Published online Jun 9, 2025. doi: 10.5409/wjcp.v14.i2.102002
Revised: February 6, 2025
Accepted: February 20, 2025
Published online: June 9, 2025
Processing time: 163 Days and 17.3 Hours
Celiac disease (CD) is an autoimmune disease triggered by the ingestion of gluten in genetically predisposed individuals. It is more commonly diagnosed in child
To assess the prevalence and types of oral manifestations in pediatric patients with CD.
We performed a comprehensive literature search in PubMed, Scielo, Cochrane Library and Lilacs databases from 2014-2024. Three independent researchers screened and extracted the information, applying the eligibility criteria and bias was assessed using Joanna Briggs Institute tools.
Of the initial 241 articles, 14 studies fulfilled the proposed objectives and were included in the review. The main oral manifestations found were recurrent aphthous stomatitis and enamel defects. Additionally, delayed tooth eruption, angular cheilitis, glossodynia and xerostomia were also reported.
Assessing oral manifestations is crucial, especially in underdiagnosed cases of children with CD. Recognizing these signs helps pediatricians or general practitioners identify them during routine exams, enabling early diagnosis and treatment to prevent negative impacts on the child’s and family’s quality of life.
Core Tip: Celiac disease is a chronic enteropathy with atypical manifestations and complex etiopathogenesis, challenging clinicians. Understanding its oral manifestations can greatly improve patients' quality of life. These manifestations occur in the non-keratinized oral mucosa, impacting feeding, speech, swallowing, and tooth brushing. Dentists play a crucial role in treating oral consequences, identifying cases early, and referring patients for medical evaluation. Their involvement is essential for timely diagnosis and management. Multidisciplinary collaboration between dentists and physicians ensures comprehensive care, addressing both oral and gastrointestinal aspects. Early detection and intervention can significantly improve outcomes and prevent long-term complications for affected individuals.
- Citation: Macho V, Rodrigues R, Pinto D, Castro R, Silva CC, Silva CC, Soares SC. Importance of celiac disease oral manifestations in pediatric patients: A systematic review. World J Clin Pediatr 2025; 14(2): 102002
- URL: https://www.wjgnet.com/2219-2808/full/v14/i2/102002.htm
- DOI: https://dx.doi.org/10.5409/wjcp.v14.i2.102002
Celiac disease (CD) is an autoimmune condition affecting approximately 1% of the pediatric population. The latest guidelines from the American College of Gastroenterology recommend performing upper gastrointestinal endoscopy with multiple duodenal biopsies to confirm the diagnosis of CD in both pediatric and adult patients with suspected disease[1]. The general recommendation from ESPGHAN 2020 for the diagnosis of CD includes total IgA. To avoid the risk of false-negative results, it is important to ensure that total IgA levels are normal. However, there is no exact reference value for total IgA described as a direct diagnostic criterion[2]. The current guideline for anti-tissue transglu
CD is a condition characterized by partial or complete villous atrophy and crypt hyperplasia, leading to the malab
The relevance of dental anomalies in diagnosing CD in the pediatric population is further emphasized by the most recent ESPGHAN guidelines[2]. These guidelines recommend screening for CD in children presenting with various symptoms, signs, and associated conditions, including recurrent aphthous ulcers (RAU) and dental enamel defects (DED)[2].
Some oral manifestations can suggest the presence of CD, so dentists can play a key role in diagnosing the disease in individuals suffering from its silent forms. Therefore, it has become important to recognize the changes that may be present in the oral cavity, as they are significant not only for the diagnosis of the disease but also for managing oral manifestations and improving oral health-related quality of life.
The primary aim of this systematic review is to inform health professionals about the importance of recognizing the oral manifestations of CD in pediatric patients.
The protocol for this systematic review was registered under number 542697 on the PROSPERO platform (ID = CRD42024542697) and adheres to the PRISMA guidelines[5]. The clinical question formulated for the purpose of this systematic review was based on the PECO strategy, commonly employed in systematic reviews to define the research question. The clinical question posed was: “Do pediatric patients with CD exhibit a higher prevalence of oral manifestations”?
Comprehensive bibliographic search was conducted in the following databases: PubMed, SciELO, Cochrane Library, and Lilacs. The search strategy combined medical subject headings (MeSH) terms: “oral manifestations”, “CD”, “pediatric dentistry”, “enamel defects” and “oral aphthous” using the Boolean operators “AND” and “OR”. The search filters included “free full text,” a time frame limit from 2014 to 2024, and the languages Portuguese, English, and Spanish.
A preliminary evaluation of the titles and abstracts of the articles was conducted to determine which articles met the study objectives, with irrelevant and duplicate articles being excluded. All full-text articles that passed the initial identification phase were reviewed to assess eligibility. Three independent investigators (Viviana Macho, Diana Pinto, Rita Rodrigues) performed the screening and data extraction, applying the eligibility criteria to the considered articles. The full texts of eligible papers that seemed to satisfy the inclusion criteria were then acquired and evaluated independently by the three reviewers. Discrepancies regarding article selection were resolved by consensus. All authors agreed to any differences after discussion.
The inclusion criteria encompassed studies conducted on children under 18 years old with confirmed CD that addressed oral manifestations; cross-sectional studies, case-control studies, and retrospective cohort studies. Exclusion criteria included studies involving adults or children without confirmed CD, studies involving children with CD but with other associated systemic conditions, as well as meta-analyses and systematic reviews.
Study design: Cross-sectional studies, case-control studies, and retrospective cohort studies.
Included participants: Pediatric population under 18 years of age with confirmed CD.
Excluded participants: Adults; children without confirmed CD; studies involving children with CD but with other associated systemic conditions; meta-analyses and systematic reviews.
Type of exposure: Presence of confirmed CD.
Primary outcome: DED, RAU, and xerostomia.
Secondary outcomes: Angular cheilitis, atrophic glossitis, geographic tongue, glossodynia, and delayed eruption.
The data extraction strategy was pre-established based on the study design and type, sample characteristics, assessed outcomes and their measurements, statistical analyses (including adjustments for confounding factors), overall results, and strength of associations. Data were extracted from the articles as described in the respective studies.
A critical appraisal of the methodological quality of all included articles was conducted using three tools developed by the Joanna Briggs Institute: The "Checklist for Analytical Cross-Sectional Studies" for cross-sectional studies, the "Checklist for Case-Control Studies" for case-control studies, and the "Checklist for Cohort Studies" for retrospective cohort studies[6].
All systematic reviews incorporate a process of critique or evaluation of the research evidence. The objective of this appraisal was to assess the methodological quality of each study and determine the extent to which the study addressed the potential for bias in its design, conduct, and analysis.
All articles selected for inclusion in the systematic review underwent rigorous evaluation by three critical reviewers (Viviana Macho, Diana Pinto, Rita Rodrigues). For the evaluation of research evidence, a form was completed using the JBI critical appraisal tools[6] by all three reviewers.
The tools consist of 8, 10, and 11 assessment parameters, respectively, guiding reviewers through each article to evaluate it comprehensively. Each parameter was categorized as follows: "Yes" for studies providing complete information regarding the assessed criterion; "No" for those lacking any reference to the criterion; "Unclear" for cases where the information was mentioned only partially; and "Not Applicable" when the parameter could not be applied to the article due to methodological determinants.
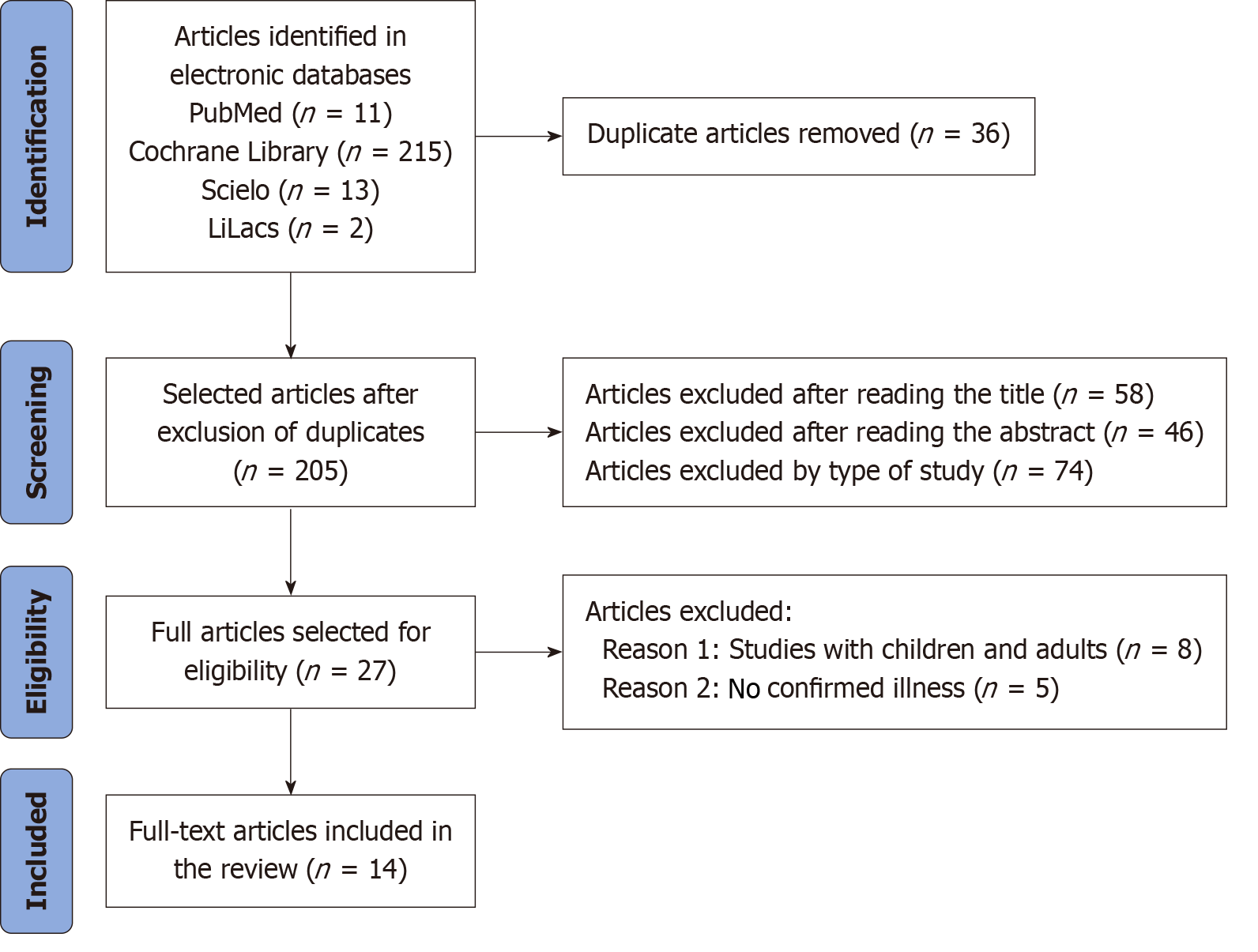
In the preliminary bibliographic search conducted across electronic databases, a total of 241 articles were identified. Following the identification of appropriate articles, a selection process was undertaken to clarify the oral manifestations of CD in pediatric patients. The PRISMA guidelines were adhered to throughout the article selection process. After the removal of 36 duplicate articles, 205 articles remained. Subsequently, 58 articles were excluded based on title review, 46 based on abstract review, and 74 based on study type, leading to the elimination of a total of 104 articles. A final selection of 27 articles was made for comprehensive reading and application of the chosen eligibility criteria. Ultimately, 14 articles were included in the review (Figure 1).
The instrument used to evaluate the risk of bias, rigor, or quality of the studies was based on a total cutoff score of ≥ 4 (4/8) for cross-sectional studies. Studies scoring below 3 were excluded, while those scoring between 4 and 6 were considered of moderate quality, and those scoring ≥ 7 were categorized as high quality[6]. It was determined that all 4 cross-sectional studies included were of moderate quality, and thus, all were incorporated into the systematic review (Table 1).
For cohort studies, a total cutoff score of ≥ 5 (5/11) was considered. Studies scoring below 4 were excluded, and those scoring between 5 and 7 were deemed to be of moderate quality, while scores ≥ 8 were classified as high quality[6]. It was found that the 2 cohort studies were both of moderate quality and were included in the review (Table 2).
In the analysis of case-control studies, a total cutoff score of ≥ 4 (4/10) was applied. Studies with scores below 3 were excluded; those scoring between 4 and 6 were classified as moderate quality, and those with scores ≥ 7 were considered high quality[6]. Of the 8 case-control studies, 5 were of moderate quality and 3 were of high quality. All 8 case-control studies were included in the review (Table 3).
| Ref. | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Q9 | Q10 | N yes |
| Alsadat et al[11], 2021 | Yes | Yes | Yes | Yes | Yes | Yes | Unclear | Yes | Not applicable | Yes | 8 |
| Shahraki et al[7], 2019 | Yes | Yes | Yes | Yes | Yes | Yes | Unclear | Yes | Not applicable | Yes | 8 |
| Alamoudi et al[23], 2020 | Yes | Yes | No | Yes | Yes | Unclear | Unclear | Yes | Not applicable | Yes | 6 |
| Macho et al[22], 2020 | Yes | Yes | Yes | Unclear | Unclear | Yes | Yes | Yes | Yes | Yes | 8 |
| Macho et al[9], 2019 | Yes | Yes | Yes | Unclear | Unclear | Unclear | Unclear | Yes | Yes | Yes | 6 |
| Zoumpoulakis et al[19], 2019 | Yes | Yes | Yes | Unclear | Unclear | Yes | Unclear | Yes | Not applicable | Yes | 6 |
| de Carvalho et al[20], 2015 | Yes | Yes | Yes | Unclear | Unclear | Yes | Unclear | Yes | Not applicable | Yes | 6 |
| Cantekin et al[21], 2015 | Yes | Yes | Yes | Unclear | Unclear | Yes | Unclear | Yes | Not applicable | Yes | 6 |
Ultimately, after identifying the appropriate articles (n = 241), a selection process was conducted to elucidate the oral manifestations of CD in pediatric patients. In total, 14 studies were found that met the eligibility criteria and proposed objectives (Table 4).
| Ref. | Objective | Study sample | Assessment technique | Results |
| Elbek-Cubukcu et al[13], 2023 | Presence of molar-incisor hypoplasia and RAU, the level of caries experience, and oral hygiene status, and measuring salivary flow, salivary buffering capacity, and cariogenic salivary microflora with Marsh types | 62 pediatric patients with CD, 64 controls | The clinical identification of hypoplasia was performed according to the criteria of the European Academy of Pediatric Dentistry. The assessment of caries experience was conducted using the DMFT index. Oral hygiene was assessed through the IHO-S record. CRT® Bacteria and Buffer Test was used to examine the cariogenic microflora of each child | The prevalence molar-incisor hypoplasia was 61%. RAU > CD. A positive relationship was found between disease duration and the severity of hypoplasia. Higher counts of S. mutans. Salivary flow was very low in CD. A positive correlation was found between poor dietary adherence and worse oral hygiene. CD group exhibited a higher DMFT index |
| Ludovichetti et al[12], 2022 | Investigate the correlation between CD and specific oral lesions | 114 pediatric patients. Three groups: Celiac patients (CD group). Patients with malabsorption without CD (non-celiac group). Healthy controls | The DED were classified according to Aine's classification. Possible lesions in oral soft tissues were investigated, along with the concomitant presence of glossodynia | The findings revealed that children with CD had a higher prevalence of DEDs compared to the control group. However, no significant difference was observed between the two groups regarding the occurrence of RUA and other oral lesions. In the CD group and the control group, the teeth most prone to dental defects were incisors and molars simultaneously (about 54% in both groups), or incisors only (about 30% in the CD group and about 36% in the control group). All oral lesions considered were more frequent in the CD group |
| Alsadat et al[11], 2021 | Compare the oral manifestations of children with CD to a healthy control group | 208 participants: 104 patients CD. 104 in the control group | The DED were evaluated according to Aine's classification. The assessment of caries experience was conducted using the DMFT index. The presence of RAU at the time of clinical evaluation was recorded, and the history of ulcers was collected during the anamnesis. The oral consequences of advanced stages of untreated caries were measured using the PUFA index. Malocclusion was assessed using the DAI index | CD presented more RAU than controls (42.3% vs 15.4%) and more DED than controls (70.2% vs 34.6%). No significant difference was found between CD cases and controls in the frequency of malocclusion. In children with CD, the caries index was lower in the primary dentition compared to the control group; in permanent dentition, the caries index was higher for the CD group. Regarding the PUFA index, in primary dentition, CD was lower than in controls, while in permanent dentition, it was higher in the CD group than in controls |
| Villemur Moreau et al[10], 2021 | Compare the frequency of DED, RAU, and dental eruption delay between children with CD and a healthy group | 28 children CD. 59 controls | DED were classified from grades I to IV according to Aine's classification. RAS were assessed through clinical observation supplemented by information from parents | The extent of DED in CD was as follows: 67.86% had Grade I defects. The difference between the groups was significant. RAU in children with CD was 3.86, compared to 0.72 in the control group |
| Alamoudi et al[23], 2020 | Assess the prevalence of dental maturity in patients with CD | 208 participants: 104 children with CD. 104 in the control group | A panoramic radiograph and a comprehensive oral examination were performed for each participant. Dental age was measured according to the Demirjian method, and dental maturity was calculated by subtracting chronological age from dental age | CD had a higher prevalence of delayed dental maturity compared to controls (62.5% vs 3%). The delay in dental maturity was greater than in the controls (7.94 ± 10.94 vs 6.99 ± 8.77). Children in the younger age group (6-7 years) had a significantly advanced dental maturity of 16.21 ± 2.58 months compared to children aged 11-14 years |
| Macho et al[22], 2020 | Evaluate the symmetry of DED in children with CD | 160 participants: 80 children with CD. 80 in the control group | The DED were evaluated according to Aine's classification (grades I to IV) | DED were found in 55% of patients with CD and 27.5% in the control group. Grade I according to Aine was the most prevalent in both groups, but it was higher in the CD group in both dentitions. Grade II was found only in the CD group. The DED in CD were symmetrical, and the most affected teeth were the permanent first molars and permanent incisors |
| Macho et al[9], 2019 | To evaluate the prevalence of soft tissue oral lesions in a pediatric CD with healthy controls. To assess the relationship between the onset of a gluten-free diet and the prevalence of oral manifestations | 80 patients with CD. 80 healthy individuals as controls | Clinical record (questionnaire) and an intraoral observational examination | Although changes in soft tissues such as angular cheilitis, atrophic glossitis, and geographic tongue were more common in the CD group, no differences were found between the two groups. The prevalence of aphthous ulcerations was higher in the CD group, with a highly significant difference between the two groups (P < 0.001) |
| Shahraki et al[7], 2019 | To investigate the frequency and distribution of DED and dental caries in children with CD and compare them to healthy group | 65 individuals with CD. 60 healthy individuals matched by age and sex | The DED were classified according to Aine's criteria, and dental caries was recorded by calculating the DMFT indices. The presence of other oral findings, such as aphthous ulcers and xerostomia, was recorded in the clinical record | Grade I was the most common enamel defect in both groups. Symmetrical and nonspecific DED were observed in 45% and 14% of individuals with CD, and in 12% and 12% of the control group (P = 0.001). The location of DED in permanent and primary teeth was more prominent in the anterior teeth, with a coronal distribution involving the incisal and middle portions of the teeth |
| Zoumpoulakis et al[19], 2019 | To assess the prevalence of oral manifestations and explore whether oral examination can be used as a diagnostic screening tool for atypical or asymptomatic forms | 45 patients with CD were classified into three categories of CD (classical, atypical, asymptomatic) 45 healthy individuals | DEDs were evaluated according to Aine's classification (grades I to IV). The assessment of caries experience was conducted using the DMFT index. The evaluation of (nonspecific atrophic glossitis, RAS, geographic tongue, and median rhomboid glossitis) included those observed clinically | The difference is statistically significant for the prevalence of DED between the groups, with systemic DED present in both groups; Grade I at (51.5%), Grade II at (39.2%), and Grade IV at (7.1%). There was a statistically significant correlation between the severity of systemic DED and the form of CD, as well as for the prevalence of RAS (40%) in the CD group. For other tissue lesions, the difference was not statistically significant |
| Bıçak et al[15], 2018 | To examine the changes in the soft and hard tissues of the oral cavity in CD | 30 CD. 30 control group | DED was evaluated according to Aine, RASU was detected through clinical assessment, and dental caries was diagnosed using WHO criteria (DMFT). After chewing paraffin wax gum, the children were asked to spit for 5 minutes to detect stimulated salivary flow rates | 20 patients with CD had DED, but none in the control group. In the CD group, all DED cases were diagnosed in permanent teeth and were specific to all the children. Grade I DED was primarily found in the incisors. The frequency of ulcers was higher in the control group, but the difference was not statistically significant. The DMFT values were relatively lower in the CD group. The stimulated salivary flow rate was significantly lower in the CD group |
| Saraceno et al[8], 2016 | To evaluate the different prevalences of oral conditions in patients with CD compared to a control group | 166 patients, between 2 and 17 years of age and of both genders patients affected by CD were compared with those of a control group | Clinical record | The most common lesions in both groups were RAU (69% in patients with CD vs 43% in the control group), followed by dental diseases (76% in patients with CD vs 65% in the control group) |
| de Carvalho et al[20], 2015 | To assess the oral manifestations of CD, the chemical composition of dental enamel, and the occurrence of CD in children with DED | 52 children with CD and 52 controls were examined for DED, RAU, DMFT, and salivary parameters. Additionally, 10 exfoliated primary molars from each group were analyzed. Another sample of 50 asymptomatic children aged 2 to 12 years with DED was included to investigate the occurrence of CD | DEDs were recorded according to Aine's category I-IV classification. The assessment of caries experience was conducted using the DMFT index. The presence of UAR at the time of clinical evaluation was recorded. The chemical composition of dental enamel was evaluated based on energy-dispersive X-ray spectroscopy and Fourier-transform infrared spectroscopy | RAU was present in 40.38% of the CD group and 17.31% of the control group, with a statistically significant difference. Regarding the analysis of salivary parameters, low salivary flow was observed in 36% of the CD group and 10% of the control group, with a significant difference between the groups. The CD group had DED in 61.54% of cases, grade I in 44.24%, and grade II in 15.38%, while only 21.15% of the control group had DED, showing a statistically significant association between the specificity of DED and CD |
| Cantekin et al[21], 2015 | Determining the presence and distribution of DED, RAU, and dental caries in children with CD and comparing the results with a healthy control group | 25 patients with CD aged 4 to 16 years. 25 healthy individuals in the control group | Caries experience was assessed using the DMFT index. RAU evaluation included clinically observed lesions. DED assessment was based on presence or absence without defined criteria | The mean DMFT values for the CD group and the control group showed a significant difference. DED were observed in 48% of the CD group and 16% of the control group, with a statistically significant difference between the groups. RAU was detected in 44% of the CD group, while no RAU was found in the control group |
| Bramanti et al[17], 2014 | To investigate hard and soft tissue oral lesions in potential celiac children in comparison to healthy controls | Group A: 50 confirmed celiac patients. Group B: 21 potential celiac patients. Group C: 54 healthy controls | DEDs were recorded according to Aine’s Category I-IV classification. RAU assessment included clinically observed lesions by the examiner and recurrent ulcerative lesions reported by parents, patients, or documented in clinical records. To assess dental maturity, conventional eruption tables for the Caucasian population were used | DEDs were present in 67% of patients in Groups A and B. Grade I: 62.5% in Group A and 50% in Group B. Grade II: 21.17% in Group A and 50% in Group B. RAU was found in 52% of Group A, 66.7% of Group B, and 7.4% of Group C. Burning tongue was recorded in 14% of Group A, 9.5% of Group B, and 5.5% of Group C. Atrophic glossitis was observed in 14% of Group A, 23.8% of Group B, and 1.85% of Group C. Angular cheilitis was recorded in 6% of Group A, 9.5% of Group B, and 3.7% of Group C. Delayed clinical dental eruption was observed in 38% of Group A, with an average delay of 1.4 years, and in 42.8% of Group B, with an average delay of 1.7 years |
Among the 14 studies included in this systematic review, 4 (28.6%) were cross-sectional studies, 2 (14.3%) were cohort studies, and the remaining 8 (57.1%) were case-control studies. Notably, the majority of the studies (n = 9) were conducted within the last 5 years. The results can be thoroughly assessed and analyzed in detail in Table 4.
Oral manifestations of CD can arise even before a formal diagnosis is made. Given the potential complications associated with delayed diagnosis, it is essential to establish guidelines that enable healthcare providers to suspect the disease early. It is vital for dentists to be knowledgeable about the various oral manifestations of CD to facilitate timely referrals to specialists. The results of this systematic review are significant for enhancing dentists' understanding of oral manifestations related to CD, particularly for pediatric dentists.
The most frequently observed oral manifestations in the pediatric population were RAU and DED.
The review of various articles confirmed a higher prevalence of RAU among celiac patients compared to those without the disease, with prevalence rates ranging from 17%[7] to 69%[8]. Macho et al[9] reported that a significantly higher proportion of celiac patients (56.3%) exhibited oral ulcers compared to healthy individuals (20%). These findings are consistent with those of other researchers. Villemur Moreau et al[10] corroborated these results, revealing that 50% of celiac patients presented with recurrent aphthous stomatitis compared to 21.82% of the control group. More recent studies have shown slightly lower prevalence rates; however, they remain consistent with previous findings. Alsadat et al[11] confirmed the occurrence of recurrent aphthous stomatitis in 42.3% of celiac patients vs 15.4% in controls, while Ludovichetti et al[12] reported that 23.7% of pediatric celiac patients experienced ulcers compared to 7.9% in the control group. Elbek-Cubukcu et al[13] found that only 30% of celiac patients presented with this manifestation, with no occurrences in the control group.
The findings of this systematic review align with those of Nieri et al[14], who reported a significantly higher prevalence of recurrent aphthous stomatitis in children with CD (46%) compared to the control group (8%).
Biçak et al[15] found that the frequency of RAU was higher in the control group compared to children with CD; however, the difference was not statistically significant. The former study reported no celiac patients with ulcers compared to healthy individuals, suggesting that this discrepancy may be due to a clinical history and physical exami
RAU is typically characterized by the presence of painful lesions that appear oval or rounded in shape. These ulcers can occur in individuals across all pediatric age groups. These lesions are defined by clear, well-marked borders and are often surrounded by an inflamed reddish area at the base. it most commonly affects the labial mucosa (the inner surface of the lips) and the lateral surfaces of the tongue[16,17]. This oral pathology predominantly manifests in the non-keratinized areas of the oral mucosa and has a significant impact on various functions, including feeding, speech production, and the swallowing process, in addition to interfering with proper oral hygiene practices such as tooth brushing. Furthermore, this condition may contribute to emotional instability in affected individuals, as it is often associated with considerable pain and discomfort, which can adversely affect their quality of life and overall well-being[16,17].
Although its etiology is not fully understood, various treatments have been proposed to manage symptoms and reduce the frequency of lesions. A systematic review conducted by Parra-Moreno evaluated the latest therapeutic approaches for RAU[18]. The authors compared different types of treatments in terms of lesion improvement, size reduction, and healing time. The authors categorized the treatment approaches into five progressive phases. The initial phase involves general measures, including optimizing oral hygiene and incorporating dietary supplements such as omega-3 fatty acids and various probiotics. The second phase consists of topical treatments, including barrier agents, topical anesthetics, amlexanox, and topical corticosteroids. The next step includes first-line systemic therapies, such as systemic corticosteroids, followed by second-line systemic treatments, including thalidomide, dapsone, and montelukast. Finally, certain biological therapies have been explored, with examples including pentoxifylline, etanercept, and adalimumab. The authors concluded that, in addition to conventional treatments for RAU, it is important to consider alternative therapeutic modalities, particularly various forms of laser therapy. This review highlights the significance of diverse therapeutic approaches in the management of recurrent aphthous stomatitis, emphasizing the effectiveness of modern techniques, such as laser therapy, in pain reduction and accelerated lesion healing[18].
Regarding DED, 12 articles reported their presence, with higher rates observed in celiac patients than in those without the disease[7,8,10-13,15,17,19-22]. The findings of this systematic review are consistent with the meta-analysis by Nieri et al[14], which included 1490 children with CD and 2,318 healthy children, reporting that 46% of children with CD had at least one tooth affected by enamel defects, compared to 14% in the control group[14]. However, these values are lower than those found in this systematic review, where eight studies reported rates between 55% and 70% among pediatric celiac patients[10-12,15,17,19,20,22]. This suggests that the prevalence of enamel defects in children has increased compared to seven years ago, likely due to a higher number of diagnosed cases.
Only one article, reported a higher prevalence of enamel defects in the control group, although this difference was not statistically significant (61% in CD patients vs 65.6% in the control group)[13]. Additionally, De Carvalho et al[20] analyzed the chemical composition of the enamel in primary teeth of patients with CD and found that the calcium/phosphorus ratio was significantly lower in these individuals.
All analyzed articles agreed that most enamel defects observed were classified as symmetric Grade I defect according to Aine’s classification, and nearly all were recognized as typical manifestations of CD. Notably, Bramanti et al[17] compared the prevalence of specific and non-specific enamel defects. They reported that non-specific enamel defects were found in 12% of celiac patients compared to 4.7% of potential celiac patients, with no statistically significant differences between the two groups. Furthermore, 5.55% of healthy individuals exhibited non-specific enamel defects, which were neither symmetric nor chronological across the dental arches. This study suggested that enamel defects could be linked to genetic alterations associated with the ALH complex (alleles DR3 and DQ2), which may lead to a specific immune response against gluten[17].
It is essential to recognize that enamel structure defects can arise during the prenatal, neonatal, and postnatal stages. The severity of these defects will be influenced by the duration and intensity of the etiological factor present during the formation of the dental crown[22]. Macho et al[22] reported a notably increased prevalence of symmetric enamel defects, primarily affecting the first permanent molars and permanent incisors. These defects were mainly observed in the permanent dentition and appeared symmetrically on both sides of the dental arch in the maxilla and mandible. CD may make patients more susceptible to enamel defects in the incisors and first molars, which is linked to the timing of odontogenesis during the active phases of the disease.
Symmetric DED are more prevalent in individuals with CD compared to the general population. As a result, indi
Angular cheilitis was studied in only three articles[9,12,13], which reported an increase in angular cheilitis among celiac patients. This condition was characterized by diffuse redness with erosive, fissured, ulcerated, or crusted surfaces. The manifestation was observed in 6% to 10% of patients with CD compared to 3.7% in the control group, with some reporting no cases (0%).
In the study by Zoumpoulakis et al[19], geographic tongue was identified in three individuals with CD, but the difference from the control group was not statistically significant. In contrast, the other three studies that reported on this condition[9,12,17] found more pronounced results. Bramanti et al[17] noted that geographic tongue appeared desquamated with atrophic surfaces, often affecting only a portion of the tongue, primarily the tip, delineated by a slightly elevated peripheral border that could be white, yellow, or grey. This manifestation was documented in 5 out of 50 (10%) celiac patients, 4 out of 21 (19%) potential celiac patients, and 2 out of 54 (3.7%) healthy individuals[17,19].
The incidence rates were even higher in the study by Ludovichetti et al[12], where geographic tongue was present in 18.4% of celiac patients vs 7.9% of the control group. Geographic tongue (benign migratory glossitis) is a common condition and may merely coincide with the presence of CD.
The surface of the tongue exhibited signs of atrophic glossitis, appearing smooth and erythematous, with patients reporting difficulties in chewing, swallowing, or speaking. Bramanti et al[17] observed this condition in 14% of diagnosed celiac patients and 23.8% of potential celiac patients, compared to 1.85% of controls. Other studies[9,12,17] corroborated these findings, indicating statistically significant differences.
The burning mouth syndrome was reported in only two articles[7,17]. In Shahraki et al[7], 12.3% of celiac patients indicated this symptom compared to 3% in the control group. These results align with those of Bramanti et al[17], who found that the burning sensation was reported in 14% of patients, 9.5% in potential celiac patients, and 5.55% in healthy individuals[7,17].
Concerning salivary flow differences, xerostomia was noted in celiac patients in three articles[7,15,20]. Two of these studies assessed samples using both unstimulated and stimulated saliva[8,16], while De Carvalho et al[20] utilized a SalivaCheck Buffer kit. In this study, 36% of patients with CD exhibited decreased salivary flow compared to 10% of the control group, revealing a significant difference (P = 0.006)[20].
In conjunction with the even mild retardation in linear growth and pubertal maturation, as well as the reduction of Body Mass Index SDS observed in patients with undiagnosed CD, alterations in the dental eruption process may also be present[16]. Delayed dental eruption was identified in four of the selected articles. Biçak et al[15] reported that 33.3% of celiac patients experienced delays in eruption, whereas no delays were found in healthy children[15]. Similarly, Bramanti et al[17] noted that 48% of celiac patients had delayed dental eruption. Alamoudi et al[23] found that children with CD had a dental development delay of 0.66 ± 0.91 years (7.94 ± 10.94 months) compared to healthy controls, who showed advanced dental maturity of 0.58 ± 0.73 years (6.99 ± 8.77 months)[23]. Conversely, Villemur Moreau et al[10] did not report any delays in dental eruption among children with CD, while such delays were noted in 3.57% of healthy children.
The various oral manifestations identified in this systematic review and their prevalence support the findings described by Coelho, Bernardes, and Mendes. In their cross-sectional study with a sample of 146 Portuguese children with CD, the most frequently reported manifestations were recurrent aphthous stomatitis (46.6%) and caries (45.6%). Other notable conditions included dental opacities (39%), delayed dental eruption (30.8%), angular cheilitis (21.9%), xerostomia (20.5%), dental hypoplasia (17.8%), alterations in the lingual mucosa (15.8%), microdontia (7.5%), and oral lichen planus (4.1%)[24].
These alterations may result from autoimmune reactions or nutritional deficiencies associated with intestinal malabsorption. Vitamin B12 deficiency is common in patients with CD due to malabsorption caused by intestinal inflammation. Additionally, vitamin D deficiency is frequently observed, which may contribute to oral manifestations such as gingival inflammation and dental sensitivity[2]. Therefore, healthcare professionals should consider CD in patients with unexplained iron deficiency anemia or oral manifestations, ensuring appropriate screening for early diagnosis and management.
CD is associated with other autoimmune disorders, such as Hashimoto’s thyroiditis and type 1 diabetes. Recent studies, including the PRECAL study, suggest that immunomodulation with calcitriol analogs can lead to the negativation of CD-associated antibodies, implying a potential role for vitamin D in primary prevention[25]. Additionally, the disease's association with vitamin deficiencies and other autoimmune disorders underscores the importance of early diagnosis and comprehensive management strategies. While there is some evidence to suggest an association between the duration and severity of CD with oral manifestations and vitamin deficiencies, more research is needed to establish clear guidelines and better understand the underlying mechanisms. The role of vitamin B12 and D deficiencies in the oral cavity in the context of CD is an important area for further investigation[25].
Oral hygiene practices are generally well implemented in children with CD, with most brushing their teeth twice daily, consuming few cariogenic foods and beverages, and regularly visiting the dentist[24].
The management of oral lesions in celiac patients aims to reduce pain, accelerate healing, and prevent secondary infections, relying heavily on the etiology, severity of lesions, and associated symptoms. Treatment options may include topical and/or systemic medications[26].
The role of the dentist in CD is fundamental, as these professional plays a crucial role in the identification and management of oral manifestations associated with the condition. Dentists are often in a position to identify early signs of CD, such as lesions in the oral mucosa, which may precede a medical diagnosis. They should be aware of oral manifestations, as these can be indicative of underlying gastrointestinal conditions.
After suspecting a gastrointestinal condition, it is crucial for dentists to refer the patient to a family physician or gastroenterologist for confirmation of diagnosis through various complementary examinations[26]. This collaboration between dentists and physicians is vital for effective and comprehensive treatment.
Dental treatment may include preventive measures, such as regular consultations to monitor and address dental issues, as well as topical treatments like corticosteroids to reduce mucosal inflammation and associated pain. Antifungal treatment may be necessary in cases of candidiasis, such as angular cheilitis[26]. Dentists also have the responsibility to educate patients about the importance of adhering to a gluten-free diet, which can help mitigate oral manifestations and improve the patient's overall health. This knowledge can empower patients to better manage their condition[27].
Pediatric patients with CD have a higher prevalence of oral manifestations. Oral clinical manifestations include aphthous ulcerations, DED, tongue alterations, burning mouth sensation, saliva alterations, angular cheilitis and delayed tooth eruption. Both the soft and hard tissues of the oral cavity can be affected in coeliac disease, but there is no pathognomonic oral manifestation of coeliac disease.
Since many pediatric patients with CD may be asymptomatic or present with non-gastrointestinal symptoms, it is crucial for pediatricians and primary care physicians to be aware of the oral manifestations of the disease. During routine physical examinations, inspecting the oral cavity can reveal signs indicative of CD. If there is clinical suspicion, serological testing, including total IgA and tTG-IgA antibodies, should be recommended. With early detection by these healthcare providers and the appropriate treatment, such as the introduction of a gluten-free diet, the negative impacts of CD on the child's health and quality of life can be minimized, preventing long-term complications. Therefore, collaboration between pediatricians and dental professionals is essential for improving the outcomes for children with CD.
| 1. | Rubio-Tapia A, Hill ID, Semrad C, Kelly CP, Greer KB, Limketkai BN, Lebwohl B. American College of Gastroenterology Guidelines Update: Diagnosis and Management of Celiac Disease. Am J Gastroenterol. 2023;118:59-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 181] [Article Influence: 90.5] [Reference Citation Analysis (0)] |
| 2. | Husby S, Koletzko S, Korponay-Szabó I, Kurppa K, Mearin ML, Ribes-Koninckx C, Shamir R, Troncone R, Auricchio R, Castillejo G, Christensen R, Dolinsek J, Gillett P, Hróbjartsson A, Koltai T, Maki M, Nielsen SM, Popp A, Størdal K, Werkstetter K, Wessels M. European Society Paediatric Gastroenterology, Hepatology and Nutrition Guidelines for Diagnosing Coeliac Disease 2020. J Pediatr Gastroenterol Nutr. 2020;70:141-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 363] [Cited by in RCA: 688] [Article Influence: 137.6] [Reference Citation Analysis (0)] |
| 3. | Leonard MM, Sapone A, Catassi C, Fasano A. Celiac Disease and Nonceliac Gluten Sensitivity: A Review. JAMA. 2017;318:647-656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 227] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 4. | Caio G, Volta U, Sapone A, Leffler DA, De Giorgio R, Catassi C, Fasano A. Celiac disease: a comprehensive current review. BMC Med. 2019;17:142. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 564] [Cited by in RCA: 560] [Article Influence: 93.3] [Reference Citation Analysis (0)] |
| 5. | Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA; PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015;350:g7647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7463] [Cited by in RCA: 8221] [Article Influence: 822.1] [Reference Citation Analysis (0)] |
| 6. | Munn Z, Barker TH, Moola S, Tufanaru C, Stern C, McArthur A, Stephenson M, Aromataris E. Methodological quality of case series studies: an introduction to the JBI critical appraisal tool. JBI Evid Synth. 2020;18:2127-2133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 565] [Article Influence: 141.3] [Reference Citation Analysis (1)] |
| 7. | Shahraki T, Omrani Mehr S, Hill ID, Shahraki M. A Comparison of the Prevalence of Dental Enamel Defects and Other Oral Findings in Children with and Without Celiac Disease. Iran J Pediatr. 2018;29:e64353. [DOI] [Full Text] |
| 8. | Saraceno R, Perugia C, Ventura A, LORè B, Chimenti S, Docimo R. Aphthous, celiac disease and other dental disorders in childhood. G Ital Dermatol Venereol. 2016;151:239-243. [PubMed] |
| 9. | Macho V, Manso M, Silva D, Andrade D. Does the introduction of gluten-free diet influence the prevalence of oral soft tissue lesions in celiac disease? J Int Oral Health. 2019;11:347. [RCA] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 10. | Villemur Moreau L, Dicky O, Mas E, Noirrit E, Marty M, Vaysse F, Olives JP. Oral manifestations of celiac disease in French children. Arch Pediatr. 2021;28:105-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 11. | Alsadat FA, Alamoudi NM, El-Housseiny AA, Felemban OM, Dardeer FM, Saadah OI. Oral and dental manifestations of celiac disease in children: a case-control study. BMC Oral Health. 2021;21:669. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 12. | Ludovichetti FS, Signoriello AG, Girotto L, Del Dot L, Piovan S, Mazzoleni S. Oro-dental Lesions in Paediatric Patients with Coeliac Disease: an Observatioanl Retrospective Clinical Study. Rev Esp Enferm Dig. 2022;114:654-659. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 13. | Elbek-Cubukcu C, Arsoy HA, Ozkaya G. Assessment of oral manifestations in pediatric patients with celiac disease in relation to marsh types. Med Oral Patol Oral Cir Bucal. 2023;28:e9-e15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 14. | Nieri M, Tofani E, Defraia E, Giuntini V, Franchi L. Enamel defects and aphthous stomatitis in celiac and healthy subjects: Systematic review and meta-analysis of controlled studies. J Dent. 2017;65:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 15. | Bıçak DA, Urgancı N, Akyüz S, Usta M, Kızılkan NU, Alev B, Yarat A. Clinical evaluation of dental enamel defects and oral findings in coeliac children. Eur Oral Res. 2018;52:150-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 16. | Macho VMP, Coelho AS, Veloso E Silva DM, de Andrade DJC. Oral Manifestations in Pediatric Patients with Coeliac Disease - A Review Article. Open Dent J. 2017;11:539-545. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 17. | Bramanti E, Cicciù M, Matacena G, Costa S, Magazzù G. Clinical Evaluation of Specific Oral Manifestations in Pediatric Patients with Ascertained versus Potential Coeliac Disease: A Cross-Sectional Study. Gastroenterol Res Pract. 2014;2014:934159. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 18. | Parra-Moreno FJ, Egido-Moreno S, Schemel-Suárez M, González-Navarro B, Estrugo-Devesa A, López-López J. Treatment of recurrent aphtous stomatitis: A systematic review. Med Oral Patol Oral Cir Bucal. 2023;28:e87-e98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 19. | Zoumpoulakis M, Fotoulaki M, Topitsoglou V, Lazidou P, Zouloumis L, Kotsanos N. Prevalence of Dental Enamel Defects, Aphthous-Like Ulcers and Other Oral Manifestations in Celiac Children and Adolescents: A Comparative Study. J Clin Pediatr Dent. 2019;43:274-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 20. | de Carvalho FK, de Queiroz AM, Bezerra da Silva RA, Sawamura R, Bachmann L, Bezerra da Silva LA, Nelson-Filho P. Oral aspects in celiac disease children: clinical and dental enamel chemical evaluation. Oral Surg Oral Med Oral Pathol Oral Radiol. 2015;119:636-643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 21. | Cantekin K, Arslan D, Delikan E. Presence and distribution of dental enamel defects, recurrent aphthous lesions and dental caries in children with celiac disease. Pak J Med Sci. 2015;31:606-609. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 22. | Macho VMP, de Barros Menéres Manso MCA, E Silva DMV, de Andrade DJC. The difference in symmetry of the enamel defects in celiac disease versus non-celiac pediatric population. J Dent Sci. 2020;15:345-350. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 23. | Alamoudi NM, Alsadat FA, El-Housseiny AA, Felemban OM, Al Tuwirqi AA, Mosli RH, Saadah OI. Dental maturity in children with celiac disease: a case-control study. BMC Oral Health. 2020;20:311. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 24. | Coelho M, Bernardo M, Mendes S. Oral Health-Related Quality of Life in Celiac Portuguese Children: a cross-sectional study. Eur Arch Paediatr Dent. 2023;24:759-767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 25. | Papadimitriou DT, Dermitzaki E, Christopoulos P, Papagianni M, Kleanthous K, Marakaki C, Papadimitriou A, Mastorakos G. Secondary Prevention of Diabetes Type 1 with Oral Calcitriol and Analogs, the PRECAL Study. Children (Basel). 2023;10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 26. | Thomas C, Dimmock M, Gilletta de Saint-Joseph C, Barres B, Paul C, Cousty S, Laurencin-Dalicieux S. How Oral Specialists Can Help Diagnose and Manage Extra-Digestive Inflammatory Bowel Disease Complications. Case Rep Gastroenterol. 2021;15:276-281. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |