Published online Nov 8, 2016. doi: 10.5409/wjcp.v5.i4.383
Peer-review started: March 18, 2016
First decision: May 19, 2016
Revised: June 30, 2016
Accepted: August 15, 2016
Article in press: August 16, 2016
Published online: November 8, 2016
Processing time: 236 Days and 8.1 Hours
To evaluate the role of zinc as add on treatment to the “recommended treatment” of nephrotic syndrome (NS) in children.
All the published literature through the major databases including Medline/Pubmed, Embase, and Google Scholar were searched till 31st December 2015. Reference lists from the articles were reviewed to identify additional pertinent articles. Retrieved papers concerning the role of zinc in childhood NS were reviewed by the authors, and the data were extracted using a standardized data collection tool. Randomized trials (RCTs) comparing zinc vs placebo was included. Effect of zinc was studied in both steroid sensitive and steroid dependent/frequent relapsing NS. The primary outcome measure was the risk of relapse in 12 mo. The secondary outcome measures were mean relapse rate per patient in 12 mo, mean relapse rate per patient in 6 mo, risk of infection associated relapse in 12 mo, cumulative dose of steroids in two groups, mean length of time to next relapse, adverse effects of therapy, and change in serum zinc levels.
Of 54 citations retrieved, a total of 6 RCTs were included. Zinc was used at a dose of 10-20 mg/d, for the duration that varied from 6-12 mo. Compared to placebo, zinc reduced the frequency of relapses, induced sustained remission/no relapse, reduced the proportion of infection episodes associated with relapse with a mild adverse event in the form of metallic taste. The GRADE evidence generated was of “very low-quality”.
Zinc may be a useful additive in the treatment of childhood NS. The evidence generated mostly was of “very low-quality”. We need more good quality RCTs in different country setting as well different subgroups of children before any firm recommendation can be made.
Core tip: Relapses in nephrotic syndrome (NS) increase morbidity and mortality. Studies have shown that zinc deficiency is common in NS. Zinc deficiency might lead to down-regulation of T-helper 1 (Th1) cytokines, a relative T-helper 2 (Th2) bias, and an increased risk of infection. The later commonly associated with relapse in NS. Zinc supplementation restores Th1-Th2 imbalance and may decrease relapse. The primary aim of this review is to evaluate the efficacy of zinc in preventing relapses in childhood NS (steroid sensitive and steroid dependent/frequent relapsing). The secondary aim is to evaluate the safety of zinc supplementation in this regard.
- Citation: Bhatt GC, Jain S, Das RR. Zinc supplementation as an adjunct to standard therapy in childhood nephrotic syndrome - a systematic review. World J Clin Pediatr 2016; 5(4): 383-390
- URL: https://www.wjgnet.com/2219-2808/full/v5/i4/383.htm
- DOI: https://dx.doi.org/10.5409/wjcp.v5.i4.383
Nephrotic syndrome (NS) is a chronic childhood illness characterized by heavy proteinuria, hypoalbuminemia and oedema. About 80%-85% of the patients with NS shows initial response to corticosteroids and labeled as steroid sensitive nephrotic syndrome (SSNS). Remaining 15%-20% of the patients, who do not respond to steroid therapy are labeled as steroid resistant nephrotic syndrome (SRNS)[1]. About 40%-50% of patients with SSNS have either frequent relapses (FRNS) or steroid dependent (SDNS) courses leading to prolonged course of illness. Relapses are associated with an increased risk of complications such as sepsis, thrombosis, dyslipidemia and malnutrition[2]. Although, relapses can be successfully treated with corticosteroids, repeated usage of high dose corticosteroids lead to significant side-effects like avascular necrosis of hip, hypertension, diabetes and behavioral disorders[3].
Relapses of NS often follow minor infections of the upper respiratory (URI) or gastrointestinal tracts, and the estimated frequency is around 50%-70% among children in developing countries[4,5]. Other infections such as urinary tract infection, diarrhea, peritonitis and skin infections have also been implicated as triggers for relapse[6]. Several theories like cytokine release, immune dysfunction, increased glomerular permeability, and podocytopathy are proposed, but none of them is conclusive[4,7-9].
A number of interventions have been tried to prevent/decrease relapses in NS. Relapses are significantly reduced when daily corticosteroids are given during onset of viral URIs[10,11] or when the maintenance doses of corticosteroids are increased at the onset of viral URIs[12]. Studies have shown that zinc supplementation reduces relapses in children with SSNS[5,13]. It is proposed that zinc deficiency might lead to down-regulation of T-helper 1 (Th1) cytokines, a relative T-helper 2 (Th2) bias, and an increased risk of infection[14,15]. As a result, zinc supplementation augments the gene expression for IL-2 and IFN-γ, thereby restoring the Th1 immune response[16]. Since, the Th1-Th2 cytokine imbalance is also believed to result in relapses of SSNS, it was proposed that the benefits of supplementation in these patients may be associated with its ability to rectify the immune defect[5]. In the present systematic review, we tried to found the role of zinc supplementation as an adjunct to standard therapy in childhood NS. To evaluate the efficacy and safety of zinc in preventing relapses in childhood NS, steroid sensitive and steroid dependent/frequent relapsing.
The review has been registered at the PROSPERO register: CRD42015026456.
Randomized controlled trials (RCTs) and quasi RCT’s comparing zinc with placebo or no additional intervention with ≥ 80% follow-up (to reduce the risk of attrition bias in the included studies in case intention-to-treat analysis has not been done).
Children of 1 to 18 years of age with frequently relapsing or steroid dependent NS were included. Studies including children with first episode NS, secondary NS, impaired renal function, SRNS, congenital NS, serious (peritonitis, Pnumonia, cellulitis) or active infections, leucopenia, thrombocytopenia, and severe anemia were excluded.
The intervention group received oral zinc supplementation regardless of the dosage and type and the control group received standard therapy alone or an oral supplementation without zinc in adjunct to standard therapy for NS.
Steroid sensitive NS.
Primary outcomes: Frequency of relapses in 12 mo.
Secondary outcomes: Frequency of relapses in 6 mo; risk of relapse per year; risk of infection associated relapse per year; cumulative dose of steroids in two groups; mean length of time to next relapse; adverse effects of therapy; change in serum zinc levels.
Primary outcomes: Frequency of relapses in 12 mo.
Secondary outcomes: Frequency of relapses in 6 mo; risk of relapse per year; risk of infection associated relapse per year; cumulative dose of steroids in two groups; mean length of time to next relapse; adverse effects of therapy; change in serum zinc levels.
Steroid sensitive: Remission is achieved within 4 wk of steroid therapy.
Relapse: It is defined as urinary protein excretion 3+/4+ on reagent strip or proteinuria > 40 mg/m2 per hour for 3 consecutive days in patient who had previously been in remission (urine albumin trace or nil or proteinuria < 4 mg/m2 per hour for 3 consecutive days). Frequent relapse is defined as ≥ 2 relapses in 6 mo of initial response or > 3 relapses in 12 mo. For the treatment of relapse, the patient is initially put on daily corticosteroids till remission and then on alternate day steroids.
Steroid dependent: Two consecutive relapses while on alternate steroids or within 14 d of its discontinuation.
Frequent relapse: ≥ 2 relapses in 6 mo of initial response or > 3 relapses in 12 mo.
Following major databases were searched systematically: Cochrane Central Register of Controlled Trials, PubMed/MEDLINE, Google Scholar, and EMBASE till 31st December 2015. Following search terms were used: [(“zinc”/exp or “zinc” or “zinc phosphate”/exp or “zinc phosphate”) and (“child”/exp or “infant”/exp or “school child”/exp or “preschool child”/exp or “toddler”/exp) and (“NS”/exp or “congenital NS”/exp or “kidney disease”/exp)] and (“randomized controlled trial”/exp or “controlled clinical trial”/exp or “clinical trial”/exp).
We also searched the major Pediatric nephrology scientific meetings and contact the authors involved in previous studies for any unpublished work. To identify unpublished trial results, we searched the United Stated National Institutes of Health, Department of Health and Human Services trials registry (http://www.clinicaltrials.gov/) and the WHO International Clinical Trials Registry Platform trial registry (http://www.who.int/ictrp/en/). No language restriction was applied. Two reviewers reviewed the search results to identify relevant original human clinical trials.
Data extraction was done using a pilot tested data extraction form. Two authors independently extracted data including author, year, study setting, type of population, exposure/intervention (dose of steroid, duration), results (outcome measures, effect, significance), and sources of funding/support. Any disagreement in the extracted data was resolved through discussion with the third author.
Two review authors independently assessed the methodological quality of the selected trials by using Cochrane risk of bias tool[17].
For assessment of the quality of evidence we used GRADE Profiler software (version 3.2)[18]. The software uses five parameters for rating the quality of evidence. The parameters used were - limitations to design of randomized controlled trials, inconsistency of results or unexplained heterogeneity, indirectness of evidence, imprecision of results, and publication bias. The rating was done as - no, serious, and very serious limitation.
The data from various studies was pooled and expressed as mean difference (MD) with 95%CI in case of continuous data, and odds ratio with 95%CI in case of categorical data. P-value < 0.05 was considered significant. Assessment of heterogeneity was done by I2 statistics. If there is a high level heterogeneity (> 50%), we tried to explore the cause. A fixed effects model was initially conducted, and if significant heterogeneity existed between the trials, potential sources of heterogeneity were considered and where appropriate, a random effects model was used. RevMan (Review Manager) version 5.2 was used for all the analyses.
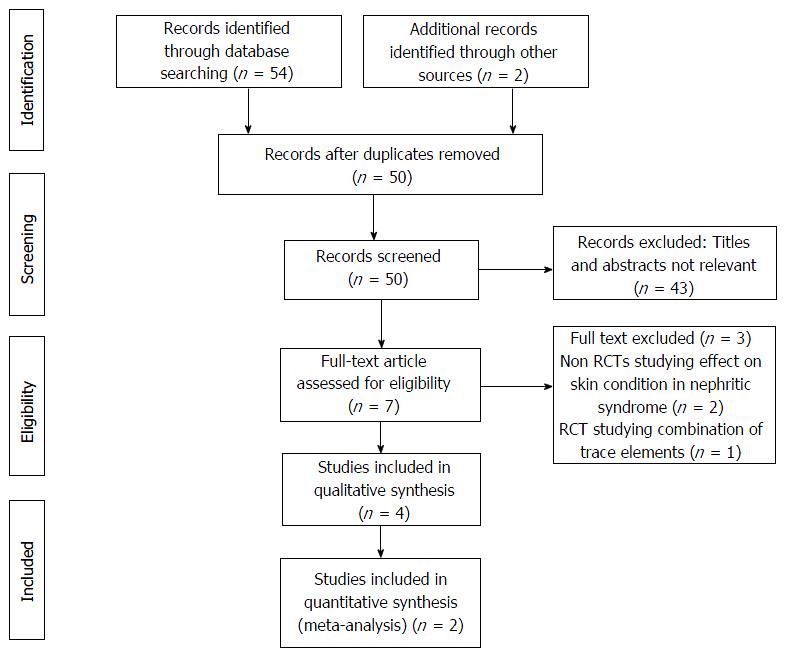
Of 56 citations retrieved, full text of 7 articles were assessed for eligibility (Figure 1). Out of these, a total of 6 RCTs were included[5,13,19,20], actually 2 RCTs evaluated both SSNS/FRNS, and SSNS/SDNS[5,19]. Out of these, 2 were conference abstracts[19,20]. We contacted the authors of these abstracts for providing the details but no reply was given, so we included data given in the abstracts only. The detailed characteristics of trials have been described in Table 1. Out of the 4 trials, 2 were conducted in India, 1 in Pakistan, and 1 in Philippines. Five trials included a total of 256 children [SSNS = 2 trials (100 children); SDNS/FRNS = 4 trials (156 children)] of 1 to 18 years age (excluding neonates < 1 mo). The dose of zinc used was 10 mg/d for a period of 12 mo in one trial[5], and 6 mo in another trial[13]. In other 2 trials, one used 20 mg/d zinc for 2 wk starting at the onset of an episode of acute infection[19], and another used zinc at the recommended daily allowance dose[20].
| Ref. | Setting, country | Participants | Intervention | Outcomes measured | Comments |
| Arun et al[5] | Hospital (out-patient), India | Number: 81 [Frequent relapse = 52 (zinc = 26; placebo = 26); Infrequent relapse = 29 (zinc = 14; placebo = 15)] Age: 1-16 yr Inclusion: SSNS with infrequent relapses or FRNS with prednisolone requirement ≤ 0.75 mg/kg on alternate days | Dose: Zinc sulfate 10 mg/d (1 h before or 2 h after meal) Duration: 12 mo | Frequency of relapses, number of relapses (mean), time to first relapse, adverse drug affects, proportion of infection associated relapses, and change in serum zinc level | Double blind placebo-controlled trial. ITT analysis not done. Small sample size (underpowered to show significant differences in the groups). Inclusion of infrequent relapsers may have diluted the significance of the findings. Authors proposed testing of a higher zinc dose along with immunological correlation |
| Sherali et al[12] | Hospital (out-patient), Pakistan | Number: 60 (zinc = 30; placebo = 30) Age: 2-15 yr Inclusion: FRNS | Dose: Zinc sulfate 10 mg/d Duration: 6 mo | Frequency of relapses, number of relapses (mean), episodes of infections, adverse drug affects, and change in serum zinc level | Double blind placebo-controlled trial. ITT analysis not done. Small sample size. Allocation concealment not clear. Post-supplementation zinc level was not measured in all subjects. Authors proposed testing of a higher zinc dose in a larger cohort |
| Afzal et al[18] | Hospital (out-patient), India | Number: 30 (zinc = 16; placebo = 14) Age (mean ± SD): 6.45 ± 2.92 yr Inclusion: FRNS (n = 24) and SDNS | Dose: Zinc 20 mg/d Duration: 2 wk starting at the onset of an episode of infection (for 12 mo) | Frequency of relapses, number of relapses (mean), episodes of infections, adverse drug affects, and change in serum and hair zinc level | Open label trial. ITT analysis not clear. Small sample size. Post-supplementation. Authors proposed testing of a higher zinc dose in a larger population |
| Pardillo et al[19] | Hospital (out-patient), Philippines | Number: 34 Age: Not clear (only children included) Inclusion: SSNS (majority) and SDNS | Dose: RDA Duration: 6 mo | Frequency of relapses, number of relapses (mean), episodes of infections, and adverse drug affects | Double blind placebo-controlled trial. ITT analysis not clear. Small sample size. Authors proposed testing of a higher zinc dose in a larger population |
Effect of Interventions: (1) steroid sensitive NS: Primary outcome measure: Frequency of relapses in 12 mo: This was reported in 1 out of the 2 trials[5]. The mean relapse rate was lower in the zinc group (1.0 ± 1.16) compared to the placebo group (1.2 ± 1.11), the pooled effect size showing 20% reduction that was not significant (MD = 0.2; 95%CI: 0.71-0.31); (2) secondary outcome measure: Frequency of relapses in 6 mo: This was reported in two trials[5,20]. The result could not be pooled as the data was not provided in 1 trial[19]. In one trial, the mean relapse rate was lower in the zinc group (0.49 ± 0.79) compared to the placebo group (0.68 ± 0.92), the pooled effect size showing 19% reduction (MD = 0.19; 95%CI: 0.57-0.19; P > 0.05). In another trial, there was significant decrease in the frequency of relapse in the zinc group[20]; risk of relapse per year: This was reported in 1 out of the 2 trials[5]. The zinc group had a 31% lower risk of relapse (RR = 0.69, 95%CI: 0.45-1.07; P > 0.05) compared to the placebo group; risk of infection associated relapse in 12 mo: This was reported in one trial, but the data was not provided; cumulative dose of steroids in two groups: This was not reported in any of the trials; mean length of time to next relapse: This was reported in one trial[5]. There was a non-significant decrease in the length of time (mo) to next relapse in the zinc group compared to the placebo group (7.9 vs 6.4; P > 0.05); adverse effects of therapy: A mild adverse event in the form of metallic taste was reported in three subjects in one trial[5]; change in serum zinc levels: One trial provided this information[5]. At enrollment, 5 children (zinc = 2; placebo = 3) were zinc deficient, but at 12 mo none was zinc deficient.
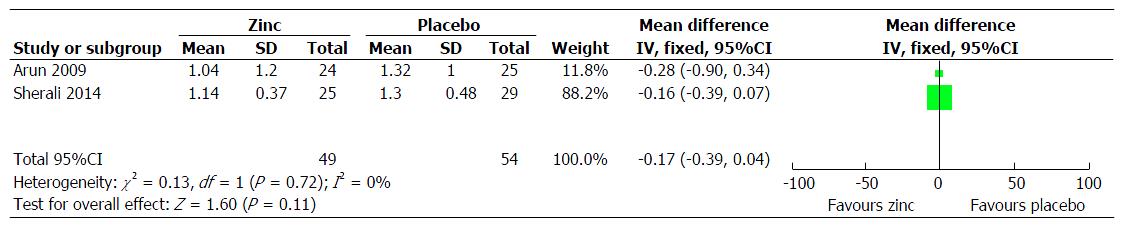
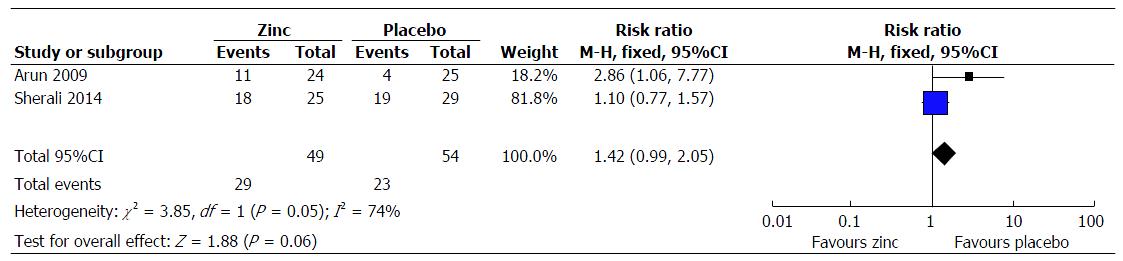
Steroid dependent/frequent relapsing NS: (1) primary outcome measure: Frequency of relapses in 12 mo: This was reported in 3 trials[5,13,19], however the result could be pooled from 2 trials[5,13]. There was decreased frequency of relapses in the zinc group compared to the placebo group (MD = 0.17; 95%CI: 0.39-0.04; P = 0.11) (Figure 2); (2) secondary outcome measure: Frequency of relapses in 6 mo: This was reported in 2 trials[5,19]. The result could not be pooled as the data was not provided in 1 trial[19]. In one trial, the mean relapse rate was lower in the zinc group (0.52 ± 0.0) compared to the placebo group (0.68 ± 0.8), the pooled effect size showing 16% reduction (MD = 0.16; 95%CI: 0.6-0.3; P > 0.05). In another trial, there was significant decrease in the frequency of relapse in the zinc group[19]; sustained remission/no relapse: This was reported in 2 trials[5,13]. The zinc group had a higher chance of going into sustained remission/no relapse compared to the compared to the placebo group (RR = 1.42; 95%CI: 0.99-2.05; P = 0.06) (Figure 3); proportion of infection episodes associated with relapse: This was reported in one trial[19]. The risk was lower in the zinc group (0.16) compared to the placebo group (0.33) (P = 0.012); cumulative dose of steroids in two groups: This was not reported in any of the trials; mean length of time to next relapse: This was not reported in any of the trials; adverse effects of therapy: A mild adverse event in the form of metallic taste was reported in three subjects in one trial[5], and in 10% of children in another trial[13]; change in serum zinc levels: Two trials provided this information[5,19]. None were zinc deficient at 12 mo.
We could not assess publication bias in the included trials because of fewer numbers.
The evidence generated was of “very low quality” for following outcomes under SDNS/FRNS the result of which could be pooled: Frequency of relapses in 12 mo, and sustained remission/no relapse (Tables 2 and 3).
| Patient or population: Patients with nephrotic syndrome | |||||
| Settings: Hospital setting | |||||
| Intervention: Zinc | |||||
| Outcomes | Illustrative comparative risks3 (95%CI) | Relative effect (95%CI) | No. of participants (studies) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| Control | Zinc | ||||
| Frequency of relapses in 12 mo Follow-up: 12 mo | The mean frequency of relapses in 12 mo in the control groups was 2% | The mean frequency of relapses in 12 mo in the intervention groups was 0.2 lower (0.71 lower to 0.31 higher) | 81 (1 study) | Very low1,2 | |
| Frequency of relapses in 6 mo Follow-up: 12 mo | The mean frequency of relapses in 6 mo in the control groups was 19% | The mean frequency of relapses in 6 mo in the intervention groups was 0.19 lower (0.57 lower to 0.19 higher) | 81 (2 studies) | Very low1,2 | |
| Risk of relapse per year Follow-up: 12 mo | 725 per 1000 | 500 per 1000 (326 to 776) | RR = 0.69 (0.45 to 1.07) | 78 (1 study) | Very low1,2 |
| Mean length of time to next relapse Follow-up: 12 mo | The mean length of time to next relapse in the control groups was 1.5 mo | The mean length of time to next relapse in the intervention groups was 1.5 higher (0 to 0 higher) | 78 (1 study) | Very low1,2 | |
| Patient or population: Patients with nephrotic syndrome | |||||
| Settings: Hospital setting | |||||
| Intervention: Zinc | |||||
| Outcomes | Illustrative comparative risks4 (95%CI) | Relative effect (95%CI) | No. of participants (studies) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| Control | Zinc | ||||
| Frequency of relapses in 12 mo Follow-up: 12 mo | The mean frequency of relapses in 12 mo in the control groups was 17% | The mean frequency of relapses in 12 mo in the intervention groups was 0.17 lower (0.39 lower to 0.04 higher) | 103 (2 studies) | Very low1,2,3 | |
| Frequency of relapses in 6 mo | The mean frequency of relapses in 6 mo in the control groups was 16% | The mean frequency of relapses in 6 mo in the intervention groups was 0.16 lower (0.6 lower to 0.3 higher) | 50 (2 studies) | Very low1,2 | |
| Sustained remission/no relapse Follow-up: 12 mo | 426 per 1000 | 605 per 1000 (422 to 873) | RR = 1.42 (0.99 to 2.05) | 103 (2 studies) | Very low1,2,3 |
| Proportion of infection episodes associated with relapse Follow-up: 12 mo | The mean proportion of infection episodes associated with relapse in the control groups was 17% | The mean proportion of infection episodes associated with relapse in the intervention groups was 0.17 lower (0 to 0 higher) | 30 (1 study) | Very low1,2 | |
After an extensive search of the literature we could find 6 trials to be eligible for inclusion. Our result indicates that, for steroid sensitive NS, zinc reduces the frequency of relapses in 12 mo and 6 mo, risk of relapse per year, mean length of time to next relapse with a mild adverse event in the form of metallic taste. For steroid dependent/frequent relapsing NS, zinc reduces the frequency of relapses in 12 mo and 6 mo, induces sustained remission/no relapse, reduces the proportion of infection episodes associated with relapse with a mild adverse event in the form of metallic taste. When we constructed the GRADE of evidence from the available evidence, it was found to be of “very low quality”.
The mechanism by which zinc is helpful as an adjunct in the treatment of childhood NS is not clear. The pathogenesis of childhood NS (e.g., SSNS, SDNS, FRNS) and the basis for relapses triggered by various infections are also unclear. There is evidence from the literature that a perturbed immune dysfunction (e.g., elevated levels of IgE and up-regulation of IL-4 and IL-13 suggest a Th2 cytokine bias[14,15]. There have been studies that show a lower blood level of zinc in childhood NS[21]. Moreover, children from developing country setting are more prone for zinc deficiency. Zinc deficiency might lead to down-regulation of Th1 cytokines, a relative Th2 bias, and increased risk of infections[14,15]. Data from various reports suggest that zinc has a therapeutic role in diarrhea and respiratory infections[22,23]. As infections are most common inciting condition leading to relapse in childhood NS, it is believable that that zinc supplementation would reduce the frequency of infections and thereby relapses. The present evidence is also in accordance with this.
Most outcomes were reported in single trials, so result could not be pooled except from few. The evidence generated was of “very low quality” (the result could be pooled for only two outcomes, high chance of publication bias, some trial also having moderate to high risk of bias because of the methods of blinding/allocation concealment). As the dose range varied among the trials, we could not determine an optimal therapeutically effective dose of zinc. No trial was conducted in a developed country setting, so it is difficult to make any generalized recommendation to all parts of the world.
More trials including a larger sample of children with FRNS or SDNS are needed in order to strengthen the evidence. A uniform dose of zinc as well different dose should be studied to find any optimal therapeutic benefit. Trials should also report about the cost-benefit ratio. The therapeutic effect of zinc in different subgroups of children should also be studied. The effect of zinc supplementation should be correlated with the immunological markers to strengthen the evidence or recommendation in this regard.
In conclusion, zinc may be a useful additive in the treatment of childhood NS. The evidence generated mostly was of “very low-quality”. We need more good quality RCTs in different country setting as well different subgroups of children and disease subtype before any firm recommendation can be made.
The authors would like to thank Dr. Nishant P Jaiswal, Scientist C, ICMR Advanced Centre for Evidence Based Child Health, PGIMER, Chandigarh for his help in the database search.
Relapses in childhood nephrotic syndrome (NS) increase morbidity and mortality. Studies have shown that zinc supplementation reduces relapses in children with steroid sensitive NS. It is proposed that zinc deficiency might lead to down-regulation of T-helper 1 (Th1) cytokines, a relative T-helper (Th2) bias, and an increased risk of infection. The later commonly leading to relapse in childhood NS. Zinc supplementation restores Th1-Th2 imbalance and may decrease relapse. The primary aim of this review is to evaluate the efficacy of zinc in preventing relapses in childhood NS (steroid sensitive and steroid dependent/frequent relapsing). The second aim is to evaluate the safety of above intervention in the prevention of relapses in childhood NS.
About 80%-85% of children with NS shows initial response to corticosteroids (SSNS), and remaining 15%-20% who do not steroid resistant NS. About 40%-50% of patients with SSNS have either frequent relapses or steroid dependent courses leading to prolonged course of illness. Relapses often follow infections (e.g., respiratory, gastrointestinal, urinary infections). Several theories like cytokine release, immune dysfunction, increased glomerular permeability, and podocytopathy are proposed, but none of them is conclusive. A number of interventions have been tried to prevent/decrease relapses. Studies have shown that zinc supplementation reduces relapses in childhood NS.
Zinc supplementation has been shown to reduce relapses in childhood NS. It is proposed that zinc deficiency might lead to down-regulation of Th1 cytokines, a relative Th2 bias, and an increased risk of infection. Zinc supplementation probably corrects the underlying immune imbalance and decreases relapse. Retrieved papers (clinical trials) concerning the utility of zinc were reviewed by the authors, and the data were extracted using a standardized collection tool.
This review suggests that zinc may be a useful additive in the treatment of childhood NS. The evidence generated mostly was of “very low-quality”. We need more good quality randomized trials in different country setting as well different subgroups of children and disease subtype before any firm recommendation can be made.
Steroid sensitive NS: Remission is achieved within 4 wk of steroid therapy. Relapse is defined as urinary protein excretion 3+/4+ on reagent strip or proteinuria > 40 mg/m2 per hour for 3 consecutive days in patient who had previously been in remission (urine albumin trace or nil or proteinuria < 4 mg/m2 per hour for 3 consecutive days). Frequent relapse is defined as ≥ 2 relapses in 6 mo of initial response or > 3 relapses in 12 mo. For the treatment of relapse, the patient is initially put on daily corticosteroids till remission and then on alternate day steroids. Steroid dependent NS: 2 consecutive relapses while on alternate steroids or within 14 d of its discontinuation. Frequent relapse NS: ≥ 2 relapses in 6 mo of initial response or > 3 relapses in 12 mo.
In this systematic review, the authors have presented a thorough and critical analysis of the utility of zinc supplementation in prevention/decrease of the frequency of relapses in childhood NS.
Manuscript source: Invited manuscript
Specialty type: Pediatrics
Country of origin: India
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Salvadori M, Tanaka H S- Editor: Qiu S L- Editor: A E- Editor: Li D
| 1. | Santín S, Bullich G, Tazón-Vega B, García-Maset R, Giménez I, Silva I, Ruíz P, Ballarín J, Torra R, Ars E. Clinical utility of genetic testing in children and adults with steroid-resistant nephrotic syndrome. Clin J Am Soc Nephrol. 2011;6:1139-1148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 158] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 2. | Webb NJA. Epidemiology and general management of childhood idiopathic nephrotic syndrome. Evidence-based Nephrology. Oxford, UK: Wiley-Blackwell 2008; . [DOI] [Full Text] |
| 3. | Hall AS, Thorley G, Houtman PN. The effects of corticosteroids on behavior in children with nephrotic syndrome. Pediatr Nephrol. 2003;18:1220-1223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 4. | MacDonald NE, Wolfish N, McLaine P, Phipps P, Rossier E. Role of respiratory viruses in exacerbations of primary nephrotic syndrome. J Pediatr. 1986;108:378-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 103] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 5. | Arun S, Bhatnagar S, Menon S, Saini S, Hari P, Bagga A. Efficacy of zinc supplements in reducing relapses in steroid-sensitive nephrotic syndrome. Pediatr Nephrol. 2009;24:1583-1586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 6. | Moorani KN. Infections are common a cause of relapse in children with Nephrotic syndrome. Pak Paed J. 2011;35:213-219. |
| 7. | Mathieson PW. Immune dysregulation in minimal change nephropathy. Nephrol Dial Transplant. 2003;18 Suppl 6:vi26-vi29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 41] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 8. | Brenchley PE. Vascular permeability factors in steroid-sensitive nephrotic syndrome and focal segmental glomerulosclerosis. Nephrol Dial Transplant. 2003;18 Suppl 6:vi21-vi25. [PubMed] |
| 9. | Holt RC, Webb NJ, Ralph S, Davies J, Short CD, Brenchley PE. Heparanase activity is dysregulated in children with steroid-sensitive nephrotic syndrome. Kidney Int. 2005;67:122-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 10. | Gulati A, Sinha A, Sreenivas V, Math A, Hari P, Bagga A. Daily corticosteroids reduce infection-associated relapses in frequently relapsing nephrotic syndrome: a randomized controlled trial. Clin J Am Soc Nephrol. 2011;6:63-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 62] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 11. | Mattoo TK, Mahmoud MA. Increased maintenance corticosteroids during upper respiratory infection decrease the risk of relapse in nephrotic syndrome. Nephron. 2000;85:343-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 37] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 12. | Sherali AR, Moorani KN, Chishty SH, Khan SI. Zinc supplement in reduction of relapses in children with steroid sensitive nephrotic syndrome. J Coll Physicians Surg Pak. 2014;24:110-113. [PubMed] |
| 13. | Shankar AH, Prasad AS. Zinc and immune function: the biological basis of altered resistance to infection. Am J Clin Nutr. 1998;68:447S-463S. [PubMed] |
| 15. | Bao B, Prasad AS, Beck FW, Godmere M. Zinc modulates mRNA levels of cytokines. Am J Physiol Endocrinol Metab. 2003;285:E1095-E1102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 133] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 16. | Higgins JPT, Green S. Cochrane handbook for systematic reviews of interventions. Version 5.1.0. The Cochrane Collaboration, 2011. . |
| 17. | Schu¨nemann H, Brozek J, Oxman A. GRADE handbook for grading quality of evidence and strength of recommendation. Version 3.2, 2008. . |
| 18. | Afzal K, Jindal S, Shahab T, Khan RA. Efficacy of zinc supplementation in reducing the frequency of relapses in frequently relapsing/steroid dependent nephrotic syndrome in children, 2012. |
| 19. | Pardillo RP, Antonio Z, Rosel M, Leon OD, Marbella MA, Imbisan CA, Manuel R. The effect of zinc supplementation in reducing relapses among steroid sensitive nephrotic syndrome and steroid dependent nephrotic syndrome in children, a randomized double blind placebo control study, 2012. |
| 20. | Reimold EW. Changes in zinc metabolism during the course of the nephrotic syndrome. Am J Dis Child. 1980;134:46-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 21. | Singh M, Das RR. Clinical potential of zinc in prophylaxis of the common cold. Expert Rev Respir Med. 2011;5:301-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 22. | Das RR. Differential effects of zinc in severe pneumonia in children. Indian J Pediatr. 2011;78:1159-1160; author reply 1160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 23. | Das RR. Zinc in acute childhood diarrhea: Is it universally effective? Indian J Pharmacol. 2012;44:140; author reply 140-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |