Published online Nov 8, 2013. doi: 10.5409/wjcp.v2.i4.31
Revised: July 1, 2013
Accepted: July 9, 2013
Published online: November 8, 2013
Processing time: 262 Days and 16.4 Hours
Growth at birth and during infancy predicts several outcomes in the immediate future as well as in the long term. Weight and height are commonly used surrogates of growth, however, infants and young children are constantly growing unlike adults. Hence, weight and height alone are insufficient measures of growth if the time component is not associated with them. Recent studies have investigated the relationship between indoor air pollution and growth using height and weight. In this commentary, I have argued using a directed acyclic graph, that a causal association between indoor pollution exposure and growth at birth cannot be established unless birth weight is adjusted for gestational age. Furthermore, to make any causal inference between growth during the first few years of life and indoor exposure, in addition to age standardization, studies must also account for fetal growth to discount any continuation of prenatal effects, which may be in the causal pathway. A careful consideration is warranted from future studies investigating these relationships.
Core tip: Prenatal and early childhood estimators of growth, such as birth weight, height etc. by themselves are inadequate measures for inter-individual comparison, unless accompanied by gestational or chronologic age. The existing evidence points toward an association between indoor air pollution and growth, however few considered age. In order to establish a causal relationship it is imperative to consider age adjusted growth.
- Citation: Ghosh R. Indoor smoke and prenatal and childhood growth: The role of (gestational) age. World J Clin Pediatr 2013; 2(4): 31-35
- URL: https://www.wjgnet.com/2219-2808/full/v2/i4/31.htm
- DOI: https://dx.doi.org/10.5409/wjcp.v2.i4.31
Reduced growth prenatally and after birth, which is associated with childhood mortality and morbidity[1] and also with chronic diseases during adulthood[2], is considered a major problem in many developing countries. Coincidentally, indoor air pollution is also high in these countries[3]. More than half of the world population, predominantly from developing countries use some form of biofuel. A few studies have presented evidence that indoor air pollution may be one of the hitherto unknown factors associated with reduced prenatal and early childhood growth. This is a commentary on the studies mostly from the last decade, focusing on residual confounding that may arise if (gestational) age is not accounted for. There are many other sources of bias that pose similar threats to internal validity, which are not the focus of this commentary.
Prenatal exposure to indoor biofuel smoke and birth weight has now been investigated in several populations. A Zimbabwean study[4] reported maternal exposure to combustion smoke from wood, dung or straw was associated with infants born with 175 g (P < 0.01) lower mean birth weight than those who resided in homes that used gas or electricity for cooking. In spite of the significant association, owing to the cross-sectional nature, it is difficult to establish if exposure preceded outcome. Furthermore, the association with birth weight may not be construed to be with prenatal growth, as it was not adjusted for gestational age.
A study on Guatemalan infants[5] reported 63 g (95%CI: 1-126) reduction in birth weight for those exposed to wood smoke compared to infants from households that used electricity or gas. This cross-sectional study accounted for some important variables but not gestational age and the possibility of exposure misclassification exists. This was one of the earliest studies and the magnitude of the point estimate is on the lower side compared to the estimates of other studies conducted subsequently.
Another Guatemalan study[6] reported 89 g (95%CI: -27-+204) higher birth weight and the association with low birth weight (LBW) was 0.74 (95%CI: 0.33-1.66) for infants born in families that used a chimney stove compared to infants born in families that used open fire. The study, though not sufficiently powered, provides higher quality of evidence owing to the longitudinal design but did not adjust for gestational age.
A population-based longitudinal study[7] from India, designed from a randomized trail reported a 105 g (95%CI: 140-69) reduction of birth weight and a RR of 1.49 (95%CI: 1.25-1.77) for LBW amongst those prenatally exposed to wood or dung smoke compared to those unexposed. The estimate was adjusted for a range of covariates including secondhand tobacco smoke (SHS) and a surrogate of socioeconomic status (SES). This study provides stronger evidence because of the design, availability of information on and adjustment for potential confounders as well as for the power to detect a significant difference in the exposed and unexposed group.
Another population based retrospective cohort study[8] from Pakistan reported adjusted OR of 1.64 (95%CI: 1.10-2.35) for LBW and -82 g (95%CI: -170-+9) reduction in birth weight (continuous) among those exposed to wood smoke compared to natural gas combustion exposure. The study accounted for a wide range of potential confounders directly, including tobacco use, maternal BMI etc. and others indirectly, using propensity scores. The association with LBW was significant, while the association with birth weight was not, even though, birth weight as a continuous variable would have more variations (than the dichotomy of LBW), and hence lend more power to the model, assuming both models had same number of observations. There was a degree of misclassification in growth when LBW (without gestational age) was considered because all LBW infants were not due to growth retardation. This dichotomy and hence the misclassification was absent in the continuous birth weight model and may have been the cause for the statistically non-significant result. Interestingly, the study from India[7] reported a RR of 1.21 (95%CI: 1.11-1.31) for small for gestational age (SGA, < 10 percentile of birth weight-for-gestational age), which was less than half of the LBW estimate (RR = 1.49) from the same study. SGA reflects retarded prenatal growth more appropriately than LBW or birth weight because it takes into account gestational age. It is appreciated that accurate gestational age measurement is a challenge in settings in which these studies were conducted.
A meta-analysis[9] pooled all the estimates from the above studies (and one more), to summarize information and increase power. However, is it prudent to pool together estimates from cross-sectional and longitudinal studies? Perhaps, a non-significant test of heterogeneity is an argument in favor. If one digs deeper, even the two longitudinal studies, the models from which the LBW/birth weight estimates were used for pooling[6,7], did not have a single covariate in common. Furthermore, it included two estimates from the Indian cohort study[7] one for term LBW and the other for preterm LBW. The original article did not anywhere associate gestational age with the LBW estimate (RR = 1.49); presumably the authors performed additional stratified analyses but the estimates (term LBW in the meta-analysis and all LBW in the original paper) are strikingly similar. The comparison in the meta-analysis is essentially between term and preterm LBW-while the former is certainly due to intrauterine growth retardation, the latter is due to early parturition and may or may not be from growth retardation? A careful approach with particular attention to study characteristics and their differences, beyond any statistical test, should be considered before choosing the meta-analytic approach, to avoid spurious results.
A few studies also investigated exposure to indoor smoke and early childhood growth. About two decades ago the first study[10] provided evidence of an adverse association between exposure to smoke produced from gas burning during cooking and height at 10 years, a 3.3 cm reduction (P < 0.03) in the exposed compared to the unexposed. The cross-sectional study on Kuwaiti and European children was a modest attempt to explore the relationship by accounting for important determinants like SHS, SES, ethnicity etc. It acknowledged that there may have been selection bias because fewer Kuwaiti families participated and amongst those who did, a larger proportion used gas. The authors did not standardize height, which made the comparison amongst participants untenable unless they were all of 10 years when height was measured.
A seven country study[11] using data from national surveys investigated exposure to biofuel smoke (wood/straw/dung vs electricity/gas/biogas/kerosene) and child’s height-for-age and reported -0.13 (95%CI: -0.19--0.07) SD units reduction in a multilevel analyses. The analytical strategy and the wide range of covariates (including population differences) make this important evidence except the fact that owing to the cross-sectional nature temporal precedence of exposure cannot be established. In a categorical analysis it reported an OR of 1.27 (95%CI: 1.02-1.59) for severe stunting, defined as -3 SD ≤Z < -2 SD, amongst exposed vs unexposed. Another study[12] using data from Indian national family health survey reported larger association for severe stunting, OR of 1.90 (95%CI: 1.49-2.42) for those using biofuels compared to those who used cleaner fuels (definition similar to the previous one). Amongst the strength was the large number of covariates used but it did not account for multistage clustered sampling and there may be some unaccounted variance in the models affecting the significance.
Two longitudinal studies provide evidence of the association between exposure to biofuel generated smoke and reduced height-for-age. The study from India[7] investigated growth at 6 mo and reported RR of 1.45 (95%CI: 1.20-1.75) for underweight (< -2 SD, weight-for-age) for those exposed to wood and dung smoke compared to those unexposed and RR of 1.30 (95%CI: 1.06-1.60) for stunting (< -2 SD, height-for-age) in the same comparison groups. As stated above, this is one of the high quality evidence we have so far. A second longitudinal study[13] measured continuous height-for-age of children 36 mo old in Caucasian children from the Czech Republic and reported 1.3 cm reduction in height of 36 mo old children exposed to coal smoke compared to those who were unexposed. Retarded growth (from biofuels exposure or something else) may be initiated prenatally; such a condition needs to be accounted if we are to estimate the magnitude of a true causal association with early childhood growth. The Czech study adjusted for growth at birth using birth weight-for-gestational age-and-sex thus discounting for any such conditions initiated prenatally.
The currently available studies are heterogeneous-one longitudinal study[13] reported continuous Z-scores while another[7] reported both continuous Z-scores and dichotomous stunting. Furthermore, the definition of stunting was different from those used in the cross-sectional studies.
The underlying question is - can exposure to indoor biofuel smoke impede normal growth before and after birth? Residual confounding is an important issue that needs to be addressed to answer this question. In case of prenatal growth without the adjustment for gestational age none of the evidence can be concluded as causal. Additionally, maternal tobacco smoking[14] and household ETS[15] exposure are also established confounders. ETS adjustment may appear to be conservative because some of the constituents of tobacco smoke and indoor biofuel smoke are similar (e.g., PAH, nitrogen oxides, PM2.5), it is still a robust approach to eliminate the possibility of type 1 error and to estimate the true magnitude of the effect. The converse argument against the idea could be that this may lead to over-adjustment, masking or attenuating a true association.
Childhood height and weight change relatively rapidly with inherent age and sex differences. Standardization for age and sex using reference populations is therefore necessary to make the results generalizable. Additionally, in the developing countries malnourishment is an important factor for retarded childhood growth and studies investigating indoor air pollution and postnatal growth in these settings should also account for factors (viz. SES and morbidity) that cause malnourishment. Interestingly, all but one childhood growth study used age-standardized measures[10], which is pertinent. If standardization by age is imperative for growth after birth, why is it not for prenatal growth, when it is well known that a week or even days of gestational age in the third trimester make a difference to birth weight? It will be inappropriate to establish causality between indoor air pollution exposure and prenatal growth, using birth weight (or LBW) without gestational age, which, at best, is a crude surrogate of growth.
Another key issue for studies on childhood growth is accounting for growth at birth. It is critical to differentiate reduction in growth due to the exogenous exposures (secondhand smoke or indoor air pollution) during childhood from that which is simply a continuation of a retarded trajectory initiated prenatally. A retarded trajectory initiated prenatally may be due to similar exposures accrued over the prenatal period or may be from other causes, e.g., malnourished mother. Either way, it is important to make this distinction for an appropriate assessment of the magnitude of the association between indoor air pollution exposure and postnatal growth.
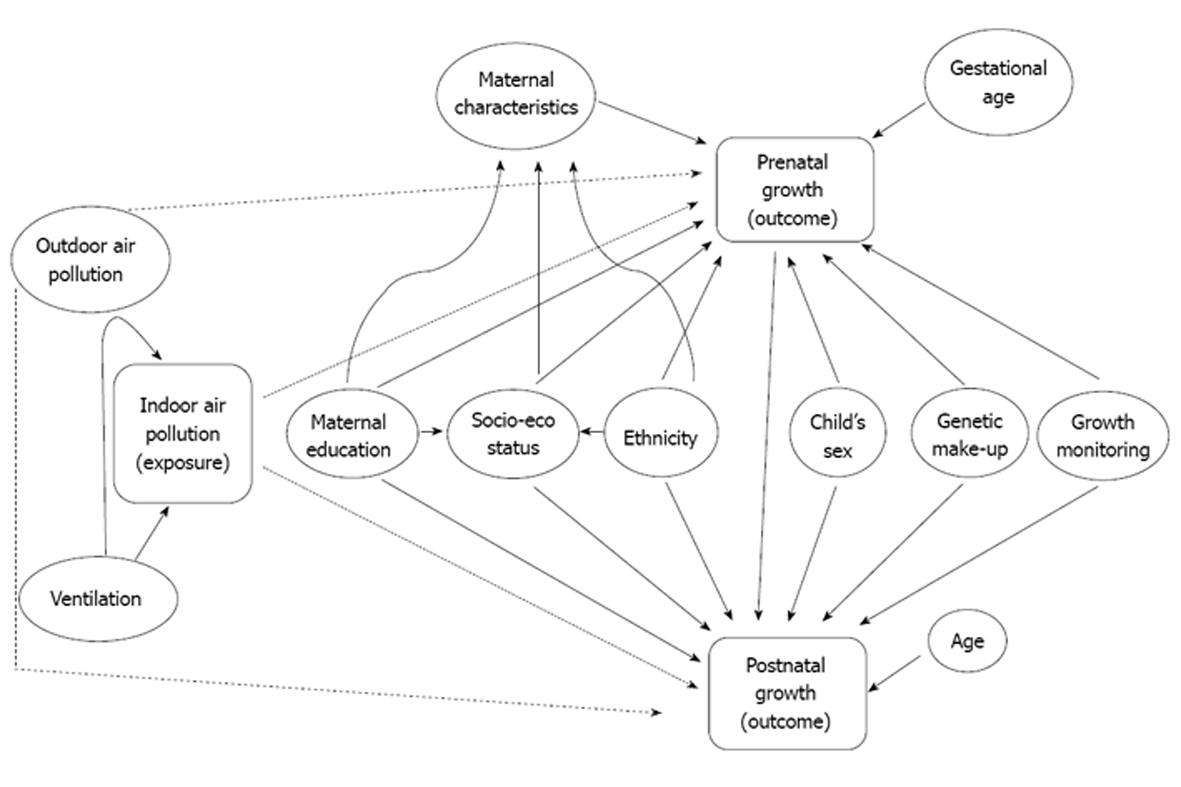
The key issues presented in the two paragraphs above can also be described using a Directed Acyclic Graph (DAG) shown in Figure 1. Age and gestational age are determinants of pre and postnatal growth, respectively and according to the definition of confounder these two variables need to be connected to exposure and outcome either through a directed or a backdoor path. Aside from the fact that higher age prolongs exposure, age or gestational age is not connected to indoor air pollution and the outcomes through any directed or unblocked backdoor paths in the DAG (Figure 1). However, age is absolutely necessary to measure growth when weight or height is used as the outcome. For example, an infant with 2800 g birth weight born at 42 wk does not have the same growth as another infant with the same weight but born at 37 wk. Therefore, it should not be mistaken that absence of any directed or backdoor path between age and exposure/outcome justifies it’s exclusion from consideration in the relationship. On the contrary, weight or height as an outcome is an insufficient measure of growth without age. Additionally, the DAG shows that postnatal growth has a backdoor path connecting prenatal growth and indoor air pollution, which suggests adjustment of prenatal growth is imperative in any model associating indoor air pollution with postnatal growth.
The other variable that deserves a mention from the DAG (Figure 1) is outdoor air pollution. The causality of the relationship between outdoor air pollution and growth is yet to be ascertained. Meanwhile, outdoor air pollution will influence indoor air and vice versa if the windows and doors are kept open for long duration. This would apparently suggest that outdoor air pollution should be adjusted while investigating the relationship between indoor air pollution and growth. However, if outdoor and indoor air has the same pollutants and under the assumption of causal relationships, adjustment for one to investigate the relationship with the other, would be taking out the very association one is interested to find.
To conclude, the evidence point towards a potential adverse association between indoor air pollution exposure and growth, and encourages further well-designed investigation with adequate power, addressing the limitations of the current ones, to estimate the true magnitude. The last trimester and early childhood is marked by steady growth, it is therefore important to adjust for gestational age or chronologic age, respectively, to eliminate any differences in growth due to age before an adverse impact can be assessed. An exercise of procuring these datasets to perform a standardized analysis can be the next step forward.
P- Reviewers: Cáceres DD, Simkhovich B S- Editor: Wen LL L- Editor: A E- Editor: Lu YJ
| 1. | McCormick MC. The contribution of low birth weight to infant mortality and childhood morbidity. N Engl J Med. 1985;312:82-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1353] [Cited by in RCA: 1307] [Article Influence: 32.7] [Reference Citation Analysis (0)] |
| 2. | Whincup PH, Kaye SJ, Owen CG, Huxley R, Cook DG, Anazawa S, Barrett-Connor E, Bhargava SK, Birgisdottir BE, Carlsson S. Birth weight and risk of type 2 diabetes: a systematic review. JAMA. 2008;300:2886-2897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 774] [Cited by in RCA: 693] [Article Influence: 40.8] [Reference Citation Analysis (0)] |
| 3. | Bruce N, Perez-Padilla R, Albalak R. Indoor air pollution in developing countries: a major environmental and public health challenge. Bull World Health Organ. 2000;78:1078-1092. [PubMed] |
| 4. | Mishra V, Dai X, Smith KR, Mika L. Maternal exposure to biomass smoke and reduced birth weight in Zimbabwe. Ann Epidemiol. 2004;14:740-747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 103] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 5. | Boy E, Bruce N, Delgado H. Birth weight and exposure to kitchen wood smoke during pregnancy in rural Guatemala. Environ Health Perspect. 2002;110:109-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 159] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 6. | Thompson LM, Bruce N, Eskenazi B, Diaz A, Pope D, Smith KR. Impact of reduced maternal exposures to wood smoke from an introduced chimney stove on newborn birth weight in rural Guatemala. Environ Health Perspect. 2011;119:1489-1494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 86] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 7. | Tielsch JM, Katz J, Thulasiraj RD, Coles CL, Sheeladevi S, Yanik EL, Rahmathullah L. Exposure to indoor biomass fuel and tobacco smoke and risk of adverse reproductive outcomes, mortality, respiratory morbidity and growth among newborn infants in south India. Int J Epidemiol. 2009;38:1351-1363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 121] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 8. | Siddiqui AR, Gold EB, Yang X, Lee K, Brown KH, Bhutta ZA. Prenatal exposure to wood fuel smoke and low birth weight. Environ Health Perspect. 2008;116:543-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 63] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 9. | Pope DP, Mishra V, Thompson L, Siddiqui AR, Rehfuess EA, Weber M, Bruce NG. Risk of low birth weight and stillbirth associated with indoor air pollution from solid fuel use in developing countries. Epidemiol Rev. 2010;32:70-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 241] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 10. | Jedrychowski W, Khogali M, Elkarim MA. Height and lung function in preadolescent children of Kuwaitis and European origin: a pilot survey on health effects of gas cooking in the Middle East. Arch Environ Health. 1991;46:361-365. [PubMed] |
| 11. | Kyu HH, Georgiades K, Boyle MH. Maternal smoking, biofuel smoke exposure and child height-for-age in seven developing countries. Int J Epidemiol. 2009;38:1342-1350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 51] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 12. | Mishra V, Retherford RD. Does biofuel smoke contribute to anaemia and stunting in early childhood? Int J Epidemiol. 2007;36:117-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 106] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 13. | Ghosh R, Amirian E, Dostal M, Sram RJ, Hertz-Picciotto I. Indoor coal use and early childhood growth. Arch Pediatr Adolesc Med. 2011;165:492-497. [PubMed] |
| 14. | Roquer JM, Figueras J, Botet F, Jiménez R. Influence on fetal growth of exposure to tobacco smoke during pregnancy. Acta Paediatr. 1995;84:118-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 51] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 15. | Salmasi G, Grady R, Jones J, McDonald SD. Environmental tobacco smoke exposure and perinatal outcomes: a systematic review and meta-analyses. Acta Obstet Gynecol Scand. 2010;89:423-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 168] [Article Influence: 11.2] [Reference Citation Analysis (0)] |