. The impact of cell source, culture methodology, culture location, and individual donors on gene expression profiles of bone marrow-derived and adipose-derived stromal cells. Stem Cells Dev. 2013;22:1086-1096. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 17. | Bivalacqua TJ, Deng W, Kendirci M, Usta MF, Robinson C, Taylor BK, Murthy SN, Champion HC, Hellstrom WJ, Kadowitz PJ. Mesenchymal stem cells alone or ex vivo gene modified with endothelial nitric oxide synthase reverse age-associated erectile dysfunction. Am J Physiol Heart Circ Physiol. 2007;292:H1278-H1290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 110] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 18. | Song YS, Lee HJ, Park IH, Kim WK, Ku JH, Kim SU. Potential differentiation of human mesenchymal stem cell transplanted in rat corpus cavernosum toward endothelial or smooth muscle cells. Int J Impot Res. 2007;19:378-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 69] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 19. | Abdel Aziz MT, El-Haggar S, Mostafa T, Atta H, Fouad H, Mahfouz S, Rashed L, Sabry D, Senbel A, Ali GA. Effect of mesenchymal stem cell penile transplantation on erectile signaling of aged rats. Andrologia. 2010;42:187-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 20. | Kendirci M, Trost L, Bakondi B, Whitney MJ, Hellstrom WJ, Spees JL. Transplantation of nonhematopoietic adult bone marrow stem/progenitor cells isolated by p75 nerve growth factor receptor into the penis rescues erectile function in a rat model of cavernous nerve injury. J Urol. 2010;184:1560-1566. [RCA] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 62] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 21. | Qiu X, Lin H, Wang Y, Yu W, Chen Y, Wang R, Dai Y. Intracavernous transplantation of bone marrow-derived mesenchymal stem cells restores erectile function of streptozocin-induced diabetic rats. J Sex Med. 2011;8:427-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 81] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 22. | Kim SJ, Choi SW, Hur KJ, Park SH, Sung YC, Ha YS, Cho HJ, Hong SH, Lee JY, Hwang TK. Synergistic effect of mesenchymal stem cells infected with recombinant adenovirus expressing human BDNF on erectile function in a rat model of cavernous nerve injury. Korean J Urol. 2012;53:726-732. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 23. | Kim SJ, Park SH, Sung YC, Kim SW. Effect of mesenchymal stem cells associated to matrixen on the erectile function in the rat model with bilateral cavernous nerve crushing injury. Int Braz J Urol. 2012;38:833-841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 24. | Sun C, Lin H, Yu W, Li X, Chen Y, Qiu X, Wang R, Dai Y. Neurotrophic effect of bone marrow mesenchymal stem cells for erectile dysfunction in diabetic rats. Int J Androl. 2012;35:601-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 59] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 25. | He Y, He W, Qin G, Luo J, Xiao M. Transplantation KCNMA1 modified bone marrow-mesenchymal stem cell therapy for diabetes mellitus-induced erectile dysfunction. Andrologia. 2014;46:479-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 26. | You D, Jang MJ, Lee J, Jeong IG, Kim HS, Moon KH, Suh N, Kim CS. Periprostatic implantation of human bone marrow-derived mesenchymal stem cells potentiates recovery of erectile function by intracavernosal injection in a rat model of cavernous nerve injury. Urology. 2013;81:104-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 46] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 27. | Ryu JK, Kim DH, Song KM, Yi T, Suh JK, Song SU. Intracavernous delivery of clonal mesenchymal stem cells restores erectile function in a mouse model of cavernous nerve injury. J Sex Med. 2014;11:411-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 28. | Terada N, Hamazaki T, Oka M, Hoki M, Mastalerz DM, Nakano Y, Meyer EM, Morel L, Petersen BE, Scott EW. Bone marrow cells adopt the phenotype of other cells by spontaneous cell fusion. Nature. 2002;416:542-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1499] [Cited by in RCA: 1375] [Article Influence: 59.8] [Reference Citation Analysis (0)] |
| 29. | Alvarez-Dolado M, Pardal R, Garcia-Verdugo JM, Fike JR, Lee HO, Pfeffer K, Lois C, Morrison SJ, Alvarez-Buylla A. Fusion of bone-marrow-derived cells with Purkinje neurons, cardiomyocytes and hepatocytes. Nature. 2003;425:968-973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1199] [Cited by in RCA: 1160] [Article Influence: 52.7] [Reference Citation Analysis (0)] |
| 30. | Yeghiazarians Y, Zhang Y, Prasad M, Shih H, Saini SA, Takagawa J, Sievers RE, Wong ML, Kapasi NK, Mirsky R. Injection of bone marrow cell extract into infarcted hearts results in functional improvement comparable to intact cell therapy. Mol Ther. 2009;17:1250-1256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 76] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 31. | Albersen M, Fandel TM, Lin G, Wang G, Banie L, Lin CS, Lue TF. Injections of adipose tissue-derived stem cells and stem cell lysate improve recovery of erectile function in a rat model of cavernous nerve injury. J Sex Med. 2010;7:3331-3340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 200] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 32. | Garcia MM, Fandel TM, Lin G, Shindel AW, Banie L, Lin CS, Lue TF. Treatment of erectile dysfunction in the obese type 2 diabetic ZDF rat with adipose tissue-derived stem cells. J Sex Med. 2010;7:89-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 101] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 33. | Huang YC, Ning H, Shindel AW, Fandel TM, Lin G, Harraz AM, Lue TF, Lin CS. The effect of intracavernous injection of adipose tissue-derived stem cells on hyperlipidemia-associated erectile dysfunction in a rat model. J Sex Med. 2010;7:1391-1400. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 81] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 34. | Lin G, Albersen M, Harraz AM, Fandel TM, Garcia M, McGrath MH, Konety BR, Lue TF, Lin CS. Cavernous nerve repair with allogenic adipose matrix and autologous adipose-derived stem cells. Urology. 2011;77:1509.e1-1509.e8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (2)] |
| 35. | Fandel TM, Albersen M, Lin G, Qiu X, Ning H, Banie L, Lue TF, Lin CS. Recruitment of intracavernously injected adipose-derived stem cells to the major pelvic ganglion improves erectile function in a rat model of cavernous nerve injury. Eur Urol. 2012;61:201-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 134] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 36. | Nishimatsu H, Suzuki E, Kumano S, Nomiya A, Liu M, Kume H, Homma Y. Adrenomedullin mediates adipose tissue-derived stem cell-induced restoration of erectile function in diabetic rats. J Sex Med. 2012;9:482-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 37. | Piao S, Kim IG, Lee JY, Hong SH, Kim SW, Hwang TK, Oh SH, Lee JH, Ra JC, Lee JY. Therapeutic effect of adipose-derived stem cells and BDNF-immobilized PLGA membrane in a rat model of cavernous nerve injury. J Sex Med. 2012;9:1968-1979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 38. | Qiu X, Fandel TM, Ferretti L, Albersen M, Orabi H, Zhang H, Lin G, Lin CS, Schroeder T, Lue TF. Both immediate and delayed intracavernous injection of autologous adipose-derived stromal vascular fraction enhances recovery of erectile function in a rat model of cavernous nerve injury. Eur Urol. 2012;62:720-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 86] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 39. | Qiu X, Villalta J, Ferretti L, Fandel TM, Albersen M, Lin G, Dai Y, Lue TF, Lin CS. Effects of intravenous injection of adipose-derived stem cells in a rat model of radiation therapy-induced erectile dysfunction. J Sex Med. 2012;9:1834-1841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 52] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 40. | Ryu JK, Tumurbaatar M, Jin HR, Kim WJ, Kwon MH, Piao S, Choi MJ, Yin GN, Song KM, Kang YJ. Intracavernous delivery of freshly isolated stromal vascular fraction rescues erectile function by enhancing endothelial regeneration in the streptozotocin-induced diabetic mouse. J Sex Med. 2012;9:3051-3065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 41. | Castiglione F, Hedlund P, Van der Aa F, Bivalacqua TJ, Rigatti P, Van Poppel H, Montorsi F, De Ridder D, Albersen M. Intratunical injection of human adipose tissue-derived stem cells prevents fibrosis and is associated with improved erectile function in a rat model of Peyronie’s disease. Eur Urol. 2013;63:551-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 131] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 42. | Jeong HH, Piao S, Ha JN, Kim IG, Oh SH, Lee JH, Cho HJ, Hong SH, Kim SW, Lee JY. Combined therapeutic effect of udenafil and adipose-derived stem cell (ADSC)/brain-derived neurotrophic factor (BDNF)-membrane system in a rat model of cavernous nerve injury. Urology. 2013;81:1108.e7-1108.14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 43. | Kim IG, Piao S, Lee JY, Hong SH, Hwang TK, Kim SW, Kim CS, Ra JC, Noh I, Lee JY. Effect of an adipose-derived stem cell and nerve growth factor-incorporated hydrogel on recovery of erectile function in a rat model of cavernous nerve injury. Tissue Eng Part A. 2013;19:14-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 46] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 44. | Nishimatsu H, Suzuki E, Nomiya A, Niimi A, Suzuki M, Fujimura T, Fukuhara H, Homma Y. Adrenomedullin and angiopoietin-1 additively restore erectile function in diabetic rats: comparison with the combination therapy of vascular endothelial growth factor and angiopoietin-1. J Sex Med. 2013;10:1707-1719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 45. | You D, Jang MJ, Lee J, Suh N, Jeong IG, Sohn DW, Kim SW, Ahn TY, Kim CS. Comparative analysis of periprostatic implantation and intracavernosal injection of human adipose tissue-derived stem cells for erectile function recovery in a rat model of cavernous nerve injury. Prostate. 2013;73:278-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 46. | Ning H, Liu G, Lin G, Yang R, Lue TF, Lin CS. Fibroblast growth factor 2 promotes endothelial differentiation of adipose tissue-derived stem cells. J Sex Med. 2009;6:967-979. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 102] [Cited by in RCA: 95] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 47. | Zhang H, Yang R, Wang Z, Lin G, Lue TF, Lin CS. Adipose tissue-derived stem cells secrete CXCL5 cytokine with neurotrophic effects on cavernous nerve regeneration. J Sex Med. 2011;8:437-446. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 62] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 48. | Kitamura K, Kangawa K, Kawamoto M, Ichiki Y, Nakamura S, Matsuo H, Eto T. Adrenomedullin: a novel hypotensive peptide isolated from human pheochromocytoma. Biochem Biophys Res Commun. 1993;192:553-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1633] [Cited by in RCA: 1585] [Article Influence: 49.5] [Reference Citation Analysis (0)] |
| 49. | Sugo S, Minamino N, Kangawa K, Miyamoto K, Kitamura K, Sakata J, Eto T, Matsuo H. Endothelial cells actively synthesize and secrete adrenomedullin. Biochem Biophys Res Commun. 1994;201:1160-1166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 423] [Cited by in RCA: 417] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 50. | Sugo S, Minamino N, Shoji H, Kangawa K, Kitamura K, Eto T, Matsuo H. Production and secretion of adrenomedullin from vascular smooth muscle cells: augmented production by tumor necrosis factor-alpha. Biochem Biophys Res Commun. 1994;203:719-726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 270] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 51. | Kubo A, Minamino N, Isumi Y, Katafuchi T, Kangawa K, Dohi K, Matsuo H. Production of adrenomedullin in macrophage cell line and peritoneal macrophage. J Biol Chem. 1998;273:16730-16738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 136] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 52. | Kim W, Moon SO, Sung MJ, Kim SH, Lee S, So JN, Park SK. Angiogenic role of adrenomedullin through activation of Akt, mitogen-activated protein kinase, and focal adhesion kinase in endothelial cells. FASEB J. 2003;17:1937-1939. [PubMed] |
| 53. | Iimuro S, Shindo T, Moriyama N, Amaki T, Niu P, Takeda N, Iwata H, Zhang Y, Ebihara A, Nagai R. Angiogenic effects of adrenomedullin in ischemia and tumor growth. Circ Res. 2004;95:415-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 96] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 54. | Tokunaga N, Nagaya N, Shirai M, Tanaka E, Ishibashi-Ueda H, Harada-Shiba M, Kanda M, Ito T, Shimizu W, Tabata Y. Adrenomedullin gene transfer induces therapeutic angiogenesis in a rabbit model of chronic hind limb ischemia: benefits of a novel nonviral vector, gelatin. Circulation. 2004;109:526-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 55] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 55. | Takahashi M, Suzuki E, Kumano S, Oba S, Sato T, Nishimatsu H, Kimura K, Nagano T, Hirata Y. Angiopoietin-1 mediates adipose tissue-derived stem cell-induced inhibition of neointimal formation in rat femoral artery. Circ J. 2013;77:1574-1584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 56. | Lin G, Qiu X, Fandel T, Banie L, Wang G, Lue TF, Lin CS. Tracking intracavernously injected adipose-derived stem cells to bone marrow. Int J Impot Res. 2011;23:268-275. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 57. | Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964-967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6624] [Cited by in RCA: 6349] [Article Influence: 226.8] [Reference Citation Analysis (1)] |
| 58. | Yin AH, Miraglia S, Zanjani ED, Almeida-Porada G, Ogawa M, Leary AG, Olweus J, Kearney J, Buck DW. AC133, a novel marker for human hematopoietic stem and progenitor cells. Blood. 1997;90:5002-5012. [PubMed] |
| 59. | Gehling UM, Ergün S, Schumacher U, Wagener C, Pantel K, Otte M, Schuch G, Schafhausen P, Mende T, Kilic N. In vitro differentiation of endothelial cells from AC133-positive progenitor cells. Blood. 2000;95:3106-3112. [PubMed] |
| 60. | Peichev M, Naiyer AJ, Pereira D, Zhu Z, Lane WJ, Williams M, Oz MC, Hicklin DJ, Witte L, Moore MA. Expression of VEGFR-2 and AC133 by circulating human CD34(+) cells identifies a population of functional endothelial precursors. Blood. 2000;95:952-958. [PubMed] |
| 61. | Gou X, He WY, Xiao MZ, Qiu M, Wang M, Deng YZ, Liu CD, Tang ZB, Li J, Chen Y. Transplantation of endothelial progenitor cells transfected with VEGF165 to restore erectile function in diabetic rats. Asian J Androl. 2011;13:332-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 43] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 62. | Vasa M, Fichtlscherer S, Aicher A, Adler K, Urbich C, Martin H, Zeiher AM, Dimmeler S. Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease. Circ Res. 2001;89:E1-E7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1571] [Cited by in RCA: 1600] [Article Influence: 66.7] [Reference Citation Analysis (0)] |
| 63. | Hill JM, Zalos G, Halcox JP, Schenke WH, Waclawiw MA, Quyyumi AA, Finkel T. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med. 2003;348:593-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2631] [Cited by in RCA: 2584] [Article Influence: 117.5] [Reference Citation Analysis (0)] |
| 64. | Tepper OM, Galiano RD, Capla JM, Kalka C, Gagne PJ, Jacobowitz GR, Levine JP, Gurtner GC. Human endothelial progenitor cells from type II diabetics exhibit impaired proliferation, adhesion, and incorporation into vascular structures. Circulation. 2002;106:2781-2786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1132] [Cited by in RCA: 1112] [Article Influence: 48.3] [Reference Citation Analysis (0)] |
| 65. | Takahashi T, Kalka C, Masuda H, Chen D, Silver M, Kearney M, Magner M, Isner JM, Asahara T. Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nat Med. 1999;5:434-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1255] [Cited by in RCA: 1375] [Article Influence: 52.9] [Reference Citation Analysis (0)] |
| 66. | Shintani S, Murohara T, Ikeda H, Ueno T, Honma T, Katoh A, Sasaki K, Shimada T, Oike Y, Imaizumi T. Mobilization of endothelial progenitor cells in patients with acute myocardial infarction. Circulation. 2001;103:2776-2779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 798] [Cited by in RCA: 763] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 67. | Foresta C, Caretta N, Lana A, Cabrelle A, Palù G, Ferlin A. Circulating endothelial progenitor cells in subjects with erectile dysfunction. Int J Impot Res. 2005;17:288-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 72] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 68. | Baumhäkel M, Werner N, Böhm M, Nickenig G. Circulating endothelial progenitor cells correlate with erectile function in patients with coronary heart disease. Eur Heart J. 2006;27:2184-2188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 50] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 69. | Esposito K, Ciotola M, Maiorino MI, Giugliano F, Autorino R, De Sio M, Jannini E, Lenzi A, Giugliano D. Circulating CD34+ KDR+ endothelial progenitor cells correlate with erectile function and endothelial function in overweight men. J Sex Med. 2009;6:107-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 51] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 70. | Dimmeler S, Aicher A, Vasa M, Mildner-Rihm C, Adler K, Tiemann M, Rütten H, Fichtlscherer S, Martin H, Zeiher AM. HMG-CoA reductase inhibitors (statins) increase endothelial progenitor cells via the PI 3-kinase/Akt pathway. J Clin Invest. 2001;108:391-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 283] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 71. | Vasa M, Fichtlscherer S, Adler K, Aicher A, Martin H, Zeiher AM, Dimmeler S. Increase in circulating endothelial progenitor cells by statin therapy in patients with stable coronary artery disease. Circulation. 2001;103:2885-2890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 704] [Cited by in RCA: 674] [Article Influence: 28.1] [Reference Citation Analysis (0)] |
| 72. | Foresta C, Ferlin A, De Toni L, Lana A, Vinanzi C, Galan A, Caretta N. Circulating endothelial progenitor cells and endothelial function after chronic Tadalafil treatment in subjects with erectile dysfunction. Int J Impot Res. 2006;18:484-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 60] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 73. | Foresta C, Caretta N, Lana A, De Toni L, Biagioli A, Vinanzi C, Ferlin A. Relationship between vascular damage degrees and endothelial progenitor cells in patients with erectile dysfunction: effect of vardenafil administration and PDE5 expression in the bone marrow. Eur Urol. 2007;51:1411-1417; discussion 1417-1419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 74. | Nolazco G, Kovanecz I, Vernet D, Gelfand RA, Tsao J, Ferrini MG, Magee T, Rajfer J, Gonzalez-Cadavid NF. Effect of muscle-derived stem cells on the restoration of corpora cavernosa smooth muscle and erectile function in the aged rat. BJU Int. 2008;101:1156-1164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 92] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 75. | Woo JC, Bae WJ, Kim SJ, Kim SD, Sohn DW, Hong SH, Lee JY, Hwang TK, Sung YC, Kim SW. Transplantation of muscle-derived stem cells into the corpus cavernosum restores erectile function in a rat model of cavernous nerve injury. Korean J Urol. 2011;52:359-363. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 76. | Kovanecz I, Rivera S, Nolazco G, Vernet D, Segura D, Gharib S, Rajfer J, Gonzalez-Cadavid NF. Separate or combined treatments with daily sildenafil, molsidomine, or muscle-derived stem cells prevent erectile dysfunction in a rat model of cavernosal nerve damage. J Sex Med. 2012;9:2814-2826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 77. | Rübe CE, Fricke A, Widmann TA, Fürst T, Madry H, Pfreundschuh M, Rübe C. Accumulation of DNA damage in hematopoietic stem and progenitor cells during human aging. PLoS One. 2011;6:e17487. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 224] [Cited by in RCA: 227] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 78. | Schuler N, Rübe CE. Accumulation of DNA damage-induced chromatin alterations in tissue-specific stem cells: the driving force of aging? PLoS One. 2013;8:e63932. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 79. | Bahk JY, Jung JH, Han H, Min SK, Lee YS. Treatment of diabetic impotence with umbilical cord blood stem cell intracavernosal transplant: preliminary report of 7 cases. Exp Clin Transplant. 2010;8:150-160. [PubMed] |
| 80. | Oishi K, Ogawa Y, Gamoh S, Uchida MK. Contractile responses of smooth muscle cells differentiated from rat neural stem cells. J Physiol. 2002;540:139-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 81. | Song Y, Mehta N, Sheh B, Saljooque F, U HS, Rajasekaran M. Transdifferentiation of rat fetal brain stem cells into penile smooth muscle cells. BJU Int. 2009;104:257-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 82. | Trentin A, Glavieux-Pardanaud C, Le Douarin NM, Dupin E. Self-renewal capacity is a widespread property of various types of neural crest precursor cells. Proc Natl Acad Sci USA. 2004;101:4495-4500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 124] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 83. | Maurer J, Fuchs S, Jäger R, Kurz B, Sommer L, Schorle H. Establishment and controlled differentiation of neural crest stem cell lines using conditional transgenesis. Differentiation. 2007;75:580-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 45] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 84. | Song YS, Lee HJ, Park IH, Lim IS, Ku JH, Kim SU. Human neural crest stem cells transplanted in rat penile corpus cavernosum to repair erectile dysfunction. BJU Int. 2008;102:220-224; discussion 224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 37] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 85. | Bochinski D, Lin GT, Nunes L, Carrion R, Rahman N, Lin CS, Lue TF. The effect of neural embryonic stem cell therapy in a rat model of cavernosal nerve injury. BJU Int. 2004;94:904-909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 124] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 86. | Jeong JO, Han JW, Kim JM, Cho HJ, Park C, Lee N, Kim DW, Yoon YS. Malignant tumor formation after transplantation of short-term cultured bone marrow mesenchymal stem cells in experimental myocardial infarction and diabetic neuropathy. Circ Res. 2011;108:1340-1347. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 305] [Cited by in RCA: 305] [Article Influence: 21.8] [Reference Citation Analysis (1)] |