Published online Sep 9, 2025. doi: 10.5409/wjcp.v14.i3.106439
Revised: April 16, 2025
Accepted: May 7, 2025
Published online: September 9, 2025
Processing time: 110 Days and 5.8 Hours
Congenital scoliosis (CS) is a spinal deformity caused by defective segmentation and development of vertebrae during early embryogenesis. It occurs in 0.5%-1% in 1000 births and may rarely occur with congenital defects affecting the heart or genitourinary system. Truncus arteriosus (TA) is a life-threatening cardiac defect in which a single arterial trunk supplies both systemic and pulmonary circulation, leading to complications such as pulmonary hypertension, heart failure, and severe hypoxia. Although rare individually, the co-occurrence of both conditions poses unique diagnostic and therapeutic challenges, with limited documentation in medical literature.
We present a 36-week preterm neonate with CS associated with TA type 1, pre
Recognizing co-existing congenital anomalies and their embryological interrelation is critical in holistic patient care, particularly during neonatal and infancy.
Core Tip: This case highlights the unusual co-occurrence of congenital scoliosis and truncus arteriosus type 1 in a preterm neonate. It emphasizes the necessity for prompt diagnosis and a coordinated, multidisciplinary approach to management, aiming to enhance survival and quality of life in patients with complex congenital anomalies.
- Citation: Omullo P, Nurani KM, Shahabi K, Emma O, Mutuku B. Congenital scoliosis with truncus arteriosus type 1 in a preterm neonate: A case report. World J Clin Pediatr 2025; 14(3): 106439
- URL: https://www.wjgnet.com/2219-2808/full/v14/i3/106439.htm
- DOI: https://dx.doi.org/10.5409/wjcp.v14.i3.106439
Congenital defects affecting the cardiovascular and musculoskeletal systems are significant causes of global morbidity and mortality[1]. Congenital scoliosis (CS) occurs in approximately 0.5%–1% of 1000 live births[2] and may present as an isolated anomaly or within syndromes such as Ehlers-Danlos, Marfan, or osteogenesis imperfecta. Isolated CS is often linked to cardiac and genitourinary anomalies due to shared embryological pathways[3].
Truncus arteriosus (TA), a rare cardiac anomaly, results from failed truncal septation during embryogenesis, leading to a single arterial trunk supplying the pulmonary, coronary, and systemic circulations[4]. Complications such as pul
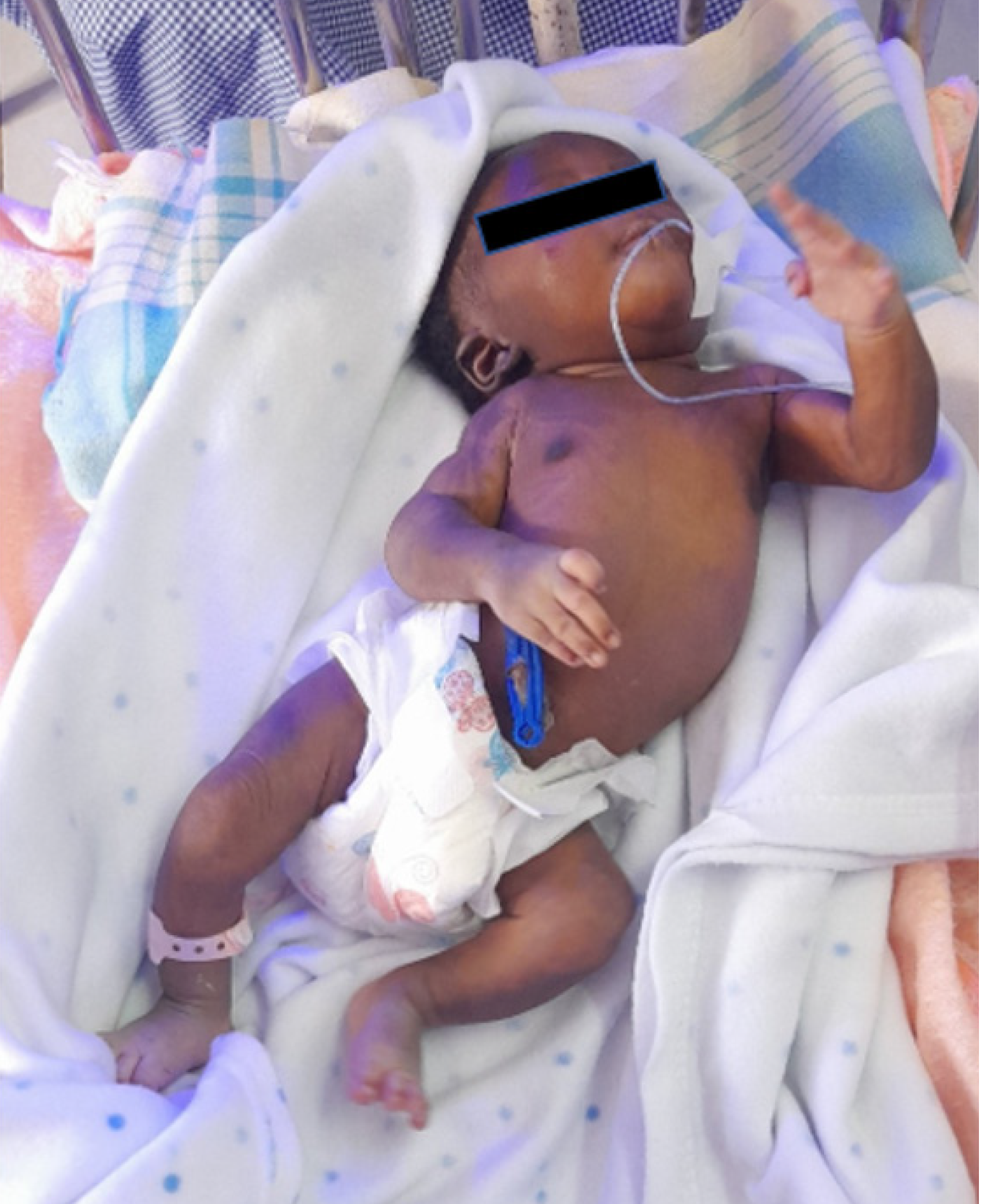
A 2-day-old female neonate was brought to the hospital with respiratory distress, cyanosis, and an apparent altered curvature of the spine.
The neonate was born at 36 weeks of gestation via spontaneous vaginal delivery, with a birth weight of 2500 grams. Apgar scores were 8, 9, and 9 at one minute, five minutes, and ten minutes, respectively. Shortly after birth, she exhibited signs of respiratory distress, including tachypnea and grunting, accompanied by central cyanosis. An abnormal spinal curvature was also noted.
The mother, gravida 5, para 2, abortus 2, had attended a single antenatal care visit at a peripheral facility without a detailed obstetric ultrasound. She reported no chronic illnesses, medication use, or exposure to teratogens during pregnancy. Also, she denied any fevers during the pregnancy.
The mother had two healthy children and two early pregnancy losses at 12 weeks. The etiology of the losses was not investigated due to socioeconomic constraints. No family history of congenital anomalies was reported.
On examination, the neonate was lethargic with labored breathing, nasal flaring, and intercostal retractions. Vital signs included a heart rate of 199 beats per minute, respiratory rate of 68 breaths per minute, temperature of 38.2 °C, and oxygen saturation of 78% on room air. Cardiovascular examination revealed a harsh holosystolic murmur. Musculoskeletal assessment confirmed a lateral curvature of the thoracic spine without limb abnormalities. Neurological examination showed normal muscle tone and an intact Moro reflex.
Included in Table 1.
| Parameter | Findings |
| Blood group | A+ |
| White blood cell count | 10000 × 109/L |
| Haemoglobin | 14.9 g/dL |
| Platelets | 253000 |
| C-reactive protein | Non-reactive |
| Blood cultures | Negative |
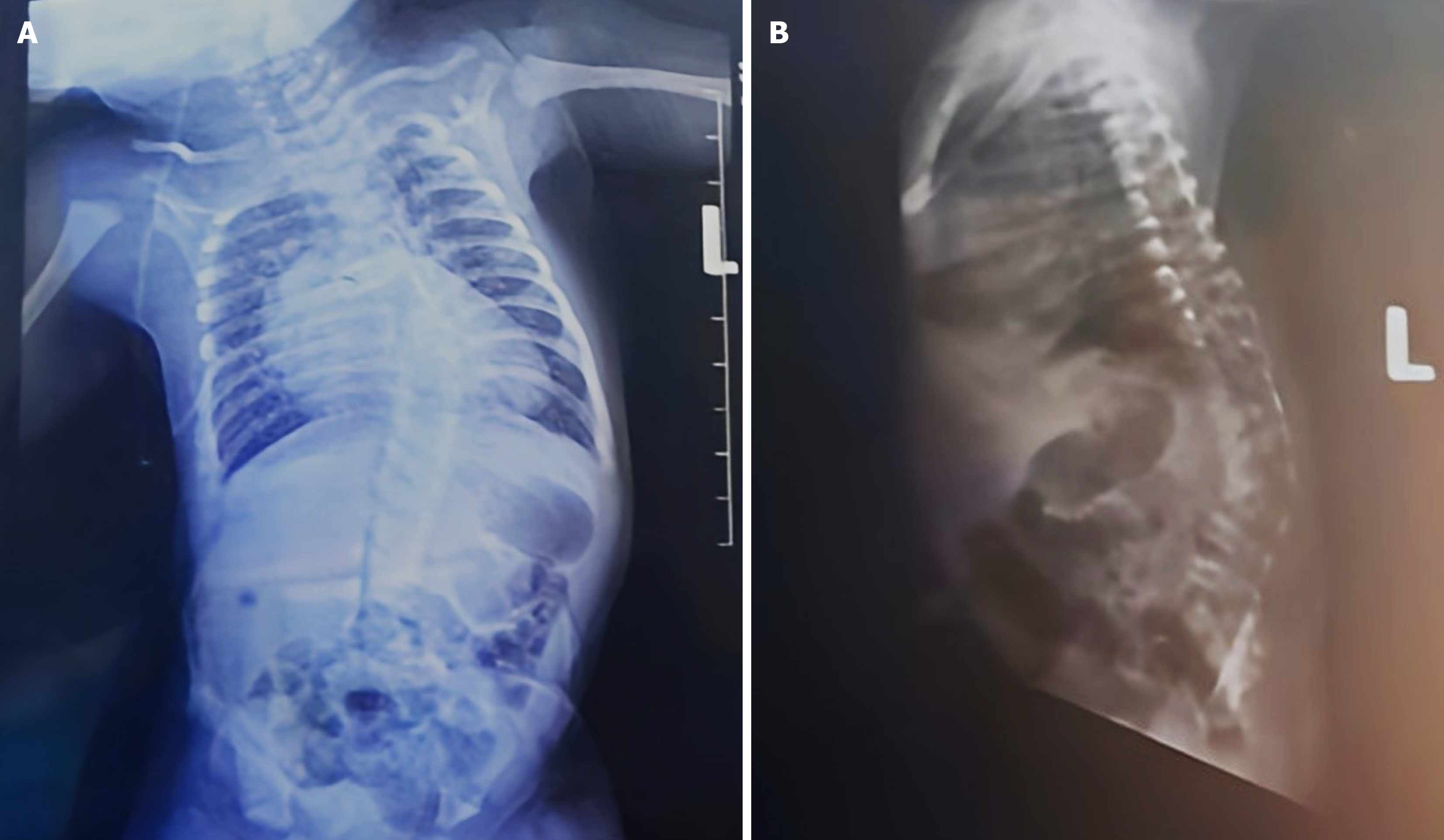
Chest radiographs demonstrated thoracic scoliosis with vertebral segmentation anomalies and a mildly enlarged cardiac silhouette (Figure 1). Echocardiography identified TA type 1, a multi-fenestrated atrial septal defect, and mild truncal valve regurgitation with preserved ventricular function (Figure 2). Cranial and kidney, ureter, and bladder ultrasounds were unremarkable.
A multidisciplinary team was convened with neonatology, cardiology, cardiothoracic surgery, orthopedics, and genetics. The consensus was to stabilize the neonate's respiratory and cardiac status, initiate medical management for heart failure, and plan for surgical interventions once the patient was optimized. Genetic evaluation was considered but deferred due to resource limitations.
The neonate was diagnosed with CS and TA type 1.
Management involved a multidisciplinary approach. Oxygen therapy via nasal prongs at 1 L/minute (inspiratory fraction of oxygen 100%) was initiated for respiratory support. Continuous positive airway pressure was considered but deferred due to the patient’s moderate distress. Intravenous antibiotics (penicillin and gentamicin) were administered while ruling out neonatal sepsis. Heart failure medications (furosemide and spironolactone) were initiated to manage pulmonary over-circulation, not for primary cardiac dysfunction. Nutritional support was provided through nasogastric feeding due to the initial poor suck reflex and risk of aspiration. Orthopedic and cardiothoracic consultations were made to guide surgical correction. Informed family counseling was prioritized, addressing the child’s complex needs, prognosis, and limitations in resource-constrained care. Genetic analysis and 22q11.2 deletion testing were planned but not performed due to limited resources.
The neonate showed clinical improvement with stable oxygen saturation and reduced respiratory distress following multidisciplinary care. Diuretic therapy managed pulmonary over-circulation, while nutritional status improved through nasogastric and oral feeds. Cardiac function remained preserved, and echocardiography confirmed mild truncal valve regurgitation without ventricular dysfunction. After family counselling, the patient was discharged in stable condition with referrals to tertiary centres for cardiothoracic surgery and orthopedic follow-up. Due to limited resources, genetic testing, including 22q11.2 deletion analysis, was deferred. Long-term monitoring for scoliosis progression, respiratory complications, and surgical timing was recommended. At one-month follow-up, the neonate was feeding well, gaining weight, and stable with no new symptoms. Ongoing multidisciplinary care was advised to optimize outcomes, particularly in a resource-constrained setting. The family was educated on signs of cardiac decompensation and the importance of adherence to follow-up visits. Surgical correction remained critical for long-term prognosis and quality of life.
CS results from underlying spinal malformations and is the most common congenital vertebral anomaly[2]. It occurs in approximately 1% in 1000 live births, with a male-to-female ratio of 1:1.4[5]. TA is an equally uncommon congenital cardiac anomaly with an incidence of 6–10 per 100000 live births[6]. No documented case reports explicitly describe patients presenting with both TA and CS. However, several case reports detail complex presentations of TA with other anomalies as summarized in Table 2[7-10]. The diverse outcomes—from survival after surgery to early neonatal mortality—underscore the heterogeneity of presentations and the need for tailored, multidisciplinary approaches to management[7-10].
| Ref. | Patient demographics | Associated anomalies | Truncus arteriosus type | Management | Outcome |
| Alizadeh et al[7], 2022 | Newborn, unspecified | Hypoplastic left heart syndrome, other cardiovascular defects | Type II | Multidisciplinary management, palliative care | Not specified |
| Takeuchi et al[8], 2018 | Infant, unspecified | Interrupted aortic arch, bronchial compression | Unspecified | Pulmonary artery stenting, surgery | Post-op bronchial issues |
| Abdelaal et al[9], 2022 | Neonate, unspecified | Coarctation of the aorta, truncal valve regurg | Common arterial trunk | Surgery | Survived post-surgery |
| Chen et al[10], 2016 | Infants to adults | Truncal valve anomalies, other cardiac issues | Mixed (I, II, III) | Surgical repair across age spectrum | Variable, mostly positive |
| Present study, 2025 | Preterm neonate, female | None confirmed, limited resources for genetic testing | Type I | Supportive care | Awaiting surgical correction |
Failure of truncal septation during embryogenesis results in a single arterial trunk supplying the coronary, systemic, and pulmonary circulations[4]. The intersection of these conditions reflects the vulnerability of early organogenesis to disruptions in genetic and environmental signaling pathways.
Fetal injury during intrauterine spinal development underpins many congenital anomalies[11]. Insults during the fifth to eighth weeks of gestation are linked to anomalies such as spinal cord dysraphism, congenital heart disease, and genitourinary defects. CS primarily arises from segmentation or formation failures, while TA is attributed to neural crest cell maldevelopment within the cardiac outflow tract[4,12]. The temporal overlap of spine and heart development suggests a shared embryological etiology.
CS is frequently associated with syndromes like vertebral, ano-rectal, cardiac, tracheo-esophageal, renal, and limb, CHARGE, and Klippel-Feil, highlighting genetic contributions[13]. Environmental factors, including maternal diabetes, hypoxia, hyperthermia, sodium valproate, and alcohol, also play a role[14]. TA’s etiology remains uncertain, but implicated factors include 22q11.2 microdeletions, maternal diabetes, and teratogens such as retinoic acid[15].
A thorough family and obstetric history is essential. Antenatal imaging is critical for identifying vertebral defects and evaluating maternal health and substance exposure[11]. While CS can be detected on physical exam, TA requires echocardiography. Advanced imaging, such as three dimensions computed tomography/magnetic resonance imaging, clarifies scoliosis, while plain X-rays measure the Cobb angle[2]. Prenatal TA diagnosis is feasible from 13 weeks via fetal echocardiography[6]. However, access remains limited in low-resource settings.
CS management aims to prevent curve progression while ensuring balance. Severe curves often require surgery, while milder deformities may be managed conservatively[2]. TA management begins with initial stabilization using heart failure medications, diuretics, and oxygen support. Definitive surgical correction is usually performed within six months[16]. The prognosis for neonates with combined congenital anomalies depends on timely cardiac management and early identification of associated defects. Successful TA repair improves outcomes, though scoliosis-related complications like restrictive lung disease require ongoing orthopedic monitoring[17].
Diagnostic delays and a lack of specialist care worsen outcomes in resource-limited settings. Enhancing antenatal services, capacity building, and integrating telemedicine could improve the management of complex neonatal conditions.
This case underscores the importance of early diagnosis, multidisciplinary management, and tailored follow-up for neonates with rare congenital anomalies. It highlights the urgent need for better prenatal screening protocols and expanded neonatal care resources in underserved regions. Further research is needed to understand the genetic and embryological underpinnings of such coexisting conditions and to optimize management strategies.
The authors thank Dr. Nick Mutisya and the Murang’a County Referral Hospital for supporting the preparation of this case report.
| 1. | Chimah OU, Emeagui KN, Ajaegbu OC, Anazor CV, Ossai CA, Fagbemi AJ, Emeagui OD. Congenital malformations: Prevalence and characteristics of newborns admitted into Federal Medical Center, Asaba. Health Sci Rep. 2022;5:e599. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 2. | Bagheri F, Razi A, Birjandinejad A, Amel Farzad S, Peivandi MT, Habibzade Shojaei SRH. Congenital Scoliosis: A Current Concepts Review. J Pediatr Rev. 2021;9:127-136. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 3. | Kaspiris A, Grivas TB, Weiss HR. Congenital scoliosis in monozygotic twins: case report and review of possible factors contributing to its development. Scoliosis. 2008;3:17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 4. | Bhansali S, Horenstein MS, Phoon C. Truncus Arteriosus. 2024 Mar 10. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. [PubMed] |
| 5. | Arlet V, Odent T, Aebi M. Congenital scoliosis. Eur Spine J. 2003;12:456-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 56] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 6. | Reller MD, Strickland MJ, Riehle-Colarusso T, Mahle WT, Correa A. Prevalence of congenital heart defects in metropolitan Atlanta, 1998-2005. J Pediatr. 2008;153:807-813. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 952] [Cited by in RCA: 798] [Article Influence: 46.9] [Reference Citation Analysis (0)] |
| 7. | Alizadeh LS, Koch V, Grünewald LD, Yel I, Mathies D, Rauschning D, Vogl TJ, Booz C. Case report of rare congenital cardiovascular anomalies associated with truncus arteriosus type 2. Heliyon. 2022;8:e11033. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 8. | Takeuchi K, Srivastava A, Steed DR. Bronchial compression as adverse effect of right pulmonary artery stenting in a patient with truncus arteriosus communis and interrupted aortic arch: A case report. Pediatr Med. 2018;1:7-7. [DOI] [Full Text] |
| 9. | Abdelaal M, Dedieu N. A rare case of common arterial trunk with coarctation of the aorta: a case report. Eur Heart J Case Rep. 2022;6:ytac257. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 10. | Chen T, Song S, Jiang H, Lian H, Hu S. Single Cell Sequencing Reveals Mechanisms of Persistent Truncus Arteriosus Formation after PDGFRα and PDGFRβ Double Knockout in Cardiac Neural Crest Cells. Genes (Basel). 2022;13:1708. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 11. | Nabizadeh N, Dimar JR. Congenital spine deformities: timing of insult during development of the spine in utero. Spine Deform. 2022;10:31-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 12. | Sadler TW. Establishing the Embryonic Axes: Prime Time for Teratogenic Insults. J Cardiovasc Dev Dis. 2017;4:15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 13. | Debnath UK, Goel V, Harshavardhana N, Webb JK. Congenital scoliosis - Quo vadis? Indian J Orthop. 2010;44:137-147. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (1)] |
| 14. | Li Z, Yu X, Shen J. Environmental aspects of congenital scoliosis. Environ Sci Pollut Res Int. 2015;22:5751-5755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 15. | Yaoita H, Kawai E, Takayama J, Iwasawa S, Saijo N, Abiko M, Suzuki K, Kimura M, Ozawa A, Tamiya G, Kure S, Kikuchi A. Genetic etiology of truncus arteriosus excluding 22q11.2 deletion syndrome and identification of c.1617del, a prevalent variant in TMEM260, in the Japanese population. J Hum Genet. 2024;69:177-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 16. | Naimo PS, Konstantinov IE. Surgery for Truncus Arteriosus: Contemporary Practice. Ann Thorac Surg. 2021;111:1442-1450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 17. | Yuan N, Skaggs DL, Dorey F, Keens TG. Preoperative predictors of prolonged postoperative mechanical ventilation in children following scoliosis repair. Pediatr Pulmonol. 2005;40:414-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 47] [Article Influence: 2.4] [Reference Citation Analysis (0)] |