Published online Nov 24, 2014. doi: 10.5410/wjcu.v3.i3.264
Revised: June 26, 2014
Accepted: July 27, 2014
Published online: November 24, 2014
Processing time: 204 Days and 18.9 Hours
Prostate cancer is the most common male malignant neoplasm. Androgens and the androgen receptor (AR) play a key role in the onset and progression of prostate cancer. The expression of the AR is still preserved in the majority of patients with castration-resistant prostate cancer (CRPC). CRPC is considered to be induced by the following mechanisms: (1) sustained AR activation by enhancing intracellular conversion of adrenal androgens to dehydrotestosterone via a de novo route; (2) AR hypersensitivity; (3) promiscuous activation of AR signaling; and (4) outlaw pathways. Recent advances in the treatment of CRPC include novel medicines targeting AR signaling pathways. In addition, functional molecular studies have shown that some of the AR-regulated genes and AR coregulators are prognostic markers and potential therapeutic targets for prostate cancer, particularly in the castration-resistant state. Therefore, identification of the AR signaling pathways responsible for establishment of CRPC is critical for developing new strategies for the treatment of CRPC.
Core tip: Prostate cancer is the most common male malignant neoplasm. Androgens and the androgen receptor (AR) play a key role in the onset and progression of prostate cancer. The expression of the AR is still preserved in the majority of patients with castration-resistant prostate cancer (CRPC). Therefore, identification of the AR signaling pathways responsible for establishment of CRPC is critical for developing new strategies for the treatment of CRPC.
- Citation: Obinata D, Fujiwara K, Yamaguchi K, Takayama KI, Urano T, Nagase H, Inoue S, Takahashi S, Fukuda N. Review of novel therapeutic medicines targeting androgen signaling in castration-resistant prostate cancer. World J Clin Urol 2014; 3(3): 264-271
- URL: https://www.wjgnet.com/2219-2816/full/v3/i3/264.htm
- DOI: https://dx.doi.org/10.5410/wjcu.v3.i3.264
Prostate cancer has been the most common male malignant neoplasm for more than 30 years and is the second leading cause of cancer-related death of men in the United States[1]. In Japan, partially because the diet seems to be becoming Westernized, the incidence of prostate cancer has been increasing. The population of older males is also becoming larger and may also be a contributor.
Androgens and the androgen receptor (AR) play a key role in the onset and progression of prostate cancer. Functional ARs are expressed during various stages of prostate carcinogenesis, from prostate intraepithelial neoplasia to locally advanced primary tumors. Approximately 80%-90% of prostate cancers are androgen-dependent at the time of diagnosis[2-4].
Since the discovery that the progression of prostate cancer could be inhibited by castration in the 1940s[5,6], hormonal therapy that specifically inhibits AR activity by using a luteinizing hormone-releasing hormone analog/antagonist, with/without anti-androgens [androgen deprivation therapy (ADT)] has become the most effective and widely used palliative method for advanced and/or metastatic prostate cancer[7-9]. In the majority of patients, although ADT leads to a biochemical response for up to 3 years, prostate cancer eventually continues to progress through cell transformation. Previously, these conditions were known by various names over the years, including hormone-resistant prostate cancer and androgen-insensitive prostate cancer. However, most recent reports indicate that AR is still expressed in the majority of ADT resistant cases, and expression of AR target genes, such as prostate-specific antigen (PSA), remains persistently high despite serum testosterone in the castrated range after surgical castration or ADT[9-11]. This condition is called castration-resistant prostate cancer (CRPC)[12]. Patients with CRPC demonstrate poor prognosis associated with a deterioration in the quality of life, and few therapeutic options are currently available[13]. Therefore, it is important to understand the AR signaling pathway to develop an effective treatment for CRPC. In this review, we summarize the roles of the AR signaling pathway and novel therapeutic medicines that target this pathway in prostate cancer. We focus in particular on functional analyses of AR targets and indicate future directions for their therapeutic use.
The AR gene is a member of the steroid hormone receptor superfamily, which includes genes encoding receptors for estrogen, progesterone, glucocorticoids, mineralocorticoids, vitamin D, retinoic acid, and the retinoid X receptor. Similar to many other steroid receptors, the AR is characterized by a modular structure consisting of distinct functional domains: a poorly conserved N-terminal domain (NTD; 555 amino acids coded by exon 1), a highly conserved DNA-binding domain (DBD; 68-amino acid coded by exon 2 and 3), a hinge region, and a moderately conserved ligand binding domain (LBD; 295 amino acids coded by exons 4-8)[14]. The AR NTD contains the major transactivation function of the AR, which is known as activation function (AF)-1, and consists of two transcriptional activation units (TAU): TAU-1 and TAU-5[15]. AF-1 interacts with the LBD, the basal transcription factors transcription factor IIF (TFIIF) and TFIIH, members of the p160 family of nuclear receptor coactivator proteins and the general coactivator cAMP response-binding protein-binding protein[16-28]. These reports also indicate that AF-1 is one of the major domains responsible for mediating AR transcriptional activity.
The DBD has important roles in mediating AR nuclear localization, homodimer formation, and specific DNA binding. The activated AR binds as a homodimer to specific DNA sequences called androgen response elements (AREs) located around the target genes[29]. These AREs can be classified into two types: canonical and non-canonical AREs[29]. Canonical AREs consist of an inverted repeat of hexameric half-sites (5’-TGTTCT-3’) with a 3-bp spacer[30]. The non-canonical AREs have an atypical motif, and their binding specificity to steroid receptors is relatively weak, resulting in their need for coregulators[31,32]. Data indicate that some important androgen-regulated genes in prostate cancer are regulated by atypical AREs. The DBD contains two zinc finger domains. The first zinc finger contains a conserved P-box motif that binds the half site of the classical ARE. The second zinc finger contains a conserved D-box motif that functions to stabilize the DNA-receptor complex and to produce a receptor homodimer[33].
The LBD is located in the C-terminal region and comprises 12 helices. The LBD assists in the binding of dihydrotestosterone (DHT), which is the first step in the androgen signaling pathway. Like the AF-1 region in the NTD, the LBD contains an AF2 that interacts with coregulators [the steroid receptor coactivator (SRC)/p160 family] to bind to the NTD[34,35]. Because AF-2 shows a higher affinity for the NTD than the coactivator, interaction with the NTD is the primary role for AF-2 rather than direct transcriptional activation. The LBD plays a key role in current ADT for prostate cancer. Although anti-androgens used in ADT, such as bicalutamide, block the activity of AF-2 by binding to the LBD[36], point mutations in the AR in prostate cancer primarily occur in the regions of the LBD that include amino acids 670-676, 701-730, and 874-919, causing resistance to anti-androgens binding and proliferation in the presence of the anti-androgens[37-39].
Androgens, the male sex steroids, regulate numerous physiological responses ranging from male sexual differentiation to the development of bone and muscle. The biological action of androgens is mediated through the AR. In prostate tissue, DHT is the primary ligand for the AR and is synthesized from testosterone by 5-reductase. The ligand-unbound AR is present primarily in the cytoplasm, where it interacts with heat shock proteins (Hsp)-90, -70, -56, cytoskeletal proteins, and other co-chaperones[40]. The AR-Hsp90 interaction is necessary to maintain the AR in a high-affinity ligand-binding conformation, suggesting that Hsp90 plays a key role in the activation of agonist-bound AR regulation of nuclear transfer, nuclear matrix binding, and transcriptional activity. Following DHT binding to the AR, the AR translocates into the nucleus, and binds to the ARE in the promoter and enhancer regions of target genes. For example, PSA is a typical product of the AR-dependent gene and an important biomarker for prostate cancer.
The AR transcriptional complex is completed by recruitment of coregulators, which ultimately results in regulation of gene expression[36]. After the discovery of SRC-1, more than 200 nuclear receptor coregulators have been identified[41,42]. Coregulators were previously classified as either enhancing (coactivators) or repressing (corepressors) AR activity, and the requirements for coregulators vary among genes[43].
The AR is also regulated by post-translational modifications generated by signal transduction pathways. These modifications can be further divided into two categories: (1) reversible modifications of specific amino acid residues of target proteins (phosphorylation and acetylation); and (2) modifications involving addition of other proteins or polypeptides (ubiquitination and sumoylation). These changes have the potential to affect AR stability, subcellular localization, and interaction with other proteins, including coregulators.
Castration resistance has been reported to be induced by: (1) sustained AR activation by enhancing intracellular conversion of adrenal androgens to DHT via a de novo route[44]; (2) AR hypersensitivity[45]; (3) promiscuous activation of AR signaling; and (4) outlaw pathways[9]. AR hypersensitivity results in the facilitation of a susceptibility to androgen by variation of a coregulator or cytokine activity and overexpression of the AR. In addition, Cai et al[46] reported that prostate cancer cells incubated with levels of androgen comparable to those seen in castration decreased AR-induced lysine-specific demethylase 1 levels, which negatively regulates AR signaling, resulting in an increase in the expression of AR and of multiple genes that contribute to increased androgen synthesis and sensitivity in CRPC[46].
Promiscuous activation of the AR signaling pathway occurs in cases of AR structural change and when the AR combines with ligands other than androgen. This phenomenon induces the anti-androgen withdrawal syndrome, i.e., anti-androgen itself serves as an accelerator of progression. Outlaw pathways include AR structural changes when androgen is absent or when cytokines other than androgen bind to the AR to activate a signaling pathway, resulting in a facilitation of an androgenic response in gene expression. The extragonadal androgen synthesized in adrenal or prostate cancer cells plays a key role in the occurrence of sustained AR activation. Androgen is a metabolite of cholesterol in the testis and adrenal gland. Most of its synthetases belong to the cytochrome P450 (CYP) family. CYP17 in particular has both 17-hydroxylase and 17,20-lyase activity that plays an important role in the synthesis of adrenal androgen. Castration does not influence the synthesis of adrenal androgen. Adrenal androgen is converted into DHT by 5-reductase in prostate cancer cells. The affinity of DHT for the ligand-binding domain on the AR is higher than testosterone as a main ligand in prostate cells. Furthermore, the CRPC cells contain increased CYP17 activity with a new metabolic pathway that converts cholesterol to androgen. Thus, the expression of androgen-dependent genes is achieved by a very small amount of testosterone under castration[47].
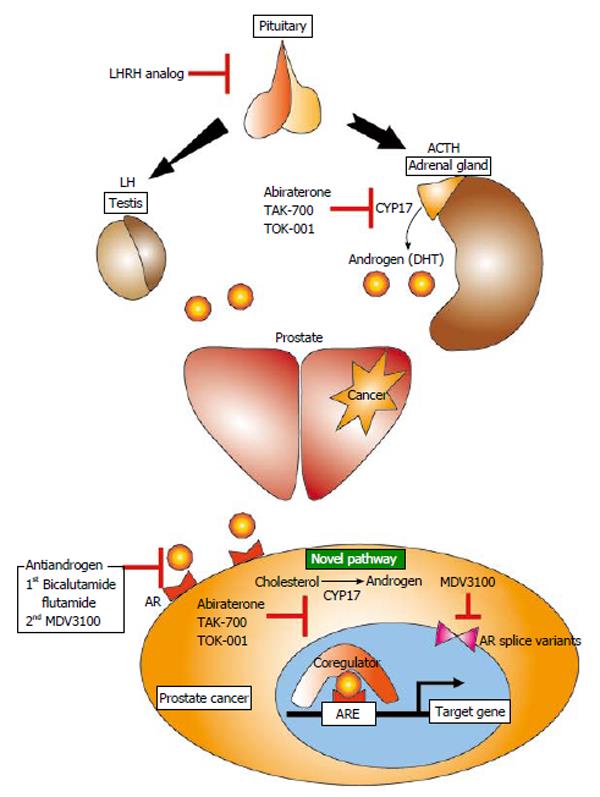
Standard therapeutic strategies for prostate cancers have recently been changed to utilize newly developed medicines for CRPCs. As described above, because androgens and the ARs remaining in CRPC cells are important for cancer progression, novel medicines should be designed to target the androgen synthesis pathway or the AR signaling pathway (Figure 1). Here, we introduce these new medicines, including compounds currently being developed in Japan.
Abiraterone is a dual inhibitor of the 17-hydroxylase and 17,20-lyase expressed in testicular, adrenal, and prostatic tumor tissues. Ketoconazole, an azole antifungal medicine that has a similar target as abiraterone, initially showed a preferable outcome in patients with CRPC[48]. Ketoconazole inhibits both testicular and adrenal androgen biosynthesis by targeting cytochrome P450 isozyme 3A4 and 17,20-lyase. However ketoconazole results in severe toxicities because it has low specificity for the CYP17 family. Based on these pieces of evidence, abiraterone was selected for development at the Institute of Cancer Research in the UK as a selective inhibitor of CYP17. The multicenter phase III randomized placebo-controlled trial COU-AA-301 evaluated the efficacy of abiraterone compared with docetaxel for men with progressive CRPC[49]. The overall survival in the abiraterone arm was significantly longer than in the docetaxel arm (14.8 vs 10.9 mo). However, abiraterone induces hyperaldosteronism by reduction of glucocorticoid following secondary adrenocorticotropic hormone overexpression. Although steroids are useful to protect against such adverse effects, the combination of steroids with abiraterone could limit its use for patients with early-stage disease and longer life expectancies.
TAK-700 is a next-generation CYP17 inhibitor that requires no steroid administration. Because TAK-700 selectively targets 17,20-lyase, and inhibition of 17α-hydroxylase and reduction of glucocorticoids were smaller than those seen in patients treated with abiraterone[47,50]. A phase III clinical trial of TAK-700 for metastatic CRPC (Evaluation of the Lyase inhibitor orteronel in Metastatic Prostate Cancer 5) is ongoing. The results of the interim analysis show that although overall survival was not significantly improved as a primary endpoint, progression-free survival was improved in the TAK-700 arm compared with the placebo arm (HR = 0.755).
The chemical composition of TOK-001 is similar to that of abiraterone. In addition to 17-hydroxylase inhibitory activity, TOK-001 also has AR antagonistic action[51-53]. Phase I/II clinical trials for CRPC are ongoing.
MDV3100 binds to the AR directly and targets multiple steps in the AR signaling pathway, including translocation to the nucleus and binding of the AR to the ARE and coregulators. Because MDV3100 differs from an agonistic anti-androgen, this compound does not induce anti-androgen withdrawal syndrome. MDV3100 induces the same action as the structural variant AR, which causes castration resistance; therefore, it is considered to be a second generation anti-androgenic agent[54]. In a phase III double-blind, placebo-controlled trial (AFFIRM Clinical Trials), MDV3100 was superior in the proportion of patients with a reduction in PSA level by 50% or more, the quality-of-life response rate, progression-free survival, and overall survival of men with metastatic CRPC after chemotherapy[55]. Based on these results, MDV3100 obtained approval for treatment of metastatic prostate cancer in Europe. In addition, recent clinical trial (PREVAIL Clinical Trials) showed that MDV3100 significantly decreased the risk of radiographic progression and prolonged overall survival in men with metastatic CRPC who have not received chemotherapy[56].
The goal of the AR signaling pathway is the transcriptional activation of target genes (androgen-responsive genes). The overexpression of the androgen-responsive genes is the main cause of cancer progression regardless of the presence or absence of castration resistance. We have reported the results of the functional analysis of novel androgen responsive genes and AR coregulators that influence the progression of prostate cancer[57-62]. Here, we introduce a part of these studies, including therapeutic medicines.
ADP ribosylation factor GTPase-activating protein 3 (ARFGAP3) is a novel androgen-regulated gene that is considered to be associated with regulation of the vesicular transport of the Golgi apparatus. In androgen-sensitive prostate cancer LNCaP cells, we observed induction of ARFGAP3 expression at the mRNA and protein levels in response to stimulation with 100 nmol/L DHT[59]. In functional analyses using LNCaP cells, the increased expression of ARFGAP3 was associated with cell growth, the G1/S cell cycle progression and cell migration. In addition, we found that ARFGAP3 interacted with paxillin as an AR coactivator, and enhanced migration activity and AR activity in LNCaP cells[59]. These findings suggest that ARFGAP3 is a novel androgen-regulated gene that can promote prostate cancer cell proliferation and migration in collaboration with paxillin.
Octamer transcription factor 1 (Oct1) is a ubiquitous member of the Pit-Oct-Unc-homeodomain family. Although Oct1 does not demonstrate androgenic responsiveness, it works as a coregulator of the AR, binding to neighbors of the ARE and regulating AR activity[29]. We found that Oct1 is expressed in the nuclei of LNCaP cells using immunocytochemistry. SiRNA silencing of Oct1 inhibited the proliferation in LNCaP cells[60]. In addition, using surgical specimens, we found a positive correlation between Oct1 immunoreactivity in samples with a high Gleason score and AR immunoreactivity. Moreover, patients with high immunoreactivities of both Oct1 and AR exhibited poorer cancer-specific survival[60]. These results demonstrate that Oct1 may be a prognostic factor for prostate cancer and a contributing factor for increased AR sensitivity and castration resistance.
Pyrrole-imidazole (PI) polyamides are small synthetic molecules that recognize and attach to the minor groove of DNA to inhibit DNA-transcription factor interactions with high affinity and sequence specificity[63-65]. DNA recognition by PI polyamides depends on a code of side-by-side pairing of pyrrole and imidazole in the hairpin structure. Various types of sequence-specific PI polyamides have recently been developed to control gene expression[66-69]. One of the most important advantages of PI polyamide is their resistance to biological degradation by nucleases and proteases compared to nucleic acid medicines, including siRNA. In addition, PI polyamides could be efficiently delivered to cell nuclei without any specific drug delivery system. Another important advantage is the safety of PI polyamides when they are injected intravenously or via peritoneal to mice or rats[66,67]. PI polyamide that recognizes AREs suppresses DHT-dependent gene expression in LNCaP cells[70]. In addition, this polyamide was reported to inhibit the binding of RNA polymerase II to the transcription start site of AR-driving genes[71]. These reports indicate that PI polyamides could be a powerful tool in the development of molecularly targeted therapeutics for androgen responsive genes/AR coregulators in prostate cancer.
The clinical challenges in prostate cancer are currently focused on controlling the action of the AR, which plays an important role in the development of hormone therapy naïve prostate cancer and also CRPC. Recent evidence shows that CRPC cells are still dependent on AR activity after ADT. Selective inhibition of the AR signaling pathway by typical ADT induced a bypass mechanism to activate the AR in low dose or in the absence of DHT, and thereby restore AR-dependent cellular proliferation. Thus, blocking the AR with second-generation AR antagonists has the potential to treat CRPC because of stronger and more durable inhibition of transcriptional activity than previous compounds. Various functional studies, including our reports, have cited androgen-regulated genes and AR collaborating factors, such as Oct1, as preferable candidates for biomarkers and therapeutic targets for CRPC. We believe future investigations of the AR signaling pathway and novel therapeutics targeting this pathway are mainstays for considering new strategies to treat CRPC.
P- Reviewer: Liu G, Pastore AL, Wu KM S- Editor: Ji FF L- Editor: Cant MR E- Editor: Liu SQ
| 1. | Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9215] [Cited by in RCA: 9856] [Article Influence: 821.3] [Reference Citation Analysis (4)] |
| 2. | Hobisch A, Culig Z, Radmayr C, Bartsch G, Klocker H, Hittmair A. Androgen receptor status of lymph node metastases from prostate cancer. Prostate. 1996;28:129-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 3. | Tilley WD, Lim-Tio SS, Horsfall DJ, Aspinall JO, Marshall VR, Skinner JM. Detection of discrete androgen receptor epitopes in prostate cancer by immunostaining: measurement by color video image analysis. Cancer Res. 1994;54:4096-4102. [PubMed] |
| 4. | van der Kwast TH, Têtu B. Androgen receptors in untreated and treated prostatic intraepithelial neoplasia. Eur Urol. 1996;30:265-268. [PubMed] |
| 5. | Huggins C. Effect of orchiectomy and irradiation on cancer of the prostate. Ann Surg. 1942;115:1192-1200. [PubMed] |
| 6. | Huggins C, Hodges CV. The effect of castration, of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. Cancer Res. 1941;1:293-297. |
| 7. | Trapman J, Brinkmann AO. The androgen receptor in prostate cancer. Pathol Res Pract. 1996;192:752-760. [PubMed] |
| 8. | Taplin ME, Balk SP. Androgen receptor: a key molecule in the progression of prostate cancer to hormone independence. J Cell Biochem. 2004;91:483-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 299] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 9. | Feldman BJ, Feldman D. The development of androgen-independent prostate cancer. Nat Rev Cancer. 2001;1:34-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1737] [Cited by in RCA: 1735] [Article Influence: 72.3] [Reference Citation Analysis (0)] |
| 10. | Denmeade SR, Isaacs JT. A history of prostate cancer treatment. Nat Rev Cancer. 2002;2:389-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 390] [Cited by in RCA: 413] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 11. | Arnold JT, Isaacs JT. Mechanisms involved in the progression of androgen-independent prostate cancers: it is not only the cancer cell’s fault. Endocr Relat Cancer. 2002;9:61-73. [PubMed] |
| 12. | Hotte SJ, Saad F. Current management of castrate-resistant prostate cancer. Curr Oncol. 2010;17 Suppl 2:S72-S79. [PubMed] |
| 13. | Kirby M, Hirst C, Crawford ED. Characterising the castration-resistant prostate cancer population: a systematic review. Int J Clin Pract. 2011;65:1180-1192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 647] [Cited by in RCA: 610] [Article Influence: 43.6] [Reference Citation Analysis (0)] |
| 14. | Tsai MJ, O’Malley BW. Molecular mechanisms of action of steroid/thyroid receptor superfamily members. Annu Rev Biochem. 1994;63:451-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2097] [Cited by in RCA: 2008] [Article Influence: 64.8] [Reference Citation Analysis (0)] |
| 15. | Jenster G, van der Korput HA, Trapman J, Brinkmann AO. Identification of two transcription activation units in the N-terminal domain of the human androgen receptor. J Biol Chem. 1995;270:7341-7346. [PubMed] |
| 16. | Ma H, Hong H, Huang SM, Irvine RA, Webb P, Kushner PJ, Coetzee GA, Stallcup MR. Multiple signal input and output domains of the 160-kilodalton nuclear receptor coactivator proteins. Mol Cell Biol. 1999;19:6164-6173. [PubMed] |
| 17. | Bevan CL, Hoare S, Claessens F, Heery DM, Parker MG. The AF1 and AF2 domains of the androgen receptor interact with distinct regions of SRC1. Mol Cell Biol. 1999;19:8383-8392. [PubMed] |
| 18. | Alen P, Claessens F, Verhoeven G, Rombauts W, Peeters B. The androgen receptor amino-terminal domain plays a key role in p160 coactivator-stimulated gene transcription. Mol Cell Biol. 1999;19:6085-6097. [PubMed] |
| 19. | Chamberlain NL, Whitacre DC, Miesfeld RL. Delineation of two distinct type 1 activation functions in the androgen receptor amino-terminal domain. J Biol Chem. 1996;271:26772-26778. [PubMed] |
| 20. | Langley E, Zhou ZX, Wilson EM. Evidence for an anti-parallel orientation of the ligand-activated human androgen receptor dimer. J Biol Chem. 1995;270:29983-29990. [PubMed] |
| 21. | Doesburg P, Kuil CW, Berrevoets CA, Steketee K, Faber PW, Mulder E, Brinkmann AO, Trapman J. Functional in vivo interaction between the amino-terminal, transactivation domain and the ligand binding domain of the androgen receptor. Biochemistry. 1997;36:1052-1064. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 152] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 22. | Ikonen T, Palvimo JJ, Jänne OA. Interaction between the amino- and carboxyl-terminal regions of the rat androgen receptor modulates transcriptional activity and is influenced by nuclear receptor coactivators. J Biol Chem. 1997;272:29821-29828. [PubMed] |
| 23. | Berrevoets CA, Doesburg P, Steketee K, Trapman J, Brinkmann AO. Functional interactions of the AF-2 activation domain core region of the human androgen receptor with the amino-terminal domain and with the transcriptional coactivator TIF2 (transcriptional intermediary factor2). Mol Endocrinol. 1998;12:1172-1183. [PubMed] |
| 24. | He B, Kemppainen JA, Voegel JJ, Gronemeyer H, Wilson EM. Activation function 2 in the human androgen receptor ligand binding domain mediates interdomain communication with the NH(2)-terminal domain. J Biol Chem. 1999;274:37219-37225. [PubMed] |
| 25. | McEwan IJ, Gustafsson J. Interaction of the human androgen receptor transactivation function with the general transcription factor TFIIF. Proc Natl Acad Sci USA. 1997;94:8485-8490. [PubMed] |
| 26. | Lee DK, Duan HO, Chang C. From androgen receptor to the general transcription factor TFIIH. Identification of cdk activating kinase (CAK) as an androgen receptor NH(2)-terminal associated coactivator. J Biol Chem. 2000;275:9308-9313. [PubMed] |
| 27. | Aarnisalo P, Palvimo JJ, Jänne OA. CREB-binding protein in androgen receptor-mediated signaling. Proc Natl Acad Sci USA. 1998;95:2122-2127. [PubMed] |
| 28. | Frønsdal K, Engedal N, Slagsvold T, Saatcioglu F. CREB binding protein is a coactivator for the androgen receptor and mediates cross-talk with AP-1. J Biol Chem. 1998;273:31853-31859. [PubMed] |
| 29. | Wang Q, Li W, Liu XS, Carroll JS, Jänne OA, Keeton EK, Chinnaiyan AM, Pienta KJ, Brown M. A hierarchical network of transcription factors governs androgen receptor-dependent prostate cancer growth. Mol Cell. 2007;27:380-392. [PubMed] |
| 30. | Mangelsdorf DJ, Thummel C, Beato M, Herrlich P, Schütz G, Umesono K, Blumberg B, Kastner P, Mark M, Chambon P. The nuclear receptor superfamily: the second decade. Cell. 1995;83:835-839. [PubMed] |
| 31. | Claessens F, Denayer S, Van Tilborgh N, Kerkhofs S, Helsen C, Haelens A. Diverse roles of androgen receptor (AR) domains in AR-mediated signaling. Nucl Recept Signal. 2008;6:e008. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 165] [Cited by in RCA: 168] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 32. | Shaffer PL, Jivan A, Dollins DE, Claessens F, Gewirth DT. Structural basis of androgen receptor binding to selective androgen response elements. Proc Natl Acad Sci USA. 2004;101:4758-4763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 274] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 33. | Umesono K, Evans RM. Determinants of target gene specificity for steroid/thyroid hormone receptors. Cell. 1989;57:1139-1146. [PubMed] |
| 34. | Heery DM, Kalkhoven E, Hoare S, Parker MG. A signature motif in transcriptional co-activators mediates binding to nuclear receptors. Nature. 1997;387:733-736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1604] [Cited by in RCA: 1566] [Article Influence: 55.9] [Reference Citation Analysis (0)] |
| 35. | Duff J, Davies P, Watt K, McEwan IJ. Structural dynamics of the human androgen receptor: implications for prostate cancer and neurodegenerative disease. Biochem Soc Trans. 2006;34:1098-1102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 36. | Dehm SM, Tindall DJ. Molecular regulation of androgen action in prostate cancer. J Cell Biochem. 2006;99:333-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 221] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 37. | Taplin ME, Bubley GJ, Shuster TD, Frantz ME, Spooner AE, Ogata GK, Keer HN, Balk SP. Mutation of the androgen-receptor gene in metastatic androgen-independent prostate cancer. N Engl J Med. 1995;332:1393-1398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 902] [Cited by in RCA: 861] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 38. | Taplin ME, Rajeshkumar B, Halabi S, Werner CP, Woda BA, Picus J, Stadler W, Hayes DF, Kantoff PW, Vogelzang NJ. Androgen receptor mutations in androgen-independent prostate cancer: Cancer and Leukemia Group B Study 9663. J Clin Oncol. 2003;21:2673-2678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 278] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 39. | Buchanan G, Greenberg NM, Scher HI, Harris JM, Marshall VR, Tilley WD. Collocation of androgen receptor gene mutations in prostate cancer. Clin Cancer Res. 2001;7:1273-1281. [PubMed] |
| 40. | Smith DF, Toft DO. Minireview: the intersection of steroid receptors with molecular chaperones: observations and questions. Mol Endocrinol. 2008;22:2229-2240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 136] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 41. | Oñate SA, Tsai SY, Tsai MJ, O’Malley BW. Sequence and characterization of a coactivator for the steroid hormone receptor superfamily. Science. 1995;270:1354-1357. [PubMed] |
| 42. | Heemers HV, Tindall DJ. Androgen receptor (AR) coregulators: a diversity of functions converging on and regulating the AR transcriptional complex. Endocr Rev. 2007;28:778-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 505] [Cited by in RCA: 523] [Article Influence: 29.1] [Reference Citation Analysis (0)] |
| 43. | Agoulnik IU, Weigel NL. Coactivator selective regulation of androgen receptor activity. Steroids. 2009;74:669-674. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 47] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 44. | Stanbrough M, Bubley GJ, Ross K, Golub TR, Rubin MA, Penning TM, Febbo PG, Balk SP. Increased expression of genes converting adrenal androgens to testosterone in androgen-independent prostate cancer. Cancer Res. 2006;66:2815-2825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 779] [Cited by in RCA: 819] [Article Influence: 43.1] [Reference Citation Analysis (0)] |
| 45. | Buchanan G, Irvine RA, Coetzee GA, Tilley WD. Contribution of the androgen receptor to prostate cancer predisposition and progression. Cancer Metastasis Rev. 2001;20:207-223. [PubMed] |
| 46. | Cai C, He HH, Chen S, Coleman I, Wang H, Fang Z, Chen S, Nelson PS, Liu XS, Brown M. Androgen receptor gene expression in prostate cancer is directly suppressed by the androgen receptor through recruitment of lysine-specific demethylase 1. Cancer Cell. 2011;20:457-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 327] [Cited by in RCA: 374] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 47. | Yamaoka M, Hara T, Kusaka M. Overcoming persistent dependency on androgen signaling after progression to castration-resistant prostate cancer. Clin Cancer Res. 2010;16:4319-4324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 69] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 48. | Small EJ, Halabi S, Dawson NA, Stadler WM, Rini BI, Picus J, Gable P, Torti FM, Kaplan E, Vogelzang NJ. Antiandrogen withdrawal alone or in combination with ketoconazole in androgen-independent prostate cancer patients: a phase III trial (CALGB 9583). J Clin Oncol. 2004;22:1025-1033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 379] [Cited by in RCA: 363] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 49. | de Bono JS, Logothetis CJ, Molina A, Fizazi K, North S, Chu L, Chi KN, Jones RJ, Goodman OB, Saad F. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364:1995-2005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3125] [Cited by in RCA: 3390] [Article Influence: 242.1] [Reference Citation Analysis (0)] |
| 50. | Kaku T, Hitaka T, Ojida A, Matsunaga N, Adachi M, Tanaka T, Hara T, Yamaoka M, Kusaka M, Okuda T. Discovery of orteronel (TAK-700), a naphthylmethylimidazole derivative, as a highly selective 17,20-lyase inhibitor with potential utility in the treatment of prostate cancer. Bioorg Med Chem. 2011;19:6383-6399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 98] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 51. | Vasaitis T, Belosay A, Schayowitz A, Khandelwal A, Chopra P, Gediya LK, Guo Z, Fang HB, Njar VC, Brodie AM. Androgen receptor inactivation contributes to antitumor efficacy of 17{alpha}-hydroxylase/17,20-lyase inhibitor 3beta-hydroxy-17-(1H-benzimidazole-1-yl)androsta-5,16-diene in prostate cancer. Mol Cancer Ther. 2008;7:2348-2357. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 127] [Cited by in RCA: 115] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 52. | Vasaitis TS, Bruno RD, Njar VC. CYP17 inhibitors for prostate cancer therapy. J Steroid Biochem Mol Biol. 2011;125:23-31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 161] [Cited by in RCA: 151] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 53. | Handratta VD, Vasaitis TS, Njar VC, Gediya LK, Kataria R, Chopra P, Newman D, Farquhar R, Guo Z, Qiu Y. Novel C-17-heteroaryl steroidal CYP17 inhibitors/antiandrogens: synthesis, in vitro biological activity, pharmacokinetics, and antitumor activity in the LAPC4 human prostate cancer xenograft model. J Med Chem. 2005;48:2972-2984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 197] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 54. | Watson PA, Chen YF, Balbas MD, Wongvipat J, Socci ND, Viale A, Kim K, Sawyers CL. Constitutively active androgen receptor splice variants expressed in castration-resistant prostate cancer require full-length androgen receptor. Proc Natl Acad Sci USA. 2010;107:16759-16765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 467] [Cited by in RCA: 509] [Article Influence: 33.9] [Reference Citation Analysis (0)] |
| 55. | Scher HI, Fizazi K, Saad F, Taplin ME, Sternberg CN, Miller K, de Wit R, Mulders P, Chi KN, Shore ND. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367:1187-1197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3198] [Cited by in RCA: 3457] [Article Influence: 265.9] [Reference Citation Analysis (0)] |
| 56. | Beer TM, Armstrong AJ, Rathkopf DE, Loriot Y, Sternberg CN, Higano CS, Iversen P, Bhattacharya S, Carles J, Chowdhury S. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med. 2014;371:424-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2205] [Cited by in RCA: 2273] [Article Influence: 206.6] [Reference Citation Analysis (1)] |
| 57. | Murata T, Takayama K, Katayama S, Urano T, Horie-Inoue K, Ikeda K, Takahashi S, Kawazu C, Hasegawa A, Ouchi Y. miR-148a is an androgen-responsive microRNA that promotes LNCaP prostate cell growth by repressing its target CAND1 expression. Prostate Cancer Prostatic Dis. 2010;13:356-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 122] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 58. | Murata T, Takayama K, Urano T, Fujimura T, Ashikari D, Obinata D, Horie-Inoue K, Takahashi S, Ouchi Y, Homma Y. 14-3-3ζ, a novel androgen-responsive gene, is upregulated in prostate cancer and promotes prostate cancer cell proliferation and survival. Clin Cancer Res. 2012;18:5617-5627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 55] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 59. | Obinata D, Takayama K, Urano T, Murata T, Ikeda K, Horie-Inoue K, Ouchi Y, Takahashi S, Inoue S. ARFGAP3, an androgen target gene, promotes prostate cancer cell proliferation and migration. Int J Cancer. 2012;130:2240-2248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 60. | Obinata D, Takayama K, Urano T, Murata T, Kumagai J, Fujimura T, Ikeda K, Horie-Inoue K, Homma Y, Ouchi Y. Oct1 regulates cell growth of LNCaP cells and is a prognostic factor for prostate cancer. Int J Cancer. 2012;130:1021-1028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 53] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 61. | Takayama K, Horie-Inoue K, Suzuki T, Urano T, Ikeda K, Fujimura T, Takahashi S, Homma Y, Ouchi Y, Inoue S. TACC2 is an androgen-responsive cell cycle regulator promoting androgen-mediated and castration-resistant growth of prostate cancer. Mol Endocrinol. 2012;26:748-761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 62. | Takayama K, Tsutsumi S, Suzuki T, Horie-Inoue K, Ikeda K, Kaneshiro K, Fujimura T, Kumagai J, Urano T, Sakaki Y. Amyloid precursor protein is a primary androgen target gene that promotes prostate cancer growth. Cancer Res. 2009;69:137-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 90] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 63. | Dervan PB, Edelson BS. Recognition of the DNA minor groove by pyrrole-imidazole polyamides. Curr Opin Struct Biol. 2003;13:284-299. [PubMed] |
| 64. | Dervan PB. Molecular recognition of DNA by small molecules. Bioorg Med Chem. 2001;9:2215-2235. [PubMed] |
| 65. | Trauger JW, Baird EE, Dervan PB. Recognition of DNA by designed ligands at subnanomolar concentrations. Nature. 1996;382:559-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 329] [Cited by in RCA: 319] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 66. | Wang X, Nagase H, Watanabe T, Nobusue H, Suzuki T, Asami Y, Shinojima Y, Kawashima H, Takagi K, Mishra R. Inhibition of MMP-9 transcription and suppression of tumor metastasis by pyrrole-imidazole polyamide. Cancer Sci. 2010;101:759-766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 49] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 67. | Matsuda H, Fukuda N, Ueno T, Katakawa M, Wang X, Watanabe T, Matsui S, Aoyama T, Saito K, Bando T. Transcriptional inhibition of progressive renal disease by gene silencing pyrrole-imidazole polyamide targeting of the transforming growth factor-β1 promoter. Kidney Int. 2011;79:46-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 62] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 68. | Murty MS, Sugiyama H. Biology of N-methylpyrrole-N-methylimidazole hairpin polyamide. Biol Pharm Bull. 2004;27:468-474. [PubMed] |
| 69. | Sato A, Nagase H, Obinata D, Fujiwara K, Fukuda N, Soma M, Yamaguchi K, Kawata N, Takahashi S. Inhibition of MMP-9 using a pyrrole-imidazole polyamide reduces cell invasion in renal cell carcinoma. Int J Oncol. 2013;43:1441-1446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 70. | Nickols NG, Dervan PB. Suppression of androgen receptor-mediated gene expression by a sequence-specific DNA-binding polyamide. Proc Natl Acad Sci USA. 2007;104:10418-10423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 165] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 71. | Yang F, Nickols NG, Li BC, Marinov GK, Said JW, Dervan PB. Antitumor activity of a pyrrole-imidazole polyamide. Proc Natl Acad Sci USA. 2013;110:1863-1868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 100] [Article Influence: 8.3] [Reference Citation Analysis (0)] |