Published online Aug 8, 2016. doi: 10.5409/wjcp.v5.i3.306
Peer-review started: March 1, 2016
First decision: March 22, 2016
Revised: March 29, 2016
Accepted: April 14, 2016
Article in press: April 18, 2016
Published online: August 8, 2016
Processing time: 159 Days and 20.7 Hours
AIM: To verify if subclinical hypothyroidism (SCH) could be associated to atopy in children.
METHODS: Seven hundred and thirty-two Caucasian children from South Italy presenting symptoms of allergic disease were enrolled and submitted to atopy, obesity, chronic low grade inflammation, and SCH work up.
RESULTS: Four hundred and forty-five out of 705 (63.12%) children affected by allergic disease were diagnosed as atopic and 260 (36.88%) as not atopic. The SCH prevalence was 6.3%. Significant higher prevalence of SCH among atopic children with average (group 2) and high (group 3) low grade chronic inflammation compared to atopic children with mild (group 1) low grade chronic inflammation was present. Moreover, group 1 and group 2 presented an OR to show SCH of 2.57 (95%CI: 1.55-6.26) and 2.96 (95%CI: 1.01-8.65), respectively. Both in atopic and not atopic children we found C3 serum levels significantly higher in group 3 respect to group 2 and group 1. Noteworthy, among atopic patients, also total immunoglobulin E (IgE) serum levels, were significantly higher in group 3 compared to group 2 and group 1 children. In atopic children, C3 and total IgE serum values increased in parallel with the increase of C-reactive protein values, while in not atopic children this phenomenon was not evident.
CONCLUSION: The possibility exists that an increasing atopic inflammation contributes to SCH occurrence. So far this is the first report in literature showing an association between SCH and atopy but further studies are needed to confirm our data.
Core tip: A high subclinical hypothyroidism (SCH) prevalence has been associated in childhood to obesity and the chronic low grade inflammation found obese children has been involved in this relationship. In our population, obesity does not influence SCH prevalence. Interestingly, we found a SCH prevalence twice higher compared to all other patients and a significant higher risk to show SCH in atopic children affected by the highest C-reactive protein values characterizing low-grade inflammation.
- Citation: Pedullà M, Fierro V, Marzuillo P, Del Tufo E, Grandone A, Perrone L, Miraglia del Giudice E. Subclinical hypothyroidism in atopic South Italian children. World J Clin Pediatr 2016; 5(3): 306-310
- URL: https://www.wjgnet.com/2219-2808/full/v5/i3/306.htm
- DOI: https://dx.doi.org/10.5409/wjcp.v5.i3.306
Subclinical hypothyroidism (SCH) is defined as a thyroid stimulating hormone (TSH) serum level above the statistically defined upper limit of the reference range despite normal serum free T4 (fT4) and free T3 (fT3) concentration. In addition to genetic cause, SCH has been associated to childhood obesity[1], with some slight myocardial dysfunction[2].
It has been recently demonstrated that atopy can be related to childhood obesity and that chronic low grade inflammation related to it was involved in this relationship[3-5].
The aim of our study is to verify if, as well as to obesity, SCH could be also associated to atopy in children.
Seven hundred thirty-two Caucasian children from South Italy (age in years 6.03 ± 3.63) presenting symptoms of allergic disease (erythema, pruritus, eczematous rash, wheals, ocular and nasal pruritus and wheezing) attending consecutively from January 2013 to July 2015 the Department of Pediatrics of the Second University of Naples were enrolled. None showed any clinical symptoms of thyroid disease. All the enrolled children were submitted to atopy, obesity, chronic low grade inflammation, and SCH work up. Body mass index (BMI) was calculated by dividing weight in kilograms by height squared in meters (kg/m2). Obesity was defined by a BMI ≥ 95th percentile[6].
In all patients, after an overnight fasting, a blood sample was obtained to evaluate C-reactive protein (CRP) and Complement C3 serum levels, measured using an Olympus AU 560 apparatus by an enzymatic colorimetric method, total and specific IgE by a fluorenzymeimmunoassay (ImmunoCap and ImmunoCap 0-100), thyroid hormones (TSH, fT3, and fT4) and anti-thyroid peroxidase antibodies (TPO-Ab) and anti-thyroglobulin antibodies (Tg-Ab), determined by high-specific solid-phase technique-chemiluminescence immunoassays (Perkinelmer, Turku, Finland).
The diagnosis of atopy suspected for clinical history and symptoms, was confirmed by levels of serum total IgE (normal value from 25 to 60 mo of age < 81 kU/L, from 61 to 156 mo of age < 101 kU/L) and specific immunoglobulin E (IgE) assay (> 0.36 kUA/L) as well as by Skin Prick Tests (SPTs). None had taken steroids or received immuno-suppressive therapy for at least 3 mo before investigation. Antihistamine therapy had been stopped at least 2 wk before SPTs were performed and serum samples were collected.
According to atopy diagnosis the children enrolled in the study were divided in two groups: Atopic and not atopic children.
Chronic low grade inflammation is diagnosed by slightly raised concentrations of inflammatory markers in the systemic circulation[7]. Therefore, to better assess the chronic low grade inflammation status we applied both in atopic and not atopic children the cut-off point described by Pearson et al[8] for assessing cardio vascular disease (CVD) risk. Thus we divided children in three different CRP serum values gradation groups: group 1 low grade (CRP < 0.1 mg/dL), group 2 average grade (CRP 0.1-0.3 mg/dL) and group 3 high grade (CRP > 0.3-< 1 mg/dL) of chronic low grade inflammation.
Twenty-seven children with CRP serum values greater than 1 mg/dL were excluded from the study given the possibility of an ongoing infection.
SCH was diagnosed when TSH value was higher than 5 mUI/mL, as indicated by the “European Thyroid Association Guidelines for the Management of Subclinical Hypothyroidism in Pregnancy and in Children”[9,10], with fT3, fT4, and antithyroid antibodies values in the normal range for age and no clinical signs or symptoms of hypothyroidism[10].
Informed consent was obtained from all enrolled children and their parents and in accordance with the approved protocol from the Institutional Review Board at the University of Naples.
Skewness and Kurtosis tests were used to evaluate if the distribution of continuous variables was normal. According to distribution, the values were expressed as mean ± SD or median and minimum-maximum values. To analyze categorical variables was used t test for unpaired data. A χ² test was also used to analyze the differences between the frequencies. Mann-Whitney U test was used for comparison of continuous variables which did not exhibit normal distribution. A P value < 0.05 was considered significant.
Odds ratio (OR) was calculated to evaluate the association of Atopy and SCH prevalence. OR was considered significant when a 95%CI excluded unity. Statistical analysis were performed using Stat-Graphics Centurion 3.0 for Windows.
On the basis of atopy work-up 459 out of 705 (62.7%) children affected by allergic disease were diagnosed as atopic and 273 (37.3%) as not atopic. The overall prevalence of SCH was 6.3%.
In Table 1 we described clinical and laboratory differences between these two groups of children. No significant differences were found.
| Atopic children | Not atopic children | P | |
| Patients, n | 459/732 (62.7%) | 273/732 (37.3%) | |
| Age expressed in years (mean age ± SD) | 6.24 ± 3.55 | 5.82 ± 3.71 | 0.128 |
| Gender (male) | 245/459 (53.37%) | 137/274 (50%) | 0.37 |
| Family history of atopy | 365/420 (86.9%) | 195/235 (80%) | 0.17 |
| Family history of thyroid disease | 191/421 (45.36%) | 94/235 (40%) | 0.18 |
| BMI (kg/m2) | 16.6 (10.9-36.8) | 16.4 (10.7-30.17) | 0.069 |
| SCH affected (%) | 33/445 (7.41%) | 13/260 (5%) | 0.21 |
| SCH obeses (%) | 1/33 (3%) | 1/13 (7.69%) | 0.48 |
| CRP (mg/dL) | 0.06 (0-10.3) | 0.08 (0-7.9) | 0.123 |
| TSH (UI/mL) | 2.33 (0.35-12.2) | 2.24 (0.52-6.98) | 0.269 |
Table 2 shows clinical and laboratory data of both atopic and not atopic children divided into 3 different groups on the basis of increasing CRP serum values: group 1 (CRP < 0.1 mg/dL), group 2 (CRP 0.1-0.3 mg/dL) and group 3 (CRP > 0.3-< 1 mg/dL). First we compared the prevalence of SCH among all the groups and no significant differences were found.
| Atopic children | P | Not atopic children | P | |||||
| Group 1 | Group 2 | Group 3 | Group 1 | Group 2 | Group 3 | |||
| CRP (mg/dL) | < 0.1 | 0.1-0.3 | > 0.3-< 1 | < 0.1 | 0.1-0.3 | > 0.3-< 1 | ||
| SCH | 18/272 (6.62%) | 7/121 (5.78%) | 8/52 (15.38%) | 10.033 OR: 2.57 (1.55-6.26) 30.04 OR 2.96 (1.01-8.65) | 8/151 (5.29%) | 3/81 (3.70%) | 2/28 (7.14%) | |
| C3 (mg/dL) | 115 (20-174) | 122 (50-172) | 138 (103-192) | 11.86e-820.027 30.00021 | 116.5 (10-170) | 126 (73-177) | 139 (109-170) | 10.000053 20.0085 30.024 |
| Total IgE (kUI/L) | 120 (100-2270) | 123.5 (100-4328) | 162.1 (124-5000) | 10.000017 30.00045 | 17.4 (1.9-98) | 22.45 (1.9-93) | 22.43 (1.9-88.9) | |
On the contrary, among atopic children, significant higher prevalence of SCH in group 3 and group 2 compared to group 1 was found (P = 0.033 and P = 0.04, Table 2). Moreover, group 3 and group 2 atopic children presented an OR to show SCH of 2.57 (95%CI: 1.55-6.26) and 2.96 (95%CI: 1.01-8.65) respectively.
Both in atopic and not atopic children we found C3 serum levels significantly higher in group 3 respect to group 2 and group 1 (Table 2). Noteworthy, among atopic patients, also total IgE serum levels, were significantly higher in group 3 compared to group 2 and group 1 children.
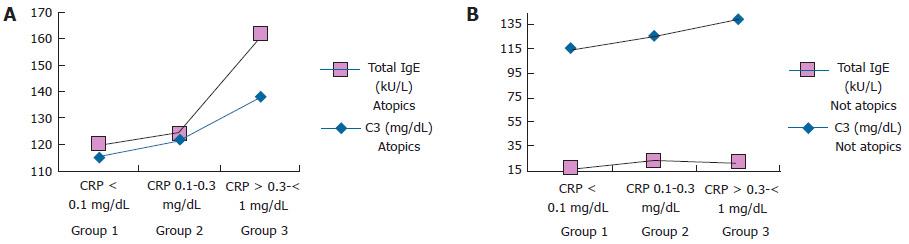
Figure 1 shows in both atopic (A) and not atopic (B) children complement C3 and total IgE serum levels values according to the different grading of CRP values.
In atopic children, C3 and total IgE serum values increased in parallel with the increase of CRP values, but no significant correlation was found. C3 and total IgE serum values in not atopic children showed a completely independent trend.
In our study atopic children showed a 7.41% prevalence of SCH while not atopic children allergic found in alike disease affected a 5% SCH prevalence. These frequencies are in line with the estimated prevalence of this thyroid disorder in the pediatric population[11,12].
A high SCH prevalence has been associated in childhood to obesity and the chronic low grade inflammation found obese children has been involved in this relationship[13]. In our population, obesity does not influence SCH prevalence.
Interestingly, we found a SCH prevalence twice higher (15.38%) compared to all other patients and a significant higher risk to show SCH [2.57 (95%CI: 1.55-6.26)] in atopic children affected by the highest CRP values characterizing low grade inflammation (group 3 atopics).
Atopy and the associated allergic disease are now regarded as systemic inflammatory disease that could affect the risk of atherosclerosis[14], impaired glucose tolerance[15], and coronary artery disease[16]. The chronic low grade inflammation involved in the pathogenesis of localized allergic disease causes a systemic inflammatory response that potentially could promote also SCH.
Moreover, in our atopic children the highest low grade chronic inflammation (CRP > 0.3-< 1 mg/dL) seems to correspond to the highest atopic inflammation as measured by total serum IgE values (Figure 1). The possibility exists that an increasing atopic inflammation contributes to SCH occurrence. A limitation of our study could be a recruitment bias because the patients enrolled were affected severe allergic disease needing of a specialist evaluation.
So far this is the first report in literature showing an association between SCH and atopy but further studies are needed to confirm our data.
Recent studies demonstrate that atopy can be associated to childhood obesity and that chronic low grade inflammation could be involved in this relationship. The aim of the study was to verify if subclinical hypothyroidism (SCH) could be also associated to atopy in children.
Important areas of research related to the study are represented by the field of pediatric endocrinology and allergology. More in particular, the study aimed to hypothesize, throughout the verification of an association, the mechanisms by which atopy could lead to SCH.
This is the first report in literature showing an association between SCH and atopy with a possible causal link represented by chronic low grade inflammation.
The study can result in future researches confirming the authors’ findings and, moreover, understanding the pathophysiological basis underlining the association between atopy and SCH.
SCH is defined as a thyroid stimulating hormone serum level above the statistically defined upper limit of the reference range despite normal serum free T4 and free T3 concentration.
The association among obesity, chronic inflammation and SCH is a very interesting topic.
Manuscript source: Invited manuscript
Specialty type: Pediatrics
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Coskun A, Svetlana V, Zhou S S- Editor: Ji FF L- Editor: A E- Editor: Wu HL
| 1. | Grandone A, Santoro N, Coppola F, Calabrò P, Perrone L, Del Giudice EM. Thyroid function derangement and childhood obesity: an Italian experience. BMC Endocr Disord. 2010;10:8. [PubMed] |
| 2. | Brienza C, Grandone A, Di Salvo G, Corona AM, Di Sessa A, Pascotto C, Calabrò R, Toraldo R, Perrone L, del Giudice EM. Subclinical hypothyroidism and myocardial function in obese children. Nutr Metab Cardiovasc Dis. 2013;23:898-902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 3. | von Mutius E, Schwartz J, Neas LM, Dockery D, Weiss ST. Relation of body mass index to asthma and atopy in children: the National Health and Nutrition Examination Study III. Thorax. 2001;56:835-838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 289] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 4. | Ford ES. C-reactive protein concentration and cardiovascular disease risk factors in children: findings from the National Health and Nutrition Examination Survey 1999-2000. Circulation. 2003;108:1053-1058. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 140] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 5. | Cibella F, Cuttitta G, La Grutta S, Melis MR, Bucchieri S, Viegi G. A cross-sectional study assessing the relationship between BMI, asthma, atopy, and eNO among schoolchildren. Ann Allergy Asthma Immunol. 2011;107:330-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 6. | Cacciari E, Milani S, Balsamo A, Spada E, Bona G, Cavallo L, Cerutti F, Gargantini L, Greggio N, Tonini G. Italian cross-sectional growth charts for height, weight and BMI (2 to 20 yr). J Endocrinol Invest. 2006;29:581-593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 554] [Cited by in RCA: 649] [Article Influence: 34.2] [Reference Citation Analysis (0)] |
| 7. | Trayhurn P, Wood IS. Adipokines: inflammation and the pleiotropic role of white adipose tissue. Br J Nutr. 2004;92:347-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1428] [Cited by in RCA: 1443] [Article Influence: 68.7] [Reference Citation Analysis (0)] |
| 8. | Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO, Criqui M, Fadl YY, Fortmann SP, Hong Y, Myers GL. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107:499-511. [PubMed] |
| 9. | Lazarus J, Brown RS, Daumerie C, Hubalewska-Dydejczyk A, Negro R, Vaidya B. 2014 European thyroid association guidelines for the management of subclinical hypothyroidism in pregnancy and in children. Eur Thyroid J. 2014;3:76-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 440] [Cited by in RCA: 433] [Article Influence: 39.4] [Reference Citation Analysis (0)] |
| 10. | Taubner K, Schubert G, Pulzer F, Pfaeffle R, Körner A, Dietz A, Thiery J, Kiess W, Kratzsch J. Serum concentrations of anti-thyroid peroxidase and anti-thyroglobulin antibodies in children and adolescents without apparent thyroid disorders. Clin Biochem. 2014;47:3-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 11. | Gawlik A, Such K, Dejner A, Zachurzok A, Antosz A, Malecka-Tendera E. Subclinical hypothyroidism in children and adolescents: is it clinically relevant? Int J Endocrinol. 2015;2015:691071. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 12. | Wu T, Flowers JW, Tudiver F, Wilson JL, Punyasavatsut N. Subclinical thyroid disorders and cognitive performance among adolescents in the United States. BMC Pediatr. 2006;6:12. [PubMed] |
| 13. | Wärnberg J, Nova E, Romeo J, Moreno LA, Sjöström M, Marcos A. Lifestyle-related determinants of inflammation in adolescence. Br J Nutr. 2007;98 Suppl 1:S116-S120. [PubMed] |
| 14. | Knoflach M, Kiechl S, Mayr A, Willeit J, Poewe W, Wick G. Allergic rhinitis, asthma, and atherosclerosis in the Bruneck and ARMY studies. Arch Intern Med. 2005;165:2521-2526. [PubMed] |
| 15. | Wang Z, Zhang H, Shen XH, Jin KL, Ye GF, Qiu W, Qian L, Li B, Zhang YH, Shi GP. Immunoglobulin E and mast cell proteases are potential risk factors of impaired fasting glucose and impaired glucose tolerance in humans. Ann Med. 2013;45:220-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 16. | Deliargyris EN, Upadhya B, Sane DC, Dehmer GJ, Pye J, Smith SC, Boucher WS, Theoharides TC. Mast cell tryptase: a new biomarker in patients with stable coronary artery disease. Atherosclerosis. 2005;178:381-386. [PubMed] |