Published online Apr 24, 2021. doi: 10.5410/wjcu.v10.i1.1
Peer-review started: September 23, 2020
First decision: December 11, 2020
Revised: December 12, 2020
Accepted: April 7, 2021
Article in press: April 7, 2021
Published online: April 24, 2021
Processing time: 203 Days and 8.5 Hours
A 66-year-old lady was referred to urology for a suspected urinoma after retroperioneoscopy done for debridement of a retroperitoneal abscess that developed following a duodenal perforation.
Serous contents of the drain sent for fluid creatinine were elevated confirming this and computed tomography urography findings suggested an upper tract injury with urinoma around the kidney. However, the antegrade nephrostogram suggested otherwise and on flexible cystoscopy and cystogram, an extraperitoneal bladder perforation was instead identified, with tip of retroperitoneal drain sitting inside the bladder.
This case identifies a limitation in the usual diagnostic approach for such injuries and emphasizes the need to exercise caution when managing them especially when they occur after several surgical procedures and in the presence of multiple surgical drains.
Core Tip: This study reports a 66-year-old lady who was referred to urology for a suspected urine leak after retroperitoneoscopy. This case identifies a limitation in the usual diagnostic approach for such injuries and emphasizes the need to exercise caution when managing them especially when they occur after several surgical procedures and in the presence of multiple surgical drains.
- Citation: Kumaran A, Yeung PM, Tiwari R. Perinephric urinoma, an unusual upper tract presentation of a lower tract injury following retroperitoneoscopy: A case report. World J Clin Urol 2021; 10(1): 1-6
- URL: https://www.wjgnet.com/2219-2816/full/v10/i1/1.htm
- DOI: https://dx.doi.org/10.5410/wjcu.v10.i1.1
Bowel perforation is an uncommon complication of endoscopy but can be associated with significant morbidity. This is especially so in the duodenum after procedures such as endoscopic retrograde cholangiopancreatography[1] and endoscopic submucosal dissection (ESD)[2]. When recognised early, primary repair can be performed but these perforations may recur and progress to form retroperitoneal collections and abscesses[3].
As these abscesses can be complicated (multiple, loculated and contain gas forming micro-organisms) as well as difficult to access, a step-up approach is employed to manage them[4]. Retroperitoneoscopy is one option that offers a minimally invasive approach through which debridement can be performed[5].
Urinary tract injury can occur during retroperitoneal surgery but is fortunately rare[6]. Drain fluid analysis for creatinine confirms the diagnosis while contrast extravasation seen on computed tomography (CT) urography localizes the site of injury. This is crucial as the prognosis and subsequent management of upper tract injuries differ greatly from lower tract ones.
A 66-year-old lady who was referred to urology for a suspected urine leak after retroperitoneoscopy.
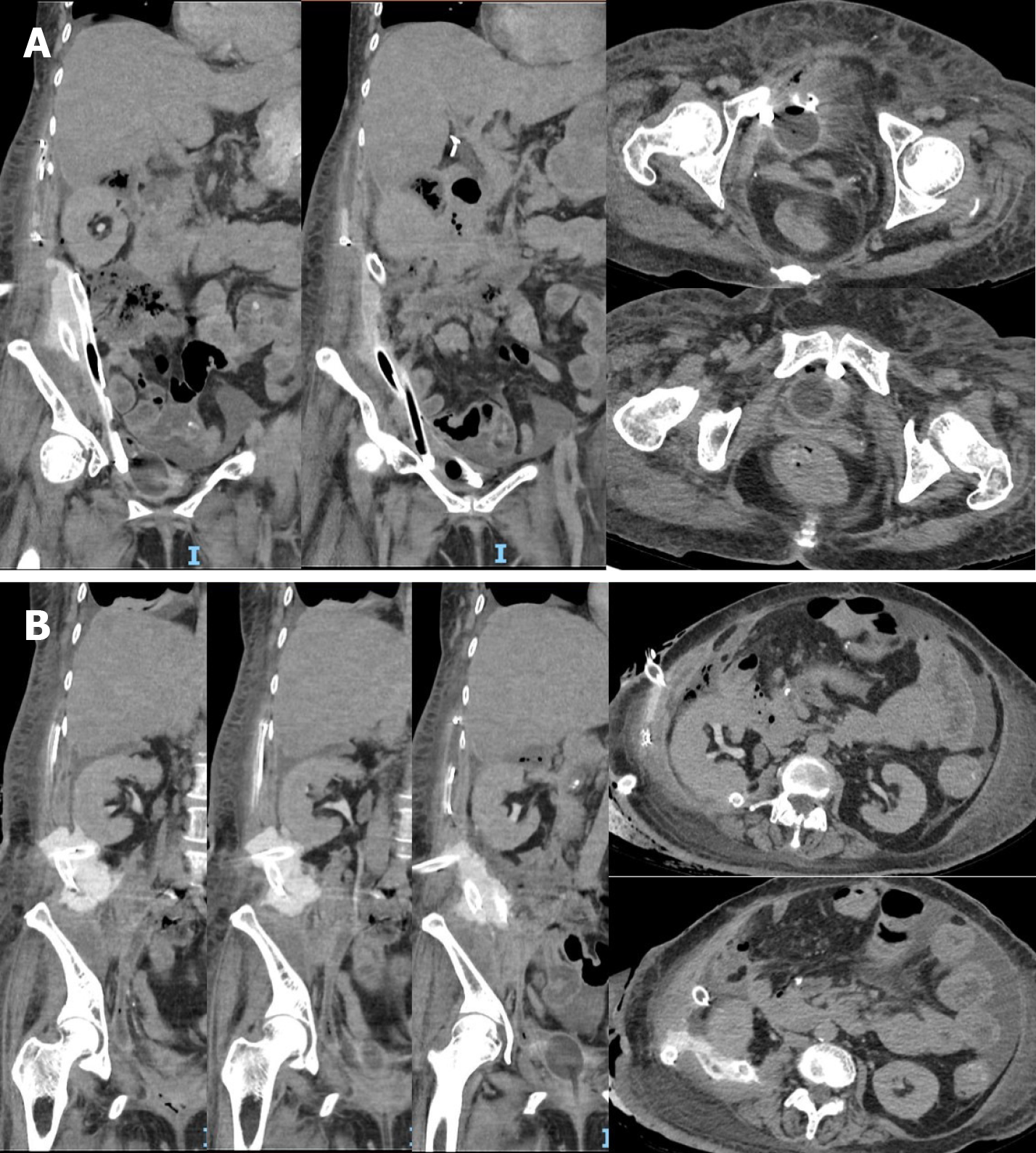
Three days following her last debridement, serous and straw-coloured fluid was observed from a 32Fr drain that was placed through her right flank directed posterior to the right kidney. Drain fluid creatinine was elevated at 650 μmol/L and a CT urography revealed a perinephric urinoma with no pooling of contrast seen around the bladder. There were two other 32Fr drains directed towards the pelvis through the right upper quadrant and flank respectively. Figures 1A and B demonstrate the location of the perinephric urinoma and surgical drains.
She underwent ESD of a duodenal polyp by general surgery 2 months prior for which the histology was a tubular adenoma with low grade dysplasia. Intra-operatively, a perforation was noted and closed with clips but unfortunately, this recurred post-operatively and a retroperitoneal abscess developed. She underwent a laparotomy, duodenojejunostomy with Roux-en-Y reconstruction and feeding jejunostomy creation.
Her post-operative recovery was stormy, requiring a 3 day stay in the intensive care unit for mechanical ventilation and inotropic support as well as percutaneous drainage by interventional radiology and further surgical debridement thrice, two of which were done endoscopically.
She has a past medical history of hypertension, hyperlipidemia, type 2 diabetes mellitus, treated pulmonary tuberculosis and lumbar spondylosis with radiculopathy. Her past surgical history includes a L5/S1 laminectomy and fusion with posterior instrumentation, cholecystectomy and hysterectomy. She neither smokes nor drinks, is independent in her activities of daily living, works as a porter in the hospital and stays with her daughter who offers good social support.
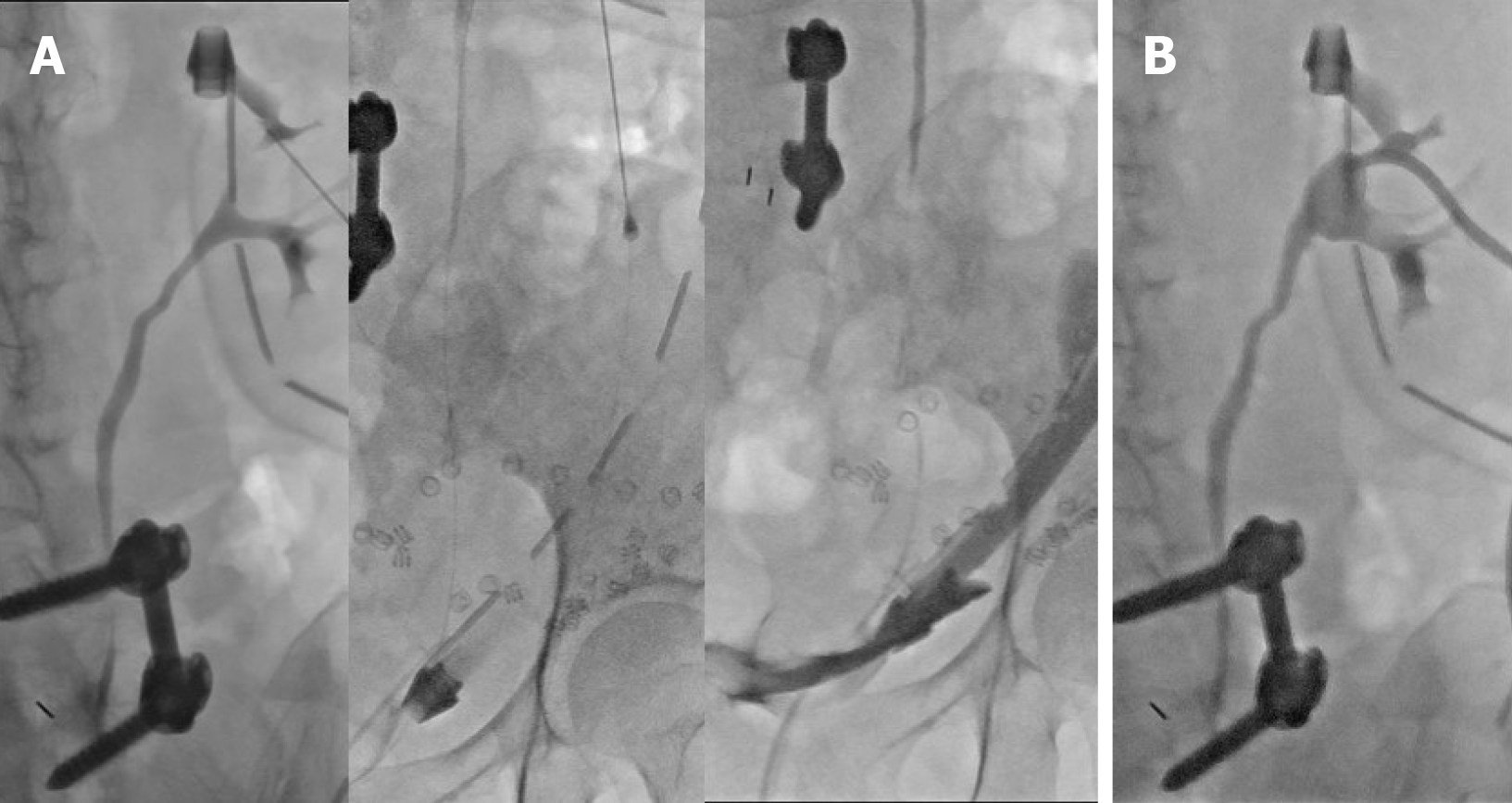
An indwelling urinary catheter (IDC) and percutaneous nephrostomy (PCN) were inserted for urinary diversion. The antegrade nephrostogram (Figure 2) performed during PCN insertion demonstrated good flow of contrast down an intact ureter into the bladder without any leakage around the kidney. In addition, contrast was seen within the lumen of one of the surgical drains in the pelvis.
Fluid from all drains were analyzed found to have raised levels of creatinine ranging from 280 μmol/L to 621 μmol/L. Serum creatinine was not elevated at 43 μmol/L.
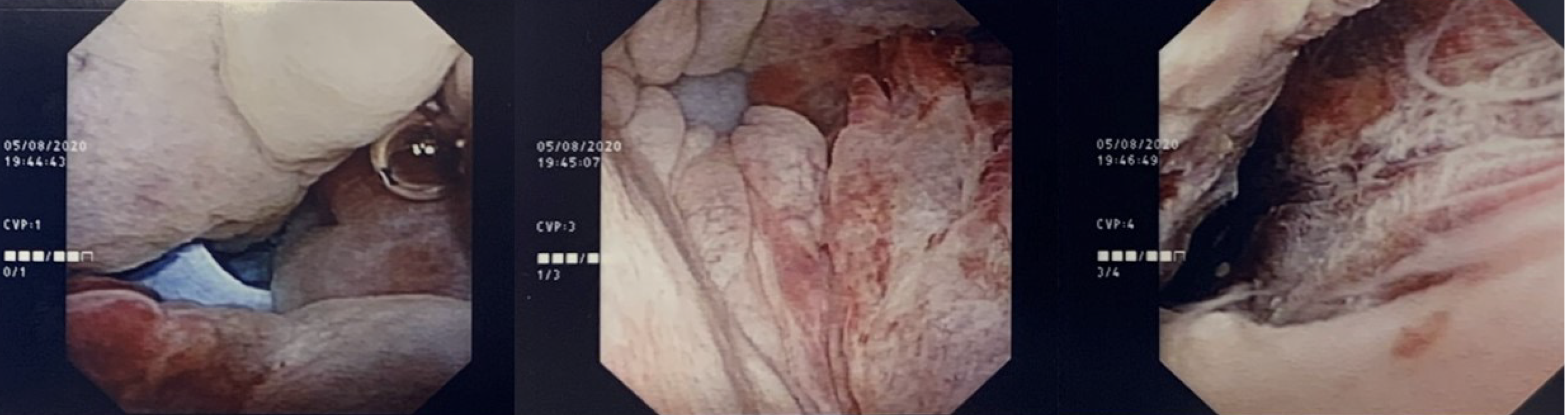
A flexible cystoscopy (Figure 3) was performed which visualized the tip of the right flank surgical drain within the bladder.
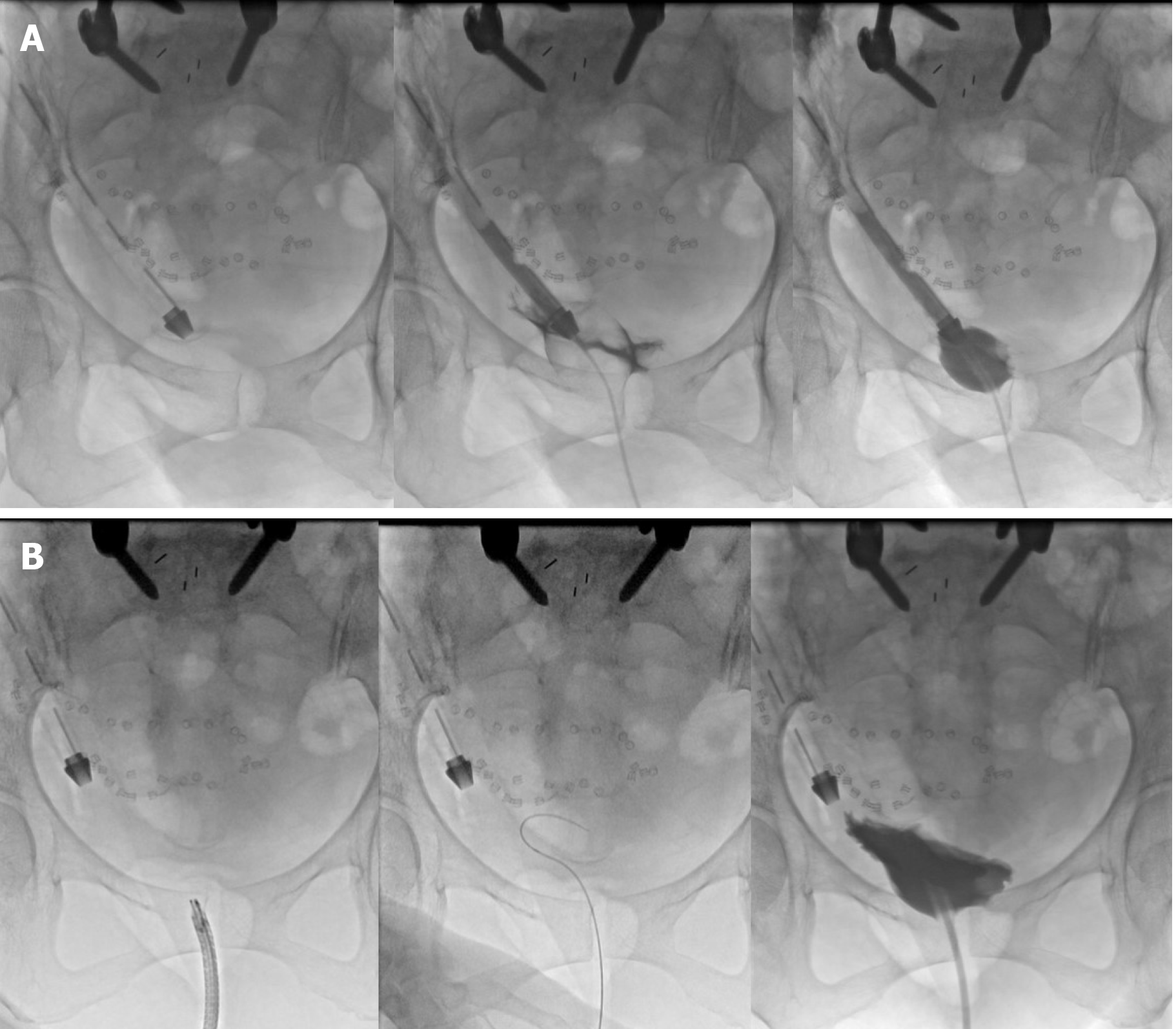
A cystogram (Figure 4A) performed in the same sitting demonstrated contrast instilled through the IDC travelled up the surgical drain.
The patient unfortunately suffered an extraperitoneal bladder perforation after retroperitoneoscopy.
Under direct vision, the right flank drain was withdrawn and the site of injury inspected. With the flexible cystoscope directed away from the site of perforation, a guide wire was passed and a new IDC inserted over it using a Seldinger technique. A repeat cystogram (Figure 4B) demonstrated contrast within the bladder which did not enter the right flank surgical drain.
The patient’s extraperitoneal bladder perforation was managed conservatively with a large 20Fr indwelling catheter left in the bladder. The retroperitoneal drain within the bladder was withdrawn cranially out of the bladder to sit cranially in the retroperitoneum. Drain outputs decreased subsequently with increased urine output in the catheter. An interval check cystogram was arranged 4 weeks after.
The patient’s initial investigations, namely, the elevated perinephric drain creatinine and urinoma suggested an upper urinary tract injury (i.e., pelvicalyceal or ureteric). This was in keeping with the lack of pooling of contrast seen within the pelvis.
However, there was no perinephric leak of contrast seen on the antegrade nephrostogram which prompted the authors to re-evaluate our provisional diagnosis. Contrast flowed freely down an intact ureter and was seen within the lumen of one of the pelvic drains, which suggested a lower urinary tract injury (i.e., bladder). This was later confirmed on flexible cystoscopy and cystogram.
Even though drain fluid from the other surgical drains did not appear like urine, samples taken showed elevated creatinine. This suggested that the leak was fairly large such that urine was being drained by all the surgical drains. Serum creatinine was also checked and not found to be elevated, which can occur in intra-peritoneal urine leaks due to peritoneal reabsorption.
The authors postulate that most of the contrast administered during CT urography travelled down the collecting system and out the surgical drain that had perforated the bladder. A small amount likely leaked around this drain and settled in the right perinephric space as it was dependent and not directly drained by another surgical drain, giving rise to the perinephric urinoma.
Creatine levels in serum and drain fluid (from all drains, even if they do not appear like urine) should be checked. When serum levels are not elevated, an intra-peritoneal urine leak is less likely. When multiple drain fluid samples have elevated creatinine, the possibility of a large leak or multiple leaks should be considered. CT urography is useful in localizing the site of urine leak but interpretation may be difficult in cases of altered anatomy and in the presence of multiple large bore surgical drains. Prior to CT scanning, all surgical drains (including IDC) should be clamped so as not to confound the results. Antegrade nephrostograms and (retrograde) cystograms are valuable in confirming the site of urine leak and can help assess the extent of injury. A flexible cystoscopy can afford direct visualization of bladder injuries and is a simple procedure under local anaesthesia that can be performed in the same setting as a cystogram.
Manuscript source: Unsolicited manuscript
Specialty type: Urology and nephrology
Country/Territory of origin: Singapore
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Balzarro M, Esposito C S-Editor: Gao CC L-Editor: A P-Editor: Wang LL
| 1. | Guerra F, Giuliani G, Coletta D, Bonapasta SA, Levi Sandri GB. Clinical outcomes of ERCP-related retroperitoneal perforations. Hepatobiliary Pancreat Dis Int. 2017;16:160-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 2. | Andromanakos N, Filippou D, Skandalakis P, Kouraklis G, Kostakis A. An extended retroperitoneal abscess caused by duodenal diverticulum perforation: report of a case and short review of the literature. Am Surg. 2007;73:85-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 3. | Ohara Y, Takimoto K, Toyonaga T, Yamaguchi T, Sakaguchi H, Kawara F, Tanaka S, Ishida T, Morita Y, Umegaki E. Enormous postoperative perforation after endoscopic submucosal dissection for duodenal cancer successfully treated with filling and shielding by polyglycolic acid sheets with fibrin glue and computed tomography-guided abscess puncture. Clin J Gastroenterol. 2017;10:524-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 4. | Eid AI, Mueller P, Thabet A, Castillo CF, Fagenholz P. A Step-Up Approach to Infected Abdominal Fluid Collections: Not Just for Pancreatitis. Surg Infect (Larchmt). 2020;21:54-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 5. | Tu Y, Jiao H, Tan X, Wang D, Du J, Sun L, Zhang W. Retroperitoneal laparoscopic debridement and drainage of infected retroperitoneal necrosis in severe acute pancreatitis. Asian J Surg. 2013;36:159-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 6. | Lombardo R, Martos R, Ribal MJ, Alcaraz A, Tubaro A, De Nunzio C. Retroperitoneoscopy in urology: a systematic review. Minerva Urol Nefrol. 2019;71:9-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |