Published online Jun 9, 2025. doi: 10.5409/wjcp.v14.i2.101982
Revised: February 19, 2025
Accepted: February 25, 2025
Published online: June 9, 2025
Processing time: 165 Days and 16.1 Hours
Anophthalmia is defined as a complete absence of one eye or both the eyes, while microphthalmia represents the presence of a small eye within the orbit. The estimated birth prevalence for anophthalmia is approximately 3 per 100000 live births, and for microphthalmia, it is around 14 per 100000 live births. However, combined evidence suggests that the prevalence of these malformations could be as high as 30 per 100000 individuals. Microphthalmia is reported to occur in 3.2% to 11.2% of blind children. Anophthalmia and microphthalmia (A/M) are part of a phenotypic spectrum alongside ocular coloboma, hypothesized to share a com
Core Tip: Anophthalmia (absence of one or both eyes) and microphthalmia (small eye within the orbit) are rare congenital conditions, affecting up to 30 per 100000 individuals. These malformations can occur independently or as a part of a syndrome, with complex genetic basis involving over 90 genes. Environmental factors like maternal infections, vitamin A deficiency, and toxin exposure also contribute to their development. Both conditions exhibit significant variability, making diagnosis and management challenging. This review discusses genetic and environmental influences on anophthalmia and microphthalmia, highlighting promising research areas such as multiomic approaches and stem-cell based models for potential therapeutic advancements.
- Citation: Goyal S, Tibrewal S, Ratna R, Vanita V. Genetic and environmental factors contributing to anophthalmia and microphthalmia: Current understanding and future directions. World J Clin Pediatr 2025; 14(2): 101982
- URL: https://www.wjgnet.com/2219-2808/full/v14/i2/101982.htm
- DOI: https://dx.doi.org/10.5409/wjcp.v14.i2.101982
Anophthalmia and microphthalmia (A/M) are rare congenital eye disorders that present significant clinical challenges due to their impact on vision and overall quality of life[1]. Anophthalmia is defined as the complete absence of the eye globe within the orbit, with no observable ocular structures’ during a macroscopic clinical examination. Microphthalmia refers to an eye that is significantly smaller and underdeveloped, characterized by an axial length of less than 19 mm in children under one year of age or less than 21 mm in adults, as measured by B-scan ultrasound. These measurements are at least two standard deviations below the mean for the individual's age[2]. These conditions can occur in isolation or as part of a broader spectrum of ocular anomalies such as anterior segment dysgenesis, ocular coloboma, cataract, vitreoretinal dysplasia and can be unilateral or bilateral[3]. The clinical presentation of A/M can range from mild cases, where the eye is small but functional, to severe cases, with no visible ocular tissue, often accompanied by other craniofacial or systemic abnormalities[4].
The global incidence of A/M is estimated to be approximately 30 per 100000 individuals[1], though the prevalence can vary based on geographic, ethnic, and environmental factors[3]. In 32%-93% of A/M cases, other organ systems are affected by additional anomalies[3,5-8]. Furthermore, 20% of children with A/M and coloboma show delayed psychomotor development[9]. Despite its rarity, A/M accounts for a significant proportion of congenital blindness and visual impairment in children, particularly in underprivileged regions where early diagnosis and interventions are often challenging[1]. The burden of A/M extends beyond the visual impairment itself, often necessitating multidisciplinary management involving ophthalmologists, geneticists, pediatricians, and other specialists to address associated anomalies and provide comprehensive care[9].
The etiology of A/M is complex and multifactorial, involving a combination of genetic and environmental factors[10]. Genetic causes of A/M include mutations in key developmental genes, chromosomal abnormalities, and syndromic associations[10]. Environmental factors, such as maternal infections, exposure to teratogens, and nutritional deficiencies, have also been implicated in the development of these conditions[1]. The interplay between genetic predisposition and environmental exposures further complicates the understanding of A/M, as similar phenotypes can result from diverse etiological pathways[11].
Advances in molecular genetics and genomics have significantly enhanced our understanding of the genetic underpinnings of A/M[12]. Next-generation sequencing technologies have facilitated the identification of novel genetic mutations, providing insights into the complex gene networks involved in ocular development[4]. These findings have a substantial influence on the diagnosis, counseling, and management of patients and their families with A/M[6]. Concurrently, research into environmental risk factors underlines the role of maternal health and harmful exposures during pregnancy (especially in first trimester) in the development of these diseases[11].
Despite these advances, many questions remain unanswered regarding the defined mechanisms by which genetic and environmental factors contribute to the development of A/M[12]. The heterogeneity of these conditions, both in terms of clinical presentation and underlying causes, poses significant challenges for research and clinical practice[12]. Moreover, the interactions between genetic predispositions and environmental influences are complex and not yet fully understood, making it difficult to predict outcomes or develop targeted prevention strategies[10].
This review aims to provide a comprehensive overview of the current understanding of the genetic and environmental factors contributing to A/M. The present review explores in detail the genetic mutations and chromosomal abnormalities associated with A/M, as well as the environmental factors that increase the risk of these disorders. It also pinpoints the interplay of genetic and environmental variables, emphasizing epigenetic alterations and gene-environment interactions[11]. Finally, recent advances in research, including developments in genetic screening, animal models, and emerging therapeutic approaches, will be discussed along with future directions for research and clinical practice in the management of A/M[6].
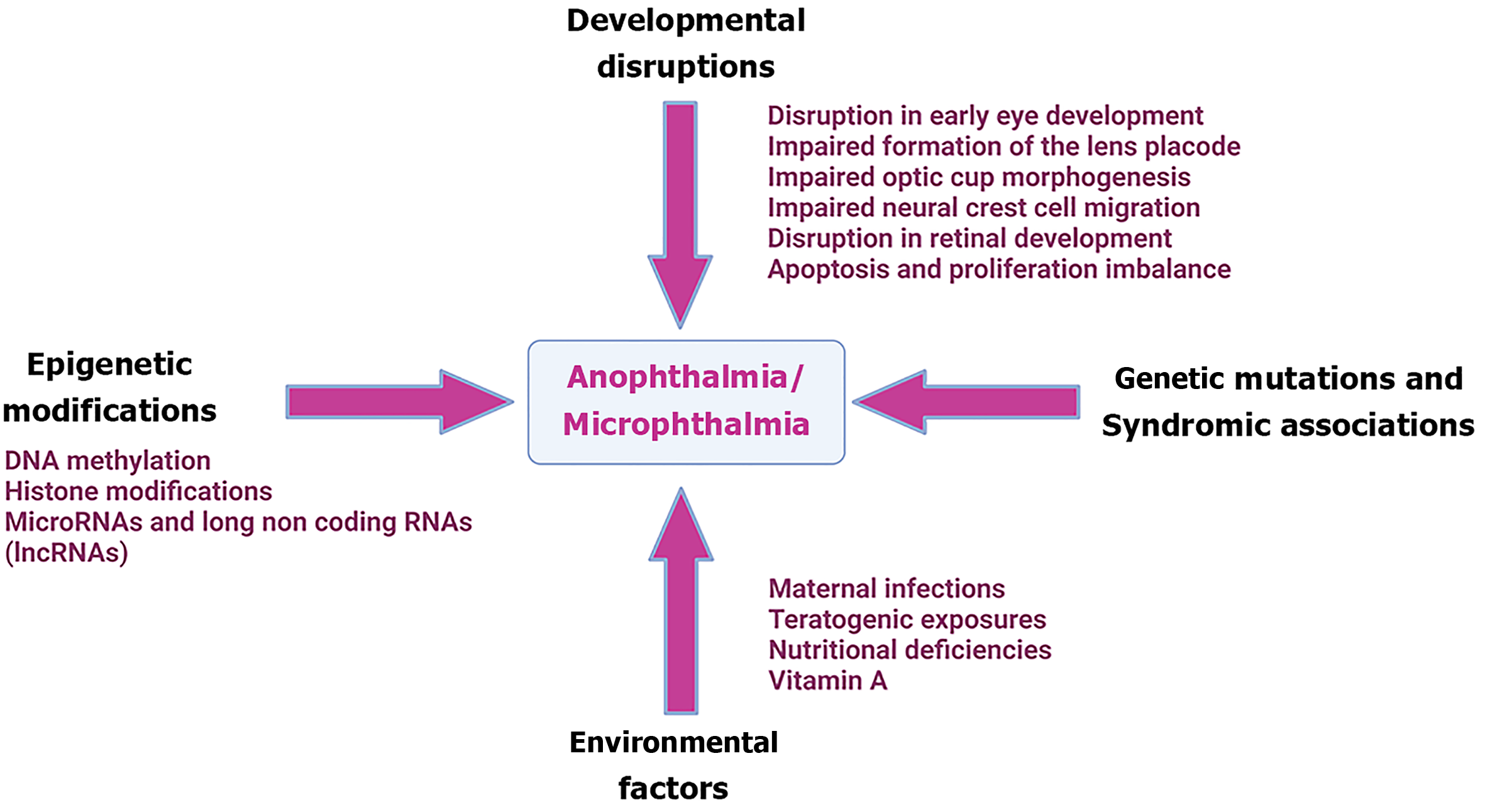
The pathophysiological mechanisms underlying A/M are complex and involve disruptions in multiple genetic and developmental pathways critical for eye formation (Figure 1).
Human eye development begins around the third week of gestation in the anterior neural plate. Orthodenticle homeobox 2 (OTX2) is crucial for specifying the forebrain and enabling eye development by regulating key genes such as sine oculis homeobox homolog 3 (SIX3), retina and anterior neural fold homeobox gene (RAX), and paired box gene 6 (PAX6)[13-17]. OTX2 and SRY-Box 2 (SOX2) work together to regulate RAX, while SIX3 inhibits WNT signaling, activating critical eye field transcription factors (EFTFs) like PAX6 and LIM homeobox 2 (LHX2). This network of EFTFs governs eye development by decreasing inhibitory signals and activating genes such as bone morphogenetic protein 4 (BMP4), which are essential for eye formation[13,16,18-20]. Studies in various animal models[13,14,16,18] such as mouse, zebrafish, and xenopus have emphasized the essential roles of these genes in initiating eye formation[21-23].
Between days 22 and 27 of gestation, SIX3 activates PAX6 and SOX2 in the surface ectoderm, crucial for forming the pre-placodal region[21,24-26]. Mice with conditional SIX3 deletion show reduced PAX6 and SOX2 levels and impaired pre-placodal thickening[4]. By days 27-28, LHX2 regulates BMP4 expression in the optic vesicle (OV)[14,18,21,24], inducing lens placode thickening[26-28]. BMP4-deficient mice fail to develop lenses, but this can be restored with exogenous BMP4, highlighting the critical function of LHX2 in regulating BMP4 and lens development[26,29].
Between days 31-33 of gestation[18,21], the lens placode releases BMP and retinoic acid, inducing invagination of the OV[30-34]. Retinoic acid from the surrounding mesenchyme further supports this process[31,34]. In mice[18,21], a lack of retinoic acid due to retinaldehyde dehydrogenase knockouts results in incomplete OV invagination, which was rescued with maternal retinoic acid[30-34]. Studies performed in mouse models reported that, filopodia from the lens ectoderm are critical for coordinating lens and OV invagination, therefore, disruption of these filopodia inhibits lens pit development[21,35].
Neural crest cells contribute to various eye structures, including the cornea, iris, and parts of the retina[36]. Impaired migration or differentiation of these cells due to mutations in genes such as PAX6, SOX10, OTX2, RAX, forkhead box (FOX) C1, BMP4, and FOXE3 can lead to malformations, resulting in microphthalmia or anophthalmia[37,38]. Additionally, conditional knockouts of Foxc2 in neural crest cells of mice exhibit microphthalmia, irregularly shaped irises, corneal vascularization and opacity[39]. Conditional knockout mice with LIM homeobox transcription factor 1-beta deletion
Between 5-8 weeks of gestation, the OV invaginates to create the optic cup, resulting in retinal development. Disruptions/mutations in genes[41-45] such as SOX2, PAX6, OTX2, visual system homeobox 2 (VSX2) (also known as CEH10 homeodomain-containing homolog), RAX, cone rod homeobox (CRX), FOXE3, sonic hedgehog (SHH), growth/differentiation factor-6 (GDF6), BMP4, stimulated by retinoic acid 6 (STRA6), microphthalmia associated transcription factor (MITF), lysine-specific methyltransferase 2D (KMT2D), T-box transcription factor 3 (TBX3)[46-50] that regulate retinal cell proliferation and differentiation can cause anophthalmia or microphthalmia[51-53].
Aberrant regulation of cell proliferation and apoptosis during eye development can result in reduced eye size or the absence of eye tissue. Genes involved in controlling these processes, such as apoptotic protease activating factor-1 (APAF1), influence the balance between cell survival and death, and their dysfunction can lead to underdevelopment of the eye[54]. In mouse models of microphthalmia, an muscle segment homeobox gene 2 (Msx2) variant led to increased apoptosis by activating the caspase-3/8 pathway, while the loss of receptor-binding motif (Rbm) removed baculoviral inhibitor of apoptosis protein repeat-containing 2's (Birc2’s) inhibition of anti-apoptotic pathways[55]. Furthermore, in vitro studies using microphthalmia patient-derived models have shown upregulation of certain pro-apoptotic and cell cycle regulatory genes, reducing OV size[56,57].
In addition to intrinsic molecular signals guiding eye development, external stimuli shape the biophysical environment needed for optic cup formation and provide extracellular signals that create a supportive microenvironment for the developing eye[58-60]. The extracellular matrix (ECM), secreted by both the surface ectoderm and neuroectodermal OV, facilitates contact between these tissues, playing a pivotal role in regulating lateral OV growth and optic cup invagination[61,62]. The ECM also influences retinal progenitor proliferation and differentiation by affecting cell cycle dynamics[60].
Disruptions to laminin subunits α, β, and γ lead to ocular malformations, impaired basement membrane integrity, cell polarity, or focal adhesion assembly[63,64]. In zebrafish, disruption of the ECM protein nidogen (also known as entactin) hinders optic cup formation, although adding exogenous nidogen partially rescued optic cup invagination and fissure closure in double-mutant zebrafish lacking neural crest cells[58,65]. The ECM protein lumican (LUM) also controls axial length, as demonstrated in Lum−/−/Fmod−/− knockout mice and lum morpholino zebrafish, both showing elongated axial lengths[66,67]. Additionally, collagen type IV variants have been linked to ocular maldevelopment, with collagen alpha chain 1/4 (COL4A1/4) regulating retinal pigment epithelium (RPE) growth and function[68,69]. Despite the essential role of the ECM in ocular development, its dysfunction remains unexplored in the context of microphthalmia.
A/M have a complex genetic background that includes Mendelian modes of inheritance i.e., autosomal dominant, autosomal recessive, and X-linked inheritance patterns, as well as rare de novo mutations. Non-penetrance and variable expressivity are commonly observed, suggesting the influence of genetic modifiers and environmental factors. Some A/M cases result from parental germline mosaicism, where mutations affect gonadal tissue but not ocular tissue. Chromosomal abnormalities, including aneuploidy, translocations, deletions, and duplications, account for 20%-30% of AM cases (Table 1)[1,70]. More than 90 genes linked with A/M (Tables 2 and 3, respectively) have been reported, majorly involved in eye development and the retinoic acid signaling pathway. Pathogenic mutations mainly include missense, nonsense, and splice-site variations.
| Type of chromosomal abnormality | Chromosome abnormality | Other features |
| Aneuploidy | Trisomy 9 mosaicism | Joint contractures, congenital heart defects, prenatal growth deficiency, learning difficulties, micrognathia, kyphoscoliosis |
| Trisomy 13 (patau syndrome) | Holoprosencephaly, moderate microcephaly, coloboma, retinal dysplasia, cyclopia, cleft lip/palate, cardiac defects, genital abnormalities, 86% die within one year | |
| Trisomy 18 (edwards syndrome) | Polyhydramnios, single umbilical artery, small placenta, low fetal activity, learning difficulties, hypertonicity, hypoplasia of skeletal muscle, subcutaneous, adipose tissue, prominent occiput, low-set malformed auricles, micrognathia, cardiac defects | |
| Triploidy | Triploidy | Large placenta with hydatidiform changes, growth deficiency, syndactyly, congenital heart defects, brain anomalies/holoprosencephaly |
| Deletion | 4p (wolf-hirschhorn syndrome) | Growth deficiency, microcephaly, ocular hypertelorism, cranial asymmetry, learning difficulties, epilepsy, cleft lip/palate, anterior segment dysgenesis |
| Deletion 7p15.1-p21.1 | Cryptophthalmos, cleft lip/palate, choanal atresia | |
| 13q-, ring 13 | Microcephaly, learning difficulties, bilateral retinoblastoma, cardiac defects, hypospadias, cryptorchidism | |
| Deletion 14q22.1-q23.2 | Pituitary hypoplasia | |
| 18q | Midface hypoplasia, small stature, learning difficulties, hypotonia, nystagmus, conductive deafness, microcephaly, midface hypoplasia, genital abnormalities | |
| Duplication | 3q syndrome (3q21-ter dup) | Learning difficulties, growth deficiency, hypertrichosis, craniosynostosis, cardiac defects, chest deformities, genital abnormalities, umbilical hernia |
| 4p syndrome | Learning difficulties, epilepsy, growth deficiency, obesity, microcephaly, characteristic faces, genital abnormalities, kyphoscoliosis | |
| 10q syndrome | Ptosis, short palpebral fissures, camptodactyly, learning difficulties, prenatal growth deficiency, microcephaly, heart and kidney malformations |
| Phenotype | Gene |
| Anophthalmia | ALDH1A3, MFRP, SOX2, RAX, OTX2, FRAS1, BMP4 |
| Unilateral anophthalmia, isolated | ARG1 |
| Anophthalmia and kidney abnormalities | BCOR |
| Anophthalmia and blepharophimosis | BMP4 |
| A/M | CHD7, OTX2, STRA6, PUF60, SIX6 |
| A/M with developmental delay | COL4A1 |
| Developmental delay with anophthalmia | EPHA6 |
| Anophthalmia and skeletal anomalies | GDF3 |
| Anophthalmia, bilateral | GDF6, VSX2, OTX2 |
| Holoprosencephaly with anophthalmia, branchial arch anomalies and central nervous system anomalies | GLI2 |
| Bilateral anophthalmia, intellectual disability and rhizomelic skeletal dysplasia | MAB21L2 |
| Waardenburg anophthalmia syndrome 4 | MITF |
| Anophthalmia and isolated growth hormone deficiency | OTX2 |
| Anophthalmia with corpus callosum hypoplasia, pituitary gland hypoplasia and vermian hypoplasia | |
| Anophthalmia, growth delays, intellectual disability, and autism | |
| Anophthalmia, hearing impairment, dysmorphic facial features and abnormal pituitary development causing growth failure | |
| Anophthalmia, pituitary hypoplasia and ear anomalies | |
| Bilateral anophthalmia, syndromic | |
| Hypopituitarism with anophthalmia, dysmorphic facies, developmental delay and patent ductus arteriosus | |
| Bilateral microphthalmia, anophthalmia or coloboma | PAX6 |
| Non-syndromic A/M | PORCN |
| Brain malformations, anophthalmia, hepatomegaly, bile duct atresia and Müllerian duct agenesis | ptk7ie (isoform of PTK7) |
| Anophthalmia and sclerocornea | RAX |
| Anophthalmia, hypopituitarism, diabetes insipidus, bilateral cleft lip and palate | |
| Microphthalmia, anophthalmia and coloboma disease | RERE, CDON, IQGAP1, CASK, MYO10, TENM3, POLR2A, WNT2B, KIF26B, MICU1, RBP4, FIG4, GDF6 |
| Waardenburg anophthalmia syndrome | SMOC1 |
| A/M, developmental delay, short stature and ataxic gait | SOX2 |
| A/M, mental retardation, short stature and ataxic gait | |
| A/M, mental retardation, short stature and unsteady gait | |
| Anophthalmia and facial dysmorphism | |
| Anophthalmia syndrome and dental anomalies | |
| Anophthalmia with status dystonicus | SOX2 |
| Anophthalmia-esophageal-genital syndrome | |
| Anophthalmia, facial dysmorphism, delayed psychomotor development and language acquisition | |
| Anophthalmia, global developmental delay, hypotonia, delayed language development and delayed gross motor development | |
| Anophthalmia, hearing loss and brain abnormalities | |
| Anophthalmia, hypogonadotropic hypogonadism and growth hormone deficiency | |
| Bilateral anophthalmia and sensorineural hearing loss | |
| Sox2 anophthalmia syndrome | |
| Bilateral anophthalmia | SOX2, GJA8 |
| Microphthalmia, anophthalmia and coloboma | SOX2, PAX6 |
| Anophthalmia and diaphragmatic defects | STRA6 |
| Anophthalmia syndrome | |
| Colobomatous microanophthalmia | STRA6, TENM3 |
| Anophthalmia and microphthalmia | VSX2, ALDH1A3, PXDN, FOXE3 |
| A/M | VSX2, SOX2, RAX, FOXE3, ALDH1A3 |
| Unilateral anophthalmia with accompanying malformations | WASHC5 |
| Pulmonary hypoplasia, diaphragmatic anomalies, A/M and cardiac defects syndrome | WNT7B |
| Phenotype | Gene |
| Microphthalmia, isolated with coloboma 7 | ABCB6 |
| Microphthalmia and cataract | ALDH1A3, SHH, RAB3GAP2, FOXC1 |
| Microphthalmia with facial clefting | ALX1 |
| Microphthalmia, renal aplasia, hearing impairment, developmental delay, micropenis and cryptorchidism | ANOS1 |
| Microphthalmia with linear skin defects | ARHGAP6 |
| Craniosynostosis-microphthalmia syndrome | BCOR |
| Intellectual disability, cortical atrophy, microphthalmia and autism spectrum disorder | |
| Lenz microphthalmia | |
| Microphthalmia and cataracts | |
| Microphthalmia with associated anomalies | |
| Microphthalmia, syndromic 2 | |
| Pediatric total cataract with microphthalmia and microcornea | |
| Anophthalmia, microphthalmia with sclerocornea and hydrocephalus | BMP4 |
| Bilateral microphthalmia and unilateral cataract | |
| Colobomatous microphthalmia | BRPF1, PUF60, TGFB2 |
| Microphthalmia, syndromic | C12orf57, RAB3GAP2, TBC1D32, SCLT1, BCOR, KMT2D, NAA10, LAMB2, SLC18A2 |
| Microphthalmia, colobomatous | C12orf57, SHH |
| Bilateral microphthalmia with accompanying malformations | CACNA1F, OSGEPL1, COL4A1, GAS2L2, IGFALS, LRP11, MAN1A1, CBR4, PKHD1L1, PRKG2, PDE6B, SUMF1, TBCE |
| Microphthalmia and/or coloboma | CAPN15 |
| A/M | CHD7, OTX2, STRA6, PUF60, SIX6 |
| Unilateral microphthalmia, isolated | CHD7, wnk4tv2 (isoform of WNK4), abcb9tv2 (isoform of ABCB9), wnk1tv2 (isoform of WNK1), FERMT1, CD22, MYO9B, ccn4tv3, EPB41, INTS11, IARS2RC3H1, ccn4tv3 (isoform of CCN4), EPB41, INTS11, IARS2RC3H1, AGRN, KRT31, PSME3IP, TUBGCP3, XKR4, MYCBP2, ABCC9, RPGRIP1L, MYH11, BHLHE22, ST18, FGF20, GRM8, ABCA13, CAPN11, NHLRC1, GRM6, MEGF10, PALLD, KMT2A, TYR, MFSD13A, HECTD2, MSRB2, ABCA1, KIFC2, FMNL2, ZP4, FRAS1, LIPH, MCCC1, P2RY14, CACNA2D3, SCN11A, GMPPA, PRKAG3, AOX1 |
| Intellectual disability with microphthalmia | CNKSR1 |
| A/M with developmental delay | COL4A1 |
| Cataracts and microphthalmia | |
| Microphthalmia with bilateral microcornea and Peters anomaly | |
| Microphthalmia with linear skin lesions | COX7B, HCCS, NDUFB11 |
| Pediatric perinuclear cataract with microphthalmia | CRYAA |
| Pediatric total cataract with microphthalmia | CRYBA4 |
| Cataract and microphthalmia | CRYBB1 |
| Cataract and microphthalmia | CRYBB3, BBS2, CRYAA |
| Nuclear cataracts with microphthalmia | CRYGC |
| Microphthalmia, fetal vasculature and vitreoretinal dysplasia | CTNNB1 |
| Microphthalmia and agenesis of corpus callosum | DIS3L2 |
| Mandibulofacial dysostosis, type Guion-Almeida, with microphthalmia, coloboma and retinal dystrophy | EFTUD2 |
| Microphthalmia with iris hypoplasia | EPHA2 |
| Colobomatous microphthalmia, ptosis, syndactyly and nephropathy | FAT1 |
| Microphthalmia, coloboma, cardiac anomalies, renal dysfunction and limb malformations | |
| Microphthalmia, and intellectual developmental disorder with dysmorphic facies and behavioral abnormalities | FBXO11 |
| Microphthalmia and choroidal osteoma | FNBP4 |
| Glaucoma, microphthalmia, iris anomalies, dysmorphic facial features, club foot, cognitive impairment and deafness | FOXC1 |
| Bilateral microphthalmia and anterior segment dysgenesis | FOXE3 |
| Microphthalmia with sclerocornea | |
| Microphthalmia, nonsyndromic bilateral | |
| Sclerocornea-microphthalmia-aphakia complex | |
| Microphthalmia and anterior segment developmental anomalies | GDF6 |
| Microphthalmia, congenital cataracts and microcornea | GJA3 |
| Congenital cataract with microcornea, microphthalmia and posterior capsule defect | GJA8 |
| Dystonia, bilateral ptosis, microphthalmia and speech and motor developmental delay | GNAL |
| Microphthalmia with linear skin lesions and autism spectrum disorder | HCCS |
| Microphthalmia, syndromic 7 | |
| Bilateral microphthalmia, isolated | hlcstv 4 (isoform of HLCS), ATRN, pax6tv32 (isoform of PAX6), TAS1R3, CERS2, NUMA1, TSFM, SLC5A11, PRKAG2, CFTR, GCK, THSD7A, |
| Colobomatous microphthalmia syndrome, X-linked | HMGB3 |
| Ocular coloboma, microphthalmia and cataract | IPO13 |
| Microphthalmia and coloboma | KIF17, MAB21L1 |
| Microcephaly, long eyelashes, microphthalmia | KMT2A |
| Microphthalmia, failure to thrive, skeletal anomalies and neurological disorders | |
| Unilateral microphthalmia with accompanying malformations | KRT23, VPS13D, MYH2, TRIM2, USP38, MYBPC3, KIAA2026, RYR2, MAP3K21, UNC13D, CHD7 |
| Microphthalmia and aniridia | MAB21L1 |
| Microphthalmia | MAB21L1, OTX2, MAB21L2, PRSS56, ALDH1A3, RAX, VSX1, PIEZO2, STRA6, SOX2, CHD7, RARB, DSC3, MFRP, RAB3GAP1, PXDN, GJA8, HMX1, MYO10, OLFM2, EPHA2, BCOR, TENM3, FOXE3, TMX3, VSX2, otx2tv5, CRYBA4, PAX6, VAX1, C12orf57, SEMA3E, RBP4, DHX38, |
| Bilateral colobomatous microphthalmia, autosomal-dominant | MAB21L2 |
| Microphthalmia/coloboma and skeletal dysplasia syndrome | |
| Syndromic microphthalmia, type 14 | |
| Cataract, anterior segment dysgenesis and microphthalmia | MAF |
| Posterior microphthalmia, non-pigmented retinitis pigmentosa, optic nerve drusen, and retinoschisis | MFRP |
| Microphthalmia with retinitis pigmentosa | MFRP, PRSS56 |
| Microphthalmia, bilateral | MIP, PAX6 |
| Coloboma, osteopetrosis, microphthalmia, macrocephaly, albinism and deafness | mitftv1 (isoform of MITF), MITF |
| Microphthalmia, bilateral with sclerocornea | NDP |
| Pediatric cataract with microphthalmia, microcornea and nystagmus | NHS |
| Pediatric total cataract with microphthalmia, microcornea and nystagmus | |
| Microcephaly, microphthalmia and cataracts | OCLN |
| Microphthalmia and coloboma | OLFM2, RAX, TMX3, PAX6, SEMA3E, ABCB6, RBP4 |
| Combined pituitary hormone deficiency, bilateral microphthalmia and agenesis of the left internal carotid artery | OTX2 |
| Combined pituitary hormone deficiency, syndromic microphthalmia and retinal dystrophy | |
| Microphthalmia, ectopic pituitary and growth hormone deficiency | |
| Microphthalmia, pituitary abnormalities and intellectual disability | |
| Microphthalmia, type 5 | |
| Microphthalmia, syndromic 5 | otx2tv5 (isoform of OTX2) |
| Syndromic microphthalmia | |
| Bilateral microphthalmia, anophthalmia or coloboma | PAX6 |
| Bilateral microphthalmia, congenital cataract and glaucoma | |
| Bilateral microphthalmia, primary aphakia, coloboma and iris hypoplasia | |
| Microphthalmia, cataracts and nystagmus | |
| Microphthalmia, late-onset keratitis and iris coloboma/aniridia | |
| Anterior segment dysgenesis and microphthalmia | PITX3 |
| Congenital cataract and microphthalmia | PITX3, CRYGC |
| Non-syndromic A/M | PORCN |
| Microphthalmia with limb anomalies | PORCN, FNBP4, SMOC1 |
| Mental retardation, microphthalmia, choroid coloboma, microcephaly, renal hypoplasia and spastic paraplegia | PQBP1 |
| Microphthalmia and Peters anomaly | PRR12 |
| Unilateral microphthalmia | |
| Unilateral microphthalmia and Peters anomaly | |
| Microphthalmia with corneal opacification | PXDN |
| Microphthalmia, sclerocornea, Peters anomaly and aphakia | |
| Microphthalmia and anterior segment dysgenesis | PXDN, GJA8, PAX6, CRYGC, CRYAA |
| Microphthalmia and diaphragmatic hernia | RARB |
| Microphthalmia and dystonia | |
| Microphthalmia, syndromic 12 | |
| Microphthalmia, isolated, with coloboma 10 | RBP4 |
| Microphthalmia, anophthalmia and coloboma disease | RERE, CDON, IQGAP1, CASK, MYO10, TENM3, POLR2A, WNT2B, |
| Lenz microphthalmia syndrome | SALL1 |
| Microphthalmia, coloboma and optic nerve hypoplasia | SALL4 |
| Microphthalmia and extensive colobomas of the globes | SIX6 |
| Coffin-Siris syndrome, microphthalmia and small-cell carcinoma of the ovary, hypercalcemic type | SMARCA4 |
| Bosma arrhinia microphthalmia syndrome | SMCHD1 |
| Alzahrani-Kuwahara syndrome and microphthalmia | SMG8 |
| A/M, developmental delay, short stature and ataxic gait | SOX2 |
| A/M, mental retardation, short stature and ataxic gait | |
| A/M, mental retardation, short stature and unsteady gait | |
| Bilateral microphthalmia, microcornea, learning disability and developmental delay | |
| Microphthalmia syndromic 3 | |
| Microphthalmia, developmental delay, hearing loss and dysmorphic features | |
| Microphthalmia, iris coloboma, anal atresia and nasal skin tag | |
| Bilateral microphthalmia | SOX2, PAX6 |
| Microphthalmia, anophthalmia and coloboma | |
| Microphthalmia 3 | SOX2, RAX |
| Mild intellectual disability with microphthalmia, coloboma, hypopituitarism, facial dysmorphology and dental anomalies | SOX3 |
| Microphthalmia, syndromic 9 | STRA6 |
| Bilateral microphthalmia, congenital cataract, microcephaly, and global developmental delay | TENM3 |
| Microphthalmia, isolated, with coloboma 9 | |
| Syndromic microphthalmia 15 | |
| Microphthalmia, congenital cataracts and microcephaly | TUBA1A |
| Microphthalmia, bilateral and coloboma | VSX2 |
| Microphthalmia, cataract and iris abnormality | |
| Anophthalmia and microphthalmia | VSX2, ALDH1A3, PXDN, FOXE3 |
| Microphthalmia and cataract | VSX2, EPHA2 |
| A/M | VSX2, SOX2, RAX, FOXE3, ALDH1A3 |
| Pulmonary hypoplasia, diaphragmatic anomalies, A/M and cardiac defects syndrome | WNT7B |
| Microcephaly, Microphthalmia, Hyperpigmentation of the skin, Global developmental delay | XPA |
| Microphthalmia and bilateral chorioretinal coloboma | YAP1 |
| Microphthalmia and bilateral uveal coloboma | |
| Microphthalmia/coloboma | YAP1, KMT2D |
Apart from genetic factors, environmental variables also play a significant role in the development of A/M by influencing embryonic eye formation through different processes. This section discusses the impact of maternal infections, teratogenic exposures, and nutritional deficiencies on A/M.
The association between maternal infections such as rubella, toxoplasmosis, cytomegalovirus (CMV), and varicella with ocular abnormalities is well-documented[70,71]. Other viruses, such as parvovirus B19[72,73], herpes simplex type 2[74], Epstein-Barr virus[75], and coxsackie A9[76], have been implicated, though with less conclusive evidence. Additionally, infections like influenza, hyperthermia, are associated with both central nervous system malformations and microphthalmia[77-80]. Animal studies provide further support for hyperthermia as a specific cause of A/M, suggesting it may also act as a co-teratogen when combined with other exposures, such as chemicals[77,81].
Additional risk factors identified in the literature for A/M or other developmental eye anomalies include solvent misuse such as per- and trichloroethylene, toluene and xylene, as well as exposure to X-rays and certain drugs, such as thalidomide, isotretinoin, warfarin, and alcohol[82-85]. Warburg[71] documented that conditions like Goldenhar, CHARGE, and VATER can occur due to maternal exposure to drugs, infections, fever, or radiation, which may disrupt neural crest cell development. Retinoic acid, a regulator of homeobox genes, can cause microphthalmos or colobomas if dysregulated. A study performed by Nichols Barber[86] reported that mouse fetuses heterozygous for anophthalmia had a higher occurrence of eye defects than homozygous normal when their mothers were treated with the teratogen trypan blue. All cases of abnormal eye development involved unilateral anophthalmia, while normal bilateral development was expected in the absence of the teratogen.
Maternal nutrition plays a vital role in fetal development, and deficiencies in essential nutrients can significantly impact the development of the eyes. In murine embryos lacking the folate-binding protein 1 gene, about 10%-12% developed anophthalmia or microphthalmia in a folate-deficient environment[87]. A report by Kochhar[88] documented vitamin A, vitamin E and zinc as the essential nutrients for eye development. Nielsen and Carlton[89] reported that vitamin E deficiency is associated with microphthalmia in rabbits, whereas zinc deficient diets in female rats results in visual abnormalities in their offspring[90].
Vitamin A is essential for normal ocular development, but excessive intake, particularly from vitamin A supplements, can be teratogenic. High vitamin A levels can lead to abnormalities in embryonic development, including microphthalmia[91]. In contrast, inadequate vitamin A during pregnancy may be associated with teratogenic consequences in humans. In a study by Hale[92], a Duroc-Jersey gilt (a pig) when fed with a vitamin A-deficient diet gave birth to eleven anophthalmic piglets. This instance emphasizes the crucial function of vitamin A in normal eye development, since its lack can result in severe congenital abnormalities such anophthalmia[92]. Sarma[93] reported a case of a baby with microcephaly and anophthalmia who survived for only 24 hours, born to a mother suffering from VAD and blindness due to xerophthalmia. Further, another case of a child born with ocular abnormalities, including microphthalmia and coloboma[94] was born to a mother suffering from VAD. World Health Organization recommends a safe maximum dosage during pregnancy is up to 10000 IU per day or 25000 IU per week, beginning after the first 60 days of gestation[95,96].
Epigenetics refers to heritable changes in gene expression that do not involve alterations to the DNA sequence. Environmental factors such as diet, exposure to toxins, and maternal infections during pregnancy can lead to epigenetic modifications, influencing the risk of congenital anomalies like A/M. These modifications occur through mechanisms such as DNA methylation, histone modification, microRNA and long non-coding RNA regulation, which alter gene activity during fetal development.
DNA methylation, primarily occurs at the CpG dinucleotides and involves the addition of a methyl group to cytosine bases, hence leading to gene silencing. This process disrupts the binding of transcription factors and prevents the recruitment of the transcriptional machinery. Methylated cytosines bind to methyl-CpG-binding domain proteins, which recruit repressive complexes that enhance chromatin compaction and suppress transcription. Additionally, methylation can affect mRNA splicing and stability, resulting in fewer mature mRNAs accessible for translation. These pathways result in stable gene silence, which is critical for controlling gene expression during development and maintaining cellular identity. Environmental exposures can cause disruptions in normal methylation processes. For example, a meta-analysis study by Joubert et al[97] reported that maternal smoking during pregnancy has been linked to altered methylation of genes important in eye development, which raises the likelihood of ocular abnormalities in kids. Similarly, folate deficiency, which plays a key role in DNA methylation, can impair ocular development. Studies have shown that reduced folate intake during pregnancy is associated with an increased risk of A/M, likely due to altered DNA methylation patterns that disrupt normal eye morphogenesis[87,98].
Prenatal alcohol exposure can lead to fetal alcohol spectrum disorder (FASD), which manifests as lifelong complications including congenital anomalies, cognitive impairments, and behavioral deficits. Among the abnormalities, microphthalmia is linked to the teratogenic effects of alcohol. While DNA methylation is critical for controlling gene expression, it is mostly unaltered in imprinted areas in FASD. However, maternal supplementation with methyl donors such as folic acid, choline, betaine, and vitamin B12 showed promising results in preventing alcohol-induced developmental problems. Providing proper preconception and prenatal nutritional support is critical for minimizing the incidence of alcohol-related abnormalities, such as microphthalmia, while maintaining normal DNA methylation patterns[99].
Histone modifications are critical epigenetic mechanisms that regulate gene expression by altering chromatin structure and accessibility of transcriptional machinery to the DNA. These modifications, which include acetylation, methylation, phosphorylation, and ubiquitination, play a significant role in the regulation of genes essential for ocular development. Dysregulation of these processes has been implicated in a variety of congenital eye disorders, including A/M. Sox2 is a transcription factor that plays a pivotal role in early eye development, and its dysregulation due to epigenetic changes such as decreased histone acetylation, can further lead to severe ocular malformations, including microphthalmia. Mutations in genes encoding histone methyltransferases, such as enhancer of zeste homolog 2 (EZH2) and KMT2D, have been linked to developmental disorders involving the eye, including A/M[100]. EZH2 is part of the polycomb repressive complex 2 (PRC2), which catalyzes the methylation of histone H3 at lysine 27 (H3K27), leading to gene silencing. Dysregulation of this process can disrupt the normal pattern of ocular development, as the repression of critical genes involved in eye formation is lifted prematurely or excessively[101]. Aref-Eshghi et al[102] documented that mutations in KMT2D (responsible for methylating H3K4), have been associated with Kabuki syndrome, a disorder that includes ocular anomalies such as coloboma and microphthalmia. Hence, these findings highlight the critical role of histone methylation in regulating gene expression during fetal eye development.
MicroRNAs (miRNAs) regulate gene expression by binding to mRNAs, inhibiting translation or promoting their degradation. Several miRNAs are involved in eye development, and disrupted regulation of microRNAs can cause ocular malformations. The miR-204 plays a key role in regulating genes important for lens and retinal development. A study by Conte et al[103] showed that miR-204 controls the expression of transcription factors like meis homeobox 2 (Meis2), crucial for eye morphogenesis. Dysregulation of miR-204 has been linked to microphthalmia in the medaka fish (Oryzias latipes) model[103].
Long non-coding RNAs (lncRNAs) regulate gene expression and play critical role in the eye development. Their dysregulation can lead to congenital eye disorders such as A/M. Six3OS lncRNA regulates the SIX3 gene, which is essential for forebrain and early eye development[104]. Rhabdomyosarcoma 2-associated transcript (RMST) is involved in retinal development and interacts with transcription factor Sox2, hence disruption of RMST may affect Sox2 regulation[105].
Noncoding RNAs, including both miRNAs and lncRNAs, regulate crucial pathways during eye development, such as the Wnt and Hedgehog signaling pathways. Dysregulation of Wnt pathway components, influenced by miRNAs like miR-204, can lead to ocular defects such as A/M[103]. Changes in the expression of non-coding RNAs associated with Hedgehog signaling have been noted in microphthalmia models[106].
Individuals with microphthalmia may preserve some visual function, but they are more likely to develop problems such as cataracts, glaucoma, or curable refractive abnormalities. Even with unilateral microphthalmia, it is critical to protect the healthy eye using glasses. Electrodiagnostic studies, such as electroretinography, electro-oculography, and visual evoked potentials, can aid in the diagnosis of optic nerve disorders, retinal dystrophy, or cortical vision abnormalities. Visual acuity should be constantly evaluated, especially in those at risk of retinal dystrophy. Surgery is frequently recommended for serious impairments, like cataracts[107].
Anophthalmia and severe microphthalmia can lead to a reduced orbital size, causing asymmetry. Socket expanders, either hydrophilic or solid, are used from birth to stimulate orbital growth, widen the palpebral fissure, and stretch the conjunctival cul-de-sacs. If an eye is present, a clear prosthesis can help in developing the bony socket and preventing asymmetry. Over time, solid prostheses of increasing size are used to expand the orbital volume, with frequent updates needed in early years to match the contralateral side. Complications may include instability or difficulty with the prosthesis, which are addressed with bespoke, hand-painted designs, and occasionally, orbital implants or socket lining may be required. Once orbital growth is complete in adulthood, changes to prostheses become less frequent[107].
Genetic diversity and variable expression complicate the diagnosis and counseling for A/M. While the recurrence risk for siblings was once estimated at 10%-15%, it is now considered lower, though it can vary significantly. Prenatal screening with ultrasound, followed by postnatal assessment, is often used when invasive methods are not possible. Identifying a genetic anomaly can improve counseling and offer options for prenatal or pre-implantation diagnosis, but counseling remains complex due to phenotypic variability and potential mosaicism.
Studying gene-environment interactions in A/M involves several challenges. The major challenge is to separate particular environmental exposure and correlating it to genetic predispositions, as variables i.e., maternal infections, dietary inadequacies, and toxin exposure frequently coexist, confounding the evaluation of their individual effects[108]. Because these situations are rare, obtaining significant sample size for rigorous statistical analysis is challenging. Furthermore, examining epigenetic regulation, which may explain these associations, requires high throughput techniques such as DNA methylation profiling and histone modification analyses. These approaches are costly and require large cohorts to get statistically significant results[97]. Furthermore, sometimes animal models may not fully reflect human ocular development, limiting the findings' relevance due to genetic variations and environmental exposure[97,109].
Whole-exome sequencing (WES) and whole-genome sequencing (WGS) have revolutionized research in A/M, resulting in the discovery of new genes and pathways involved in eye development. These high throughput technologies helped researchers to identify rare genetic mutations that previously remained undetected due to traditional approaches, therefore, offering more insight into the complex genetic architecture of A/M[43,110].
WES has been useful in identifying disease causing mutations in genes such as SOX2, OTX2, and VSX2, responsible for early fetal eye development[43,110]. Furthermore, WGS has higher benefits than WES, as it can detect structural variants, variants present in the non-coding regions, and regulatory factors that might contribute to congenital eye anomalies. WGS can also identify copy number variants (CNVs) that alter genes critical for eye development, assisting in the elucidation of new pathogenic pathways. As our understanding of genetic diversity grows, researchers are better able to examine how these interactions may impact the development of A/M. Future research may improve the significance of WES and WGS, especially as sequencing prices fall and larger-scale studies become feasible[108].
Animal models, especially mice and zebrafish, have greatly advanced our knowledge of A/M. These animal models share similar eye morphology with humans and can be genetically modified, enabling researchers to investigate the role of specific genes in eye development. Because of the similarities in ocular development and physiology between mice and humans, many mouse lines exhibiting microphthalmia have been created, primarily by disrupting genes associated with the condition in humans. These genes include Sox2, Otx2, Rax, Vsx2, Pax6, Stra6, Foxe3, Bmp4, Bmp7, sparc-related modular calcium-binding protein 1 (Smoc1), Shh, porcupine o-acyltransferase (Porcn), forkead box C1 (Foxc1), fraser extracellular matrix complex subunit 1 (Fras1), fras1-related extracellular matrix protein 1 (Frem1), tectonic family member 2 (Tctn2), collagen type IV alpha 1 (Col4a1), TBC1 domain family, member 32 (Tbc1d32), protease, serine 56 (Prss56), peroxidasin (Pxdn), paired-like homeodomain transcription factor 2 (Pitx) 2, Pitx3, Mitf, alpha A crystallin (Cryaa), Frem2, retinitis pigmentosa GTPase regulator-interacting protein (Rpgrip1), SMG9 nonsense-mediated mRNA decay factor (Smg9), sorting nexin 3 (Snx3), dystroglycan 1
Stem cell research offers potential for treating A/M. Induced pluripotent stem cells (iPSCs) can generate retinal cells and other eye tissues, providing opportunities for regenerative medicine[111]. The iPSC-based models help study disease-specific mutations and their impact on eye development. A human induced pluripotent stem cell (hiPSC) line UCLi013-Awas established from fibroblasts obtained from a 34-year-old donor with severe microphthalmia and aniridia, carrying a heterozygous missense mutation in the PAX6 gene [c.372C>A, p.(Asn124 Lys)], confirmed via Sanger sequencing. This cell line may offer a valuable platform for studying microphthalmia and aniridia, identifying therapeutic targets, and screening potential drugs[112]. Similarly, Eintracht et al[113] used iPSC-derived OVs from two microphthalmia patients and healthy controls. RNA sequencing identified upregulated apoptosis and ECM genes. Increased expression of LUM, nidogen, and collagen type IV suggested ECM overproduction. The authors further reported that pharmacological inhibition of caspase-8 with Z-IETD-FMK decreased apoptosis in one patient model, indicating a possible therapeutic strategy. Though still in its early stages, stem cell therapy may one day restore vision or regenerate ocular structures in individuals with severe eye malformations[114].
The rapid advancements in genetic and genomic research have significantly enhanced the diagnostic landscape for A/M. Clinical applications of genetic discoveries in A/M include molecular diagnostics, personalized therapeutic interventions, and emerging gene therapies.
Genetic testing for precision diagnosis: WES and WGS have revolutionized the ability to diagnose A/M, particularly in non-syndromic cases where clinical features alone are insufficient. CNV analysis and chromosomal microarray are instrumental in detecting larger structural variations associated with A/M. In a mixed microphthalmia, anophthalmia and coloboma (MAC) cohort, Harding et al[115] using targeted gene panels, WGS, and microarray comparative genomic hybridization, identified pathogenic mutations in approximately 33% of cases, with a diagnostic yield that was consistent for both unilateral and bilateral cases. Systemic associations were observed in 34% of patients, emphasizing the need for a multidisciplinary approach. Imaging techniques such as magnetic resonance imaging and B-scan ultrasound are crucial for evaluating ocular structure and associated abnormalities. Novel findings, including the association of EPHA2 with microphthalmia and FOXE3 with hearing loss and kidney anomalies, contribute to expanding current knowledge. Harding et al[115] underscored the importance of comprehensive phenotyping, genetic testing, and individualized management to improve patient outcomes.
The integration of these genetic tools into clinical practice allows for earlier and more accurate diagnosis, guiding genetic counseling and reproductive decision-making for affected families. WES is increasingly used in prenatal diagnosis when fetal anomalies are detected on ultrasound but conventional genetic tests like karyotyping and microarray fail to provide a diagnosis. Studies have shown that WES improves the diagnostic yield in such cases, with rates ranging from 6.2% to 57%[116-118]. Prenatal WES has been successfully used in detecting SOX2, OTX2, and RAX mutations, leading to early intervention and improved perinatal care[119]. A study investigating five cases of fetal ultrasound abnormalities using WES at a single institution reported a diagnostic yield of 80%, with four out of these five cases yielding a confirmed genetic diagnosis. In one of these cases, anophthalmia and a choroid plexus cyst were identified at 19 weeks of gestation[120]. Given the known genetic associations, WES confirmed a de novo SOX2 pathogenic variant, a known cause of syndromic anophthalmia[41]. The pregnancy was terminated before WES results, and autopsy confirmed anophthalmia. This represents a rare instance of prenatal diagnosis of syndromic microphthalmia[120].
Personalized therapeutic approaches: With advances in molecular biology, personalized medicine for A/M is becoming a possibility. The identification of patient-specific mutations enables targeted interventions, including pharmacological treatments that modulate affected developmental pathways. For instance, retinoic acid derivatives have been explored for PAX6 and ALDH1A3-related microphthalmia, given their role in ocular morphogenesis[14,121].
Gene therapy and stem cell-based interventions: Gene therapy for A/M is currently an area of active research. IPSCs derived from patients with microphthalmia have been used to generate retinal organoids, offering a potential source for regenerative therapies[122]. The hiPSCs with a homozygous VSX2 mutation (p.Arg200Gln) associated with microphthalmia were differentiated into early optic cups, showing normal initial development but disrupted optic cup formation due to reduced proliferation and increased RPE differentiation[57]. RNA-seq revealed upregulation of Wnt/transforming growth factor signaling (WNT11, BMP8A) and downregulation of fibroblast growth factor (FGF) signaling (FGF19) in mutant vesicles[57]. Further studies confirmed dysregulated Wnt signaling and abnormal VSX2/MITF co-expression, disrupting neural retina/RPE differentiation in microphthalmia[56]. Wnt inhibition partially rescued neuroretina formation but failed to restore the RPE layer, while Wnt activation in wild-type vesicles replicated the microphthalmia phenotype[56].
A/M prevalence and clinical outcomes vary significantly across regions, particularly in low-resource settings, where early diagnosis and genetic testing remain largely unavailable. While high-income countries benefit from advanced diagnostic tools such as WGS and prenatal imaging, many low-income and middle-income countries (LMICs) rely solely on clinical examination, leading to underdiagnosis and mismanagement[123,124]. Due to limited access to healthcare, advanced prenatal clinics and unaffordability, early detection of MAC spectrum disorders remains a challenge in LMICs. Severe microphthalmos and anophthalmos with or without cysts which can easily be detected by prenatal ultrasound are often missed due to lack of adequate healthcare[125,126].
The largest study of MAC disorders published was conducted as a collaborative study in three tertiary eye care centers from North India to understand the clinical spectrum of non-syndromic MAC disorders in India in a one-year study period[127]. Total 545 children (0-18 years of age) were included in the study; however, despite the enormous disease burden, genetic investigations were not carried out to decipher the molecular causation. This is due to the steep costs of genetic investigations in LMICs and the lack of support by insurance agencies in carrying out personalized genetic tests. A trend of segregation of severe types of MAC was seen in a particular region where, co-incidentally, VAD related corneal disease was common. Nutritional deficiencies such as VAD are considered a significant public health concern, with a relatively high prevalence, where studies report a considerable percentage of the population suffering from subclinical or clinical VAD, often linked to inadequate dietary intake of vitamin A-rich foods[128]. Considering the evidence in literature regarding the interplay between Vitamin A pathway genes and MAC disorders[94], a relationship between the maternal VAD and incidence of MAC could be hypothesized (Subhedkar et al, data unpublished). Both VAD and MAC are more common in the lower socio-economic classes[129,130] making them a serious health concern in LMICs. The MAC disorders have also been found to be more common in rice-dependent communities and consanguineous populations[131,132].
A study in Colombia examined the causes of eye loss among patients, revealing that over 60% of cases were from low socioeconomic backgrounds. The study highlighted a significant gap in healthcare coverage, as the national health system did not cover expenses related to ocular prostheses. Additionally, more than 75% of patients had been using the same ocular prosthesis for over a decade, reflecting a lack of follow-up care and limited resources for maintenance[133].
These cases underscore the critical need for improved access to specialized healthcare, early diagnostic tools, and comprehensive rehabilitation services in LMICs.
Efforts to bridge the gap in A/M management include: (1) Low-cost genetic testing initiatives: Programs such as the Human Heredity and Health in Africa (H3Africa) project aim to expand access to affordable genetic testing[134]; (2) Telemedicine and artificial intelligence-based diagnostics: Artificial intelligence-driven imaging platforms are being developed to facilitate remote diagnosis and genetic counseling in underprivileged areas[135]; and (3) Community-based genetic screening: In countries such as India and Brazil, public health initiatives are integrating community-based genetic screening programs, reducing the burden of undiagnosed congenital conditions[136].
Future studies on A/M should integrate genetic and environmental data to investigate their complex relationships. Building comprehensive databases with genomic, transcriptomic, proteomic, and environmental information would allow researchers to investigate gene-environment interactions more effectively. More epidemiological studies should be performed that focusses on maternal exposures during pregnancy such as infections, drugs, and dietary variables. These studies may give crucial data for developing public health policy to protect at-risk populations from these hazards. This information might lead to customized medical approaches, allowing early identification of high-risk people and individualized therapy tactics that target both genetic and environmental effects[108].
The future of preventing A/M depends on integrating genetic counseling with public health measures that address environmental risks. Genetic counseling for A/M is difficult due to the large range of associated genes and phenotypic heterogeneity. The key gene found is SOX2, and most mutations arise de novo. It is difficult to predict recurrence risk, especially when there is mosaicism or varied penetrance in monogenic cases. If the mechanism of inheritance is identified, targeted counseling can be offered, with a 10%-15% risk to siblings when no apparent reason or family history is evident[43,137]. Chromosomal abnormalities often present with distinct syndromes, and risk to siblings depends on whether the parents carry any chromosomal rearrangements[1,110]. Furthermore, advances in prenatal genetic testing, especially non-invasive prenatal testing, may allow for early diagnosis of certain mutations, aiding informed decision-making during pregnancy[43].
Public health strategies aimed at reducing environmental risks are also crucial. For example, improving maternal health through nutritional supplementation-especially with folic acid, which has been shown to reduce the risk of neural tube defects, may also reduce ocular malformations-and thus could help decrease the incidence of A/M[87]. In addition, public health campaigns aimed at reducing exposure to teratogens, such as alcohol, isotretinoin, and infections like CMV and rubella, can play an important role in prevention. Several years ago in England, there was public concern about potential clusters of A/M cases. The pesticide benomyl, and later its derivative carbendazim, were suspected of being linked to these clusters[138].
Recent advances in gene therapy, stem cell research, and regenerative medicine offer promising avenues for treating A/M. While these approaches are still in the early stages of development, they hold great potential for the future. Gene therapy, for instance, has shown success in treating retinal degenerative diseases, and similar techniques may eventually be adapted to target genetic defects responsible for A/M[114]. Correcting mutations in genes like SOX2 or OTX2 through gene editing technologies like clustered regularly interspaced short palindromic repeats-Cas9 could offer a future cure for these conditions[108]. Stem cell research also holds promise, particularly through the use of iPSCs to generate retinal cells and other ocular tissues for transplantation. Stem cell-based therapies could potentially replace lost or malformed tissues in patients with A/M, offering a regenerative approach to treatment[111]. However, significant challenges remain, including ensuring the safety and efficacy of these treatments, as well as addressing ethical concerns surrounding genetic manipulation and stem cells use.
While these therapeutic interventions offer exciting possibilities, more research is needed to fully understand their potential and to develop safe, effective treatments for A/M. Ethical considerations, such as access to these advanced therapies and the long-term consequences of gene editing, will also need to be addressed as the field progresses[114].
A/M, as severe congenital eye disorders have garnered significant attention due to their complex etiologies involving both genetic and environmental factors. Over the last decade, advances in genetic screening techniques, such as whole-exome and WGS, have significantly expanded our understanding of the genetic basis of A/M. Mutations in key developmental genes, including SOX2, OTX2, RAX, and VSX2, have been identified as primary contributors to the pathogenesis of these conditions[43,110]. Additionally, emerging evidence highlights the role of non-coding RNAs, such as miR-204 and Six3OS, in regulating critical pathways during eye development, further emphasizing the complexity of genetic regulation in A/M[103,104]. At the same time, maternal environmental exposures-including infections, teratogens, and nutritional deficiencies-have been implicated in the development of A/M. Studies have shown that maternal infections like rubella, CMV, and toxoplasmosis, as well as exposure to alcohol, isotretinoin, and folate deficiencies, can increase the risk of ocular malformations[38,87]. These findings underscore the critical need for a comprehensive approach that integrates research at both genetic and environmental level. By focusing on both prevention through public health measures and innovative therapeutic interventions, we can hope to improve outcomes for individuals affected by these rare but severe ocular malformations.
| 1. | Verma AS, Fitzpatrick DR. Anophthalmia and microphthalmia. Orphanet J Rare Dis. 2007;2:47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 280] [Cited by in RCA: 260] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 2. | Richardson R, Sowden J, Gerth-Kahlert C, Moore AT, Moosajee M. Clinical utility gene card for: Non-Syndromic Microphthalmia Including Next-Generation Sequencing-Based Approaches. Eur J Hum Genet. 2017;25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 3. | Roos L, Jensen H, Grønskov K, Holst R, Tümer Z. Congenital Microphthalmia, Anophthalmia and Coloboma among Live Births in Denmark. Ophthalmic Epidemiol. 2016;23:324-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 4. | Chassaing N, Causse A, Vigouroux A, Delahaye A, Alessandri JL, Boespflug-Tanguy O, Boute-Benejean O, Dollfus H, Duban-Bedu B, Gilbert-Dussardier B, Giuliano F, Gonzales M, Holder-Espinasse M, Isidor B, Jacquemont ML, Lacombe D, Martin-Coignard D, Mathieu-Dramard M, Odent S, Picone O, Pinson L, Quelin C, Sigaudy S, Toutain A, Thauvin-Robinet C, Kaplan J, Calvas P. Molecular findings and clinical data in a cohort of 150 patients with anophthalmia/microphthalmia. Clin Genet. 2014;86:326-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 85] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 5. | Chambers TM, Agopian AJ, Lewis RA, Langlois PH, Danysh HE, Weber KA, Shaw GM, Mitchell LE, Lupo PJ. Epidemiology of anophthalmia and microphthalmia: Prevalence and patterns in Texas, 1999-2009. Am J Med Genet A. 2018;176:1810-1818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 6. | Slavotinek AM. Eye development genes and known syndromes. Mol Genet Metab. 2011;104:448-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 86] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 7. | Spagnolo A, Bianchi F, Calabro A, Calzolari E, Clementi M, Mastroiacovo P, Meli P, Petrelli G, Tenconi R. Anophthalmia and benomyl in Italy: a multicenter study based on 940,615 newborns. Reprod Toxicol. 1994;8:397-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 19] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 8. | Tucker S, Jones B, Collin R. Systemic anomalies in 77 patients with congenital anophthalmos or microphthalmos. Eye (Lond). 1996;10 (Pt 3):310-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 33] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 9. | Morrison D, FitzPatrick D, Hanson I, Williamson K, van Heyningen V, Fleck B, Jones I, Chalmers J, Campbell H. National study of microphthalmia, anophthalmia, and coloboma (MAC) in Scotland: investigation of genetic aetiology. J Med Genet. 2002;39:16-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 192] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 10. | Williamson KA, FitzPatrick DR. The genetic architecture of microphthalmia, anophthalmia and coloboma. Eur J Med Genet. 2014;57:369-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 180] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 11. | Plaisancié J, Ceroni F, Holt R, Zazo Seco C, Calvas P, Chassaing N, Ragge NK. Genetics of anophthalmia and microphthalmia. Part 1: Non-syndromic anophthalmia/microphthalmia. Hum Genet. 2019;138:799-830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 70] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 12. | Shah SP, Taylor AE, Sowden JC, Ragge N, Russell-Eggitt I, Rahi JS, Gilbert CE; Surveillance of Eye Anomalies Special Interest Group. Anophthalmos, microphthalmos, and Coloboma in the United kingdom: clinical features, results of investigations, and early management. Ophthalmology. 2012;119:362-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 13. | Sinn R, Wittbrodt J. An eye on eye development. Mech Dev. 2013;130:347-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 96] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 14. | Chow RL, Lang RA. Early eye development in vertebrates. Annu Rev Cell Dev Biol. 2001;17:255-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 458] [Cited by in RCA: 468] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 15. | Zagozewski JL, Zhang Q, Eisenstat DD. Genetic regulation of vertebrate eye development. Clin Genet. 2014;86:453-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 16. | Zuber ME, Gestri G, Viczian AS, Barsacchi G, Harris WA. Specification of the vertebrate eye by a network of eye field transcription factors. Development. 2003;130:5155-5167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 409] [Cited by in RCA: 378] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 17. | Loosli F, Winkler S, Wittbrodt J. Six3 overexpression initiates the formation of ectopic retina. Genes Dev. 1999;13:649-654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 180] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 18. | Graw J. Eye development. Curr Top Dev Biol. 2010;90:343-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 184] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 19. | Danno H, Michiue T, Hitachi K, Yukita A, Ishiura S, Asashima M. Molecular links among the causative genes for ocular malformation: Otx2 and Sox2 coregulate Rax expression. Proc Natl Acad Sci U S A. 2008;105:5408-5413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 79] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 20. | Medina-Martinez O, Amaya-Manzanares F, Liu C, Mendoza M, Shah R, Zhang L, Behringer RR, Mahon KA, Jamrich M. Cell-autonomous requirement for rx function in the mammalian retina and posterior pituitary. PLoS One. 2009;4:e4513. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 21. | Fuhrmann S. Eye morphogenesis and patterning of the optic vesicle. Curr Top Dev Biol. 2010;93:61-84. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 326] [Cited by in RCA: 275] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 22. | Zagozewski JL, Zhang Q, Pinto VI, Wigle JT, Eisenstat DD. The role of homeobox genes in retinal development and disease. Dev Biol. 2014;393:195-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 45] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 23. | Bazin-Lopez N, Valdivia LE, Wilson SW, Gestri G. Watching eyes take shape. Curr Opin Genet Dev. 2015;32:73-79. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 24. | Cvekl A, Ashery-Padan R. The cellular and molecular mechanisms of vertebrate lens development. Development. 2014;141:4432-4447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 168] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 25. | Liu W, Lagutin OV, Mende M, Streit A, Oliver G. Six3 activation of Pax6 expression is essential for mammalian lens induction and specification. EMBO J. 2006;25:5383-5395. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 147] [Cited by in RCA: 135] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 26. | Cvekl A, McGreal R, Liu W. Lens Development and Crystallin Gene Expression. Prog Mol Biol Transl Sci. 2015;134:129-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 53] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 27. | Kim HT, Kim JW. Compartmentalization of vertebrate optic neuroephithelium: external cues and transcription factors. Mol Cells. 2012;33:317-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 28. | Furuta Y, Hogan BL. BMP4 is essential for lens induction in the mouse embryo. Genes Dev. 1998;12:3764-3775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 321] [Cited by in RCA: 334] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 29. | Yun S, Saijoh Y, Hirokawa KE, Kopinke D, Murtaugh LC, Monuki ES, Levine EM. Lhx2 links the intrinsic and extrinsic factors that control optic cup formation. Development. 2009;136:3895-3906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 77] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 30. | Hyer J, Kuhlman J, Afif E, Mikawa T. Optic cup morphogenesis requires pre-lens ectoderm but not lens differentiation. Dev Biol. 2003;259:351-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 85] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 31. | Cvekl A, Wang WL. Retinoic acid signaling in mammalian eye development. Exp Eye Res. 2009;89:280-291. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 152] [Cited by in RCA: 146] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 32. | Mic FA, Molotkov A, Molotkova N, Duester G. Raldh2 expression in optic vesicle generates a retinoic acid signal needed for invagination of retina during optic cup formation. Dev Dyn. 2004;231:270-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 76] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 33. | Molotkov A, Molotkova N, Duester G. Retinoic acid guides eye morphogenetic movements via paracrine signaling but is unnecessary for retinal dorsoventral patterning. Development. 2006;133:1901-1910. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 175] [Cited by in RCA: 167] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 34. | Matt N, Ghyselinck NB, Pellerin I, Dupé V. Impairing retinoic acid signalling in the neural crest cells is sufficient to alter entire eye morphogenesis. Dev Biol. 2008;320:140-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 83] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 35. | Chauhan BK, Disanza A, Choi SY, Faber SC, Lou M, Beggs HE, Scita G, Zheng Y, Lang RA. Cdc42- and IRSp53-dependent contractile filopodia tether presumptive lens and retina to coordinate epithelial invagination. Development. 2009;136:3657-3667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 79] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 36. | Trainor PA. Craniofacial birth defects: The role of neural crest cells in the etiology and pathogenesis of Treacher Collins syndrome and the potential for prevention. Am J Med Genet A. 2010;152A:2984-2994. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 148] [Cited by in RCA: 137] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 37. | Shaham O, Menuchin Y, Farhy C, Ashery-Padan R. Pax6: a multi-level regulator of ocular development. Prog Retin Eye Res. 2012;31:351-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 179] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 38. | Harding P, Moosajee M. The Molecular Basis of Human Anophthalmia and Microphthalmia. J Dev Biol. 2019;7:16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 39. | Seo S, Chen L, Liu W, Zhao D, Schultz KM, Sasman A, Liu T, Zhang HF, Gage PJ, Kume T. Foxc1 and Foxc2 in the Neural Crest Are Required for Ocular Anterior Segment Development. Invest Ophthalmol Vis Sci. 2017;58:1368-1377. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 40. | Liu P, Johnson RL. Lmx1b is required for murine trabecular meshwork formation and for maintenance of corneal transparency. Dev Dyn. 2010;239:2161-2171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 41. | Fantes J, Ragge NK, Lynch SA, McGill NI, Collin JR, Howard-Peebles PN, Hayward C, Vivian AJ, Williamson K, van Heyningen V, FitzPatrick DR. Mutations in SOX2 cause anophthalmia. Nat Genet. 2003;33:461-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 365] [Cited by in RCA: 378] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 42. | Glaser T, Walton DS, Maas RL. Genomic structure, evolutionary conservation and aniridia mutations in the human PAX6 gene. Nat Genet. 1992;2:232-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 426] [Cited by in RCA: 441] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 43. | Ragge NK, Brown AG, Poloschek CM, Lorenz B, Henderson RA, Clarke MP, Russell-Eggitt I, Fielder A, Gerrelli D, Martinez-Barbera JP, Ruddle P, Hurst J, Collin JR, Salt A, Cooper ST, Thompson PJ, Sisodiya SM, Williamson KA, Fitzpatrick DR, van Heyningen V, Hanson IM. Heterozygous mutations of OTX2 cause severe ocular malformations. Am J Hum Genet. 2005;76:1008-1022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 237] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 44. | Ferda Percin E, Ploder LA, Yu JJ, Arici K, Horsford DJ, Rutherford A, Bapat B, Cox DW, Duncan AM, Kalnins VI, Kocak-Altintas A, Sowden JC, Traboulsi E, Sarfarazi M, McInnes RR. Human microphthalmia associated with mutations in the retinal homeobox gene CHX10. Nat Genet. 2000;25:397-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 185] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 45. | Voronina VA, Kozhemyakina EA, O'Kernick CM, Kahn ND, Wenger SL, Linberg JV, Schneider AS, Mathers PH. Mutations in the human RAX homeobox gene in a patient with anophthalmia and sclerocornea. Hum Mol Genet. 2004;13:315-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 150] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 46. | Freund CL, Wang QL, Chen S, Muskat BL, Wiles CD, Sheffield VC, Jacobson SG, McInnes RR, Zack DJ, Stone EM. De novo mutations in the CRX homeobox gene associated with Leber congenital amaurosis. Nat Genet. 1998;18:311-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 195] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 47. | Reis LM, Sorokina EA, Dudakova L, Moravikova J, Skalicka P, Malinka F, Seese SE, Thompson S, Bardakjian T, Capasso J, Allen W, Glaser T, Levin AV, Schneider A, Khan A, Liskova P, Semina EV. Comprehensive phenotypic and functional analysis of dominant and recessive FOXE3 alleles in ocular developmental disorders. Hum Mol Genet. 2021;30:1591-1606. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 48. | Roessler E, Belloni E, Gaudenz K, Jay P, Berta P, Scherer SW, Tsui LC, Muenke M. Mutations in the human Sonic Hedgehog gene cause holoprosencephaly. Nat Genet. 1996;14:357-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 883] [Cited by in RCA: 817] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 49. | Asai-Coakwell M, French CR, Berry KM, Ye M, Koss R, Somerville M, Mueller R, van Heyningen V, Waskiewicz AJ, Lehmann OJ. GDF6, a novel locus for a spectrum of ocular developmental anomalies. Am J Hum Genet. 2007;80:306-315. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 88] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 50. | Bakrania P, Efthymiou M, Klein JC, Salt A, Bunyan DJ, Wyatt A, Ponting CP, Martin A, Williams S, Lindley V, Gilmore J, Restori M, Robson AG, Neveu MM, Holder GE, Collin JR, Robinson DO, Farndon P, Johansen-Berg H, Gerrelli D, Ragge NK. Mutations in BMP4 cause eye, brain, and digit developmental anomalies: overlap between the BMP4 and hedgehog signaling pathways. Am J Hum Genet. 2008;82:304-319. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 207] [Cited by in RCA: 199] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 51. | Pasutto F, Sticht H, Hammersen G, Gillessen-Kaesbach G, Fitzpatrick DR, Nürnberg G, Brasch F, Schirmer-Zimmermann H, Tolmie JL, Chitayat D, Houge G, Fernández-Martínez L, Keating S, Mortier G, Hennekam RC, von der Wense A, Slavotinek A, Meinecke P, Bitoun P, Becker C, Nürnberg P, Reis A, Rauch A. Mutations in STRA6 cause a broad spectrum of malformations including anophthalmia, congenital heart defects, diaphragmatic hernia, alveolar capillary dysplasia, lung hypoplasia, and mental retardation. Am J Hum Genet. 2007;80:550-560. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 283] [Cited by in RCA: 265] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 52. | Tassabehji M, Read AP, Newton VE, Harris R, Balling R, Gruss P, Strachan T. Waardenburg's syndrome patients have mutations in the human homologue of the Pax-3 paired box gene. Nature. 1992;355:635-636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 512] [Cited by in RCA: 477] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 53. | Ng SB, Bigham AW, Buckingham KJ, Hannibal MC, McMillin MJ, Gildersleeve HI, Beck AE, Tabor HK, Cooper GM, Mefford HC, Lee C, Turner EH, Smith JD, Rieder MJ, Yoshiura K, Matsumoto N, Ohta T, Niikawa N, Nickerson DA, Bamshad MJ, Shendure J. Exome sequencing identifies MLL2 mutations as a cause of Kabuki syndrome. Nat Genet. 2010;42:790-793. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1075] [Cited by in RCA: 976] [Article Influence: 65.1] [Reference Citation Analysis (0)] |
| 54. | Cecconi F, Alvarez-Bolado G, Meyer BI, Roth KA, Gruss P. Apaf1 (CED-4 homolog) regulates programmed cell death in mammalian development. Cell. 1998;94:727-737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 679] [Cited by in RCA: 682] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 55. | Yu Z, Yu W, Liu J, Wu D, Wang C, Zhang J, Zhao J. Lens-specific deletion of the Msx2 gene increased apoptosis by enhancing the caspase-3/caspase-8 signaling pathway. J Int Med Res. 2018;46:2843-2855. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 56. | Capowski EE, Wright LS, Liang K, Phillips MJ, Wallace K, Petelinsek A, Hagstrom A, Pinilla I, Borys K, Lien J, Min JH, Keles S, Thomson JA, Gamm DM. Regulation of WNT Signaling by VSX2 During Optic Vesicle Patterning in Human Induced Pluripotent Stem Cells. Stem Cells. 2016;34:2625-2634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 57. | Phillips MJ, Perez ET, Martin JM, Reshel ST, Wallace KA, Capowski EE, Singh R, Wright LS, Clark EM, Barney PM, Stewart R, Dickerson SJ, Miller MJ, Percin EF, Thomson JA, Gamm DM. Modeling human retinal development with patient-specific induced pluripotent stem cells reveals multiple roles for visual system homeobox 2. Stem Cells. 2014;32:1480-1492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 95] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 58. | Bryan CD, Casey MA, Pfeiffer RL, Jones BW, Kwan KM. Optic cup morphogenesis requires neural crest-mediated basement membrane assembly. Development. 2020;147:dev181420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 59. | Casey MA, Lusk S, Kwan KM. Build me up optic cup: Intrinsic and extrinsic mechanisms of vertebrate eye morphogenesis. Dev Biol. 2021;476:128-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 60. | Serjanov D, Bachay G, Hunter DD, Brunken WJ. Laminin β2 Chain Regulates Retinal Progenitor Cell Mitotic Spindle Orientation via Dystroglycan. J Neurosci. 2018;38:5996-6010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 61. | Lee JH, Park HS, Holmes DP. Elastic Instabilities Govern the Morphogenesis of the Optic Cup. Phys Rev Lett. 2021;127:138102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 62. | Oltean A, Huang J, Beebe DC, Taber LA. Tissue growth constrained by extracellular matrix drives invagination during optic cup morphogenesis. Biomech Model Mechanobiol. 2016;15:1405-1421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 63. | Bryan CD, Chien CB, Kwan KM. Loss of laminin alpha 1 results in multiple structural defects and divergent effects on adhesion during vertebrate optic cup morphogenesis. Dev Biol. 2016;416:324-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 64. | Guo Y, Wang P, Ma JH, Cui Z, Yu Q, Liu S, Xue Y, Zhu D, Cao J, Li Z, Tang S, Chen J. Modeling Retinitis Pigmentosa: Retinal Organoids Generated From the iPSCs of a Patient With the USH2A Mutation Show Early Developmental Abnormalities. Front Cell Neurosci. 2019;13:361. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 58] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 65. | Carrara N, Weaver M, Piedade WP, Vöcking O, Famulski JK. Temporal characterization of optic fissure basement membrane composition suggests nidogen may be an initial target of remodeling. Dev Biol. 2019;452:43-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 66. | Yeh LK, Liu CY, Kao WW, Huang CJ, Hu FR, Chien CL, Wang IJ. Knockdown of zebrafish lumican gene (zlum) causes scleral thinning and increased size of scleral coats. J Biol Chem. 2010;285:28141-28155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 43] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 67. | Song Y, Zhang F, Zhao Y, Sun M, Tao J, Liang Y, Ma L, Yu Y, Wang J, Hao J. Enlargement of the Axial Length and Altered Ultrastructural Features of the Sclera in a Mutant Lumican Transgenic Mouse Model. PLoS One. 2016;11:e0163165. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 68. | Deml B, Reis LM, Maheshwari M, Griffis C, Bick D, Semina EV. Whole exome analysis identifies dominant COL4A1 mutations in patients with complex ocular phenotypes involving microphthalmia. Clin Genet. 2014;86:475-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 69. | Eamegdool SS, Sitiwin EI, Cioanca AV, Madigan MC. Extracellular matrix and oxidative stress regulate human retinal pigment epithelium growth. Free Radic Biol Med. 2020;146:357-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 70. | Lambert S, Hoyt C. Ocular manifestations of intrauterine infections. Paediatric Ophthalmology Boston: Blackwell Scientific, 1990: 91-102. |
| 71. | Warburg M. Update of sporadic microphthalmos and coloboma. Non-inherited anomalies. Ophthalmic Paediatr Genet. 1992;13:111-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 72. | Hartwig NG, Vermeij-Keers C, Van Elsacker-Niele AM, Fleuren GJ. Embryonic malformations in a case of intrauterine parvovirus B19 infection. Teratology. 1989;39:295-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 34] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 73. | Weiland HT, Vermey-Keers C, Salimans MM, Fleuren GJ, Verwey RA, Anderson MJ. Parvovirus B19 associated with fetal abnormality. Lancet. 1987; 1: 682-683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 76] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 74. | Von Herzen JL, Benirschke K. Unexpected disseminated herpes simplex infection in a newborn. Obstet Gynecol. 1977;50:728-730. [PubMed] |
| 75. | Eye BM. Birth defects encyclopaedia. Massachusetts: Center for Birth Defects Information Services; 1990. |
| 76. | Leck I. Book Reviews. Int J Epidemiol. 1992;21:427-428. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 77. | Edwards MJ. Hyperthermia as a teratogen: a review of experimental studies and their clinical significance. Teratog Carcinog Mutagen. 1986;6:563-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 163] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 78. | Jones KL, Jones MC, Del Campo M. Smith's recognizable patterns of human malformation. Acta Peadiatrica Promoting Child Health. 2009;98:205. |
| 79. | Spraggett K. Teratogenicity of maternal fever in women‐a retrospective study. Teratology. 1982;25:78A. |
| 80. | Fraser FC, Skelton J. Possible teratogenicity of maternal fever. Lancet. 1978;2:634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 49] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 81. | Sulik KK, Cook CS, Webster WS. Teratogens and craniofacial malformations: relationships to cell death. Development. 1988;103 Suppl:213-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 238] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 82. | Donald JM, Hooper K, Hopenhayn-Rich C. Reproductive and developmental toxicity of toluene: a review. Environ Health Perspect. 1991;94:237-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 23] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 83. | Običan S, Scialli AR. Teratogenic exposures. Am J Med Genet C Semin Med Genet. 2011;157C:150-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 84. | Taskinen H, Kyyrönen P, Hemminki K, Hoikkala M, Lajunen K, Lindbohm ML. Laboratory work and pregnancy outcome. J Occup Med. 1994;36:311-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 68] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 85. | Windham GC, Shusterman D, Swan SH, Fenster L, Eskenazi B. Exposure to organic solvents and adverse pregnancy outcome. Am J Ind Med. 1991;20:241-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 44] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 86. | Barber AN. The effects of maternal hypoxia on inheritance of recessive blindness in mice. Am J Ophthalmol. 1957;44:94-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 87. | Spiegelstein O, Cabrera RM, Bozinov D, Wlodarczyk B, Finnell RH. Folate-regulated changes in gene expression in the anterior neural tube of folate binding protein-1 (Folbp1)-deficient murine embryos. Neurochem Res. 2004;29:1105-1112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 88. | Kochhar D. Retinoids and fetal malformations. In: Sharma RP, editor. Dietary factors and birth defects. United States: Semantic Scholar, 1993:134-230. |
| 89. | Nielsen JN, Carlton WW. Colobomatous microphthalmos in a New Zealand white rabbit, arising from a colony with suspected vitamin E deficiency. Lab Anim Sci. 1995;45:320-322. [PubMed] |
| 90. | Russell RM, Cox ME, Solomons N. Zinc and the special senses. Ann Intern Med. 1983;99:227-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 45] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 91. | Dibley MJ, Jeacocke DA. Safety and Toxicity of Vitamin A Supplements in Pregnancy. Food Nutr Bull. 2001;22:248-266. [RCA] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 92. | Hale F. Pigs Born without Eye Balls. In: Persaud TVN, editor. Problems of Birth Defects. Berlin: Springer, 1933: 166-167. [DOI] [Full Text] |
| 93. | SARMA V. Maternal vitamin A deficiency and fetal microcephaly and anophthalmia; report of a case. Obstet Gynecol. 1959;13:299-301. [PubMed] |
| 94. | Lamba PA, Sood NN. Congenital Microphthalmos and Colobomata in Maternal Vitamin A Deficiency. J Pediatr Ophthalmol Strabismus. 1968;5:115-117. [DOI] [Full Text] |
| 95. | World Health Organization Nutrition Unit; Organization WH. Safe vitamin A dosage during pregnancy and lactation: recommendations and report of a consultation. Geneva Switzerland Who Programme of Nutrition. 1998;. |
| 96. | McGuire S. WHO Guideline: Vitamin A supplementation in pregnant women. Geneva: WHO, 2011; WHO Guideline: Vitamin A supplementation in postpartum women. Geneva: WHO, 2011. Adv Nutr. 2012;3:215-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 97. | Joubert BR, Felix JF, Yousefi P, Bakulski KM, Just AC, Breton C, Reese SE, Markunas CA, Richmond RC, Xu CJ, Küpers LK, Oh SS, Hoyo C, Gruzieva O, Söderhäll C, Salas LA, Baïz N, Zhang H, Lepeule J, Ruiz C, Ligthart S, Wang T, Taylor JA, Duijts L, Sharp GC, Jankipersadsing SA, Nilsen RM, Vaez A, Fallin MD, Hu D, Litonjua AA, Fuemmeler BF, Huen K, Kere J, Kull I, Munthe-Kaas MC, Gehring U, Bustamante M, Saurel-Coubizolles MJ, Quraishi BM, Ren J, Tost J, Gonzalez JR, Peters MJ, Håberg SE, Xu Z, van Meurs JB, Gaunt TR, Kerkhof M, Corpeleijn E, Feinberg AP, Eng C, Baccarelli AA, Benjamin Neelon SE, Bradman A, Merid SK, Bergström A, Herceg Z, Hernandez-Vargas H, Brunekreef B, Pinart M, Heude B, Ewart S, Yao J, Lemonnier N, Franco OH, Wu MC, Hofman A, McArdle W, Van der Vlies P, Falahi F, Gillman MW, Barcellos LF, Kumar A, Wickman M, Guerra S, Charles MA, Holloway J, Auffray C, Tiemeier HW, Smith GD, Postma D, Hivert MF, Eskenazi B, Vrijheid M, Arshad H, Antó JM, Dehghan A, Karmaus W, Annesi-Maesano I, Sunyer J, Ghantous A, Pershagen G, Holland N, Murphy SK, DeMeo DL, Burchard EG, Ladd-Acosta C, Snieder H, Nystad W, Koppelman GH, Relton CL, Jaddoe VW, Wilcox A, Melén E, London SJ. DNA Methylation in Newborns and Maternal Smoking in Pregnancy: Genome-wide Consortium Meta-analysis. Am J Hum Genet. 2016;98:680-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 738] [Cited by in RCA: 631] [Article Influence: 70.1] [Reference Citation Analysis (0)] |
| 98. | Hsiao TH, Lee GH, Chang YS, Chen BH, Fu TF. The Incoherent Fluctuation of Folate Pools and Differential Regulation of Folate Enzymes Prioritize Nucleotide Supply in the Zebrafish Model Displaying Folate Deficiency-Induced Microphthalmia and Visual Defects. Front Cell Dev Biol. 2021;9:702969. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 99. | Breton-Larrivée M, Elder E, Legault LM, Langford-Avelar A, MacFarlane AJ, McGraw S. Mitigating the detrimental developmental impact of early fetal alcohol exposure using a maternal methyl donor-enriched diet. FASEB J. 2023;37:e22829. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 7] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 100. | Basinski BW, Balikov DA, Aksu M, Li Q, Rao RC. Ubiquitous Chromatin Modifiers in Congenital Retinal Diseases: Implications for Disease Modeling and Regenerative Medicine. Trends Mol Med. 2021;27:365-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 101. | Iida A, Iwagawa T, Baba Y, Satoh S, Mochizuki Y, Nakauchi H, Furukawa T, Koseki H, Murakami A, Watanabe S. Roles of histone H3K27 trimethylase Ezh2 in retinal proliferation and differentiation. Dev Neurobiol. 2015;75:947-960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 102. | Aref-Eshghi E, Schenkel LC, Lin H, Skinner C, Ainsworth P, Paré G, Rodenhiser D, Schwartz C, Sadikovic B. The defining DNA methylation signature of Kabuki syndrome enables functional assessment of genetic variants of unknown clinical significance. Epigenetics. 2017;12:923-933. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 76] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 103. | Conte I, Carrella S, Avellino R, Karali M, Marco-Ferreres R, Bovolenta P, Banfi S. miR-204 is required for lens and retinal development via Meis2 targeting. Proc Natl Acad Sci U S A. 2010;107:15491-15496. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 139] [Cited by in RCA: 141] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 104. | Geng X, Acosta S, Lagutin O, Gil HJ, Oliver G. Six3 dosage mediates the pathogenesis of holoprosencephaly. Development. 2016;143:4462-4473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 105. | Ng SY, Bogu GK, Soh BS, Stanton LW. The long noncoding RNA RMST interacts with SOX2 to regulate neurogenesis. Mol Cell. 2013;51:349-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 327] [Cited by in RCA: 341] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 106. | La Torre A, Georgi S, Reh TA. Conserved microRNA pathway regulates developmental timing of retinal neurogenesis. Proc Natl Acad Sci U S A. 2013;110:E2362-E2370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 171] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 107. | Ragge NK, Subak-Sharpe ID, Collin JR. A practical guide to the management of anophthalmia and microphthalmia. Eye (Lond). 2007;21:1290-1300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 80] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 108. | Reis LM, Semina EV. Conserved genetic pathways associated with microphthalmia, anophthalmia, and coloboma. Birth Defects Res C Embryo Today. 2015;105:96-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 54] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 109. | Vargesson N. Thalidomide-induced teratogenesis: history and mechanisms. Birth Defects Res C Embryo Today. 2015;105:140-156. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 422] [Cited by in RCA: 517] [Article Influence: 51.7] [Reference Citation Analysis (0)] |
| 110. | Kelberman D, de Castro SC, Huang S, Crolla JA, Palmer R, Gregory JW, Taylor D, Cavallo L, Faienza MF, Fischetto R, Achermann JC, Martinez-Barbera JP, Rizzoti K, Lovell-Badge R, Robinson IC, Gerrelli D, Dattani MT. SOX2 plays a critical role in the pituitary, forebrain, and eye during human embryonic development. J Clin Endocrinol Metab. 2008;93:1865-1873. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 135] [Cited by in RCA: 109] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 111. | Lamba DA, Karl MO, Ware CB, Reh TA. Efficient generation of retinal progenitor cells from human embryonic stem cells. Proc Natl Acad Sci U S A. 2006;103:12769-12774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 508] [Cited by in RCA: 507] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 112. | Harding P, Lima Cunha D, Méjécase C, Eintracht J, Toualbi L, Sarkar H, Moosajee M. Generation of human iPSC line (UCLi013-A) from a patient with microphthalmia and aniridia, carrying a heterozygous missense mutation c.372C>A p.(Asn124Lys) in PAX6. Stem Cell Res. 2021;51:102184. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 113. | Eintracht J, Owen N, Harding P, Moosajee M. Disruption of common ocular developmental pathways in patient-derived optic vesicle models of microphthalmia. Stem Cell Reports. 2024;19:839-858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 114. | Shirai H, Mandai M, Matsushita K, Kuwahara A, Yonemura S, Nakano T, Assawachananont J, Kimura T, Saito K, Terasaki H, Eiraku M, Sasai Y, Takahashi M. Transplantation of human embryonic stem cell-derived retinal tissue in two primate models of retinal degeneration. Proc Natl Acad Sci U S A. 2016;113:E81-E90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 246] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 115. | Harding P, Gore S, Malka S, Rajkumar J, Oluonye N, Moosajee M. Real-world clinical and molecular management of 50 prospective patients with microphthalmia, anophthalmia and/or ocular coloboma. Br J Ophthalmol. 2023;107:1925-1935. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 15] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 116. | Alamillo CL, Powis Z, Farwell K, Shahmirzadi L, Weltmer EC, Turocy J, Lowe T, Kobelka C, Chen E, Basel D, Ashkinadze E, D'Augelli L, Chao E, Tang S. Exome sequencing positively identified relevant alterations in more than half of cases with an indication of prenatal ultrasound anomalies. Prenat Diagn. 2015;35:1073-1078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 73] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 117. | Best S, Wou K, Vora N, Van der Veyver IB, Wapner R, Chitty LS. Promises, pitfalls and practicalities of prenatal whole exome sequencing. Prenat Diagn. 2018;38:10-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 241] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 118. | Carss KJ, Hillman SC, Parthiban V, McMullan DJ, Maher ER, Kilby MD, Hurles ME. Exome sequencing improves genetic diagnosis of structural fetal abnormalities revealed by ultrasound. Hum Mol Genet. 2014;23:3269-3277. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 123] [Cited by in RCA: 139] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 119. | Harding P, Brooks BP, FitzPatrick D, Moosajee M. Anophthalmia including next-generation sequencing-based approaches. Eur J Hum Genet. 2020;28:388-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 120. | Yadava SM, Ashkinadze E. Whole exome sequencing for prenatal diagnosis in cases with fetal anomalies: Criteria to improve diagnostic yield. J Genet Couns. 2019;28:251-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 121. | Fares-Taie L, Gerber S, Chassaing N, Clayton-Smith J, Hanein S, Silva E, Serey M, Serre V, Gérard X, Baumann C, Plessis G, Demeer B, Brétillon L, Bole C, Nitschke P, Munnich A, Lyonnet S, Calvas P, Kaplan J, Ragge N, Rozet JM. ALDH1A3 mutations cause recessive anophthalmia and microphthalmia. Am J Hum Genet. 2013;92:265-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 83] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 122. | Eintracht J, Toms M, Moosajee M. The Use of Induced Pluripotent Stem Cells as a Model for Developmental Eye Disorders. Front Cell Neurosci. 2020;14:265. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 123. | Alkatan HM, Bedaiwi KM, Al-Faky YH, Maktabi AMY. Demographics and histopathological characteristics of enucleated microphthalmic globes. Sci Rep. 2022;12:5283. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 124. | Diomande IA, Toure A, Koffi KV, Diomande GF, Djiguimde WP, Habib N, Ahnoux-Zabsonre A. Anophthalmia and serious microphthalmia: a summary of the problems associated with antenatal diagnosis and therapeutic refunding in Sub-Saharan Africa. Int Med Case Rep J. 2015;8:287-290. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 125. | Suman S, Kumar A, Rathod HU, Yadav T. Bilateral severe microphthalmos with bilateral colobomatus orbitopalpebral cyst: accessibility of speciality eye-care and rehabilitation services in low and middle-income countries. BMJ Case Rep. 2021;14:e241783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 126. | Onebunne EO, Ugalahi MO, Olusanya BA, Baiyeroju AM. Bilateral Congenital Anophthalmia. NJM. 2022;31:106-109. [RCA] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 127. | Tibrewal S, Subhedar K, Sen P, Mohan A, Singh S, Shah C, Nischal KK, Ganesh S; Bodhya Eye Consortium. Clinical spectrum of non-syndromic microphthalmos, anophthalmos and coloboma in the paediatric population: a multicentric study from North India. Br J Ophthalmol. 2021;105:897-903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 128. | Song P, Adeloye D, Li S, Zhao D, Ye X, Pan Q, Qiu Y, Zhang R, Rudan I; Global Health Epidemiology Research Group (GHERG). The prevalence of vitamin A deficiency and its public health significance in children in low- and middle-income countries: A systematic review and modelling analysis. J Glob Health. 2023;13:04084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 129. | Hanson C, Lyden E, Abresch C, Anderson-Berry A. Serum Retinol Concentrations, Race, and Socioeconomic Status in of Women of Childbearing Age in the United States. Nutrients. 2016;8:508. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 130. | Das AV, Rauniyar D, Chaurasia S, Jalali S, Padhi TR. Clinical and Demographic Profile of Uveal Coloboma: A Hospital-Based Study of 14,371 Eyes of 9557 Indian Patients. Am J Ophthalmol. 2022;242:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 131. | Hornby SJ, Ward SJ, Gilbert CE, Dandona L, Foster A, Jones RB. Environmental risk factors in congenital malformations of the eye. Ann Trop Paediatr. 2002;22:67-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 35] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 132. | Hornby SJ, Ward SJ, Gilbert CE. Eye birth defects in humans may be caused by a recessively-inherited genetic predisposition to the effects of maternal vitamin A deficiency during pregnancy. Med Sci Monit. 2003;9:HY23-HY26. [PubMed] |
| 133. | Moreno-Caviedes FH, Velez Cuellar N, Caicedo Zapata M, Triana Reina G, Sánchez A. Characterization of Eyeball Loss in Four Cities of Colombia. Cureus. 2017;9:e1677. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 134. | Mulder N, Abimiku A, Adebamowo SN, de Vries J, Matimba A, Olowoyo P, Ramsay M, Skelton M, Stein DJ. H3Africa: current perspectives. Pharmgenomics Pers Med. 2018;11:59-66. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 98] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 135. | Perez K, Wisniewski D, Ari A, Lee K, Lieneck C, Ramamonjiarivelo Z. Investigation into Application of AI and Telemedicine in Rural Communities: A Systematic Literature Review. Healthcare (Basel). 2025;13:324. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 136. | World Health Organization. Community genetics services: report of a WHO consultation on community genetics in low-and middle-income countries [Internet]. Geneva: World Health Organization, 2010. |
| 137. | Faivre L, Williamson KA, Faber V, Laurent N, Grimaldi M, Thauvin-Robinet C, Durand C, Mugneret F, Gouyon JB, Bron A, Huet F, Hayward C, Heyningen Vv, Fitzpatrick DR. Recurrence of SOX2 anophthalmia syndrome with gonosomal mosaicism in a phenotypically normal mother. Am J Med Genet A. 2006;140:636-639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 44] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 138. | Mariman EC. Clustering of anophthalmia and microphthalmia. No clustering has been found-but a link seems to exist with population density. BMJ. 1998;317:895-896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |