Published online Feb 8, 2016. doi: 10.5409/wjcp.v5.i1.1
Peer-review started: June 2, 2015
First decision: August 22, 2015
Revised: September 10, 2015
Accepted: December 13, 2015
Article in press: December 15, 2015
Published online: February 8, 2016
Processing time: 244 Days and 8.1 Hours
Cardiovascular magnetic resonance is a non-invasive imaging modality which is emerging as important tool for the investigation and management of pediatric cardiovascular disease. In this review we describe the key technical and practical differences between scanning children and adults, and highlight some important considerations that must be taken into account for this patient population. Using case examples commonly seen in clinical practice, we discuss the important clinical applications of cardiovascular magnetic resonance, and briefly highlight key future developments in this field.
Core tip: Cardiovascular magnetic resonance is playing an increasingly important role in the investigation and management of pediatric cardiovascular disease. However, imaging this patient population brings its own unique set of challenges. This article describes some of the key differences between scanning children and adults, discusses the important clinical applications of cardiovascular magnetic resonance in pediatrics, and highlights some of the key future developments in this field.
- Citation: Mitchell FM, Prasad SK, Greil GF, Drivas P, Vassiliou VS, Raphael CE. Cardiovascular magnetic resonance: Diagnostic utility and specific considerations in the pediatric population. World J Clin Pediatr 2016; 5(1): 1-15
- URL: https://www.wjgnet.com/2219-2808/full/v5/i1/1.htm
- DOI: https://dx.doi.org/10.5409/wjcp.v5.i1.1
Cardiovascular magnetic resonance (CMR) is a non-invasive imaging technique that uses magnetic resonance imaging (MRI) to provide clear delineation of cardiovascular anatomy, detailed tissue characterization, and a comprehensive evaluation of cardiac function. In recent decades there has been a significant increase in use of CMR for a variety of purposes in both congenital and acquired heart disease in children.
Overall, the basic sequences and imaging strategies used in children are similar to those used in adults. Adolescents with normal intellectual and emotional development can usually be successfully imaged using adult techniques[1]. However, younger children may not be able to comply with breath-holding during image acquisition and their faster heart and respiratory rates provide technological challenges. Additionally, their anatomy is smaller and, in cases of congenital heart disease, often unique and complex. Pediatric imaging is therefore more demanding in terms of sequence optimization and each scan requires an individualized approach[2,3].
Despite these challenges, CMR remains a useful tool to assist with investigation and management of a wide range of cardiovascular pathology in children. It can be used for diagnostic and screening purposes to define anatomy and assess function, to monitor disease progression as part of serial follow-up, and to plan and evaluate the outcomes of surgery and other therapeutic interventions. This article provides an overview of the main applications of CMR in children and discusses some of the specific considerations for this patient population.
CMR uses magnetic fields and radiofrequency energy to produce tomographic images of the human body. It is based on the phenomenon of “nuclear magnetic resonance” - the ability of some atomic nuclei to selectively absorb then later re-emit radiofrequency energy. The emitted energy can then be captured and transformed into an image. Only nuclei with an odd number of protons and neutrons (thus possessing a “net magnetic moment”) are capable of exhibiting this phenomenon[4]. Several examples of such nuclei are present in biological tissues[5], however the hydrogen (1H) atom is the primary choice for clinical imaging due to its abundance in water and fat.
Under normal conditions, hydrogen nuclei in tissues behave as tiny bar magnets randomly oriented in space such that the net magnetization of the tissue is zero. When placed in a strong magnetic field (created by the large superconducting magnet of the scanner), the nuclei align in the direction of the magnetic field creating a net tissue magnetization oriented along the axis of the scanner. The nuclei spin (precess) around the direction of the magnetic field at a frequency specific to the magnetic field strength[4]. The field strengths of clinical scanners can vary from 0.15 to 7 tesla (T) although CMR is typically performed at 1.5 T, approximately 20000 times the magnetic field strength of the earth[6].
External radiofrequency transmitter coils are used to apply radiofrequency energy to the tissue at a specific “resonance” frequency. Hydrogen nuclei absorb this energy and flip their orientation within the magnetic field, going from a stable low energy state to an unstable high energy state. When the radiofrequency transmission ceases, the nuclei relax back to the lower energy state and re-emit the absorbed energy, which is detected by a receiver coil as radiofrequency signals. The signals are electronically amplified by a computer and the intensity of each signal is plotted on a grey-scale in order to build up a cross-sectional image of the tissue. The resulting image is a representation of the spatially-resolved signals[4,5].
In order to localize the part of the tissue from which these emitted radiofrequency signals originate, gradient coils driven by pulses of electricity are used to produce small field gradients in multiple planes within the wider magnetic field of the scanner magnet. These gradients cause a predictable variation in both the magnetic field strength and resonant frequency in different parts of the patient. By varying the times at which gradient fields are switched on and off in relation to the application of the radiofrequency pulses, then analyzing the properties of the emitted signal (in terms of frequency and phase), the computer is able to reconstruct an image of the patient[6].
The hydrogen nucleus relaxes back to the lower energy state by two main processes: Longitudinal relaxation with relaxation time T1, and transverse relaxation with relaxation time T2[7]. The relative proportions of T1 and T2 relaxation times vary between different tissues. By altering the timing of radiofrequency pulses, strength of the gradient fields, and through use of contrast agents and magnetization preparation pulses (such as inversion recovery, saturation recovery, fat-suppression and blood-nulling sequences), the differences in T1 and T2 values between tissues can be exploited to enable detailed tissue characterization, producing images that highlight the tissue of interest[8].
Echocardiography is the mainstay of cardiovascular imaging in children. It is cheap, quick, accessible, non-invasive and particularly informative in neonates and infants for whom it is possible to achieve good acoustic windows. However, it is operator dependent and provides only limited views of extra-cardiac vascular structures[9,10]. Cardiac catheterization provides useful hemodynamic information and permits concurrent therapeutic intervention. However, it is invasive with rare but potentially fatal complications, involves exposure to ionizing radiation and is dependent on the use of iodine-based contrast agents[11-13]. CMR on the other hand, is non-invasive and radiation-free. This is particularly relevant to children, for whom the risk of risk of radiation-induced malignancy is significantly higher than in adults[14]. Thus CMR is amenable to being used for serial assessments, such as pre- and post-procedure or for ongoing follow-up to monitor disease progression. CMR reduces the requirement for invasive study in certain cases and enables the assessment of anatomy and function where echocardiographic views are sub-optimal. Non-contrast imaging provides excellent soft tissue contrast resolution permitting detailed tissue characterization, and superior structural and functional information, including the determination of extra-cardiac anatomy and hemodynamic parameters[15].
However, CMR scanners are rarely mobile and availability is limited compared to echocardiography. Even in a centre that offers conventional MRI, CMR requires significant software, training and expertise[16]. The data acquisition time is long, typically 20-50 min depending on what information is required, and the space within the magnet is limited. It can be claustrophobic, and for children unable to co-operate with the scanning procedure, general anesthesia may be required. It is also less suitable for clinically unstable patients requiring intensive monitoring, and in the event of a cardiopulmonary arrest, the patient must be removed from the magnet environment of the CMR scanner before advanced life support can commence. All monitoring equipment used during a CMR scan must be MRI compatible, requiring a switch to compatible pumps before the patient enters the scanner.
Computerized tomography (CT) is often used as an alternative to CMR - it permits acquisition of a high resolution data set in a much shorter time period and is therefore useful for children and unstable patients unable to tolerate a lengthy CMR scan. In the pediatric population, it is considered superior to CMR when evaluating airway anatomy in cases of vascular rings, when assessing pulmonary vasculature (particularly in thromboembolic disease of the pulmonary arteries, where breath-holding capability is often compromised), and when determining the presence or absence of any major aorto-pulmonary collateral arteries (MAPCAs)[17,18]. CT can also be used as an alternative where CMR is contraindicated due to the presence of implanted devices, foreign bodies or claustrophobia. However, it is less detailed in terms of tissue characterization and exposes patients to high doses of ionizing radiation - particularly in the case of serial imaging where the cumulative radiation dose is significant[19].
Despite its shortcomings, CMR remains a versatile tool with distinct advantages over other modalities. However, outcomes are most successful when it is used in conjunction with other imaging technologies in a directed manner to obtain an answer to a specific clinical question.
CMR pulse sequences represent the co-ordinated actions of turning on and off the gradient coils and transmitted radiofrequency pulses in order to highlight specific features of the tissue being imaged[15]. The basic principles of these sequences are similar for adult and pediatric CMR. As with adult imaging, sequences used in the pediatric setting must be carefully selected in order to best answer the clinical question. However, in children specific adaptations must be made in order to accommodate the smaller patient size (demanding a higher spatial resolution), and faster heart rates (demanding a higher temporal resolution)[20]. Also, in cases of congenital heart disease where the anatomy is complex, a more individualized approach to the scan is required[2].
Spin echo pulse sequences produce images that are acquired during one fixed point of the cardiac cycle (Figure 1A and B). They are static images in which blood appears black and the surrounding stationary tissue appears in shades of grey[16]. These images are useful for providing anatomical information[21] and they permit excellent tissue characterization (particularly when magnetization preparation pulses are used), achieving good visualization of pathology for conditions such as myocarditis, pericarditis, cardiomyopathies, vasculitis and cardiac tumours[22]. Acquisition time is long, and although faster variants exist, they result in poorer spatial and temporal resolution[20].
Gradient echo cine imaging enables the generation of short “movies” depicting motion of the heart throughout the cardiac cycle. This is achieved by dividing the cardiac cycle into multiple segments (frames) to produce a series of 2D images that can then be laced together into a cinematic display. Blood appears bright and the resulting “cines’’ are useful for assessing the dynamic function of the heart - such as blood flow, valvular function, ventricular volumes, ventricular mass, ejection fraction and motion of the ventricular walls. CMR volume measurements are considered more accurate and reproducible compared to echocardiographic measurements[15,23,24], and normal values for both atrial and ventricular volumes have been widely published in children[25-28] with studies demonstrating good reproducibility[29].
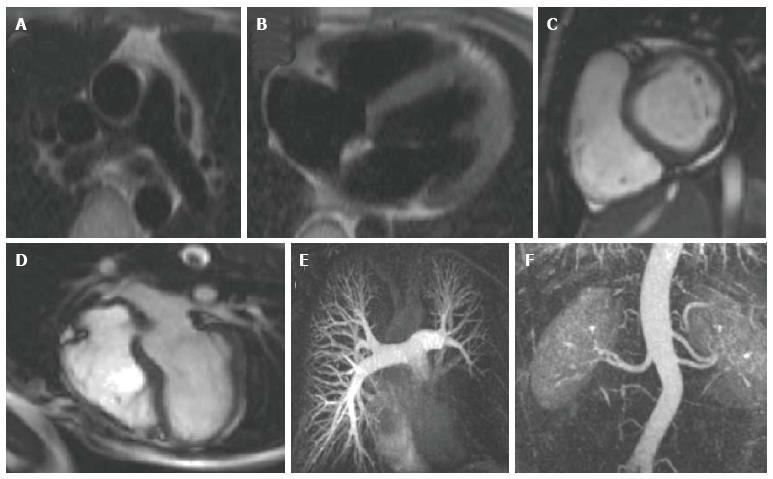
Gradient echo cine imaging can be performed using a standard spoiled gradient echo pulse sequence, or the more recent steady state free precession sequence (SSFP) (Figure 1C and D). SSFP has generally surpassed the use of spoiled gradient echo for cine imaging as it is faster and provides superior contrast between blood and myocardium[20]. It can also be adapted for 3D imaging, enabling acquisition of a high resolution 3D anatomical dataset of the heart and thoracic vasculature without the requirement for intravenous contrast[30-32]. However, SSFP is more prone to artefact when there is inhomogeneity in the magnetic field[20], so both types of sequence still form the basis of multiple cardiac imaging applications. Spoiled gradient echo sequences are still widely used for late gadolinium enhancement (LGE) imaging to detect myocardial fibrosis, 3D contrast- enhanced magnetic resonance angiography (MRA), velocity encoded phase contrast imaging for assessment of in-vivo blood flow, and first-pass perfusion imaging for evaluation of myocardial perfusion.
LGE imaging: Gadolinium-based contrast agents (GBCAs) distribute in greater volumes in fibrosed myocardium and demonstrate slower washout times compared to normal myocardium. Using spoiled gradient echo sequences, it is possible to demonstrate the abnormal deposition of contrast late after contrast injection as focal regions of fibrosis become hyperenhanced[16]. Validation studies have strongly correlated the finding of LGE with the presence and extent of myocardial fibrosis[33,34]. In children, LGE is typically seen along areas of reconstruction post-surgically following repair of congenital cardiac lesions[35-38]. The presence of LGE is known to be associated with arrhythmias and poorer ventricular function and studies in adults have demonstrated that it is associated with a poorer prognosis in dilated cardiomyopathy[39], hypertrophic cardiomyopathy[40,41] and valvular heart disease[42].
3D contrast-enhanced MRA: Running a spoiled gradient echo pulse sequence during administration of intravenous GBCAs enables detailed, high resolution vascular imaging (Figure 1E and F). Gadolinium reduces the T1 relaxation time of blood, enhancing the contrast between blood and the surrounding tissue[43]. By varying the time delay between contrast administration and the pulse sequence, it is possible to alter the portion of the thoracic vasculature imaged. In this manner, clear images of the aorta and its branches, the pulmonary vessels, systemic veins, collateral vessels and any shunts, conduits or vascular grafts can be obtained[44-49]. The collected data can be formatted to generate 2D slices in any orientation, or volume-rendered into a 3D image, often negating the requirement for invasive diagnostic catheterization.
Velocity encoded phase contrast imaging: When hydrogen nuclei (such as in blood) flow through specially designed magnetic field gradients, the signal they emit accumulates a phase shift relative to the signal from the surrounding tissue that is proportional to their velocity. Velocity encoded phase contrast sequences capture data encoding this velocity information in addition to data encoding information about the surrounding tissue. Software can then be used to obtain measurements of flow rates within individual vessels by contouring the vessel in a cross-sectional plane and calculating volume of blood passing through the plane as a product of velocity and cross-sectional area[50-52]. Using this technique, it is possible to assess flow in large and small arteries and the systemic and pulmonary veins. It is also possible to quantify cardiac output and intra- and extra-cardiac shunts, measure pressure gradients across areas of stenosis and calculate valvular regurgitant fractions[53-56].
First-pass perfusion imaging: Performed using spoiled gradient echo or SSFP sequences, this technique involves the administration of a GBCA followed by dynamic imaging of the passage of contrast through the myocardium in order to detect zones of decreased perfusion[57]. Normally perfused myocardium gives a bright signal and areas of poor perfusion appear darker. Images are typically acquired both at rest and under pharmacological stress (induced via administration of a coronary artery vasodilator such as adenosine) in order to accentuate the difference between the perfusion of myocardium supplied by normal coronary arteries compared to myocardium supplied by abnormal vessels[16,20]. Examples of uses in children include the investigation of chest pain, congenital heart disease with anomalous coronary artery origins, post-surgery involving coronary artery re-implantation and in acquired abnormalities of the coronaries such as aneurysms in Kawasaki disease[17,58-60]. CMR offers distinct advantages compared to the traditional nuclear perfusion imaging in terms of improved spatial resolution and lack of ionizing radiation[61-64]. Dobutamine instead of adenosine is also used for stress imaging in certain circumstances[65,66].
In addition to the technological challenges with regards to performing CMR in children, there are a number of specific practical considerations to take into account. These include the strategies employed in order to minimize both generalized and cardio-respiratory motion artefact (both accentuated in children due their reduced ability to co-operate with the scanning procedure, and their elevated heart rate and respiratory rate in comparison with adult patients), the equipment used, and some of the additional preparation steps that can be taken with children in order to facilitate the scanning process.
In terms of generalized motion artefact, older children (typically greater than 7 years of age) with normal development are often capable of lying still and following instructions such that adequate quality images can be obtained. However, for neonates, infants, younger children and patients with developmental delay, specific strategies must be employed in order to minimize motion artefact. Approaches will vary depending on the age of the child, their clinical condition, and the expertise and resources available. For infants less than 6 mo, it may be possible to perform the scan during natural sleep after feeding[67], however early awakening will likely compromise the scan. Deep sedation using sedative medications is an option[68], but is avoided where possible due to risks of hypoventilation and aspiration. Thus, for children unable to breath-hold, the preferred approach is endotracheal intubation and mechanical ventilation under general anesthesia (GA). CMR under GA is resource intensive, requiring a pediatric anesthetist with cardiac experience and CMR compatible equipment. It is also challenging since intensive monitoring is required despite limited access to the child during the scan. However, with trained personnel, good communication and a comprehensive emergency plan in place, it has an excellent safety profile[69-71]. Additionally, it presents the opportunity to perform other invasive investigations during a single GA, for example trans-oesophageal echocardiography and endoscopic procedures. However, the decision for a child to undergo CMR under GA is not taken lightly and is usually made in discussion with the wider multi-disciplinary team. There should be careful consideration of the age and maturity of the child, the parents’ perception of the child’s ability to co-operate with a non-GA procedure, their clinical condition, relevant past experiences, the length of the scanning protocol, the risks of anesthesia and the benefits of the scan in terms of diagnosis and patient management[2,72].
In order to minimize the effect of cardio-respiratory motion on image quality, specific strategies are employed. For cardiac motion, the techniques used are broadly similar for adults and children. To obtain images of acceptable quality, CMR data is acquired over multiple heart beats, synchronizing the data acquisition to a particular time point in the cardiac cycle. MRI compatible electrodes and leads are applied to the patient’s chest and specific software detects the ECG trace, synchronizing the CMR pulse sequence (and thus data acquisition) to the R wave. In this manner, with each cardiac cycle there is a new repetition of the pulse sequence. Images can be obtained either at a single time point in the cardiac cycle for still imaging, or at multiple time points for cine imaging and the resulting images can are laced together in a cinematic display. Two main ECG synchronization techniques exist: Prospective triggering and retrospective gating. Typically for still imaging using spin echo sequences, prospective triggering is used, whereas for cine imaging using gradient echo sequences, either technique can be used[6,73]. For respiratory motion, most pulse sequences enable data acquisition to be completed within a single breath-hold, thus older children can be taught to breath-hold with practice. Breath-holding can also be achieved under general anesthetic, with the anesthetist strategically pausing the ventilator at specific times. In sedated infants and small children, breathing tends to be shallow and regular so a technique employing multiple signal averages can be used to average out respiratory motion artefact at the expense of reduced spatial resolution. Alternatively, respiratory gating strategies can be employed to synchronize data acquisition to the respiratory cycle such as using a navigator beam to track the motion of the diaphragm and gating data acquisition to a point in end expiration when the diaphragm is relatively still[73,74].
For pediatric CMR, the use of smaller coils placed directly on top of or underneath the child significantly improves image quality. Adolescent children can be imaged using a standard adult surface coil, while for younger children, infants and neonates, better image quality can be obtained with a smaller surface coil. Specific pediatric coils are commercially available for imaging the brain and spine, and while pediatric thoracic coils are becoming increasingly available, often adult coils designed for other applications (such as adult orthopedic extremity coils) are used for this purpose[75].
On a practical level, better outcomes are achieved with children when there is thorough planning and preparation prior to the scan. Some practical considerations and tips for achieving a successful outcome are described in Table 1.
| Before the scan |
| Begin preparation for the scan well in advance of the appointment |
| If multiple children from the same family require scans (such as when screening for hereditary conditions), where possible arrange for all children to be scanned at a similar time (ideally on the same day) so they can prepare together |
| Discuss the procedure with the child in an age-appropriate manner and provide parents with a detailed description of the procedure so they can be of assistance |
| Play therapists can help prepare the child by talking them through pictures of the scan, and using dummy scanners to practice lying still and breath-holding |
| Arranging a pre-scan visit to the CMR department and allowing the child to see the scanner before their scheduled appointment may help reduce anxiety |
| Perform a full metal screen on parents so that they can demonstrate going into the scanner if the child is anxious, and so that they can remain in the room for the duration of the scan to reassure the child if necessary |
| Some modern scanners have MRI compatible audio-visual equipment, where this is available allow the child to pre-select their own music or movie to play during the scan (ideally bringing a favourite one from home) – this may help them tolerate longer scanning times |
| Within reason, allocate a lengthier appointment for the scan to give the child time to get accustomed to the magnet, coils, ear protection and breath-holding instructions |
| During the scan |
| Be patient and flexible |
| Minimize the time the child must spend in the scanner by only running sequences that will directly answer the relevant clinical questions |
| Run the most essential sequences first bearing in mind that the child may not tolerate the whole scan |
| For a breath-holding child, use short sequences only as they may struggle with a long breath-hold |
| An inspiratory breath-hold is easier for a child to understand and achieve compared to an end-expiratory breath-hold |
| For stress perfusion studies provide the child with a stress ball that can be repeatedly squeezed during administration of the stress agent to minimize side effects (61) |
| After the scan |
| Praise and reward the child with stickers and certificates even if the scan was not entirely successful, bearing in mind that for many conditions repeat scanning may be required in future so all attempts to alleviate bad experiences should be made |
It is essential that all patients and any accompanying persons (such as parents) undergo thorough screening for the presence any implanted medical devices or foreign bodies - these include pacemakers, implantable defibrillators, neurostimulators, stents, cerebro-spinal fluid shunts, cerebrovascular clips and coils, cochlear implants, orthopedic devices, shrapnel, bullets and metal fragments[76]. Where the history is unreliable, plain radiographs can be used to aid the screening process. The strong magnetic fields of the scanner may disrupt the function of some electrically, magnetically or mechanically activated devices, and ferromagnetic objects risk becoming dislodged during the scan causing local tissue damage[77]. Many modern devices are designed to be MRI compatible - they may cause artefact but are not ferromagnetic and will not overheat or fail in the presence of the magnetic field. Older devices, on the other hand, are less likely to be MRI compatible, therefore it is essential to thoroughly check the safety information for each specific device and follow all recommendations made by the manufacturer.
The use of GBCAs can also raise issues. Although the incidence of complications relating to the use of these agents is low, children are susceptible to all the adverse effects experienced by adults. These include feelings of coldness or warmth on injection, nausea, vomiting, headache, paresthesia, dizziness, itching, extravasation of contrast agent and allergic reactions ranging from a simple rash to anaphylaxis[78-80]. A serious complication of GBCAs is nephrogenic systemic fibrosis (NSF), a progressive, incurable and often fatal condition that involves widespread fibrosis of the skin, subcutaneous tissue, joints, skeletal muscles, and organs such as the eyes, lungs, heart and liver. It typically occurs in the context of renal dysfunction when GFR is less than 30 mL/min per 1.73 m2, additional risk factors include the requirement for renal replacement therapy, concurrent hepatic disease and a pro-inflammatory state[81,82]. NSF is exceedingly rare, and even more so in children compared to adults[83]. This is surprising given the immature renal function of neonates and infants. Nevertheless, all patients should be screened for risk factors and renal function should be checked before GBCAs are administered[84]. If renal dysfunction is identified, local or national guidelines should be consulted and steps should be taken to minimize relevant risk factors with contrast-enhanced scanning only proceeding after careful consideration of the risks and benefits[85]. In light of this screening process, and the introduction of safer agents bound to a cyclic chelate[83], the incidence of GBCA-related NSF has fallen significantly in recent years[86].
Additional CMR safety considerations, especially with relation to neonates and infants, include the use of ear protection in order to prevent hearing damage from the acoustic noise of the scanner[87], and the requirement for close monitoring of body temperature. Scanning rooms are deliberately kept cool to reduce overheating of the electrical equipment however local heating of the coils in close proximity to the patient can still occur. Thus, small children, infants and neonates with reduced ability to control their body temperature are at risk of both hypothermia and hyperthermia during CMR[88].
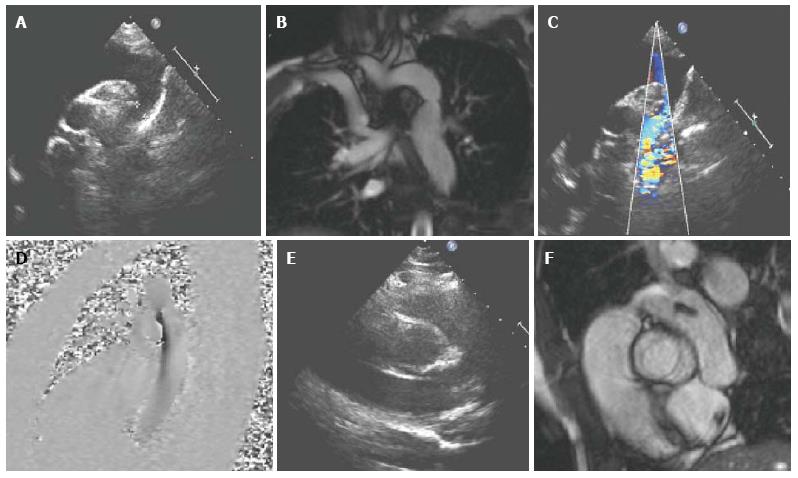
Conditions amenable to assessment with CMR include coarctation, interrupted aortic arch, vascular rings and congenital connective tissue diseases[2]. Echocardiography is usually sufficient for diagnostic purposes for these conditions in neonates and infants, however CMR can be a useful adjunct in older children with poor acoustic windows, especially with regards to planning surgical or catheter intervention (Figure 2). CMR is also used first-line for post-intervention follow-up[89-91]. The use of black blood sequences along with contrast-enhanced 3D MRA and non-contrast 3D SSFP allows delineation of arch geometry and morphology, evaluation of the presence of collaterals, the site and size of areas of stenosis, the extent of any aneurysm formation, and characterization of coarctation stents[92-97]. SSFP cine sequences are also useful for assessing aortic valve morphology (often bicuspid), and left ventricular function. Velocity encoded phase contrast imaging can be used to quantify collateral flow[98]. CMR can also be used for monitoring aortic dimensions, aortic root dilation and aortic regurgitation in cases of connective tissue disease such as Marfan’s in order determine optimum time for intervention[99,100].
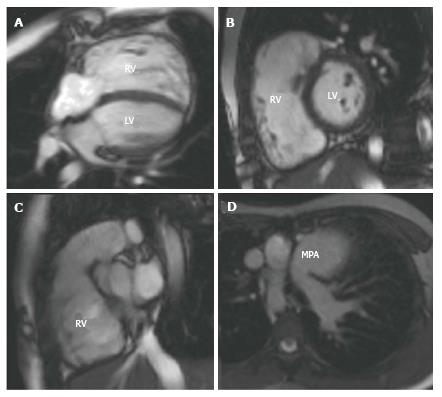
For tetralogy of Fallot (ToF) CMR is usually only performed pre-operatively if there are associated situs and aortic arch anomalies, however it is the preferred tool for the post-operative serial follow-up of these patients[101]. Pulmonary regurgitation, right ventricular outflow tract (RVOT) obstruction and pulmonary artery stenosis are common post-operatively and lead to right ventricular volume and pressure overload. This is initially well tolerated in childhood and adolescence, but may ultimately cause right ventricular dysfunction, arrhythmias and premature death[102,103]. Intervention is required in most cases, either with a surgical pulmonary valve replacement or percutaneous pulmonary valve implantation (PPVI) and the decision about when to intervene is controversial[104]. CMR can assist the decision making process[105,106]. LGE CMR contributes to risk stratification of these patients, SSFP cines enable accurate right ventricular volumetric and functional analysis (Figure 3), and velocity encoded phase contrast sequences can be used for flow assessment in relation to the pulmonary regurgitation and stenosis. Contrast-enhanced 3D MRA and 3D SSFP can also be used to delineate RVOT anatomy for intervention planning[107]. Similarly, with transposition of the great arteries (TGA), echocardiography is usually sufficient to define anatomy pre-operatively and the predominant role of CMR is for the investigation of late post-operative complications. These will vary depending on the corrective procedure performed, however for the most commonly performed arterial switch operation (ASO), complications include RVOT stenosis, supravalvular pulmonary artery stenosis, branch pulmonary artery stenosis, coronary ostial stenosis, dilatation of the neo-aortic root and neo-aortic valve regurgitation[108-110]. Spin echo, gradient echo and phase contrast sequences are used for assessment of anatomy, stenosis and valvular function and 3D MRA and 3D SSFP and first-pass perfusion sequences are useful for assessing the patency of the re-implanted coronary arteries[111-113].
CMR is considered the first-line imaging modality for complex congenital heart disease (CHD) as it achieves superior delineation of anatomy and enables a concurrent hemodynamic assessment[2,101]. Specific congenital anomalies amenable to assessment with CMR include viscero-atrial situs anomalies, abnormal atrio-ventricular and/or ventriculo-arterial connections, septal defects, outflow tract malformations, abnormal extra-cardiac thoracic vessels and any associated tracheo-bronchial anomalies. CMR can also play a key role in the staged palliation of univentricular hearts[114]. Initially, it can help determine the approach required particularly in borderline cases where there is debate over whether to perform a single ventricular or bi-ventricular repair[115], and subsequently CMR can be used in conjunction with echocardiography and catheterization to perform an anatomical and functional assessment at each stage of the palliation process for evaluating outcomes and planning subsequent interventions[116-119].
Although echocardiography remains the gold-standard modality for valvular morphological assessment, CMR can play a complementary role when acoustic windows are poor. SSFP cine imaging can be used to determine the functional consequences of valvular lesions, particularly in terms of the effect on ventricular volumes and myocardial mass, and velocity encoded phase contrast imaging permits visualization and quantification of regurgitant and stenotic jets[15]. In terms of the pulmonary vasculature, contrast-enhanced 3D MRA and 3D SSFP sequences provide good visualization of the morphology and dimensions of the pulmonary arteries and veins, revealing anomalous connections and areas of stenosis, and velocity encoded phase contrast cines enable quantitative measurements of blood flow within these vessels[2]. CMR also permits evaluation cardiac shunts in terms of their location, flow direction, magnitude and functional consequences such as volume loading of any of the cardiac chambers whilst providing detailed anatomical information. Shunt quantification is performed using velocity encoded phase contrast cines to assess the ratio of pulmonary (Qp) to systemic flow (Qs), and this important hemodynamic parameter is often used as a determining factor when planning surgical or interventional management[120].
Imaging the coronary vessels is challenging due to small vessel size and an increased susceptibility to cardio-respiratory motion artefact. Indications for coronary artery imaging in children include presence of congenital anomalous coronary arteries, vasculitis (in particular Kawasaki disease), before any surgery or interventional procedure close to the proximal course of the coronary arteries, and post-operatively for procedures involving transfer and re-implantation of the coronary arteries[2]. Although cardiac catheterization is considered gold standard for assessment of the coronary arteries, 3D SSFP CMR sequences can being increasingly used for this purpose[121-125]. First-pass perfusion and LGE imaging are also useful for the assessment of myocardial viability in the context of coronary artery pathology, and cine imaging can provide information about the functional consequences of myocardial ischemia where this is suspected.
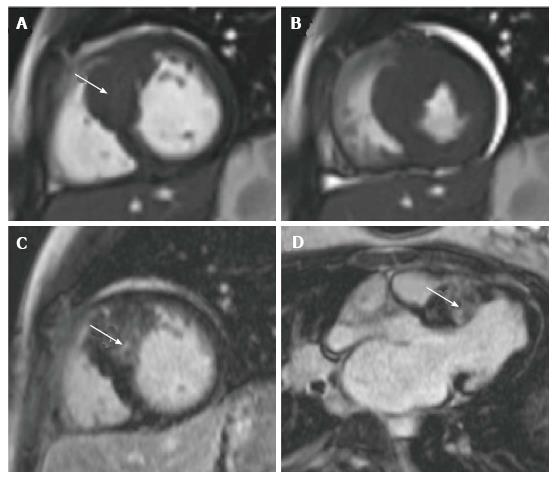
CMR permits detailed in vivo myocardial tissue characterization and therefore can play a key role in the diagnosis, risk-stratification and ongoing management of these patients[126]. Dilated cardiomyopathy (DCM) and hypertrophic cardiomyopathy (HCM) are the most commonly encountered cardiomyopathies in children[127]. In DCM, the extent of both left and right ventricular dilation can be easily visualized and quantified using CMR. LGE imaging can help distinguish between DCM of ischemic and non-ischemic etiology[126], and SSFP cine sequences can be used to provide valuable information about global ventricular function especially in terms of contractility, relaxation impairment and wall motion anomalies. DCM in children is also known to be associated with anomalous coronary arteries, the presence of which can be determined using CMR[121]. Where myocarditis is suspected, spin echo sequences are useful in revealing the extent of inflammatory change[128]. Since left ventricular hypertrophy is generally considered an independent risk factor for cardiac events[129], accurate assessment of the magnitude and distribution of hypertrophy is essential in order to appropriately risk stratify and manage affected patients. Echocardiography is the most commonly used modality for the diagnosis and follow-up of HCM, however it has been shown that CMR can detect hypertrophy missed by echocardiography[130,131]. Ventricular mass and function in HCM can also be well characterized using CMR (Figure 4) and it has been demonstrated that with LGE imaging in HCM, the extent of myocardial fibrosis is closely correlated with the development of left ventricular dilatation and failure, and the risk of sudden cardiac death[40,132,133]. Where myocardial hypertrophy is related to a metabolic defect such as in Pompe’s disease or Fabry’s disease, CMR can play a useful role in the diagnosis of these conditions and in monitoring response to enzyme replacement therapies[134,135]. CMR can also be used in the assessment of iron overload cardiomyopathy. This predominantly affects children with inherited severe anemias such as thalassemia and sickle cell disease, where the excess iron load from regular blood transfusions deposits in the tissues of organs such as the heart and liver. In the myocardium, this causes fibrosis, systolic impairment ultimately cardiac failure. The measurement of T2*, a CMR relaxation parameter derived from local magnetic field inhomogeneities[6], has been shown to accurately reflect tissue iron load[136,137], thus CMR can be used as a means of evaluating the requirement for and response to chelation regimens[138-140].
The most common cardiac tumours in children are benign fibromas or rhabdomyomas. Malignant secondaries from leukemia, lymphoma, neuroblastoma and nephroblastoma are rare, and malignant primaries, typically cardiac sarcomas, are rarer still[141]. Using spin echo, SSFP cine, first-pass perfusion and LGE sequences it is possible to assess the size, site and malignant potential of the tumour through detailed tissue characterization, and assess hemodynamic relevance in terms of how any obstructive mass effects may impair myocardial or valvular function[142-144].
Technological advances in CMR hardware and software are continually occurring, resulting in faster sequences with shorter acquisition times, and improved image quality with greater spatial and temporal resolution. Such advances have permitted the development of real-time imaging, involving rapid and continuous data acquisition with nearly instantaneous image reconstruction and a reduced requirement for cardio-respiratory motion compensation - which is particularly advantageous in pediatrics[145-147]. Real-time imaging has also paved the way for the growing field of interventional CMR, whereby CMR performed using open magnets can be used to guide cardiac catheterization procedures, thus avoiding exposure to ionizing radiation[148]. However, although the concept of purely CMR guided interventional procedures is promising, a number of obstacles still exist that prevent it translating into routine clinical practice[149-151]. Thus, interventional CMR in current practice falls into the realm of hybrid CMR/X-ray cardiac catheter (XMR) laboratories. In these laboratories both modalities are present in the same room and the patient can be rapidly moved between them during the imaging process permitting cross-modality image integration. Additional emerging techniques include time-resolved 3D MRA permitting direct visualization of complex flow dynamics in vessels[152] and time-resolved 3D (4D) velocity encoded phase contrast imaging allowing quantification of flow parameters in multiple planes[153]. Higher field strength 3T scanners also exist that yield a higher signal-to-noise ratio and better spatial resolution. This is particularly beneficial when imaging small children. However, these scanners have their own limitations and are not yet compatible with all CMR sequences[154].
CMR is emerging as helpful imaging tool in pediatric cardiology and is becoming increasingly available for a wide range of range of both congenital and acquired cardiac disease. Its non-invasiveness and lack of exposure to ionizing radiation are particular advantages with regards to the pediatric population, and CMR is well tolerated in children of all ages with an excellent safety profile when performed in specialist centres by experienced personnel. Outcomes are most successful when scans are undertaken using an individualized approach specifically tailored to the clinical question. In these circumstances, CMR is capable of providing detailed anatomical and functional information, and it is well suited to serial imaging for long-term follow-up and as a means of planning and evaluating surgical and interventional management. In light of all the technological developments currently taking place in the field of CMR, it will be interesting to see what the future holds for this modality in the world of pediatric cardiology.
P- Reviewer: Sertoglu E S- Editor: Qiu S L- Editor: A E- Editor: Lu YJ
| 1. | Occleshaw C. Technical aspects of pediatric cardiac MR. Principles and practice of cardiac magnetic resonance in congenital heart disease: form, function and flow. USA: Wiley-Blackwell 2010; 17-32. |
| 2. | Valsangiacomo Buechel ER, Grosse-Wortmann L, Fratz S, Eichhorn J, Sarikouch S, Greil GF, Beerbaum P, Bucciarelli-Ducci C, Bonello B, Sieverding L. Indications for cardiovascular magnetic resonance in children with congenital and acquired heart disease: an expert consensus paper of the Imaging Working Group of the AEPC and the Cardiovascular Magnetic Resonance Section of the EACVI. Eur Heart J Cardiovasc Imaging. 2015;16:281-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 101] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 3. | Fratz S, Hess J, Schuhbaeck A, Buchner C, Hendrich E, Martinoff S, Stern H. Routine clinical cardiovascular magnetic resonance in paediatric and adult congenital heart disease: patients, protocols, questions asked and contributions made. J Cardiovasc Magn Reson. 2008;10:46. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 35] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 4. | Plewes DB, Kucharczyk W. Physics of MRI: a primer. J Magn Reson Imaging. 2012;35:1038-1054. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 98] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 5. | Cardiovascular magnetic resonance. Clin Privil White Pap. 2011;1-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 6. | Ridgway JP. Cardiovascular magnetic resonance physics for clinicians: part I. J Cardiovasc Magn Reson. 2010;12:71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 147] [Cited by in RCA: 111] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 7. | Weishaupt D, Kochli VD, Marincek B, Kim EE. How does MRI Work? An introduction to the physics and function of magnetic resonance imaging. 2nd ed. Germany: Springer 2007; 1910. |
| 8. | Biglands JD, Radjenovic A, Ridgway JP. Cardiovascular magnetic resonance physics for clinicians: Part II. J Cardiovasc Magn Reson. 2012;14:66. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 63] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 9. | Lai WW, Geva T, Shirali GS, Frommelt PC, Humes RA, Brook MM, Pignatelli RH, Rychik J. Guidelines and standards for performance of a pediatric echocardiogram: a report from the Task Force of the Pediatric Council of the American Society of Echocardiography. J Am Soc Echocardiogr. 2006;19:1413-1430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 499] [Cited by in RCA: 611] [Article Influence: 32.2] [Reference Citation Analysis (0)] |
| 10. | Lai WW, Mertens LL, Geva T, Cohen MS. Echocardiography in pediatric and congenital heart disease: from fetus to adult. 2nd ed. USA: Wiley-Blackwell 2008; . |
| 11. | Zeevi B, Berant M, Fogelman R, Galit BM, Blieden LC. Acute complications in the current era of therapeutic cardiac catheterization for congenital heart disease. Cardiol Young. 1999;9:266-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 41] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 12. | Mehta R, Lee KJ, Chaturvedi R, Benson L. Complications of pediatric cardiac catheterization: a review in the current era. Catheter Cardiovasc Interv. 2008;72:278-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 130] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 13. | Bennett D, Marcus R, Stokes M. Incidents and complications during pediatric cardiac catheterization. Paediatr Anaesth. 2005;15:1083-1088. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 14. | Kleinerman RA. Cancer risks following diagnostic and therapeutic radiation exposure in children. Pediatr Radiol. 2006;36 Suppl 2:121-125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 390] [Cited by in RCA: 411] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 15. | Pennell DJ, Sechtem UP, Higgins CB, Manning WJ, Pohost GM, Rademakers FE, van Rossum AC, Shaw LJ, Yucel EK. Clinical indications for cardiovascular magnetic resonance (CMR): Consensus Panel report. Eur Heart J. 2004;25:1940-1965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 545] [Cited by in RCA: 481] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 16. | Fratz S, Chung T, Greil GF, Samyn MM, Taylor AM, Valsangiacomo Buechel ER, Yoo SJ, Powell AJ. Guidelines and protocols for cardiovascular magnetic resonance in children and adults with congenital heart disease: SCMR expert consensus group on congenital heart disease. J Cardiovasc Magn Reson. 2013;15:51. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 334] [Cited by in RCA: 346] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 17. | Ntsinjana HN, Hughes ML, Taylor AM. The role of cardiovascular magnetic resonance in pediatric congenital heart disease. J Cardiovasc Magn Reson. 2011;13:51. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 112] [Cited by in RCA: 103] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 18. | Taylor AM. Cardiac imaging: MR or CT? Which to use when. Pediatr Radiol. 2008;38 Suppl 3:S433-S438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 38] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 19. | Brenner D, Elliston C, Hall E, Berdon W. Estimated risks of radiation-induced fatal cancer from pediatric CT. AJR Am J Roentgenol. 2001;176:289-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2366] [Cited by in RCA: 2183] [Article Influence: 91.0] [Reference Citation Analysis (0)] |
| 20. | Simonetti OP, Cook S. Technical aspects of pediatric CMR. J Cardiovasc Magn Reson. 2006;8:581-593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 21. | Geva T, Vick GW, Wendt RE, Rokey R. Role of spin echo and cine magnetic resonance imaging in presurgical planning of heterotaxy syndrome. Comparison with echocardiography and catheterization. Circulation. 1994;90:348-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 57] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 22. | Finn JP, Nael K, Deshpande V, Ratib O, Laub G. Cardiac MR imaging: state of the technology. Radiology. 2006;241:338-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 152] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 23. | Grothues F, Smith GC, Moon JC, Bellenger NG, Collins P, Klein HU, Pennell DJ. Comparison of interstudy reproducibility of cardiovascular magnetic resonance with two-dimensional echocardiography in normal subjects and in patients with heart failure or left ventricular hypertrophy. Am J Cardiol. 2002;90:29-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1033] [Cited by in RCA: 1075] [Article Influence: 46.7] [Reference Citation Analysis (0)] |
| 24. | Margossian R, Schwartz ML, Prakash A, Wruck L, Colan SD, Atz AM, Bradley TJ, Fogel MA, Hurwitz LM, Marcus E. Comparison of echocardiographic and cardiac magnetic resonance imaging measurements of functional single ventricular volumes, mass, and ejection fraction (from the Pediatric Heart Network Fontan Cross-Sectional Study). Am J Cardiol. 2009;104:419-428. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 185] [Cited by in RCA: 170] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 25. | Buechel EV, Kaiser T, Jackson C, Schmitz A, Kellenberger CJ. Normal right- and left ventricular volumes and myocardial mass in children measured by steady state free precession cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2009;11:19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 155] [Cited by in RCA: 151] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 26. | Robbers-Visser D, Boersma E, Helbing WA. Normal biventricular function, volumes, and mass in children aged 8 to 17 years. J Magn Reson Imaging. 2009;29:552-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 92] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 27. | Sarikouch S, Peters B, Gutberlet M, Leismann B, Kelter-Kloepping A, Koerperich H, Kuehne T, Beerbaum P. Sex-specific pediatric percentiles for ventricular size and mass as reference values for cardiac MRI: assessment by steady-state free-precession and phase-contrast MRI flow. Circ Cardiovasc Imaging. 2010;3:65-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 28. | Lorenz CH. The range of normal values of cardiovascular structures in infants, children, and adolescents measured by magnetic resonance imaging. Pediatr Cardiol. 2000;21:37-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 88] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 29. | Mooij CF, de Wit CJ, Graham DA, Powell AJ, Geva T. Reproducibility of MRI measurements of right ventricular size and function in patients with normal and dilated ventricles. J Magn Reson Imaging. 2008;28:67-73. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 252] [Cited by in RCA: 230] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 30. | Razavi RS, Hill DL, Muthurangu V, Miquel ME, Taylor AM, Kozerke S, Baker EJ. Three-dimensional magnetic resonance imaging of congenital cardiac anomalies. Cardiol Young. 2003;13:461-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 33] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 31. | Sørensen TS, Körperich H, Greil GF, Eichhorn J, Barth P, Meyer H, Pedersen EM, Beerbaum P. Operator-independent isotropic three-dimensional magnetic resonance imaging for morphology in congenital heart disease: a validation study. Circulation. 2004;110:163-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 32. | Srichai MB, Kim S, Axel L, Babb J, Hecht EM. Non-gadolinium-enhanced 3-dimensional magnetic resonance angiography for the evaluation of thoracic aortic disease: a preliminary experience. Tex Heart Inst J. 2010;37:58-65. [PubMed] |
| 33. | Amado LC, Gerber BL, Gupta SN, Rettmann DW, Szarf G, Schock R, Nasir K, Kraitchman DL, Lima JA. Accurate and objective infarct sizing by contrast-enhanced magnetic resonance imaging in a canine myocardial infarction model. J Am Coll Cardiol. 2004;44:2383-2389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 389] [Cited by in RCA: 373] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 34. | Kehr E, Sono M, Chugh SS, Jerosch-Herold M. Gadolinium-enhanced magnetic resonance imaging for detection and quantification of fibrosis in human myocardium in vitro. Int J Cardiovasc Imaging. 2008;24:61-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 85] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 35. | Harris MA, Johnson TR, Weinberg PM, Fogel MA. Delayed-enhancement cardiovascular magnetic resonance identifies fibrous tissue in children after surgery for congenital heart disease. J Thorac Cardiovasc Surg. 2007;133:676-681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 27] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 36. | Babu-Narayan SV, Goktekin O, Moon JC, Broberg CS, Pantely GA, Pennell DJ, Gatzoulis MA, Kilner PJ. Late gadolinium enhancement cardiovascular magnetic resonance of the systemic right ventricle in adults with previous atrial redirection surgery for transposition of the great arteries. Circulation. 2005;111:2091-2098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 37. | Babu-Narayan SV, Kilner PJ, Li W, Moon JC, Goktekin O, Davlouros PA, Khan M, Ho SY, Pennell DJ, Gatzoulis MA. Ventricular fibrosis suggested by cardiovascular magnetic resonance in adults with repaired tetralogy of fallot and its relationship to adverse markers of clinical outcome. Circulation. 2006;113:405-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 449] [Cited by in RCA: 433] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 38. | Rathod RH, Prakash A, Powell AJ, Geva T. Myocardial fibrosis identified by cardiac magnetic resonance late gadolinium enhancement is associated with adverse ventricular mechanics and ventricular tachycardia late after Fontan operation. J Am Coll Cardiol. 2010;55:1721-1728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 149] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 39. | Gulati A, Jabbour A, Ismail TF, Guha K, Khwaja J, Raza S, Morarji K, Brown TD, Ismail NA, Dweck MR. Association of fibrosis with mortality and sudden cardiac death in patients with nonischemic dilated cardiomyopathy. JAMA. 2013;309:896-908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 775] [Cited by in RCA: 890] [Article Influence: 74.2] [Reference Citation Analysis (0)] |
| 40. | Moon JC, McKenna WJ, McCrohon JA, Elliott PM, Smith GC, Pennell DJ. Toward clinical risk assessment in hypertrophic cardiomyopathy with gadolinium cardiovascular magnetic resonance. J Am Coll Cardiol. 2003;41:1561-1567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 575] [Cited by in RCA: 511] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 41. | Green JJ, Berger JS, Kramer CM, Salerno M. Prognostic value of late gadolinium enhancement in clinical outcomes for hypertrophic cardiomyopathy. JACC Cardiovasc Imaging. 2012;5:370-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 343] [Cited by in RCA: 331] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 42. | Dweck MR, Joshi S, Murigu T, Alpendurada F, Jabbour A, Melina G, Banya W, Gulati A, Roussin I, Raza S. Midwall fibrosis is an independent predictor of mortality in patients with aortic stenosis. J Am Coll Cardiol. 2011;58:1271-1279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 370] [Cited by in RCA: 421] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 43. | Manning WJ, Pennell DJ. Cardiovascular Magnetic Resonance. 2nd ed. USA: Churchill Livingstone 2010; . |
| 44. | Masui T, Katayama M, Kobayashi S, Ito T, Seguchi M, Koide M, Nozaki A, Sakahara H. Gadolinium-enhanced MR angiography in the evaluation of congenital cardiovascular disease pre- and postoperative states in infants and children. J Magn Reson Imaging. 2000;12:1034-1042. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 45. | Prasad SK, Soukias N, Hornung T, Khan M, Pennell DJ, Gatzoulis MA, Mohiaddin RH. Role of magnetic resonance angiography in the diagnosis of major aortopulmonary collateral arteries and partial anomalous pulmonary venous drainage. Circulation. 2004;109:207-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 75] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 46. | Valsangiacomo Büchel ER, DiBernardo S, Bauersfeld U, Berger F. Contrast-enhanced magnetic resonance angiography of the great arteries in patients with congenital heart disease: an accurate tool for planning catheter-guided interventions. Int J Cardiovasc Imaging. 2005;21:313-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 47. | Oosterhof T, Mulder BJ. Magnetic resonance angiography for anatomical evaluation of the great arteries. Int J Cardiovasc Imaging. 2005;21:323-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 48. | Geva T, Greil GF, Marshall AC, Landzberg M, Powell AJ. Gadolinium-enhanced 3-dimensional magnetic resonance angiography of pulmonary blood supply in patients with complex pulmonary stenosis or atresia: comparison with x-ray angiography. Circulation. 2002;106:473-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 49. | Greil GF, Powell AJ, Gildein HP, Geva T. Gadolinium-enhanced three-dimensional magnetic resonance angiography of pulmonary and systemic venous anomalies. J Am Coll Cardiol. 2002;39:335-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 90] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 50. | Powell AJ, Geva T. Blood flow measurement by magnetic resonance imaging in congenital heart disease. Pediatr Cardiol. 2000;21:47-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 67] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 51. | Powell AJ, Maier SE, Chung T, Geva T. Phase-velocity cine magnetic resonance imaging measurement of pulsatile blood flow in children and young adults: in vitro and in vivo validation. Pediatr Cardiol. 2000;21:104-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 149] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 52. | Chai P, Mohiaddin R. How we perform cardiovascular magnetic resonance flow assessment using phase-contrast velocity mapping. J Cardiovasc Magn Reson. 2005;7:705-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 53. | Hundley WG, Li HF, Hillis LD, Meshack BM, Lange RA, Willard JE, Landau C, Peshock RM. Quantitation of cardiac output with velocity-encoded, phase-difference magnetic resonance imaging. Am J Cardiol. 1995;75:1250-1255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 93] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 54. | Hundley WG, Li HF, Lange RA, Pfeifer DP, Meshack BM, Willard JE, Landau C, Willett D, Hillis LD, Peshock RM. Assessment of left-to-right intracardiac shunting by velocity-encoded, phase-difference magnetic resonance imaging. A comparison with oximetric and indicator dilution techniques. Circulation. 1995;91:2955-2960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 55. | Kilner PJ, Gatehouse PD, Firmin DN. Flow measurement by magnetic resonance: a unique asset worth optimising. J Cardiovasc Magn Reson. 2007;9:723-728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 142] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 56. | Myerson SG. Heart valve disease: investigation by cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2012;14:7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 120] [Cited by in RCA: 115] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 57. | Gebker R, Schwitter J, Fleck E, Nagel E. How we perform myocardial perfusion with cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2007;9:539-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 32] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 58. | Buechel ER, Balmer C, Bauersfeld U, Kellenberger CJ, Schwitter J. Feasibility of perfusion cardiovascular magnetic resonance in paediatric patients. J Cardiovasc Magn Reson. 2009;11:51. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 39] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 59. | Gerber BL, Raman SV, Nayak K, Epstein FH, Ferreira P, Axel L, Kraitchman DL. Myocardial first-pass perfusion cardiovascular magnetic resonance: history, theory, and current state of the art. J Cardiovasc Magn Reson. 2008;10:18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 174] [Cited by in RCA: 155] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 60. | Prakash A, Powell AJ, Krishnamurthy R, Geva T. Magnetic resonance imaging evaluation of myocardial perfusion and viability in congenital and acquired pediatric heart disease. Am J Cardiol. 2004;93:657-661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 59] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 61. | Bucciarelli-Ducci C, Daubeney PE, Kilner PJ, Seale A, Reyes E, Wage R, Pennell DJ. Images in cardiovascular medicine: Perfusion cardiovascular magnetic resonance in a child with ischemic heart disease: potential advantages over nuclear medicine. Circulation. 2010;122:311-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 62. | Schwitter J, Nanz D, Kneifel S, Bertschinger K, Büchi M, Knüsel PR, Marincek B, Lüscher TF, von Schulthess GK. Assessment of myocardial perfusion in coronary artery disease by magnetic resonance: a comparison with positron emission tomography and coronary angiography. Circulation. 2001;103:2230-2235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 496] [Cited by in RCA: 438] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 63. | Schwitter J, Wacker CM, van Rossum AC, Lombardi M, Al-Saadi N, Ahlstrom H, Dill T, Larsson HB, Flamm SD, Marquardt M. MR-IMPACT: comparison of perfusion-cardiac magnetic resonance with single-photon emission computed tomography for the detection of coronary artery disease in a multicentre, multivendor, randomized trial. Eur Heart J. 2008;29:480-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 493] [Cited by in RCA: 456] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 64. | Schwitter J, Wacker CM, Wilke N, Al-Saadi N, Sauer E, Huettle K, Schönberg SO, Debl K, Strohm O, Ahlstrom H. Superior diagnostic performance of perfusion-cardiovascular magnetic resonance versus SPECT to detect coronary artery disease: The secondary endpoints of the multicenter multivendor MR-IMPACT II (Magnetic Resonance Imaging for Myocardial Perfusion Assessment in Coronary Artery Disease Trial). J Cardiovasc Magn Reson. 2012;14:61. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 117] [Cited by in RCA: 110] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 65. | Strigl S, Beroukhim R, Valente AM, Annese D, Harrington JS, Geva T, Powell AJ. Feasibility of dobutamine stress cardiovascular magnetic resonance imaging in children. J Magn Reson Imaging. 2009;29:313-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 66. | Paetsch I, Jahnke C, Wahl A, Gebker R, Neuss M, Fleck E, Nagel E. Comparison of dobutamine stress magnetic resonance, adenosine stress magnetic resonance, and adenosine stress magnetic resonance perfusion. Circulation. 2004;110:835-842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 203] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 67. | Windram J, Grosse-Wortmann L, Shariat M, Greer M-L, Crawford MW, Yoo S-J. Cardiovascular MRI without sedation or general anesthesia using a feed-and-sleep technique in neonates and infants. Pediatr Radiol. 2012;42:183-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 89] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 68. | Fogel MA, Weinberg PM, Parave E, Harris C, Montenegro L, Harris MA, Concepcion M. Deep sedation for cardiac magnetic resonance imaging: a comparison with cardiac anesthesia. J Pediatr. 2008;152:534-539, 539.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 32] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 69. | Odegard KC, DiNardo JA, Tsai-Goodman B, Powell AJ, Geva T, Laussen PC. Anaesthesia considerations for cardiac MRI in infants and small children. Paediatr Anaesth. 2004;14:471-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 70] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 70. | Stockton E, Hughes M, Broadhead M, Taylor A, McEwan A. A prospective audit of safety issues associated with general anesthesia for pediatric cardiac magnetic resonance imaging. Paediatr Anaesth. 2012;22:1087-1093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 71. | Sarikouch S, Schaeffler R, Körperich H, Dongas A, Haas NA, Beerbaum P. Cardiovascular magnetic resonance imaging for intensive care infants: safe and effective? Pediatr Cardiol. 2009;30:146-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 72. | Dorfman AL, Odegard KC, Powell AJ, Laussen PC, Geva T. Risk factors for adverse events during cardiovascular magnetic resonance in congenital heart disease. J Cardiovasc Magn Reson. 2007;9:793-798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 46] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 73. | Ferreira PF, Gatehouse PD, Mohiaddin RH, Firmin DN. Cardiovascular magnetic resonance artefacts. J Cardiovasc Magn Reson. 2013;15:41. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 120] [Cited by in RCA: 115] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 74. | Krishnamurthy R, Cheong B, Muthupillai R. Tools for cardiovascular magnetic resonance imaging. Cardiovasc Diagn Ther. 2014;4:104-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 75. | Myerson S, Francis J, Neubauer S. Cardiovascular magnetic resonance. Oxford: Oxford Univeristy Press 2013; 62-63. |
| 76. | Shellock FG, Spinazzi A. MRI safety update 2008: part 2, screening patients for MRI. AJR Am J Roentgenol. 2008;191:1140-1149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 102] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 77. | Levine GN, Gomes AS, Arai AE, Bluemke DA, Flamm SD, Kanal E, Manning WJ, Martin ET, Smith JM, Wilke N. Safety of magnetic resonance imaging in patients with cardiovascular devices: an American Heart Association scientific statement from the Committee on Diagnostic and Interventional Cardiac Catheterization, Council on Clinical Cardiology, and the Council on Cardiovascular Radiology and Intervention: endorsed by the American College of Cardiology Foundation, the North American Society for Cardiac Imaging, and the Society for Cardiovascular Magnetic Resonance. Circulation. 2007;116:2878-2891. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 369] [Cited by in RCA: 317] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 78. | Runge VM. Safety of approved MR contrast media for intravenous injection. J Magn Reson Imaging. 2000;12:205-213. [PubMed] [DOI] [Full Text] |
| 79. | Dillman JR, Ellis JH, Cohan RH, Strouse PJ, Jan SC. Frequency and severity of acute allergic-like reactions to gadolinium-containing i.v. contrast media in children and adults. AJR Am J Roentgenol. 2007;189:1533-1538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 214] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 80. | Prince MR, Zhang H, Zou Z, Staron RB, Brill PW. Incidence of immediate gadolinium contrast media reactions. AJR Am J Roentgenol. 2011;196:W138-W143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 204] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 81. | Kaewlai R, Abujudeh H. Nephrogenic systemic fibrosis. AJR Am J Roentgenol. 2012;199:W17-W23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 82] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 82. | Sadowski EA, Bennett LK, Chan MR, Wentland AL, Garrett AL, Garrett RW, Djamali A. Nephrogenic systemic fibrosis: risk factors and incidence estimation. Radiology. 2007;243:148-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 745] [Cited by in RCA: 643] [Article Influence: 35.7] [Reference Citation Analysis (0)] |
| 83. | Mendichovszky IA, Marks SD, Simcock CM, Olsen OE. Gadolinium and nephrogenic systemic fibrosis: time to tighten practice. Pediatr Radiol. 2008;38:489-496; quiz 602-603. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 72] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 84. | Prince MR, Zhang HL, Roditi GH, Leiner T, Kucharczyk W. Risk factors for NSF: a literature review. J Magn Reson Imaging. 2009;30:1298-1308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 89] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 85. | Shellock FG, Spinazzi A. MRI safety update 2008: part 1, MRI contrast agents and nephrogenic systemic fibrosis. AJR Am J Roentgenol. 2008;191:1129-1139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 128] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 86. | Zou Z, Ma L. Nephrogenic systemic fibrosis: review of 408 biopsy-confirmed cases. Indian J Dermatol. 2011;56:65-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 87. | Radomskij P, Schmidt MA, Heron CW, Prasher D. Effect of MRI noise on cochlear function. Lancet. 2002;359:1485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 48] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 88. | Kussman BD, Mulkern RV, Holzman RS. Iatrogenic hyperthermia during cardiac magnetic resonance imaging. Anesth Analg. 2004;99:1053-1055, table of contents. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 89. | Konen E, Merchant N, Provost Y, McLaughlin PR, Crossin J, Paul NS. Coarctation of the aorta before and after correction: the role of cardiovascular MRI. AJR Am J Roentgenol. 2004;182:1333-1339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 39] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 90. | Riquelme C, Laissy JP, Menegazzo D, Debray MP, Cinqualbre A, Langlois J, Schouman-Claeys E. MR imaging of coarctation of the aorta and its postoperative complications in adults: assessment with spin-echo and cine-MR imaging. Magn Reson Imaging. 1999;17:37-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 91. | Muzzarelli S, Meadows AK, Ordovas KG, Higgins CB, Meadows JJ. Usefulness of cardiovascular magnetic resonance imaging to predict the need for intervention in patients with coarctation of the aorta. Am J Cardiol. 2012;109:861-865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 92. | Nielsen JC, Powell AJ, Gauvreau K, Marcus EN, Prakash A, Geva T. Magnetic resonance imaging predictors of coarctation severity. Circulation. 2005;111:622-628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 95] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 93. | Greil GF, Kramer U, Dammann F, Schick F, Miller S, Claussen CD, Sieverding L. Diagnosis of vascular rings and slings using an interleaved 3D double-slab FISP MR angiography technique. Pediatr Radiol. 2005;35:396-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 94. | Beekman RP, Hazekamp MG, Sobotka MA, Meijboom EJ, de Roos A, Staalman CR, Beek FJ, Ottenkamp J. A new diagnostic approach to vascular rings and pulmonary slings: the role of MRI. Magn Reson Imaging. 1998;16:137-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 95. | Prince MR, Narasimham DL, Jacoby WT, Williams DM, Cho KJ, Marx MV, Deeb GM. Three-dimensional gadolinium-enhanced MR angiography of the thoracic aorta. AJR Am J Roentgenol. 1996;166:1387-1397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 163] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 96. | Oshinski JN, Parks WJ, Markou CP, Bergman HL, Larson BE, Ku DN, Mukundan S, Pettigrew RI. Improved measurement of pressure gradients in aortic coarctation by magnetic resonance imaging. J Am Coll Cardiol. 1996;28:1818-1826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 49] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 97. | Nordmeyer J, Gaudin R, Tann OR, Lurz PC, Bonhoeffer P, Taylor AM, Muthurangu V. MRI may be sufficient for noninvasive assessment of great vessel stents: an in vitro comparison of MRI, CT, and conventional angiography. AJR Am J Roentgenol. 2010;195:865-871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 98. | Steffens JC, Bourne MW, Sakuma H, O’Sullivan M, Higgins CB. Quantification of collateral blood flow in coarctation of the aorta by velocity encoded cine magnetic resonance imaging. Circulation. 1994;90:937-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 114] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 99. | Alpendurada F, Wong J, Kiotsekoglou A, Banya W, Child A, Prasad SK, Pennell DJ, Mohiaddin RH. Evidence for Marfan cardiomyopathy. Eur J Heart Fail. 2010;12:1085-1091. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 92] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 100. | Kaemmerer H, Oechslin E, Seidel H, Neuhann T, Neuhann IM, Mayer HM, Hess J. Marfan syndrome: what internists and pediatric or adult cardiologists need to know. Expert Rev Cardiovasc Ther. 2005;3:891-909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 101. | Kilner PJ, Geva T, Kaemmerer H, Trindade PT, Schwitter J, Webb GD. Recommendations for cardiovascular magnetic resonance in adults with congenital heart disease from the respective working groups of the European Society of Cardiology. Eur Heart J. 2010;31:794-805. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 331] [Cited by in RCA: 288] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 102. | Geva T, Sandweiss BM, Gauvreau K, Lock JE, Powell AJ. Factors associated with impaired clinical status in long-term survivors of tetralogy of Fallot repair evaluated by magnetic resonance imaging. J Am Coll Cardiol. 2004;43:1068-1074. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 457] [Cited by in RCA: 460] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 103. | Gatzoulis MA, Balaji S, Webber SA, Siu SC, Hokanson JS, Poile C, Rosenthal M, Nakazawa M, Moller JH, Gillette PC. Risk factors for arrhythmia and sudden cardiac death late after repair of tetralogy of Fallot: a multicentre study. Lancet. 2000;356:975-981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1288] [Cited by in RCA: 1201] [Article Influence: 48.0] [Reference Citation Analysis (0)] |
| 104. | Geva T. Indications and timing of pulmonary valve replacement after tetralogy of Fallot repair. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2006;11-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 177] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 105. | Holmes KW. Timing of pulmonary valve replacement in tetralogy of fallot using cardiac magnetic resonance imaging: an evolving process. J Am Coll Cardiol. 2012;60:1015-1017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 106. | Oosterhof T, van Straten A, Vliegen HW, Meijboom FJ, van Dijk AP, Spijkerboer AM, Bouma BJ, Zwinderman AH, Hazekamp MG, de Roos A. Preoperative thresholds for pulmonary valve replacement in patients with corrected tetralogy of Fallot using cardiovascular magnetic resonance. Circulation. 2007;116:545-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 455] [Cited by in RCA: 439] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 107. | Helbing WA, de Roos A. Clinical applications of cardiac magnetic resonance imaging after repair of tetralogy of Fallot. Pediatr Cardiol. 2000;21:70-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 66] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 108. | Warnes CA. Transposition of the great arteries. Circulation. 2006;114:2699-2709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 399] [Cited by in RCA: 367] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 109. | Hardy CE, Helton GJ, Kondo C, Higgins SS, Young NJ, Higgins CB. Usefulness of magnetic resonance imaging for evaluating great-vessel anatomy after arterial switch operation for D-transposition of the great arteries. Am Heart J. 1994;128:326-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 25] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 110. | Losay J, Touchot A, Capderou A, Piot JD, Belli E, Planché C, Serraf A. Aortic valve regurgitation after arterial switch operation for transposition of the great arteries: incidence, risk factors, and outcome. J Am Coll Cardiol. 2006;47:2057-2062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 103] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 111. | Ntsinjana HN, Tann O, Taylor AM. Trends in pediatric cardiovascular magnetic resonance imaging. Acta Radiol. 2013;54:1063-1074. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 112. | Gutberlet M, Boeckel T, Hosten N, Vogel M, Kühne T, Oellinger H, Ehrenstein T, Venz S, Hetzer R, Bein G. Arterial switch procedure for D-transposition of the great arteries: quantitative midterm evaluation of hemodynamic changes with cine MR imaging and phase-shift velocity mapping-initial experience. Radiology. 2000;214:467-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 48] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 113. | Taylor AM, Dymarkowski S, Hamaekers P, Razavi R, Gewillig M, Mertens L, Bogaert J. MR coronary angiography and late-enhancement myocardial MR in children who underwent arterial switch surgery for transposition of great arteries. Radiology. 2005;234:542-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 87] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 114. | Gewillig M. The Fontan circulation. Heart. 2005;91:839-846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 327] [Cited by in RCA: 312] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 115. | Grosse-Wortmann L, Yun TJ, Al-Radi O, Kim S, Nii M, Lee KJ, Redington A, Yoo SJ, van Arsdell G. Borderline hypoplasia of the left ventricle in neonates: insights for decision-making from functional assessment with magnetic resonance imaging. J Thorac Cardiovasc Surg. 2008;136:1429-1436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 78] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 116. | Brown DW, Gauvreau K, Powell AJ, Lang P, del Nido PJ, Odegard KC, Geva T. Cardiac magnetic resonance versus routine cardiac catheterization before bidirectional Glenn anastomosis: long-term follow-up of a prospective randomized trial. J Thorac Cardiovasc Surg. 2013;146:1172-1178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 117. | Fogel MA. Cardiac magnetic resonance of single ventricles. J Cardiovasc Magn Reson. 2006;8:661-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 118. | Ro PS, Rychik J, Cohen MS, Mahle WT, Rome JJ. Diagnostic assessment before Fontan operation in patients with bidirectional cavopulmonary anastomosis: are noninvasive methods sufficient? J Am Coll Cardiol. 2004;44:184-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 37] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 119. | Yoo SJ, Kim YM, Choe YH. Magnetic resonance imaging of complex congenital heart disease. Int J Card Imaging. 1999;15:151-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 120. | Wang ZJ, Reddy GP, Gotway MB, Yeh BM, Higgins CB. Cardiovascular shunts: MR imaging evaluation. Radiographics. 2003;23 Spec No:S181-S194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 73] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 121. | Beerbaum P, Sarikouch S, Laser KT, Greil G, Burchert W, Körperich H. Coronary anomalies assessed by whole-heart isotropic 3D magnetic resonance imaging for cardiac morphology in congenital heart disease. J Magn Reson Imaging. 2009;29:320-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 42] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 122. | Rajiah P, Setser RM, Desai MY, Flamm SD, Arruda JL. Utility of free-breathing, whole-heart, three-dimensional magnetic resonance imaging in the assessment of coronary anatomy for congenital heart disease. Pediatr Cardiol. 2011;32:418-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 123. | Greil GF, Seeger A, Miller S, Claussen CD, Hofbeck M, Botnar RM, Sieverding L. Coronary magnetic resonance angiography and vessel wall imaging in children with Kawasaki disease. Pediatr Radiol. 2007;37:666-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 44] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 124. | Greil GF, Desai MY, Fenchel M, Miller S, Pettigrew RI, Sieverding L, Stuber M. Reproducibility of free-breathing cardiovascular magnetic resonance coronary angiography. J Cardiovasc Magn Reson. 2007;9:49-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 125. | Bunce NH, Lorenz CH, Keegan J, Lesser J, Reyes EM, Firmin DN, Pennell DJ. Coronary artery anomalies: assessment with free-breathing three-dimensional coronary MR angiography. Radiology. 2003;227:201-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 131] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 126. | Alpendurada F, O’Hanlon R, Prasad SK. Cardiovascular magnetic resonance of cardiomyopathies. Curr Cardiol Rep. 2009;11:61-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 127. | Hong YM. Cardiomyopathies in children. Korean J Pediatr. 2013;56:52-59. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 128. | Gagliardi MG, Bevilacqua M, Di Renzi P, Picardo S, Passariello R, Marcelletti C. Usefulness of magnetic resonance imaging for diagnosis of acute myocarditis in infants and children, and comparison with endomyocardial biopsy. Am J Cardiol. 1991;68:1089-1091. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 85] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 129. | Messerli FH, Ketelhut R. Left ventricular hypertrophy: an independent risk factor. J Cardiovasc Pharmacol. 1991;17 Suppl 4:S59-S66; discussion S66-S67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 130. | Rickers C, Wilke NM, Jerosch-Herold M, Casey SA, Panse P, Panse N, Weil J, Zenovich AG, Maron BJ. Utility of cardiac magnetic resonance imaging in the diagnosis of hypertrophic cardiomyopathy. Circulation. 2005;112:855-861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 392] [Cited by in RCA: 367] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 131. | Moon JC, Fisher NG, McKenna WJ, Pennell DJ. Detection of apical hypertrophic cardiomyopathy by cardiovascular magnetic resonance in patients with non-diagnostic echocardiography. Heart. 2004;90:645-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 276] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 132. | Moon JC, Reed E, Sheppard MN, Elkington AG, Ho SY, Burke M, Petrou M, Pennell DJ. The histologic basis of late gadolinium enhancement cardiovascular magnetic resonance in hypertrophic cardiomyopathy. J Am Coll Cardiol. 2004;43:2260-2264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 507] [Cited by in RCA: 511] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 133. | O’Hanlon R, Grasso A, Roughton M, Moon JC, Clark S, Wage R, Webb J, Kulkarni M, Dawson D, Sulaibeekh L. Prognostic significance of myocardial fibrosis in hypertrophic cardiomyopathy. J Am Coll Cardiol. 2010;56:867-874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 589] [Cited by in RCA: 625] [Article Influence: 41.7] [Reference Citation Analysis (0)] |
| 134. | Moon JC, Sachdev B, Elkington AG, McKenna WJ, Mehta A, Pennell DJ, Leed PJ, Elliott PM. Gadolinium enhanced cardiovascular magnetic resonance in Anderson-Fabry disease. Evidence for a disease specific abnormality of the myocardial interstitium. Eur Heart J. 2003;24:2151-2155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 318] [Cited by in RCA: 335] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 135. | Barker PC, Pasquali SK, Darty S, Ing RJ, Li JS, Kim RJ, DeArmey S, Kishnani PS, Campbell MJ. Use of cardiac magnetic resonance imaging to evaluate cardiac structure, function and fibrosis in children with infantile Pompe disease on enzyme replacement therapy. Mol Genet Metab. 2010;101:332-337. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 136. | Anderson LJ, Holden S, Davis B, Prescott E, Charrier CC, Bunce NH, Firmin DN, Wonke B, Porter J, Walker JM. Cardiovascular T2-star (T2*) magnetic resonance for the early diagnosis of myocardial iron overload. Eur Heart J. 2001;22:2171-2179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1169] [Cited by in RCA: 1212] [Article Influence: 50.5] [Reference Citation Analysis (0)] |
| 137. | Pepe A, Positano V, Santarelli MF, Sorrentino F, Cracolici E, De Marchi D, Maggio A, Midiri M, Landini L, Lombardi M. Multislice multiecho T2* cardiovascular magnetic resonance for detection of the heterogeneous distribution of myocardial iron overload. J Magn Reson Imaging. 2006;23:662-668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 149] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 138. | Wood JC, Origa R, Agus A, Matta G, Coates TD, Galanello R. Onset of cardiac iron loading in pediatric patients with thalassemia major. Haematologica. 2008;93:917-920. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 86] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 139. | Anderson LJ, Westwood MA, Holden S, Davis B, Prescott E, Wonke B, Porter JB, Walker JM, Pennell DJ. Myocardial iron clearance during reversal of siderotic cardiomyopathy with intravenous desferrioxamine: a prospective study using T2* cardiovascular magnetic resonance. Br J Haematol. 2004;127:348-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 308] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 140. | Anderson LJ, Wonke B, Prescott E, Holden S, Walker JM, Pennell DJ. Comparison of effects of oral deferiprone and subcutaneous desferrioxamine on myocardial iron concentrations and ventricular function in beta-thalassaemia. Lancet. 2002;360:516-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 305] [Cited by in RCA: 307] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 141. | Uzun O, Wilson DG, Vujanic GM, Parsons JM, De Giovanni JV. Cardiac tumours in children. Orphanet J Rare Dis. 2007;2:11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 191] [Cited by in RCA: 181] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 142. | Beroukhim RS, Prakash A, Buechel ER, Cava JR, Dorfman AL, Festa P, Hlavacek AM, Johnson TR, Keller MS, Krishnamurthy R. Characterization of cardiac tumors in children by cardiovascular magnetic resonance imaging: a multicenter experience. J Am Coll Cardiol. 2011;58:1044-1054. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 122] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 143. | Kiaffas MG, Powell AJ, Geva T. Magnetic resonance imaging evaluation of cardiac tumor characteristics in infants and children. Am J Cardiol. 2002;89:1229-1233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 48] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 144. | O’Donnell DH, Abbara S, Chaithiraphan V, Yared K, Killeen RP, Cury RC, Dodd JD. Cardiac tumors: optimal cardiac MR sequences and spectrum of imaging appearances. AJR Am J Roentgenol. 2009;193:377-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 166] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 145. | Kaji S, Yang PC, Kerr AB, Tang WH, Meyer CH, Macovski A, Pauly JM, Nishimura DG, Hu BS. Rapid evaluation of left ventricular volume and mass without breath-holding using real-time interactive cardiac magnetic resonance imaging system. J Am Coll Cardiol. 2001;38:527-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 51] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 146. | Lurz P, Muthurangu V, Schievano S, Nordmeyer J, Bonhoeffer P, Taylor AM, Hansen MS. Feasibility and reproducibility of biventricular volumetric assessment of cardiac function during exercise using real-time radial k-t SENSE magnetic resonance imaging. J Magn Reson Imaging. 2009;29:1062-1070. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 46] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 147. | Muthurangu V, Lurz P, Critchely JD, Deanfield JE, Taylor AM, Hansen MS. Real-time assessment of right and left ventricular volumes and function in patients with congenital heart disease by using high spatiotemporal resolution radial k-t SENSE. Radiology. 2008;248:782-791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 57] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 148. | Razavi R, Hill DL, Keevil SF, Miquel ME, Muthurangu V, Hegde S, Rhode K, Barnett M, van Vaals J, Hawkes DJ. Cardiac catheterisation guided by MRI in children and adults with congenital heart disease. Lancet. 2003;362:1877-1882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 227] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 149. | Moore P. MRI-guided congenital cardiac catheterization and intervention: the future? Catheter Cardiovasc Interv. 2005;66:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 150. | Saikus CE, Lederman RJ. Interventional cardiovascular magnetic resonance imaging: a new opportunity for image-guided interventions. JACC Cardiovasc Imaging. 2009;2:1321-1331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 45] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 151. | Geva T, Marshall AC. Magnetic resonance imaging-guided catheter interventions in congenital heart disease. Circulation. 2006;113:1051-1052. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 152. | Blackham KA, Passalacqua MA, Sandhu GS, Gilkeson RC, Griswold MA, Gulani V. Applications of time-resolved MR angiography. AJR Am J Roentgenol. 2011;196:W613-W620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 153. | Markl M, Chan FP, Alley MT, Wedding KL, Draney MT, Elkins CJ, Parker DW, Wicker R, Taylor CA, Herfkens RJ. Time-resolved three-dimensional phase-contrast MRI. J Magn Reson Imaging. 2003;17:499-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 286] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 154. | Oshinski JN, Delfino JG, Sharma P, Gharib AM, Pettigrew RI. Cardiovascular magnetic resonance at 3.0 T: current state of the art. J Cardiovasc Magn Reson. 2010;12:55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 71] [Article Influence: 4.7] [Reference Citation Analysis (0)] |