Published online Nov 24, 2014. doi: 10.5410/wjcu.v3.i3.184
Revised: June 26, 2014
Accepted: July 25, 2014
Published online: November 24, 2014
Processing time: 217 Days and 17 Hours
Benign prostatic hyperplasia (BPH) is a pathologic condition of the prostate described as a substantial increase in its number of epithelial and stromal cells. BPH may significantly reduce the quality of life due to the initiation of bladder outlet obstruction and lower urinary tract syndromes. Current medical therapies mostly consist of inhibitors of 5α-reductase or α1-adrenergic blockers; their efficacy is often insufficient. Antagonistic analogs of neuropeptide hormones are novel candidates for the management of BPH. At first, antagonists of luteinizing hormone-releasing hormone (LHRH) have been introduced to the therapy aimed to reduce serum testosterone levels. However, they have also been found to produce an inhibitory activity on local LHRH receptors in the prostate as well as impotence and other related side effects. Since then, several preclinical and clinical studies reported the favorable effects of LHRH antagonists in BPH. In contrast, antagonists of growth hormone-releasing hormone (GHRH) and gastrin-releasing peptide (GRP) have been tested only in preclinical settings and produce significant reduction in prostate size in experimental models of BPH. They act at least in part, by blocking the action of respective ligands produced locally on prostates through their respective receptors in the prostate, and by inhibition of autocrine insulin-like growth factors-I/II and epidermal growth factor production. GHRH and LHRH antagonists were also tested in combination resulting in a cumulative effect that was greater than that of each alone. This article will review the numerous studies that demonstrate the beneficial effects of antagonistic analogs of LHRH, GHRH and GRP in BPH, as well as suggesting a potential role for somatostatin analogs in experimental therapies.
Core tip: A new, effective treatment for benign prostatic hyperplasia (BPH) is critically needed. Present side effects of therapy include impotence, decreased libido, abnormal ejaculation, dizziness, weakness, blurred vision and insomnia. Preclinical data suggest that antagonists of neuropeptides growth hormone-releasing hormone, luteinizing hormone-releasing hormone and gastrin-releasing peptide are effective in shrinking prostates in part by suppressing growth factors and inflammatory cytokines. Their effect is exerted through a decrease in levels of circulating hormones and also on a direct action on their respective prostatic receptors. These analogs seem to have the same clinical effects as the currently available BPH medical therapies but possess greater efficacy and have fewer or no side effects.
- Citation: Popovics P, Schally AV, Block NL, Rick FG. Preclinical therapy of benign prostatic hyperplasia with neuropeptide hormone antagonists. World J Clin Urol 2014; 3(3): 184-194
- URL: https://www.wjgnet.com/2219-2816/full/v3/i3/184.htm
- DOI: https://dx.doi.org/10.5410/wjcu.v3.i3.184
Benign prostatic hyperplasia (BPH) is an age-dependent condition which may start as early as 40 years of age and its prevalence increases to 50%-60% in men in their 60’s[1,2]. The BPH-associated growth in prostatic volume arises from the increase in epithelial and stromal cell number occurring mainly in the transition zone of the prostate[3]. In some cases, histologic BPH remains asymptomatic and the patient does not require clinical treatment. However, the prostate gland frequently becomes substantially enlarged, resulting in compression of the diameter of the urethra thus leading to bladder outlet obstruction[1]. Lower urinary tract symptoms (LUTS) that are often associated with BPH are developed in response to the increased resistance of the urethra and the consequently elevated pressure in the bladder[4,5]. Unfortunately, the current medical modalities aimed at treating BPH are not completely effective[6]. These include therapies targeting 5α-reductase activity to inhibit the production of dihydrotestosterone as well as compounds that reduce the adrenergic tone at the bladder outlet, these collectively known as α1-adrenergic blockers[7-9]. When an intervention is required, either a minimally invasive technique (such as transurethral needle ablation or microwave thermotherapy) or surgery (transurethral resection of the prostate or “open prostatectomy”) is performed to reduce the volume of the prostate and its restriction in outlet flow[10-12].
The pathogenesis of BPH is not completely understood although it has been suggested that a decrease in the rate of cell death is more critical for the hyperplastic behavior than a rise in cell proliferation[13]. Various factors, such as a discrepancy in androgen and estrogen levels[14-17], altered autocrine regulation by growth factors [most importantly fibroblast growth factors-2 (FGF-2) and FGF-7][18] or cytokines released by infiltrated inflammatory cells[19] have been found to contribute to the development of BPH. It has also emerged that mesenchymal transition of epithelial and endothelial cells directed by the transforming growth factor (TGF)-β/Smad pathway may play a key role in the pathogenesis of BPH[20]. Most recently, neuropeptide hormones were also found to play a major role in this process, not only by indirectly controlling their classical hormonal targets but also as local regulators in the prostate[21-25]. Consequently, their receptors became potential targets for the development of new treatment strategies for BPH. These include the potential therapeutic utilization of antagonistic analogs of luteinizing hormone-releasing hormone (LHRH), growth hormone-releasing hormone (GHRH) and gastrin-releasing peptide (GRP). The utilization of these analogs in experimental BPH also improved our knowledge on the physiological role of neuropeptides and their receptors in the pathogenesis of BPH. The blockade of these receptors by specific antagonists inhibits the proliferation of stromal and epithelial cells and reduces the release of cytokines and growth factors[6,20,22,24,25] indicating the participation of the native neuropeptides in these processes. As new antagonistic analogs of neuropeptides have recently become available for clinical practice as well others are currently being developed for human trials, we felt that a review of recent findings related to their use in BPH is timely. This review therefore focuses exclusively on preclinical and clinical studies where neuropeptide antagonists were tested against BPH. Additionally, the use of somatostatin agonists is also suggested based on previous findings in prostate cancer with the hope it will facilitate their experimental and clinical testing.
Initially, LHRH antagonists were developed for the purpose of contraception using reduction of the mid-cycle pituitary follicule-stimulating hormone and luteinizing hormone (LH) release thus preventing ovulation[26,27]. Early antagonistic analogs of LHRH demonstrated low potency and significant side effects due to a substantial histamine release[28]. Since those first attempts, many antagonistic analogs of LHRH have been synthetized with higher potency and greatly decreased histamine-releasing activity[29,30]. Cetrorelix[29] was the first antagonistic analog of LHRH that was approved for use in clinical practice as part of the hormonal therapy of in vitro fertilization used to prevent premature LH surges[31]. Numerous clinical trials have been conducted with Cetrotide brand of cetrorelix for the treatment of ovarian cancer, endometriosis, ovarian hyperstimulation syndrome and uterine leiyomyoma[32-35]. Cetrorelix was also tested in patients with prostate cancer[36,37]. The most advanced LHRH antagonist, degarelix, that has been approved for patients with advanced prostate cancer has an improved formula that allows the slow tonic release of the peptide, and moreover, has the lowest histamine-releasing activity among the LHRH antagonists[38,39].
The utilization of LHRH antagonists in the treatment of BPH is suggested by several previous findings. Hormonal therapy with the 5-α reductase inhibitors has long been used to treat BPH and has been shown to shrink prostate volume and improve urinary outflow[16]. This suggests a dihydrotestosterone-dependent pathology of the disease. However, only 30%-50% of patients respond to this treatment[40] highlighting the need for the development of a more effective intervention, such as a systematic suppression of testosterone levels. Cetrorelix (300 μg) was able to reduce serum testosterone levels by 80% at 12 h after administration in men with a mean age of 24[41]. This finding encouraged the clinical testing of cetrorelix in BPH.
In a study by Gonzalez-Barcena et al[36], 11 patients were recruited with symptomatic BPH. Subjects were treated with 500 μg cetrorelix every 12 h for 4 wk in an open label study. Improvements were seen in urinary flow just after the first week of treatment and it became normal after 4 wk. Also, the level of serum acid phosphatases reached normal levels at the end of treatment. Free testosterone levels either dropped immediately after the first cetrorelix injection or decreased gradually throughout the 4 wk, however, in 4 patients it remained similar to pretreatment values. In all cases, prostatic volume decreased significantly which suggests a testosterone-independent action of cetrorelix on the prostate in patients where testosterone level had not been reduced significantly[36]. In a subsequent Phase I/II clinical trial, 13 patients with moderate to severe BPH were treated with a loading dose of 5 mg cetrorelix twice daily for 2 d and then with 1 mg daily for two months[42]. In this study, testosterone fell to castrate levels during the initial high dose therapy and increased to approximately 30% of the normal serum level during the 2 mo of maintenance therapy. On week 8, the International Prostate Symptom Score (IPSS) was significantly reduced and there was a 27% decline in prostate volume.
A decade after these pilot studies, a placebo-controlled phase II trial explored the effects of a 4-wk treatment at 3 different dose levels of cetrorelix in 140 patients with symptomatic BPH[43]. LUTS were significantly improved in all treatment groups compared to placebo which effect occurred rapidly, by week 4 (time point of the first evaluation). Prostate size was also significantly reduced in two of the treatment groups and the overall reduction of symptoms lasted 16 wk after the termination of the treatment (time point of last evaluation). In a further study, cetrorelix pamoate was administered as a 60 mg sustained release formulation, in a double-blind, randomized, multicenter study[44]. One subsequent administration of cetrorelix (30 mg, sustained release) resulted in a 4-point improvement in IPSS and the significant advancement was sustained for 26 wk after the last dose was given. In these latter studies it was shown that the suppression of testosterone levels by cetrorelix was moderate and transient[43,44].
Despite the success of these studies, the phase III clinical trials conducted in the United States and in Europe by AEterna Zentaris[45,46] failed to confirm a significant improvement in IPSS in response to cetrorelix treatment compared to the placebo group. In the United States study, there were no significant changes after either 3 or 4 doses of cetrorelix administered during an 18-wk period, however, cetrorelix was beneficial in a subgroup of patients with substantially enlarged prostates[47]. Although the phase III trial failed, all previous attempts were successful which encouraged the initiation of new clinical testing with the more potent LHRH antagonist, degarelix. This compound has greatly reduced histamine-releasing activity and upon subcutaneous administration it aggregates into a slow-release complex[38,39]. A Phase-II study has been completed with this compound but results have not yet been released[48].
Initially, the concept of the management of prostate cancer and BPH by LHRH antagonists was based on their action on pituitary LHRH receptors (LHRHR) leading to suppression of gonadal testosterone production, however, there is a growing body of evidence that they also act directly in the prostate. This idea is supported by a number of studies showing the presence of LHRH receptor in the prostate[49-51]. In an early study by Kadar et al[49], a high affinity low capacity binding site for D-TRP-6-LHRH in prostate samples from patients with BPH and prostate cancer was found. A similar binding site and one with low affinity high capacity were also detected in Dunning prostate tumors[50]. In a more recent study, LHRHR was detected by reverse transcription polymerase chain reaction in 60% of patients with BPH[51].
A second line of evidence for the local action of LHRH antagonists in the prostate is derived from a number of studies where cetrorelix was tested in vitro on human BPH cell lines expressing LHRHR. Siejka et al[21] showed that cetrorelix inhibits proliferation of the immortalized human BPH cell line (BPH-1) and reduces the protein expression of proliferating cell nuclear antigen (PCNA), epidermal growth factor (EGF), EGF receptor, most abundant adrenergic receptor in the prostate (α1AAR) and LHRHR in a concentration-dependent manner[21]. Proliferation was also inhibited by cetrorelix after cells were stimulated with growth factors insulin-like growth factors (IGF)-I, IGF-II or FGF-2. Additionally, the activation of signal transducer and activator of transcription 3 (STAT3) by phosphorylation, an event associated with increased proliferation in many cells[52], was suppressed by cetrorelix[21]. The downregulation of α1AAR by cetrorelix might be of particular interest since an increase in α1AAR expression induced by prolonged administration of α1A-adrenergic blockers might be responsible for development of the therapeutic tolerance to this treatment seen in clinical practice[53]. Rick et al[22] utilized a rat model of BPH in which the growth of prostate was induced by repeated administration of testosterone[54]. In this study, prostate size was reduced by cetrorelix in a dose-dependent manner compared to controls treated by testosterone only. In addition, the expression of various proinflammatory cytokines and growth factors that have been implicated in the pathogenesis of BPH were found to be reduced following cetrorelix treatment[22]. A significant reduction in serum levels of dihydrotestosterone and LH was also observed. Interestingly, cetrorelix treatment reversed testosterone-induced morphological changes to resemble the histology of the normal prostate, including a decrease in epithelial height[22]. In addition, AR and 5α-reductase levels were reduced by cetrorelix[22]. Unfortunately, the testosterone-induced BPH model has its limitations due to the complexity of the pathogenesis of BPH. Testosterone-induced hyperplasia selectively appears in the ventral prostate lobe in rats that might be the result of the distinct anatomy of this model from humans[55]. Also, the efficacy of testosterone to induce prostatic hyperplasia varies among different rat strains[56]. In addition to the noted disadvantages of the model, only the proliferation of epithelial cells is triggered by the addition of testosterone[56], whereas stromal-epithelial interactions are believed to be crucial in the pathogenesis of BPH[57,58]. Siejka et al[59] further examined this interaction using the BPH-1 cells and an immortalized stromal myofibroblast cell line, WPMY-1. Growth medium collected from either of the cell lines stimulated the growth of the other. This strongly supports the crucial role of epithelial-stromal cross-talk in the proliferative activity of these cells. This effect seemed to be directed through the mitogen-activated protein kinase (phosphorylation of ERK1/2) and STAT pathways. Cetrorelix inhibited the proliferation of both cell lines on its own and also after the BPH cells were stimulated with WPMY-1-conditioned medium[59].
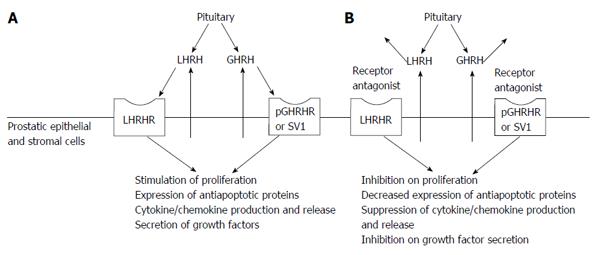
The above mentioned studies shed light on the existence of a local autocrine/paracrine LHRH feedback in the prostate that might contribute to the pathogenesis of BPH (Figure 1). Since both LHRH ligand and its receptor are expressed in BPH[22] and the LHRH antagonist, cetrorelix, is able to decrease the proliferative activity of BPH cells in vitro[22], this feedback loop might act as a local stimulatory signal for cell proliferation or survival. It is also known that hormone-refractory prostate carcinomas express higher levels of LHRHR than BPH and hormone-dependent prostate cancer[60]. Consequently, LHRHR levels might gain prognostic value in the future.
Antagonistic analogs of GHRH have been found to reduce the growth of various tumors[61-69] including prostate cancer in xenograft models of nude mice[70-74]. IGF-1 is a well-known growth-promoting factor for various tumors[70,75]. By blocking GHRH receptors on pituitary somatotropes, these antagonists suppress the production and secretion of growth hormone (GH) thereby decreasing circulating levels of IGF1. The full length pituitary type receptor (pGHRHR) and its main splice variant, splice variant 1, are expressed in various extrapituitary sites of normal and malignant tissues, including prostate[71,76,77]. GHRH is also secreted locally in normal and malignant prostate tissue, suggesting that it serves as an autocrine/paracrine regulator which process might be involved in the pathogenesis as well as the progression of prostate cancer[23,78,79]. Both in vivo tumor growth and in vitro cell proliferation are inhibited by GHRH antagonists in experimental androgen-dependent and-independent prostate cancers further indicating that, apart from their action in the pituitary, these peptides also function directly in the prostate[80].
Two studies have investigated the effect of GHRH antagonist monotherapy in experimental BPH models. They confirmed that both pGHRHR and GHRH are present in BPH-1 cells and in rat prostates[23,24]. Rick et al[23] reported also that the levels of pGHRHR and GHRH were increased following the induction of prostate growth by testosterone, indicating the importance of this autocrine/paracrine circuit in the pathogenesis of BPH (Figure 1). In the same study, GHRH antagonists were found to significantly reduce relative prostate weights better than finasteride, an 5-α reductase inhibitor. GHRH antagonists downregulated the mRNA and protein levels of various cytokines and growth factors that were elevated after testosterone treatment and also decreased proliferation and increased apoptosis in the prostate. GHRH antagonists decreased the transcriptional expression of growth hormones such as IGF-2, TGF-α, TGF-β1 and -β2, EGF, FGF-2, vascular endothelial growth factor (VEGF)-A, that have been found to contribute to the pathogenesis of BPH[81]. Cytokines interleukin (IL)-1α, IL-1β, IL-13, IL-15, and IL-17β, that have been downregulated by GHRH antagonists, otherwise promote T-lymphocyte infiltration and inflammation in BPH[82]. Interestingly, in this study, serum GH and IGF-1 levels were not affected significantly by the GHRH antagonist treatment, that might indicate the crucial role of their direct action in the prostate rather than through the pituitary axis. Intriguingly, prostates of testosterone-treated rats contained increased levels of the antiapoptotic molecule, B-cell lymphoma 2 (BCL-2), a process may explain the increased survival of cells implicated in the development of BPH[20]. Additionally, GHRH antagonist significantly dowregulated BCL-2 levels and simultaneously elevated the expression of the proapoptotic factor, BCL-2-associated X protein (BAX), and the tumor suppressor, p53, which events may underlie the strong apoptotic effect of these peptides.
In the study by Siejka et al[21] GHRH antagonists inhibited the proliferation of BPH-1 cells in vitro. The existence of the local GHRH/GHRHR loop was further supported by this study; incubation of the cells with GHRH resulted in an increased rate of proliferation which was then inhibited by the simultaneous addition of GHRH antagonist. Their study also revealed that GHRH triggers the phosphorylation of ERK 1/2, Janus kinase 2 (JAK2) and STAT3, signaling molecules that are known to be involved in the pathogenesis of BPH[83,84].
Since the existence of autocrine/paracrine systems of regulation by both LHRH and GHRH are strongly suggested in BPH, the simultaneous blockade of their receptors would be expected to result in a more effective therapy. Rick et al[85] studied the combination of cetrorelix plus a highly potent GHRH antagonist, JMR-132, in the testosterone-induced rat BPH model. They found that combination of LHRH and GHRH antagonists resulted in a greater decrease in prostate-specific antigen (PSA) and prostatic STEAP (six-transmembrane epithelial antigen of the prostate) protein levels than either of the peptides alone. Relative prostate weights were reduced to the control level by the combination therapy. Antagonists of GHRH and LHRH administered together were also more effective in inducing apoptosis as measured by changes in the levels of BCL-2, BAX, p53, nuclear factor (NF)-κB and cyclooxygenase-2 (COX-2). The combination therapy therefore has a great prospect in reducing hyperplastic prostate volume by triggering apoptotic cell death. In addition, chronic inflammation has been linked to the development and worsening of BPH; COX-2 has been proposed to play a key role in this process[86]. Hence, coadministration of GHRH and LHRH antagonists may also improve clinical outcome by reducing the expression of inflammation-related proteins such as NF-κB and COX-2[87]. In a subsequent study[88], the cumulative effect of cetrorelix plus JMR-132 was also superior to their individual inhibition on the proliferation of BPH-1 and WPMY-1 cells in vitro. Only the combination of JMR-132 and cetrorelix increased the proportion of cells in the S-phase significantly with a simultaneous decrease in the number of cells in G0/G1 and G2/M phases in BPH-1 cells. A decrease in the expression of several genes was detected in response to the combination treatment in the rat testosterone-induced BPH model; these included growth factors (EGF, FGF-1, -2, -7, -8 and -14, IGF-1 and-2, BMP5 and -7, VEGF-A, etc.), genes implicated in inflammatory response (chemokines, chemokine receptors, cytokines and cytokine receptors), and members of the Wnt, Hedgehog, PI3-kinase/AKT, JAK-STAT, Phospholipase C and low-density lipoprotein (LDL) pathways. According to the authors, among these changes, the downregulation of IGF-1 is of particular interest, since it has been linked to the development of BPH in diabetic men[89]. Also, inflammation-related chemokine/cytokine release has been shown to trigger the production of growth factors leading to the hyperplastic behavior of prostatic cells[90]. We therefore believe that combination therapy with antagonists of GHRH and LHRH might provide a highly beneficial approach to the management of BPH.
GRP is a bombesin-related hormone first isolated for porcine stomach and named for its ability to trigger the secretion of gastrin[91,92]. Among the three receptor subtypes that had been described for bombesin-like peptides, GRP binds to the first type (GRPR) with high affinity, and to the second type (neuromedin-B receptor) with a relatively low activity[93]. GRPR expression has been found in a variety of tissues where it regulates the secretion of gastric acid and stimulates exocrine function of the pancreas as well as triggering smooth muscle contraction in the stomach, gall bladder and urinary bladder[94,95]. In the prostate, GRP and bombesin have been shown to display mitogenic activity, affect cell migration and induce contraction in bladder and left ventral prostate[95,96]. In addition, GRPR has been implicated in the neurophysiology of memory and fear-related behavior, and the processing of pruritus and penile reflexes[97]. In small-cell lung carcinoma xenografted into nude mice, an antibody against the GRPR receptor significantly inhibited tumor growth suggesting the crucial role of a GRP/GRPR autocrine/paracrine loop[98]. Soon after, the existence of this feedback regulation was demonstrated in various tumors, such as glioblastoma, colon cancer, hepatic cancer, prostate and gynecologic cancers[99-104]. Several antagonistic analogs that target GRPR have been synthetized by our group; among these RC-3940-II possesses the highest affinity for GRPR combined with an increased antitumor efficacy[105].
GRPR is expressed in prostates from healthy patients as well as in those diagnosed with BPH and malignant prostate[106,107]. A study by Rick et al[25] using the testosterone-induced rat model, investigated the role of GRP/GRPR in BPH in greater depth. They demonstrated that GRPR and its ligand are expressed in prostates of normal as well as testosterone-induced rats and also in the human BPH-1 and WPMY-1 cell lines. A single high-affinity binding site was also identified, in control rat prostates and human cell lines, with a radioligand binding assay using 125I-labeled [Tyr4]bombesin. In this study, the GRP antagonist, RC-3940-II, inhibited the proliferation of BPH-1 and WPMY-1 cells in vitro. It also significantly decreased cell volume and triggered S-phase cell cycle arrest in these cells. The GRP antagonist dose-dependently decreased prostate size in vivo in testosterone-treated rats. The proteomic analysis of rat prostates revealed that treatment with RC-3940-II reversed the testosterone-induced elevation in NF-κB phosphorylation and expression of androgen receptor and PCNA. Also, it decreased the mean epithelial area and induced apoptosis in testosterone-treated prostates. Analysis of the transcriptional changes in the different treatment groups identified several genes responsible for the beneficial effects of RC-3940-II. Changes were found in the levels of growth factors, inflammatory chemokines, cytokines and their receptors; attempts to identify key signaling pathways for this process resulted in the implication of the Wnt, Hedgehog, TGF-β, NF-κB, JAK-STAT and LDL pathways. Accordingly, GRP antagonists may represent an important tool for the management of BPH, either alone or in combination with LHRH and/or GHRH antagonists.
Somatostatin inhibits the release of GH from the pituitary and also possesses inhibitory action in the gastrointestinal-tract and pancreas as shown by suppression of secretion of gastrin and glucagon, respectively[108,109]. There is much evidence that analogs of somatostatin can inhibit growth of various experimental tumors including prostate cancer[110]. Kadar et al[111] identified a single binding site for somatostatin using somatostatin analog RC-160 in rat prostate adenocarcinoma. In normal and pathologic prostate, findings deciphering the expression pattern of somatostatin receptors are contradictory. According to Dizeyi et al[112], among the five somatostatin subtypes (SSTRs), SSTR1-3 is expressed in the epithelium of normal and malignant prostate cancer, whereas SSTR4 was found only in epithelial cells. Specific neuroendocrine cells expressing SSTRs have also been identified. In a study by Tatoud et al[113], SSTR1 was found in most of the epithelial and stromal cell lines tested whereas SSTR2 was only detected in one BPH stromal cell line. By using fluorescent in situ hybridization techniques, SSTR4 mRNA expression was found only in the epithelium whereas SSTR2 was mainly detected in stromal cells of BPH and carcinoma[114]. Nevertheless, the expression of SSTRs in the prostate suggested that the use of somatostatin analogs in pathologic conditions of the prostate by inhibiting the autoregulatory loop of GHRH/GHRHR might be beneficial. In accord with this hypothesis, somatostatin analogs were shown to decrease the proliferation of androgen sensitive and androgen independent prostate cancer cells by elevating p27 and p21 protein levels, decreasing cyclin E expression and ERK1/2 phosphorylation and the secretion of IGF-1 and IGF-2[113,115,116]. The inhibitory activity of somatostatin analog on the production of growth factors, IGF-1 and IGF-2, is of particular interest since these powerful octapeptides have been linked to the pathogenesis of BPH[90].
Somatostatin analogs have also been tested clinically in patients with androgen-independent prostate cancer. A study by Maulard et al[117] showed improvement in PSA levels and achieved a reduction in bone pain. A Phase-I study demonstrated the favorable toxicity profile of somatostatin analog lanreotide, and showed its inhibitory effect on plasma IGF-1 levels. In contrast, no clinical improvement has been noted with this analog in advanced metastatic androgen-independent prostate cancer[118]. In a study by Berruti et al[119], lanreotide was also able to decrease plasma levels of IGF-1 and of the prognostic marker, chromogranin-A, but had no effect on serum PSA levels in patients with advanced prostate cancer. The poor or no inhibition of tumor growth to somatostatin analogs found in these clinical trials is thought to be due to differences in the receptor subtype-specific binding of the analogs. Consequently, the utilization of a non-receptor selective somatostatin analog has been suggested[120]. According to Cariaga-Martinez et al[121], whereas SSTR2 is expressed in benign prostatic hyperplasia, in most cases, it is repressed or absent in malignant prostate tissue. Conversely, the profound expression of somatostatin receptors in non-malignant prostate tissue indicates the need for preclinical and clinical testing of its analogs in BPH. This suggests that monotherapy with a somatostatin analog or a combination treatment with antagonists of GHRH and/or LHRH might represent a promising strategy for the treatment of BPH which should be investigated in the future.
The development of novel therapies for BPH is undoubtedly required. Whereas the beneficial effects of LHRH antagonists in pathological conditions of the prostate are already confirmed in clinical setting, other peptide analogs (antagonists of GHRH and GRP) have only been tested in experimental BPH models. We hope that the present review of findings on this topic will accelerate the further experimental and clinical investigation of these compounds. It appears that the local actions of various analogs in the prostate are more crucial for their beneficial influence on BPH than are their systemic effects on hormonal levels. By affecting the activation of multiple signaling pathways, LHRH, GHRH and GRP regulate cell cycle, apoptosis, cytokine and chemokine release as well as local immune response. Monotherapy or combination therapy with antagonists of LHRH, GHRH and GRP are suggested to represent an improved treatment compared to the currently available medical modalities.
P- Reviewer: Azadzoi K, Simone G S- Editor: Ji FF L- Editor: A E- Editor: Liu SQ
| 1. | Roehrborn CG. Benign prostatic hyperplasia: an overview. Rev Urol. 2005;7 Suppl 9:S3-S14. [PubMed] |
| 2. | Berry SJ, Coffey DS, Walsh PC, Ewing LL. The development of human benign prostatic hyperplasia with age. J Urol. 1984;132:474-479. [PubMed] |
| 3. | McNeal JE. Origin and evolution of benign prostatic enlargement. Invest Urol. 1978;15:340-345. [PubMed] |
| 4. | Levin RM, Monson FC, Haugaard N, Buttyan R, Hudson A, Roelofs M, Sartore S, Wein AJ. Genetic and cellular characteristics of bladder outlet obstruction. Urol Clin North Am. 1995;22:263-283. [PubMed] |
| 5. | Levin RM, Haugaard N, O’Connor L, Buttyan R, Das A, Dixon JS, Gosling JA. Obstructive response of human bladder to BPH vs rabbit bladder response to partial outlet obstruction: a direct comparison. Neurourol Urodyn. 2000;19:609-629. [PubMed] |
| 6. | Rick FG, Saadat SH, Szalontay L, Block NL, Kazzazi A, Djavan B, Schally AV. Hormonal manipulation of benign prostatic hyperplasia. Curr Opin Urol. 2013;23:17-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 7. | Slater S, Dumas C, Bubley G. Dutasteride for the treatment of prostate-related conditions. Expert Opin Drug Saf. 2012;11:325-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | Lepor H, Kazzazi A, Djavan B. α-Blockers for benign prostatic hyperplasia: the new era. Curr Opin Urol. 2012;22:7-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 73] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 10. | Berardinelli F, Hinh P, Wang R. Minimally invasive surgery in the management of benign prostatic hyperplasia. Minerva Urol Nefrol. 2009;61:269-289. [PubMed] |
| 11. | Hoffman RM, Monga M, Elliott SP, Macdonald R, Langsjoen J, Tacklind J, Wilt TJ. Microwave thermotherapy for benign prostatic hyperplasia. Cochrane Database Syst Rev. 2012;9:CD004135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 12. | Simforoosh N, Abdi H, Kashi AH, Zare S, Tabibi A, Danesh A, Basiri A, Ziaee SA. Open prostatectomy versus transurethral resection of the prostate, where are we standing in the new era? A randomized controlled trial. Urol J. 2010;7:262-269. [PubMed] |
| 13. | Roehrborn CG. Benign prostatic hyperplasia: etiology, pathophysiology, epidemiology, and natural history. Campbell-Walsh Urology. 10th ed. Philadelphia: Elsevier Saunders 2012; 2556-2596. |
| 14. | Isaacs JT. Antagonistic effect of androgen on prostatic cell death. Prostate. 1984;5:545-557. [PubMed] |
| 15. | McConnell JD. Prostatic growth: new insights into hormonal regulation. Br J Urol. 1995;76 Suppl 1:5-10. [PubMed] |
| 16. | Nicholson TM, Ricke WA. Androgens and estrogens in benign prostatic hyperplasia: past, present and future. Differentiation. 2011;82:184-199. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 257] [Cited by in RCA: 228] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 17. | Tennakoon JB, Shi Y, Han JJ, Tsouko E, White MA, Burns AR, Zhang A, Xia X, Ilkayeva OR, Xin L. Androgens regulate prostate cancer cell growth via an AMPK-PGC-1α-mediated metabolic switch. Oncogene. 2013;Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 140] [Cited by in RCA: 179] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 18. | Ropiquet F, Giri D, Lamb DJ, Ittmann M. FGF7 and FGF2 are increased in benign prostatic hyperplasia and are associated with increased proliferation. J Urol. 1999;162:595-599. [PubMed] |
| 19. | Theyer G, Kramer G, Assmann I, Sherwood E, Preinfalk W, Marberger M, Zechner O, Steiner GE. Phenotypic characterization of infiltrating leukocytes in benign prostatic hyperplasia. Lab Invest. 1992;66:96-107. [PubMed] |
| 20. | Alonso-Magdalena P, Brössner C, Reiner A, Cheng G, Sugiyama N, Warner M, Gustafsson JA. A role for epithelial-mesenchymal transition in the etiology of benign prostatic hyperplasia. Proc Natl Acad Sci USA. 2009;106:2859-2863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 141] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 21. | Siejka A, Schally AV, Block NL, Barabutis N. Mechanisms of inhibition of human benign prostatic hyperplasia in vitro by the luteinizing hormone-releasing hormone antagonist cetrorelix. BJU Int. 2010;106:1382-1388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 22. | Rick FG, Schally AV, Block NL, Halmos G, Perez R, Fernandez JB, Vidaurre I, Szalontay L. LHRH antagonist Cetrorelix reduces prostate size and gene expression of proinflammatory cytokines and growth factors in a rat model of benign prostatic hyperplasia. Prostate. 2011;71:736-747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 66] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 23. | Rick FG, Schally AV, Block NL, Nadji M, Szepeshazi K, Zarandi M, Vidaurre I, Perez R, Halmos G, Szalontay L. Antagonists of growth hormone-releasing hormone (GHRH) reduce prostate size in experimental benign prostatic hyperplasia. Proc Natl Acad Sci USA. 2011;108:3755-3760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 65] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 24. | Siejka A, Schally AV, Block NL, Barabutis N. Antagonists of growth hormone-releasing hormone inhibit the proliferation of human benign prostatic hyperplasia cells. Prostate. 2010;70:1087-1093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 25. | Rick FG, Abi-Chaker A, Szalontay L, Perez R, Jaszberenyi M, Jayakumar AR, Shamaladevi N, Szepeshazi K, Vidaurre I, Halmos G. Shrinkage of experimental benign prostatic hyperplasia and reduction of prostatic cell volume by a gastrin-releasing peptide antagonist. Proc Natl Acad Sci USA. 2013;110:2617-2622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 26. | Canales ES, Montvelinsky H, Zárate A, Kastin AJ, Coy DH, Schally AV. Suppressive effect of an inhibitory LHRH analog on the gonadotropin response to LHRH in normal women. Int J Fertil. 1980;25:190-192. [PubMed] |
| 27. | Zarate A, Canales ES, Sthory I, Coy DH, Comaru-Schally AM, Schally AV. Anovulatory effect of a LHRH antagonist in women. Contraception. 1981;24:315-320. [PubMed] |
| 28. | Schmidt F, Sundaram K, Thau RB, Bardin CW. [Ac-D-NAL(2)1,4FD-Phe2,D-Trp3,D-Arg6]-LHRH, a potent antagonist of LHRH, produces transient edema and behavioral changes in rats. Contraception. 1984;29:283-289. [PubMed] |
| 29. | Bajusz S, Csernus VJ, Janaky T, Bokser L, Fekete M, Schally AV. New antagonists of LHRH. II. Inhibition and potentiation of LHRH by closely related analogues. Int J Pept Protein Res. 1988;32:425-435. [PubMed] |
| 30. | Rick FG, Block NL, Schally AV. Agonists of luteinizing hormone-releasing hormone in prostate cancer. Expert Opin Pharmacother. 2013;14:2237-2247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 31. | Reissmann T, Schally AV, Bouchard P, Riethmiiller H, Engel J. The LHRH antagonist cetrorelix: a review. Hum Reprod Update. 2000;6:322-331. [PubMed] |
| 32. | Finas D, Hornung D, Diedrich K, Schultze-Mosgau A. Cetrorelix in the treatment of female infertility and endometriosis. Expert Opin Pharmacother. 2006;7:2155-2168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 33. | Hosseini MA, Mahdavi A, Aleyasin A, Safdarian L, Bahmaee F. Treatment of ovarian hyperstimulation syndrome using gonadotropin releasing hormone antagonist: a pilot study. Gynecol Endocrinol. 2012;28:853-855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 34. | Verschraegen CF, Westphalen S, Hu W, Loyer E, Kudelka A, Völker P, Kavanagh J, Steger M, Schulz KD, Emons G. Phase II study of cetrorelix, a luteinizing hormone-releasing hormone antagonist in patients with platinum-resistant ovarian cancer. Gynecol Oncol. 2003;90:552-559. [PubMed] |
| 35. | Gonzalez-Barcena D, Alvarez RB, Ochoa EP, Cornejo IC, Comaru-Schally AM, Schally AV, Engel J, Reissmann T, Riethmüller-Winzen H. Treatment of uterine leiomyomas with luteinizing hormone-releasing hormone antagonist Cetrorelix. Hum Reprod. 1997;12:2028-2035. [PubMed] |
| 36. | Gonzalez-Barcena D, Vadillo-Buenfil M, Gomez-Orta F, Fuentes Garcia M, Cardenas-Cornejo I, Graef-Sanchez A, Comaru-Schally AM, Schally AV. Responses to the antagonistic analog of LH-RH (SB-75, Cetrorelix) in patients with benign prostatic hyperplasia and prostatic cancer. Prostate. 1994;24:84-92. [PubMed] |
| 37. | Gonzalez-Barcena D, Vadillo-Buenfil M, Cortez-Morales A, Fuentes-Garcia M, Cardenas-Cornejo I, Comaru-Schally AM, Schally AV. Luteinizing hormone-releasing hormone antagonist cetrorelix as primary single therapy in patients with advanced prostatic cancer and paraplegia due to metastatic invasion of spinal cord. Urology. 1995;45:275-281. [PubMed] |
| 38. | Koechling W, Hjortkjaer R, Tankó LB. Degarelix, a novel GnRH antagonist, causes minimal histamine release compared with cetrorelix, abarelix and ganirelix in an ex vivo model of human skin samples. Br J Clin Pharmacol. 2010;70:580-587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 39. | Rick FG, Block NL, Schally AV. An update on the use of degarelix in the treatment of advanced hormone-dependent prostate cancer. Onco Targets Ther. 2013;6:391-402. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 40. | Andersen JT. Alpha 1-blockers vs 5 alpha-reductase inhibitors in benign prostatic hyperplasia. A comparative review. Drugs Aging. 1995;6:388-396. [PubMed] |
| 41. | Gonzalez-Barcena D, Vadillo-Buenfil M, Guerra-Arguero L, Carreno J, Comaru-Schally AM, Schally AV. Potent antagonistic analog of LH-RH (SB-75) inhibits LH, FSH and testosterone levels in human beings. 1990;Abstr No. 1318, 354. |
| 42. | Comaru-Schally AM, Brannan W, Schally AV, Colcolough M, Monga M. Efficacy and safety of luteinizing hormone-releasing hormone antagonist cetrorelix in the treatment of symptomatic benign prostatic hyperplasia. J Clin Endocrinol Metab. 1998;83:3826-3831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 43. | Debruyne F, Gres AA, Arustamov DL. Placebo-controlled dose-ranging phase 2 study of subcutaneously administered LHRH antagonist cetrorelix in patients with symptomatic benign prostatic hyperplasia. Eur Urol. 2008;54:170-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 44. | Debruyne F, Tzvetkov M, Altarac S, Geavlete PA. Dose-ranging study of the luteinizing hormone-releasing hormone receptor antagonist cetrorelix pamoate in the treatment of patients with symptomatic benign prostatic hyperplasia. Urology. 2010;76:927-933. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 45. | AEterna Zentaris. Cetrorelix Pamoate in Patients With Symptomatic Benign Prostatic Hypertrophy (BPH). In: ClinicalTrials.gov [Internet].. Bethesda (MD): National Library of Medicine (US) 2010; Available from: http: // www.clinicaltrials.gov/ct2/show/study/NCT00663858?term=cetrorelix AND BPH&rank=3§=X6015 NLM Identifier: NCT00663858. |
| 46. | AEterna Zentaris. Cetrorelix Pamoate Regimens in Patients With Symptomatic Benign Prostatic Hypertrophy (BPH). In: ClinicalTrials.gov [Internet].. Bethesda (MD): National Library of Medicine (US) 2011; Available from: http: // www.clinicaltrials.gov/ct2/show/study/NCT00449150?term=cetrorelix AND BPH&rank=2§=X36015 NLM Identifier: NCT00449150. |
| 49. | Kadar T, Ben-David M, Pontes JE, Fekete M, Schally AV. Prolactin and luteinizing hormone-releasing hormone receptors in human benign prostatic hyperplasia and prostate cancer. Prostate. 1988;12:299-307. [PubMed] |
| 50. | Fekete M, Redding TW, Comaru-Schally AM, Pontes JE, Connelly RW, Srkalovic G, Schally AV. Receptors for luteinizing hormone-releasing hormone, somatostatin, prolactin, and epidermal growth factor in rat and human prostate cancers and in benign prostate hyperplasia. Prostate. 1989;14:191-208. [PubMed] |
| 51. | Rózsa B, Juhász A, Treszl A, Tóth G, Flaskó T, Dezsö B, Block NL, Schally AV, Halmos G. Expression of mRNA for human type-I LHRH receptor transcript forms in human benign prostatic hyperplasia. Int J Oncol. 2009;35:1053-1059. [PubMed] |
| 52. | Ihle JN. The Stat family in cytokine signaling. Curr Opin Cell Biol. 2001;13:211-217. [PubMed] |
| 53. | Kojima Y, Sasaki S, Hayashi Y, Tsujimoto G, Kohri K. Subtypes of alpha1-adrenoceptors in BPH: future prospects for personalized medicine. Nat Clin Pract Urol. 2009;6:44-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 54. | Maggi CA, Manzini S, Giuliani S, Meli A. Infravesical outflow obstruction in rats: a comparison of two models. Gen Pharmacol. 1989;20:345-349. [PubMed] |
| 55. | Mahapokai W, Van Sluijs FJ, Schalken JA. Models for studying benign prostatic hyperplasia. Prostate Cancer Prostatic Dis. 2000;3:28-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 56. | Scolnik MD, Servadio C, Abramovici A. Comparative study of experimentally induced benign and atypical hyperplasia in the ventral prostate of different rat strains. J Androl. 1994;15:287-297. [PubMed] |
| 57. | Li W, Wu CL, Febbo PG, Olumi AF. Stromally expressed c-Jun regulates proliferation of prostate epithelial cells. Am J Pathol. 2007;171:1189-1198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 44] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 58. | Webber MM, Trakul N, Thraves PS, Bello-DeOcampo D, Chu WW, Storto PD, Huard TK, Rhim JS, Williams DE. A human prostatic stromal myofibroblast cell line WPMY-1: a model for stromal-epithelial interactions in prostatic neoplasia. Carcinogenesis. 1999;20:1185-1192. [PubMed] |
| 59. | Siejka A, Schally AV, Barabutis N. The effect of LHRH antagonist cetrorelix in crossover conditioned media from epithelial (BPH-1) and stromal (WPMY-1) prostate cells. Horm Metab Res. 2014;46:21-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 60. | Straub B, Müller M, Krause H, Schrader M, Goessl C, Heicappell R, Miller K. Increased incidence of luteinizing hormone-releasing hormone receptor gene messenger RNA expression in hormone-refractory human prostate cancers. Clin Cancer Res. 2001;7:2340-2343. [PubMed] |
| 61. | Hohla F, Schally AV, Szepeshazi K, Varga JL, Buchholz S, Köster F, Heinrich E, Halmos G, Rick FG, Kannadka C. Synergistic inhibition of growth of lung carcinomas by antagonists of growth hormone-releasing hormone in combination with docetaxel. Proc Natl Acad Sci USA. 2006;103:14513-14518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 62. | Hohla F, Buchholz S, Schally AV, Seitz S, Rick FG, Szalontay L, Varga JL, Zarandi M, Halmos G, Vidaurre I. GHRH antagonist causes DNA damage leading to p21 mediated cell cycle arrest and apoptosis in human colon cancer cells. Cell Cycle. 2009;8:3149-3156. [PubMed] |
| 63. | Kovács M, Schally AV, Hohla F, Rick FG, Pozsgai E, Szalontay L, Varga JL, Zarándi M. A correlation of endocrine and anticancer effects of some antagonists of GHRH. Peptides. 2010;31:1839-1846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 64. | Jaszberenyi M, Schally AV, Block NL, Zarandi M, Cai RZ, Vidaurre I, Szalontay L, Jayakumar AR, Rick FG. Suppression of the proliferation of human U-87 MG glioblastoma cells by new antagonists of growth hormone-releasing hormone in vivo and in vitro. Target Oncol. 2013;8:281-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 65. | Papadia A, Schally AV, Halmos G, Varga JL, Seitz S, Buchholz S, Rick F, Zarandi M, Bellyei S, Treszl A. Growth hormone-releasing hormone antagonists inhibit growth of human ovarian cancer. Horm Metab Res. 2011;43:816-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 66. | Perez R, Schally AV, Vidaurre I, Rincon R, Block NL, Rick FG. Antagonists of growth hormone-releasing hormone suppress in vivo tumor growth and gene expression in triple negative breast cancers. Oncotarget. 2012;3:988-997. [PubMed] |
| 67. | Pozsgai E, Schally AV, Hocsak E, Zarandi M, Rick F, Bellyei S. The effect of a novel antagonist of growth hormone releasing hormone on cell proliferation and on the key cell signaling pathways in nine different breast cancer cell lines. Int J Oncol. 2011;39:1025-1032. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 68. | Rick FG, Seitz S, Schally AV, Szalontay L, Krishan A, Datz C, Stadlmayr A, Buchholz S, Block NL, Hohla F. GHRH antagonist when combined with cytotoxic agents induces S-phase arrest and additive growth inhibition of human colon cancer. Cell Cycle. 2012;11:4203-4210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 69. | Seitz S, Rick FG, Schally AV, Treszl A, Hohla F, Szalontay L, Zarandi M, Ortmann O, Engel JB, Buchholz S. Combination of GHRH antagonists and docetaxel shows experimental effectiveness for the treatment of triple-negative breast cancers. Oncol Rep. 2013;30:413-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 70. | Schally AV. New approaches to the therapy of various tumors based on peptide analogues. Horm Metab Res. 2008;40:315-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 65] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 71. | Schally AV, Varga JL, Engel JB. Antagonists of growth-hormone-releasing hormone: an emerging new therapy for cancer. Nat Clin Pract Endocrinol Metab. 2008;4:33-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 172] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 72. | Heinrich E, Schally AV, Buchholz S, Rick FG, Halmos G, Mile M, Groot K, Hohla F, Zarandi M, Varga JL. Dose-dependent growth inhibition in vivo of PC-3 prostate cancer with a reduction in tumoral growth factors after therapy with GHRH antagonist MZ-J-7-138. Prostate. 2008;68:1763-1772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 73. | Rick FG, Schally AV, Szalontay L, Block NL, Szepeshazi K, Nadji M, Zarandi M, Hohla F, Buchholz S, Seitz S. Antagonists of growth hormone-releasing hormone inhibit growth of androgen-independent prostate cancer through inactivation of ERK and Akt kinases. Proc Natl Acad Sci USA. 2012;109:1655-1660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 59] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 74. | Stangelberger A, Schally AV, Rick FG, Varga JL, Baker B, Zarandi M, Halmos G. Inhibitory effects of antagonists of growth hormone releasing hormone on experimental prostate cancers are associated with upregulation of wild-type p53 and decrease in p21 and mutant p53 proteins. Prostate. 2012;72:555-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 75. | Westley BR, May FE. Insulin-like growth factors: the unrecognised oncogenes. Br J Cancer. 1995;72:1065-1066. [PubMed] |
| 76. | Havt A, Schally AV, Halmos G, Varga JL, Toller GL, Horvath JE, Szepeshazi K, Köster F, Kovitz K, Groot K. The expression of the pituitary growth hormone-releasing hormone receptor and its splice variants in normal and neoplastic human tissues. Proc Natl Acad Sci USA. 2005;102:17424-17429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 100] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 77. | Rekasi Z, Czompoly T, Schally AV, Halmos G. Isolation and sequencing of cDNAs for splice variants of growth hormone-releasing hormone receptors from human cancers. Proc Natl Acad Sci USA. 2000;97:10561-10566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 138] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 78. | Halmos G, Schally AV, Czompoly T, Krupa M, Varga JL, Rekasi Z. Expression of growth hormone-releasing hormone and its receptor splice variants in human prostate cancer. J Clin Endocrinol Metab. 2002;87:4707-4714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 56] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 79. | Chopin LK, Herington AC. A potential autocrine pathway for growth hormone releasing hormone (GHRH) and its receptor in human prostate cancer cell lines. Prostate. 2001;49:116-121. [PubMed] |
| 80. | Fahrenholtz CD, Rick FG, Garcia MI, Zarandi M, Cai RZ, Block NL, Schally AV, Burnstein KL. Preclinical efficacy of growth hormone-releasing hormone antagonists for androgen-dependent and castration-resistant human prostate cancer. Proc Natl Acad Sci USA. 2014;111:1084-1089. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 81. | Lucia MS, Lambert JR. Growth factors in benign prostatic hyperplasia: basic science implications. Curr Urol Rep. 2008;9:272-278. [PubMed] |
| 82. | Steiner GE, Stix U, Handisurya A, Willheim M, Haitel A, Reithmayr F, Paikl D, Ecker RC, Hrachowitz K, Kramer G. Cytokine expression pattern in benign prostatic hyperplasia infiltrating T cells and impact of lymphocytic infiltration on cytokine mRNA profile in prostatic tissue. Lab Invest. 2003;83:1131-1146. [PubMed] |
| 83. | Bromberg J, Wang TC. Inflammation and cancer: IL-6 and STAT3 complete the link. Cancer Cell. 2009;15:79-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 400] [Cited by in RCA: 445] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 84. | Rodríguez-Berriguete G, Prieto A, Fraile B, Bouraoui Y, de Bethencourt FR, Martínez-Onsurbe P, Olmedilla G, Paniagua R, Royuela M. Relationship between IL-6/ERK and NF-κB: a study in normal and pathological human prostate gland. Eur Cytokine Netw. 2010;21:241-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 85. | Rick FG, Szalontay L, Schally AV, Block NL, Nadji M, Szepeshazi K, Vidaurre I, Zarandi M, Kovacs M, Rekasi Z. Combining growth hormone-releasing hormone antagonist with luteinizing hormone-releasing hormone antagonist greatly augments benign prostatic hyperplasia shrinkage. J Urol. 2012;187:1498-1504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 86. | Sciarra A, Mariotti G, Salciccia S, Autran Gomez A, Monti S, Toscano V, Di Silverio F. Prostate growth and inflammation. J Steroid Biochem Mol Biol. 2008;108:254-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 119] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 87. | Barnes PJ, Karin M. Nuclear factor-kappaB: a pivotal transcription factor in chronic inflammatory diseases. N Engl J Med. 1997;336:1066-1071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3573] [Cited by in RCA: 3589] [Article Influence: 128.2] [Reference Citation Analysis (0)] |
| 88. | Rick FG, Schally AV, Block NL, Abi-Chaker A, Krishan A, Szalontay L. Mechanisms of synergism between antagonists of growth hormone-releasing hormone and antagonists of luteinizing hormone-releasing hormone in shrinking experimental benign prostatic hyperplasia. Prostate. 2013;73:873-883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 89. | Wang Z, Olumi AF. Diabetes, growth hormone-insulin-like growth factor pathways and association to benign prostatic hyperplasia. Differentiation. 2011;82:261-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 90. | McLaren ID, Jerde TJ, Bushman W. Role of interleukins, IGF and stem cells in BPH. Differentiation. 2011;82:237-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 45] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 91. | McDonald TJ, Jörnvall H, Nilsson G, Vagne M, Ghatei M, Bloom SR, Mutt V. Characterization of a gastrin releasing peptide from porcine non-antral gastric tissue. Biochem Biophys Res Commun. 1979;90:227-233. [PubMed] |
| 92. | McDonald TJ, Nilsson G, Vagne M, Ghatei M, Bloom SR, Mutt V. A gastrin releasing peptide from the porcine nonantral gastric tissue. Gut. 1978;19:767-774. [PubMed] |
| 93. | Jensen RT, Battey JF, Spindel ER, Benya RV. International Union of Pharmacology. LXVIII. Mammalian bombesin receptors: nomenclature, distribution, pharmacology, signaling, and functions in normal and disease states. Pharmacol Rev. 2008;60:1-42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 458] [Cited by in RCA: 424] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 94. | Ohki-Hamazaki H, Iwabuchi M, Maekawa F. Development and function of bombesin-like peptides and their receptors. Int J Dev Biol. 2005;49:293-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 118] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 95. | Watts SW, Cohen ML. Effect of bombesin, bradykinin, substance P and CGRP in prostate, bladder body and neck. Peptides. 1991;12:1057-1062. [PubMed] |
| 96. | Flores DG, Lenz G, Roesler R, Schwartsmann G. Gastrin-releasing peptide receptor signaling in Cancer. Cancer Ther. 2009;7:331-345. |
| 97. | Weber HC. Regulation and signaling of human bombesin receptors and their biological effects. Curr Opin Endocrinol Diabetes Obes. 2009;16:66-71. [PubMed] |
| 98. | Cuttitta F, Carney DN, Mulshine J, Moody TW, Fedorko J, Fischler A, Minna JD. Bombesin-like peptides can function as autocrine growth factors in human small-cell lung cancer. Nature. 1985;316:823-826. [PubMed] |
| 99. | Hohla F, Schally AV. Targeting gastrin releasing peptide receptors: New options for the therapy and diagnosis of cancer. Cell Cycle. 2010;9:1738-1741. [PubMed] |
| 100. | Pinski J, Schally AV, Halmos G, Szepeshazi K, Groot K. Somatostatin analogues and bombesin/gastrin-releasing peptide antagonist RC-3095 inhibit the growth of human glioblastomas in vitro and in vivo. Cancer Res. 1994;54:5895-5901. [PubMed] |
| 101. | Glover SC, Tretiakova MS, Carroll RE, Benya RV. Increased frequency of gastrin-releasing peptide receptor gene mutations during colon-adenocarcinoma progression. Mol Carcinog. 2003;37:5-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 102. | Szepeshazi K, Schally AV, Rick FG, Block NL, Vidaurre I, Halmos G, Szalontay L. Powerful inhibition of in-vivo growth of experimental hepatic cancers by bombesin/gastrin-releasing peptide antagonist RC-3940-II. Anticancer Drugs. 2012;23:906-913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 103. | Pinski J, Reile H, Halmos G, Groot K, Schally AV. Inhibitory effects of somatostatin analogue RC-160 and bombesin/gastrin-releasing peptide antagonist RC-3095 on the growth of the androgen-independent Dunning R-3327-AT-1 rat prostate cancer. Cancer Res. 1994;54:169-174. [PubMed] |
| 104. | Rick FG, Buchholz S, Schally AV, Szalontay L, Krishan A, Datz C, Stadlmayr A, Aigner E, Perez R, Seitz S. Combination of gastrin-releasing peptide antagonist with cytotoxic agents produces synergistic inhibition of growth of human experimental colon cancers. Cell Cycle. 2012;11:2518-2525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 105. | Cai R, Qin Y, Ertl T, Schally A. New pseudononapeptide bombesin antagonists with C-terminal leu-psi(ch2n)tac-nh2 show high binding-affinity to bombesin/grp receptors on cfpac-1 human pancreatic-cancer cells. Int J Oncol. 1995;6:1165-1172. [PubMed] |
| 106. | Sun B, Halmos G, Schally AV, Wang X, Martinez M. Presence of receptors for bombesin/gastrin-releasing peptide and mRNA for three receptor subtypes in human prostate cancers. Prostate. 2000;42:295-303. [PubMed] |
| 107. | Markwalder R, Reubi JC. Gastrin-releasing peptide receptors in the human prostate: relation to neoplastic transformation. Cancer Res. 1999;59:1152-1159. [PubMed] |
| 108. | Guillemin R, Gerich JE. Somatostatin: physiological and clinical significance. Annu Rev Med. 1976;27:379-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 160] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 109. | Janecka A, Zubrzycka M, Janecki T. Somatostatin analogs. J Pept Res. 2001;58:91-107. [PubMed] |
| 110. | Ruscica M, Arvigo M, Steffani L, Ferone D, Magni P. Somatostatin, somatostatin analogs and somatostatin receptor dynamics in the biology of cancer progression. Curr Mol Med. 2013;13:555-571. [PubMed] |
| 111. | Kadar T, Redding TW, Ben-David M, Schally AV. Receptors for prolactin, somatostatin, and luteinizing hormone-releasing hormone in experimental prostate cancer after treatment with analogs of luteinizing hormone-releasing hormone and somatostatin. Proc Natl Acad Sci USA. 1988;85:890-894. [PubMed] |
| 112. | Dizeyi N, Konrad L, Bjartell A, Wu H, Gadaleanu V, Hansson J, Helboe L, Abrahamsson PA. Localization and mRNA expression of somatostatin receptor subtypes in human prostatic tissue and prostate cancer cell lines. Urol Oncol. 2002;7:91-98. [PubMed] |
| 113. | Tatoud R, Degeorges A, Prévost G, Hoepffner JL, Gauvillé C, Millot G, Thomas F, Calvo F. Somatostatin receptors in prostate tissues and derived cell cultures, and the in vitro growth inhibitory effect of BIM-23014 analog. Mol Cell Endocrinol. 1995;113:195-204. [PubMed] |
| 114. | Hansson J, Bjartell A, Gadaleanu V, Dizeyi N, Abrahamsson PA. Expression of somatostatin receptor subtypes 2 and 4 in human benign prostatic hyperplasia and prostatic cancer. Prostate. 2002;53:50-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 40] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 115. | Ruscica M, Arvigo M, Gatto F, Dozio E, Feltrin D, Culler MD, Minuto F, Motta M, Ferone D, Magni P. Regulation of prostate cancer cell proliferation by somatostatin receptor activation. Mol Cell Endocrinol. 2010;315:254-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 116. | Ruscica M, Magni P, Steffani L, Gatto F, Albertelli M, Rametta R, Valenti L, Ameri P, Magnaghi V, Culler MD. Characterization and sub-cellular localization of SS1R, SS2R, and SS5R in human late-stage prostate cancer cells: effect of mono- and bi-specific somatostatin analogs on cell growth. Mol Cell Endocrinol. 2014;382:860-870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 117. | Maulard C, Richaud P, Droz JP, Jessueld D, Dufour-Esquerré F, Housset M. Phase I-II study of the somatostatin analogue lanreotide in hormone-refractory prostate cancer. Cancer Chemother Pharmacol. 1995;36:259-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 35] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 118. | Figg WD, Thibault A, Cooper MR, Reid R, Headlee D, Dawson N, Kohler DR, Reed E, Sartor O. A phase I study of the somatostatin analogue somatuline in patients with metastatic hormone-refractory prostate cancer. Cancer. 1995;75:2159-2164. [PubMed] |
| 119. | Berruti A, Dogliotti L, Mosca A, Tarabuzzi R, Torta M, Mari M, Gorzegno G, Fontana D, Angeli A. Effects of the somatostatin analog lanreotide on the circulating levels of chromogranin-A, prostate-specific antigen, and insulin-like growth factor-1 in advanced prostate cancer patients. Prostate. 2001;47:205-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 120. | Berruti A, Vignani F, Russo L, Bertaglia V, Tullio M, Tucci M, Poggio M, Dogliotti L. Prognostic role of neuroendocrine differentiation in prostate cancer, putting together the pieces of the puzzle. Open Access J Urol. 2010;2:109-124. [PubMed] |
| 121. | Cariaga-Martinez AE, Lorenzati MA, Riera MA, Cubilla MA, De La Rossa A, Giorgio EM, Tiscornia MM, Gimenez EM, Rojas ME, Chaneton BJ. Tumoral prostate shows different expression pattern of somatostatin receptor 2 (SSTR2) and phosphotyrosine phosphatase SHP-1 (PTPN6) according to tumor progression. Adv Urol. 2009;723831. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 1.0] [Reference Citation Analysis (0)] |