Published online Jun 9, 2025. doi: 10.5409/wjcp.v14.i2.102049
Revised: February 12, 2025
Accepted: February 27, 2025
Published online: June 9, 2025
Processing time: 162 Days and 1.1 Hours
Although critically ill pediatric patients can benefit from the use of sedation, it can cause side effects and even iatrogenic complications. Since pediatric patients cannot adequately express the intensity or location of the pain, discriminating the cause of their irritability and agitation can be more complicated than in adults. Thus, sedation therapy for children requires more careful attention.
To evaluate the association of the internal parental care protocol and the reduction in pediatric intensive care unit (PICU) postoperatively.
This retrospective cohort study was carried out in the PICU of the tertiary medical center in Kazakhstan. The internal parental care protocol was developed and implemented by critical care team. During the pandemic, restrictions were also placed on parental presence in the PICU. We compare two groups: During restriction and after return to normal. The level of agitation was evaluated using the Richmond Agitation-Sedation Scale. Univariate and multivariate logistic regression analyses were performed to examine associations of parental care with sedation therapy.
A total of 289 patients were included in the study. Of them, 167 patients were hospitalized during and 122 after the restrictions of parental care. In multivariate analysis, parental care was associated with lower odds of prescribing diazepam (odds ratio = 0.11, 95% confidence interval: 0.05-0.25), controlling for age, sex, cerebral palsy, and type of surgery.
The results of this study show that parental care was associated only with decreased odds of prescribing sedative drugs, while no differences were observed for analgesics.
Core Tip: Parental presence in the pediatric intensive care unit (PICU) may serve as a nonpharmacologic intervention to reduce the need for sedation in postoperative pediatric patients. This retrospective cohort study shows that children whose parents were present during their PICU stay were less likely to receive diazepam, suggesting that parental care may help reduce anxiety and agitation. The findings highlight the therapeutic role of family-centered care in the PICU and emphasize the need for further research on structured parental involvement as a strategy to optimize sedation practices and improve patient recovery.
- Citation: Sazonov V, Issanov A, Turar S, Tobylbayeva Z, Mironova O, Saparov A, Viderman D. Parental presence in the pediatric intensive care unit reduces postoperative sedative requirements: A retrospective study. World J Clin Pediatr 2025; 14(2): 102049
- URL: https://www.wjgnet.com/2219-2808/full/v14/i2/102049.htm
- DOI: https://dx.doi.org/10.5409/wjcp.v14.i2.102049
Pediatric intensive care units (PICUs) remain one of the most critical departments in hospitals. Children who require care in the PICU often undergo intensive and invasive procedures essential for their survival. Therefore, it is crucial to ensure both physical and emotional well-being for the child, his family and the medical staff[1]. A common challenge faced in the PICU is managing postoperative discomfort and distress. Along with the pain management, sedation is a crucial intervention in this setting to reduce anxiety, and ensure safety during mechanical ventilation or certain invasive procedures[2]. Postoperative sedation can play a critical role in the pediatric intensive care setting, serving several important functions. First, it helps reduce the perception of pain, which improves the child’s overall comfort and promotes a smoother recovery process. In addition, sedation reduces anxiety and distress, which can have long-term psychological consequences if left untreated[3]. In addition, for mechanically ventilated children, appropriate sedation is essential to ensure patient safety by preventing unintentional extubation or interference with critical medical equipment[4]. Despite its benefits, sedation carries significant risks. Prolonged or excessive use may result in respiratory depression, hemodynamic instability, and delayed recovery. Certain sedatives also carry a risk of dependence and may cause withdrawal symptoms when discontinued[5]. In addition, adverse effects, drug interactions, and the potential for overdose underscore the need for cautious use. Given these concerns, exploring alternative or adjunctive strategies to minimize the need for sedation is critical to optimizing patient safety and recovery. Parental care plays a critical role in the recovery of pediatric patients in the PICU. The deep emotional bond between parents and their children provides a unique opportunity for therapeutic intervention. When appropriately integrated, parental presence can serve as a powerful non-pharmacological strategy, providing comfort through touch, voice, and warmth. This reassurance can significantly reduce a child’s anxiety and distress, thereby reducing the need for sedative medications[6]. Incorporating parental care into standard medical protocols not only addresses the child’s physiological needs, but also promotes emotional well-being, ultimately contributing to a smoother recovery process. The purpose of this study is to determine whether parental care can reduce the need for sedation in PICU patients.
This retrospective cohort study was conducted in the PICU of the University Medical Center, a tertiary medical center in Astana, Kazakhstan. The 12-bed PICU provides specialized care for critically ill pediatric patients, including those undergoing surgical, medical, and oncohematologic treatments. The internal parental care protocol was developed and implemented by the University Medical Center critical care team in early 2018[7]. The protocol allows parents/guardians to remain with their child in the PICU without time restrictions and, after appropriate training, to participate in certain aspects of patient care. During the coronavirus disease 2019 pandemic, parental presence in the PICU was limited. The study compares patient outcomes in two cohorts: one admitted during the period of restricted parental care (June-December 2019), and another admitted after the restoration of unrestricted parental presence (January-June 2022).
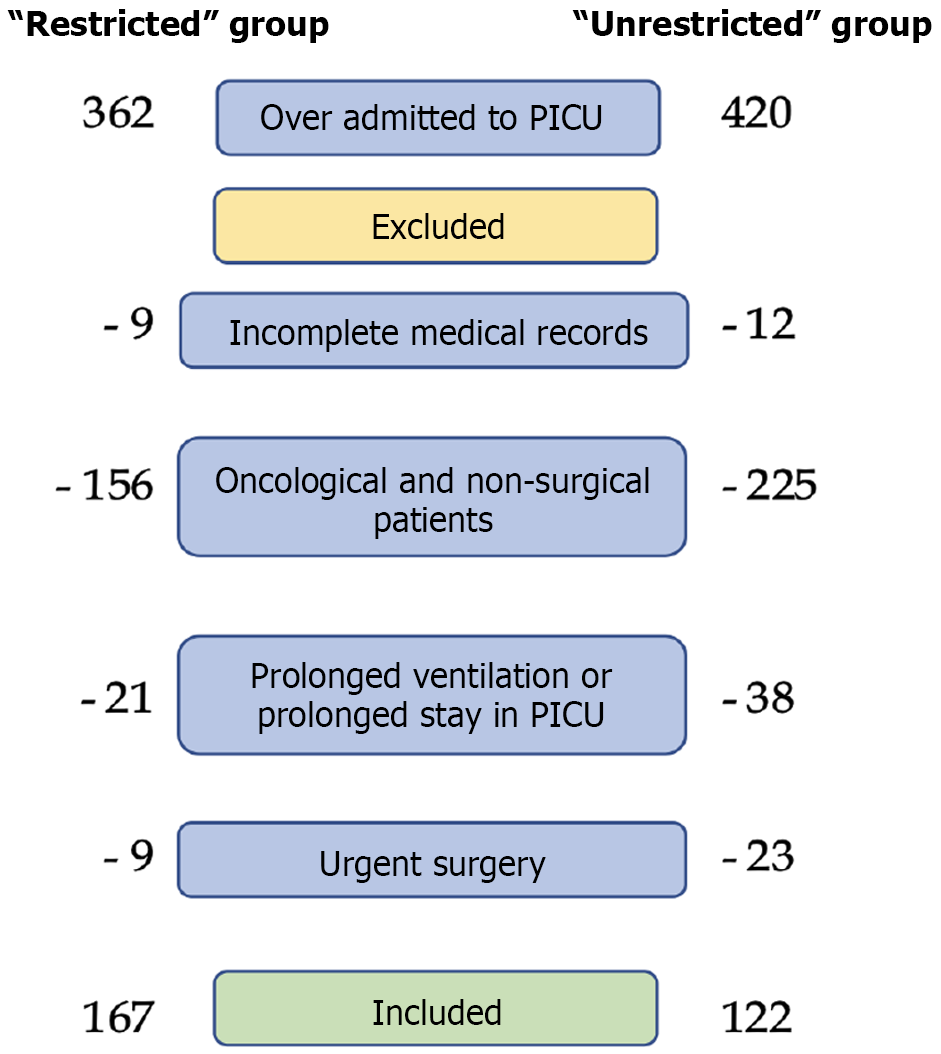
This study focused on pediatric patients who underwent elective surgery and were subsequently admitted to the PICU for postoperative monitoring and supportive care within the specified timeframes. To ensure a homogeneous study population, only patients undergoing elective orthopedic or abdominal surgery were included. These patients required intensive monitoring and specialized care in the immediate postoperative period, but were not expected to have prolonged PICU stays or require prolonged mechanical ventilation. Patients were eligible for inclusion if they met the following criteria: The age is up to 18 years, who underwent elective orthopedic or abdominal surgery. Inclusion required PICU admission for postoperative monitoring and supportive care, although they were not expected to require prolonged intensive care. Patients must have complete medical records, including detailed documentation of analgesic and sedative prescriptions. To minimize confounding factors and ensure a well-defined study population, several patient groups were excluded. Patients undergoing non-elective, emergent procedures were excluded because their postoperative course is inherently more variable and complex. Patients who were transferred to the PICU due to deterioration without undergoing surgery were not included. Patients with oncology were excluded due to their complex medical conditions and different pain management strategies. Any patient requiring mechanical ventilation beyond the immediate postoperative period was excluded, as prolonged sedation requirements could significantly affect study results. Patients whose records were missing essential information, including sedation, analgesia, or pain assessment data, were also excluded to ensure data integrity and completeness (Figure 1).
We retrospectively reviewed and analyzed electronic medical records of all eligible pediatric patients admitted to the PICU during the defined study periods. Baseline characteristics of all enrolled patients were obtained from hospital records, including age, sex, and body weight at the time of PICU admission. In addition, information on the presence of pre-existing medical conditions, including comorbidities and neurological conditions such as cerebral palsy, was recorded to account for potential differences in pain perception and sedation requirements. For each patient, detailed information on the surgical procedure was obtained. The timing and conditions of PICU admission were also recorded, including date of surgery, date of PICU transfer, and total PICU and hospital length of stay (LOS). Because parental presence may influence early postoperative recovery, a comparison of LOS between the two groups was an essential component of the analysis.
For anesthesia management, inhalational anesthetic or propofol was used for induction and maintenance, followed by intubation with induction of age-appropriate rocuronium (0.3-0.5 mg/kg). Analgesia was maintained during surgery with 0.05% fentanyl. The average duration of surgery was less than two hours. After surgery, patients were transferred to the PICU for further management and observation. Patients were extubated with return of reflexes, spontaneous breathing, and upper airway clearance. Given the extent of the surgery, analgesia was prescribed. The PICU provided supportive infusion therapy, adequate analgesia, antibacterial therapy (as indicated), and symptomatic therapy. Enteral nutrition was resumed as soon as possible.
Postoperative pain and agitation were systematically assessed by trained medical personnel using validated pain and sedation scales to ensure standardized assessment across patients. In older children who were able to self-report pain, the numerical rating scale was used to quantify pain intensity[8]. For younger children (3 years and younger) and nonverbal patients, the revised Face, Legs, Activity, Cry, Consolability scale[9] and the Wong-Baker Faces scale for children 3 years and older[10] were used. Agitation was assessed using the Richmond Agitation-Sedation Scale[11]. In cases where agitation reached a Richmond Agitation-Sedation Scale score of ≥ 1, additional assessments were performed to identify and address non-pharmacologic causes, such as pain, hunger, or environmental discomfort, before sedation therapy was considered.
Since the primary focus of this study was to evaluate the impact of parental care on the use of sedation, a comprehensive dataset of postoperative pain and sedation management was extracted from medical records. For postoperative pain management, we use paracetamol (acetaminophen) orally or per rectum (suppositories) for mild to moderate pain in a dosage of 15-20 mg/kg; or non-steroidal anti-inflammatory drugs, including doses of ibuprofen 10 mg/kg/every 6-8 hours. If the pain intensity reaches 4 points on the numerical rating scale or higher, we add morphine/trimeperidine - age differences (dose) 0.05 mg/kg up to 0.2 mg/kg in children older than 12 months (maximum 5-10 mg) For postoperative sedation, we use diazepam (0.1-0.2 mg/kg) intravenously/intramuscularly and rarely dexmedetomidine intravenously (0.5-0.7 mcg/kg/hour).
All statistical analyzes were performed using Stata version 16.1. Continuous variables were described either as means and standard deviations or as medians and interquartile ranges (for non-normally distributed variables). In descriptive statistics, categorical variables were presented as frequencies and percentages. To compare continuous data between two groups, an independent t-test or Mann-Whitney U-test was used. The χ2 test was utilized to examine associations between categorical variables. Univariate and multivariate logistic regression analyses were performed to examine associations of parental care with sedation therapy.
A total of 289 patients were included in the study. Of these, 167 patients were hospitalized during the restrictions of parental care and 122 after the parental care protocol back (Table 1). In the “unrestricted” group, the patients were statistically significantly younger (versus the “restricted” group) 3.5 (1.3-10.8) years vs 7.5 (2.8-13.7) years, respectively; had lower body weight of 15 (9.5-28.8) kg vs 19.6 (12-39) kg. The most common reason for admission to the ICU was postoperative care after pediatric orthopedic surgeries. The surgical procedures were performed successfully in all cases.
| Variables | Total (n = 289) | Restricted parental care (n = 167) | Unrestricted parental care (n = 122) | P value |
| Age (years), median (IQR) | 5.6 (2.0-12.8) | 7.5 (2.8-13.7) | 3.5 (1.3-10.8) | < 0.01 |
| Sex, male | 134 (46.4) | 69 (41.3) | 65 (53.3) | 0.04 |
| Weight (kg), median (IQR) | 17.4 (11-32.4) | 19.6 (12-39) | 15 (9.5-28.8) | < 0.01 |
| Comorbidities, yes | 37 (12.8) | 26 (15.) | 11 (9.0) | 0.10 |
| Presence of cerebral palsy, yes | 13 (4.5) | 11 (6.7) | 2 (1.6) | 0.05 |
| Type of surgery | ||||
| Orthopedic | 134 (46.4) | 90 (53.9) | 44 (36.1) | < 0.01 |
| Abdominal | 91 (31.5) | 47 (28.1) | 44 (36.1) | - |
| Other | 64 (22.1) | 30 (18.0) | 34 (27.8) | - |
| LOS in ICU (days), median (IQR) | 2 (2-3) | 2 (2-3) | 2 (2-4) | 0.04 |
| Hospital LOS, mean ± SD | 16.0 ± 6.5 | 14.6 ± 6.0 | 18.2 ± 6.5 | < 0.001 |
| RASS, mean ± SD | 1.1 ± 0.3 | 1.2 ± 0.5 | 1.0 ± 0.2 | < 0.01 |
| Received diazepam | 56 (19.4) | 45 (27.0) | 11 (9.0) | < 0.001 |
| Diazepam dose (mg/kg), median (IQR) | 0.1 (0.1-0.1) | 0.1 (0.1-0.1) | 0.1 (0.1-0.1) | 0.74 |
| Received dexmedetomidine | 3 (1.0) | 1 (0.6) | 2 (1.6) | 0.58 |
| Dexmedetomidine dose (mg/kg), median (IQR) | 0.7 (0.7-0.7) | 0.7 (0.7-0.7) | 0.7 (0.7-0.7) | 1.00 |
| Received trimeperidine | 209 (72.3) | 133 (79.6) | 76 (62.3) | < 0.01 |
| Trimeperidine dose (mg/kg), median (IQR) | 0.2 (0.15-0.25) | 0.2 (0.2-0.25) | 0.2 (0.15-0.25) | 1.00 |
| Received morphine | 86 (29.8) | 35 (21.0) | 51 (41.8) | < 0.001 |
| Morphine dose (mg/kg), median (IQR) | 0.1 (0.1-0.1) | 0.1 (0.1-0.1) | 0.1 (0.1-0.1) | 0.81 |
As shown in Table 2, 45 (27.0%) patients in the “restricted” group received diazepam compared to 11 (9.0%) in the “unrestricted” group. The dose of diazepam was not significantly different between the two groups of patients who received diazepam. One hundred and thirty-three patients (79.6%) received trimeperidine in the “restricted” group compared to 76 (62.3%), however, the dose of trimeperidine in those patients who received it did not differ significantly between the groups. The frequency of morphine use was significantly lower in the “restricted” group 35 (21.0%) than in the “unrestricted” group 51 (41.8%) and the dose between the patients received it was not statistically significant. In the bivariate analysis, younger age, lower body weight, presence of cerebral palsy, and abdominal surgery were associated with higher rates of prescription for diazepam in both groups (Table 2). Older age, higher body weight, presence of cerebral palsy and comorbidities, and orthopedic surgery were associated with higher rates of trimeperidine prescriptions in both groups (Table 2). Trimeperidine prescription was associated with a reduction in LOS in the ICU. Diazepam and morphine prescriptions were associated with an increase in LOS in the ICU. In multivariate analysis, parental care was associated with lower odds of prescribing diazepam [odds ratio (OR) = 0.11, 95% confidence interval (CI): 0.05-0.25], controlling for age, sex, cerebral palsy, and type of surgery (Table 3). However, cerebral palsy was independently associated with the prescribing diazepam (OR = 21.0, 95%CI: 3.4-129.1). Age was inversely related to the prescription of diazepam and morphine, while older pediatric patients were likely to receive trimeperidine (OR = 2.22, 95%CI: 1.69-2.92), controlling for covariates.
| Variables | Diazepam | Trimeperidine | Morphine | |||
| Yes | No | Yes | No | Yes | No | |
| Age (years), median (IQR) | 1.9 (1.0-3.1) | 7.5 (3.0-13.6)c | 9.2 (4.5-14.0) | 1.0 (0.7-1.6)c | 1.1 (0.7-1.8) | 9.3 (4.7-14.0)c |
| Sex, male | 31 (55.4) | 103 (44.2) | 90 (43.1) | 44 (55.0) | 46 (53.5) | 88 (43.4) |
| Weight (kg), median (IQR) | 11.2 ± 5.2 | 26.7 ± 17.5c | 29.2 ± 16.7 | 9.6 ± 6.1c | 10.1 ± 9.2 | 29.6 ± 16.8c |
| Comorbidities, yes | 13 (23.2) | 24 (10.3)a | 29 (13.9) | 8 (10.0) | 8 (9.3) | 29 (14.3) |
| Presence of cerebral palsy, yes | 6 (10.7) | 7 (3.0%)a | 12 (5.7) | 1 (1.3) | 1 (1.2) | 12 (5.9) |
| Type of surgery | ||||||
| Orthopedic | 14 (25.0) | 120 (51.5)b | 126 (60.3) | 8 (10.0)c | 12 (14.0) | 122 (60.1)c |
| Abdominal | 26 (46.4) | 65 (27.9) | 54 (25.8) | 37 (46.3) | 39 (45.3) | 52 (25.6) |
| Other | 16 (28.6) | 48 (20.6) | 29 (13.9) | 35 (43.7) | 35 (40.7) | 29 (14.3) |
| LOS in ICU (days), median (IQR) | 3 (2-4.5) | 2 (2-3)c | 2 (2-3) | 3 (2-4)c | 3 (2-4) | 2 (2-3)c |
| Hospital LOS, mean ± SD | 16.3 ± 6.9 | 15.9 ± 6.4 | 15.4 ± 6.3 | 17.4 ± 6.7a | 17.4 ± 6.6 | 15.4 ± 6.3a |
| RASS, mean ± SD | 1.3 ± 0.4 | 1.0 ± 0.1c | 1.1 ± 0.4 | 1.1 ± 0.3 | 1.1 ± 0.3 | 1.1 ± 0.4 |
| Variables | Diazepam | Trimeperidine | Morphine | |||
| Unadj. OR (95%CI) | Adj. OR (95%CI) | Unadj. OR (95%CI) | Adj. OR (95%CI) | Unadj. OR (95%CI) | Adj. OR (95%CI) | |
| Parental care | 0.28 (0.14-0.56)c | 0.11 (0.05-0.25)c | 0.43 (0.25-0.72)b | 0.73 (0.33-1.61) | 2.69 (1.61-4.50)c | 1.84 (0.88-3.84) |
| Age | 0.75 (0.68-0.83)c | 0.66 (0.56-0.77)c | 2.57 (1.94-3.40)c | 2.22 (1.69-2.92)c | 0.47 (0.38-0.59)c | 0.52 (0.41-0.64)c |
| Sex, female | 0.64 (0.36-1.15) | 0.86 (0.40-1.83) | 1.61 (0.96-2.70) | 0.93 (0.41-2.13) | 0.67 (0.40-1.10) | 1.12 (0.52-2.41) |
| Cerebral palsy | 3.89 (1.30-11.6)a | 21.0 (3.4-129.1)b | 3.35 (0.60-18.6) | 1.77 (0.02-147.2) | 0.27 (0.05-1.49) | 0.42 (0.01-13.9) |
The results of this study indicate that parental presence in the PICU was significantly associated with a reduction in diazepam prescriptions, while no significant differences were observed in the use of trimeperidine or morphine. This suggests that parental involvement may serve as an effective non-pharmacological strategy to minimize the use of certain sedatives in the postoperative period, potentially contributing to shorter PICU stays and improved patient outcomes. In critically ill pediatric patients, intensive care management involves a variety of invasive procedures, including intravenous catheterization, tracheostomy, mechanical ventilation, and surgical interventions. These procedures often cause significant discomfort and distress, necessitating the use of sedation to manage pain, anxiety, and agitation. The dosage and selection of sedative agents depend on individual patient factors such as age, metabolic rate, physiologic characteristics, and drug sensitivity[12]. While sedation plays a critical role in maintaining patient comfort and safety, prolonged or excessive use is associated with numerous adverse effects, including cognitive impairment, memory deficits, emotional instability, and respiratory depression[13]. In addition, ineffective or excessive sedation has been associated with longer ICU stays, increased risk of hospital-acquired infections, and higher rates of unplanned extubation[14]. Another major concern is drug tolerance, where prolonged exposure to sedatives leads to decreased efficacy and the need for escalating doses to achieve the same therapeutic effect[3]. In addition to pharmacologic risks, prolonged immobilization due to deep sedation further worsens patient outcomes. Reduced mobility contributes to muscle atrophy, delayed recovery, and prolonged dependence on mechanical ventilation. Early mobilization and nutritional intake are critical to accelerating recovery, and excessive sedation can significantly impede these processes. Evidence suggests that reducing unnecessary sedation can lead to shorter duration of mechanical ventilation, shorter hospital stays, and improved survival[15]. The overuse and underuse of sedation remains a widespread challenge in both adult and PICUs. However, sedation management in pediatric patients is particularly complex, even for experienced intensivists, due to the wide variability in age, developmental stage, and different physiological, pharmacokinetic, and pharmacodynamic profiles[2]. Unlike adults, children often have difficulty communicating the intensity or location of pain, making it difficult to distinguish between pain-related distress, procedural discomfort, and agitation. This diagnostic challenge requires a more cautious and individualized approach to sedation therapy[16]. While sedation provides critical benefits in the management of pain and agitation in the pediatric ICU patient, it is not without risk. Inappropriate sedation strategies - whether excessive or inadequate - can lead to significant complications[5,17]. Inadequate sedation may expose the child to physical and psychological distress, with possible long-term consequences such as posttraumatic stress disorder. Conversely, excessive sedation increases the risk of delirium, neuromuscular atrophy, prolonged weakness, and delayed rehabilitation, further complicating the recovery process[12]. Given these risks, achieving an optimal sedation balance is essential to ensure both comfort and safety in critically ill pediatric patients. Pediatric patients are particularly vulnerable to emotional distress in the ICU because they often struggle to understand their condition, the procedures being performed, and the presence of unfamiliar healthcare providers. This uncertainty and lack of control over their environment can lead to heightened anxiety, fear, and emotional instability. In addition, young patients are highly sensitive to the distress of those around them and are more likely to respond to the deterioration of other patients in the ICU[18]. Common psychological reactions in hospitalized children include sleep disturbances (insomnia, nightmares), refusal to eat, and difficulties in socialization and communication with medical staff. Many children experience severe separation anxiety, fear of strangers, distress over excessive medical attention, and even an emerging fear of death. The severity of these psychological reactions is influenced by age, parental separation, length of hospital stay, and the intensity of their illness[19]. Ensuring a supportive environment, incorporating parental presence, and employing individualized sedation strategies can help alleviate the emotional distress of critically ill children while optimizing their overall well-being. The Centers for Disease Control and Prevention has recognized that nonopioid therapies can be effective in the management of acute to moderate pain, including postoperative pain[20]. As a result, it is recommended that nonpharmacologic methods be incorporated into daily pain management protocols alongside pharmacologic treatments[21]. While nonpharmacological approaches do not directly eliminate pain as medications do, they play a critical role in making pain more tolerable, reducing distress, and giving children a sense of control over their experience. These interventions are often referred to as mind-body therapies[22]. Pölkki et al[23] categorized non-pharmacological pain management strategies into four key areas: Cognitive-behavioral methods, physical methods with emotional support strategies, creating a comfortable environment, and preparation. Cognitive-behavioral techniques aim to redirect the child’s attention away from the pain by engaging them in imaginative storytelling, listening to their favorite music, or incorporating interactive distraction techniques. Breathing exercises are often taught in a playful format, making them more engaging and effective for young patients[24]. Preprocedural preparation focuses on reducing fear and anxiety by explaining daily routines and upcoming medical procedures in advance. Children often experience distress due to fear of the unknown, and providing clear, age-appropriate information can help reduce anxiety[25]. Physical methods combined with emotional support can further enhance comfort. These may include gentle massage (when medically appropriate), a reassuring touch on the shoulder or head, and other comforting gestures that provide a sense of security and reassurance[26]. Successful implementation of nonpharmacologic pain management strategies requires consistent attention and active participation by healthcare providers. However, in many healthcare settings, these methods remain underutilized due to several barriers, including limited staff training, time constraints, and the heavy workload of healthcare professionals. In the ICU, patient care involves a multidisciplinary team, including physicians, surgeons, anesthesiologists, radiologists, nutritionists, and clinical psychologists, all of whom play a role in the treatment and recovery process[27]. Of these, nurses have the most frequent and direct interactions with patients and their families, positioning them as key facilitators of communication and emotional support. By engaging with families and providing reassurance, nurses can play a critical role in reducing patient anxiety, improving psychological well-being, and fostering a more supportive care environment. Their ability to bridge communication between medical teams and families makes them an integral part of holistic, patient-centered care in the PICU[28]. In contemporary pediatric care, family-centered care has emerged as a widely accepted model that integrates various non-pharmacological methods to support both patients and their families. This approach has been endorsed by leading professional organizations, including the Institute of Medicine, the American College of Critical Care Medicine, and the American Academy of Pediatrics[29]. The American Academy of Pediatrics defines patient- and family-centered care as “an innovative approach to planning, delivering, and evaluating health care that is based on a mutually beneficial partnership among patients, families, and health care providers that recognizes the essential role of the family in a child’s well-being”[30]. One of the most effective family-centered strategies for reducing stress in the pediatric ICU is parental presence. This presence can take two forms: Active and passive involvement. Active involvement, in which parents are trained by medical staff to assist with aspects of their child’s care, such as positioning, hygiene, and feeding. Passive involvement, in which parents provide emotional and psychological support without directly participating in medical care[19]. Despite its benefits, parental presence is not universally implemented in PICUs. A major concern is the risk of transmission of infection from parents to critically ill patients, necessitating strict infection control measures. These concerns were particularly heightened during the coronavirus disease 2019 pandemic, leading to widespread restrictions on parental visitation and limited family involvement in patient care[30]. While these precautions were necessary, they also highlighted the importance of balancing infection control with the emotional and psychological needs of pediatric patients. Despite the well-documented benefits of parental presence in the PICU, not all PICUs allow open family visitation. One of the main concerns is the significant amount of medical staff time required to manage family interactions, address questions, and mitigate potential conflicts among family members. In addition, prolonged parental presence may increase caregiver stress, potentially impacting healthcare workflow and decision making[31]. Nevertheless, the primary benefit of parental involvement is its ability to reduce patient stress and anxiety. To balance this benefit with infection control measures, hospitals often implement strict visitation policies that regulate the number of visitors, duration of visits, frequency of parental presence, and pre-screening requirements such as medical tests to minimize the risk of community-acquired infections[31]. A 2010 study by Vagnoli et al[32] compared several methods to reduce preoperative anxiety in children, including parental presence, guest clowns, and additional sedation. Their results showed that parental presence had a significant calming effect and improved the child’s cooperation with medical staff. However, an important observation was that the child’s emotional state often mirrored that of the parent. This highlights the need for caregiver preparation, as anxious or distressed parents may inadvertently transfer their emotions to their child, negating the intended calming effect. Parents of critically ill pediatric patients also face significant emotional stress and often experience a disruption in their daily lives due to their child’s hospitalization. To minimize shock and distress, healthcare providers should ensure that parents are adequately prepared before entering the PICU. This includes educating them about their child’s condition, familiarizing them with medical equipment (e.g., tracheostomies, nasogastric tubes, mechanical ventilators, catheters), and setting appropriate expectations. Proper preparation helps parents cope with the intensive care environment and ensures that they can provide meaningful emotional support without becoming overwhelmed[33].
The restrictive policies that limit parental presence in the PICU are often driven by several concerns, including infection control, privacy and confidentiality risks, potential emotional distress for both patients and parents, and logistical challenges such as space and staffing limitations[34]. While these concerns are valid, they can also create barriers to implementing family-centered care, which has been shown to improve patient outcomes. In hospitals of our country, there are several institutional and practical barriers to full implementation of open visitation policies. Some PICU teams remain reluctant to allow unrestricted family presence, fearing that parents may inadvertently interfere with medical equipment or disrupt clinical workflow by raising non-urgent concerns. In addition, logistical issues such as the need for additional protective equipment (e.g., privacy screens) and the potential psychological distress parents may experience in the high-stress ICU environment contribute to the reluctance to implement broader parental access policies[34]. Beyond these logistical challenges, deeper interpersonal and environmental barriers also limit the effectiveness of family-centered care. A study conducted by Malepe et al[35] in a Gauteng hospital identified poor communication between nurses and caregivers, as well as hospital infrastructure limitations, as key challenges to effective family involvement in pediatric care. To overcome these barriers and promote a more holistic approach to pediatric intensive care, it is essential to improve nurse-caregiver communication, provide structured guidelines for parental involvement, and adapt hospital policies to create a more family-inclusive environment. Perception of parents and ICU personnel. With proper training, parents can actively participate in simple caregiving tasks such as assisting with positioning, hygiene and basic monitoring, ultimately reducing the burden on healthcare professionals. This involvement not only saves time for healthcare providers, but also significantly improves parental well-being by providing transparency and reassurance about their child’s condition. In addition, parental presence has been shown to have a positive impact on a child’s emotional state, leading to improved mood, reduced anxiety and better psychological resilience. Although there is a growing emphasis on minimizing the use of sedatives in the PICU, protocol-based sedation remains an essential component of critical care. Structured sedation strategies play a key role in facilitating early mobilization, reducing the risk of delirium, and promoting positive family interactions to ensure both patient comfort and recovery[1]. Overall, the integration of parental care in the PICU has been well received by both families and healthcare providers. In our experience, no adverse effects associated with parental involvement have been observed, reinforcing the benefits of a family-centered approach to pediatric critical care.
The limitations of this study are the single-center study, a relatively small sample size, and a retrospective nonexperimental study design. Additional limitations include heterogeneity in the patient population, age, body mass, surgical procedures, drugs administered in the perioperative period, presence of comorbidities, neurological and developmental abnormalities, and parental care. In addition, although our institutional protocol allows parents to remain with their child in the PICU without time restrictions, the exact duration of parental presence per day was not systematically documented in the medical records. Based on our clinical experience, parents typically stayed with their infants during waking hours but were absent during nighttime rest periods. However, in the absence of precise recorded data on the duration of parental presence, its potential influence on the need for sedation could not be fully assessed. Future prospective studies should include structured monitoring of parental stay time to better quantify its impact on patient outcomes and sedation practices.
This study highlights the potential benefits of parental presence in the PICU, particularly in reducing the need for sedation and its associated adverse effects, such as drug-related complications and prolonged ICU and hospital stays. By integrating family-centered care, PICUs can improve patient outcomes while optimizing sedation practices. Beyond sedation reduction, our findings suggest that parental involvement plays a critical role in facilitating early patient activation, which in turn supports earlier initiation of enteral nutrition and contributes to shorter ICU stays. These benefits underscore the importance of incorporating structured parental care protocols into routine pediatric intensive care. Overall, the implementation of parental care in the ICU may promote a more natural, patient-centered approach that improves both the physical recovery and emotional well-being of critically ill children. Future research should explore standardized frameworks for parental involvement to maximize its effectiveness while addressing potential challenges.
| 1. | Balit CR, LaRosa JM, Ong JSM, Kudchadkar SR. Sedation protocols in the pediatric intensive care unit: fact or fiction? Transl Pediatr. 2021;10:2814-2824. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 2. | Barnes S, Yaster M, Kudchadkar SR. Pediatric Sedation Management. Pediatr Rev. 2016;37:203-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 3. | Royer AS, Busari JO. A systematic review of the impact of intensive care admissions on post discharge cognition in children. Eur J Pediatr. 2021;180:3443-3454. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 4. | Lin PH, Chen CF, Chiu HW, Tai HP, Lee DL, Lai RS. Outcomes of unplanned extubation in ordinary ward are similar to those in intensive care unit: A STROBE-compliant case-control study. Medicine (Baltimore). 2019;98:e14841. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 5. | Zhou Y, Yang J, Wang B, Wang P, Wang Z, Yang Y, Liang G, Jing X, Jin X, Zhang Z, Deng Y, Hu C, Liao X, Yin W, Tang Z, Tian Y, Tao L, Kang Y. Sequential use of midazolam and dexmedetomidine for long-term sedation may reduce weaning time in selected critically ill, mechanically ventilated patients: a randomized controlled study. Crit Care. 2022;26:122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 16] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 6. | Alcaraz Garcia-Tejedor G, Le M, Tackey T, Watkins J, Caldeira-Kulbakas M, Matava C. Experiences of Parental Presence in the Induction of Anesthesia in a Canadian Tertiary Pediatric Hospital: A Cross-Sectional Study. Cureus. 2023;15:e36246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 7. | Saparov AI, Sazonov VG, Tobylbaeva ZS, Karina GB, Kurochkin MN, Beremzhanova DK, Mұstafa AZ. The First Successful Implementation of Family-Centered Health Care in Pediatric Intensive Care Unit in Republic of Kazakhstan (Report). General Reanimatology. 2019;15:21-25. [DOI] [Full Text] |
| 8. | Miró J, Castarlenas E, Huguet A. Evidence for the use of a numerical rating scale to assess the intensity of pediatric pain. Eur J Pain. 2009;13:1089-1095. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 141] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 9. | Felemban OM, Alshamrani RM, Aljeddawi DH, Bagher SM. Effect of virtual reality distraction on pain and anxiety during infiltration anesthesia in pediatric patients: a randomized clinical trial. BMC Oral Health. 2021;21:321. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 51] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 10. | Scher C, Meador L, Van Cleave JH, Reid MC. Moving Beyond Pain as the Fifth Vital Sign and Patient Satisfaction Scores to Improve Pain Care in the 21st Century. Pain Manag Nurs. 2018;19:125-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 101] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 11. | Ely EW, Truman B, Shintani A, Thomason JW, Wheeler AP, Gordon S, Francis J, Speroff T, Gautam S, Margolin R, Sessler CN, Dittus RS, Bernard GR. Monitoring sedation status over time in ICU patients: reliability and validity of the Richmond Agitation-Sedation Scale (RASS). JAMA. 2003;289:2983-2991. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1055] [Cited by in RCA: 1136] [Article Influence: 51.6] [Reference Citation Analysis (0)] |
| 12. | MacDonald I, Perez MH, Amiet V, Trombert A, Ramelet AS. Quality of clinical practice guidelines and recommendations for the management of pain, sedation, delirium and iatrogenic withdrawal in pediatric intensive care: a systematic review protocol. BMJ Paediatr Open. 2022;6:e001293. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 13. | Xiao A, Feng Y, Yu S, Xu C, Chen J, Wang T, Xiao W. General anesthesia in children and long-term neurodevelopmental deficits: A systematic review. Front Mol Neurosci. 2022;15:972025. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 25] [Reference Citation Analysis (0)] |
| 14. | Arcanjo ABB, Beccaria LM. Factors associated with extubation failure in an intensive care unit: a case-control study. Rev Lat Am Enfermagem. 2023;31:e3864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 15. | Hyun DG, Ahn JH, Gil HY, Nam CM, Yun C, Lim CM. Longitudinal trajectories of sedation level and clinical outcomes in patients who are mechanically ventilated based on a group-based trajectory model: a prospective, multicentre, longitudinal and observational study in Korea. BMJ Open. 2023;13:e072628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 16. | Beaulieu FP, Zuckerberg G, Coletti K, Mapelli E, Flibotte J, Sampath S, Hwang M, Drum ET. Sedation and anesthesia for imaging of the infant and neonate-a brief review. Pediatr Radiol. 2024;54:1579-1588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 17. | Schultheis JM, Heath TS, Turner DA. Association Between Deep Sedation from Continuous Intravenous Sedatives and Extubation Failures in Mechanically Ventilated Patients in the Pediatric Intensive Care Unit. J Pediatr Pharmacol Ther. 2017;22:106-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 18. | Woodruff AG, Choong K. Long-Term Outcomes and the Post-Intensive Care Syndrome in Critically Ill Children: A North American Perspective. Children (Basel). 2021;8:254. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 19. | Quadir A, Festa M, Gilchrist M, Thompson K, Pride N, Basu S. Long-term follow-up in pediatric intensive care-a narrative review. Front Pediatr. 2024;12:1430581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 20. | Dowell D, Ragan KR, Jones CM, Baldwin GT, Chou R. CDC Clinical Practice Guideline for Prescribing Opioids for Pain - United States, 2022. MMWR Recomm Rep. 2022;71:1-95. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 569] [Cited by in RCA: 844] [Article Influence: 281.3] [Reference Citation Analysis (0)] |
| 21. | Pölkki T, Pietilä AM, Vehviläinen-Julkunen K, Laukkala H, Kiviluoma K. Imagery-induced relaxation in children's postoperative pain relief: a randomized pilot study. J Pediatr Nurs. 2008;23:217-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 31] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 22. | Moll-Bertó A, López-Rodrigo N, Montoro-Pérez N, Mármol-López MI, Montejano-Lozoya R. A Systematic Review of the Effectiveness of Non-Pharmacological Therapies Used by Nurses in Children Undergoing Surgery. Pain Manag Nurs. 2024;25:195-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 23. | Pölkki T, Vehviläinen-Julkunen K, Pietilä AM. Nonpharmacological methods in relieving children's postoperative pain: a survey on hospital nurses in Finland. J Adv Nurs. 2001;34:483-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 65] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 24. | Murphy G. Distraction techniques for venepuncture: a review. Paediatr Nurs. 2009;21:18-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 25. | Wennström B, Hallberg LR, Bergh I. Use of perioperative dialogues with children undergoing day surgery. J Adv Nurs. 2008;62:96-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 26. | So PS, Jiang Y, Qin Y. Touch therapies for pain relief in adults. Cochrane Database Syst Rev. 2008;CD006535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 42] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 27. | Ervin JN, Kahn JM, Cohen TR, Weingart LR. Teamwork in the intensive care unit. Am Psychol. 2018;73:468-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 141] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 28. | Adams A, Mannix T, Harrington A. Nurses' communication with families in the intensive care unit - a literature review. Nurs Crit Care. 2017;22:70-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 63] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 29. | Committee on Hospital Care and Institute for Patient- and Family-Centered Care. Patient- and family-centered care and the pediatrician's role. Pediatrics. 2012;129:394-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 590] [Cited by in RCA: 663] [Article Influence: 51.0] [Reference Citation Analysis (0)] |
| 30. | Miller L, Richard M, Krmpotic K, Kennedy A, Seabrook J, Slumkoski C, Walls M, Foster J. Parental presence at the bedside of critically ill children in the pediatric intensive care unit: A scoping review. Eur J Pediatr. 2022;181:823-831. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 31. | Bergerat M, De Saint Blanquat L, Milesi C, Grimaud M, Le Bourgeois F, Renolleau S, Pouletty M, Oualha M, Leteurtre S, Recher M, Béranger A. Visiting Policies and Parental Presence During PICU Admission: A Survey in French Units. Pediatr Crit Care Med. 2023;24:e498-e504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 32. | Vagnoli L, Caprilli S, Messeri A. Parental presence, clowns or sedative premedication to treat preoperative anxiety in children: what could be the most promising option? Paediatr Anaesth. 2010;20:937-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 89] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 33. | Debelić I, Mikolčić A, Tihomirović J, Barić I, Lendić Đ, Nikšić Ž, Šencaj B, Lovrić R. Stressful Experiences of Parents in the Paediatric Intensive Care Unit: Searching for the Most Intensive PICU Stressors. Int J Environ Res Public Health. 2022;19:11450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 34. | Meert KL, Schim SM, Briller SH. Parental bereavement needs in the pediatric intensive care unit: review of available measures. J Palliat Med. 2011;14:951-964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 35. | Malepe TC, Havenga Y, Mabusela PD. Barriers to family-centred care of hospitalised children at a hospital in Gauteng. Health SA. 2022;27:1786. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |