Published online Jun 9, 2025. doi: 10.5409/wjcp.v14.i2.101663
Revised: December 15, 2024
Accepted: February 21, 2025
Published online: June 9, 2025
Processing time: 176 Days and 17.3 Hours
Cow's milk allergy (CMA) is a common condition in infants, requiring alternative protein sources in their diets. Soya milk has become a popular substitute, espe
To determine how often infants diagnosed with CMA also had soya cross-allergy and to examine the symptoms and outcomes of these infants at 2 years of age.
Data from two pediatric centers were analyzed, looking at clinical records of children under 2 years old diagnosed with CMA from August 2015 to July 2023, divided into two four-year periods.
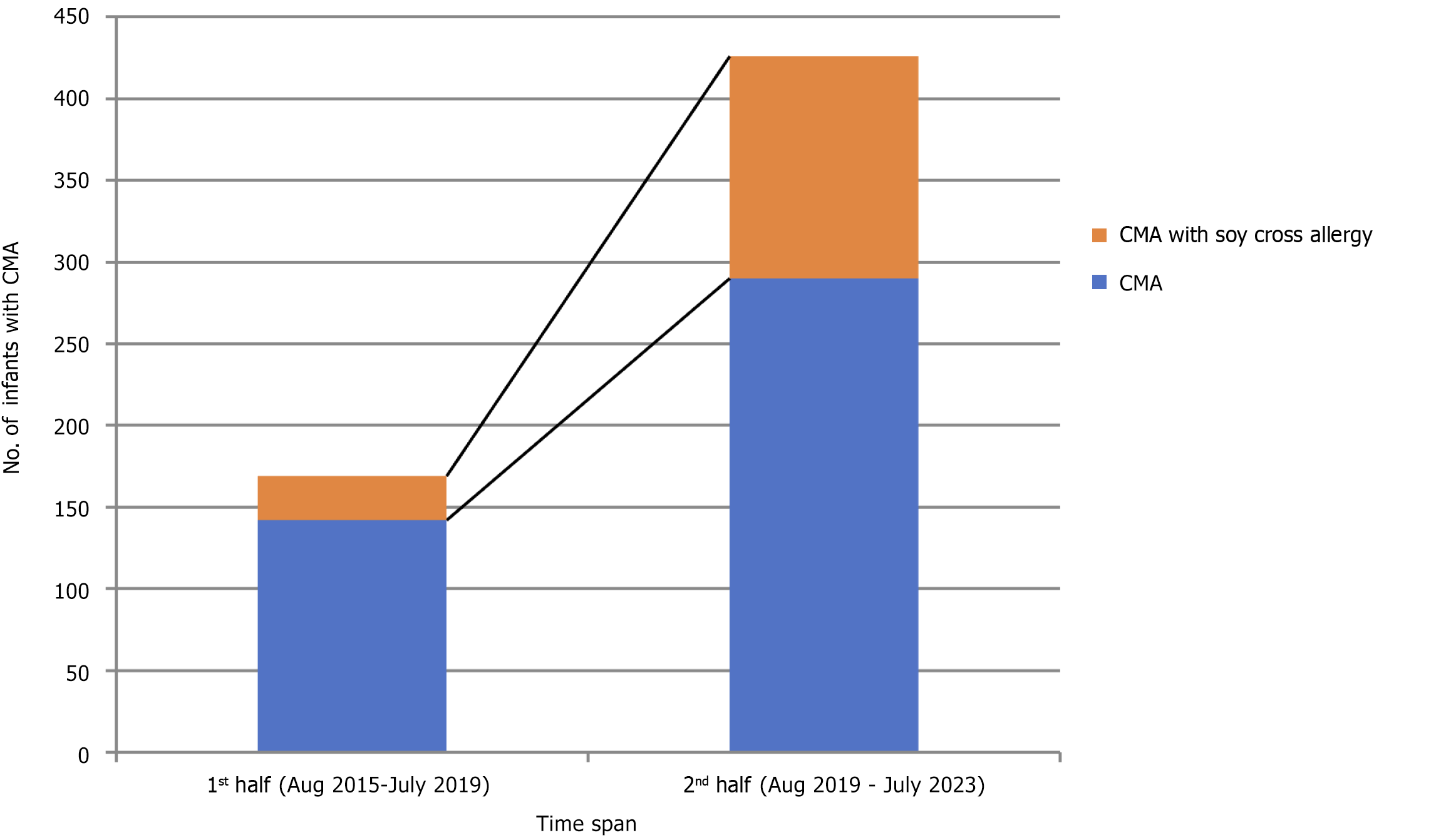
The records of 432 infants with CMA were analyzed. In the first four-year period from August 2015 to July 2019, 142 infants were studied, with 27 (19%) found to have soya-protein allergy as well. In the second four-year period, a total of 290 infants were studied, and soya allergy was found in 136 babies (47%). This represents a significant increase (P < 0.0001) in cases of soya protein cross-allergy among infants with CMA. The most common symptoms observed were gastro
In conclusion, this study emphasizes the importance of being cautious when using soy protein in infants with cow's milk protein allergy, especially in areas where cost is a major concern.
Core Tip: This is a unique retrospective observational study showing a rising trend of soya cross allergy. Caution is advised when using soy milk as a substitute for infants with cow's milk allergy (CMA). This recent study shows a significant increase in soy protein cross-allergies among CMA infants, affecting nearly 47% of them. It is crucial to regularly monitor, accurately diagnose, and create personalized feeding plans for the best outcomes.
- Citation: Acharyya BC, Mukhopadhyay M. Escalation of soya cross-allergy in infants with cow's milk allergy. World J Clin Pediatr 2025; 14(2): 101663
- URL: https://www.wjgnet.com/2219-2808/full/v14/i2/101663.htm
- DOI: https://dx.doi.org/10.5409/wjcp.v14.i2.101663
Cow's milk allergy (CMA) is a prevalent condition affecting infants, necessitating alternative protein sources in their diets. In India, the awareness of CMA is low, leading to misdiagnosis and delay in diagnosis. The prevalence of CMA peaks in infancy (1.5%-3%) and falls below 1% around 6 years of age. Soya milk had emerged as a common substitute. It was being widely used in developing countries as a cheaper option compared to the expensive hypoallergic feeds for infants facing insufficient breast milk supply. However, soya protein is not hypoallergic[1]. The incidence of soy protein allergy in soy formula-fed infants is comparable to that of CMA in cow’s milk formula-fed infants[2]. A recent observation indicated a notable rise in soya cross-allergic reactions among infants with CMA at our center.
So, this multi-center retrospective study was planned to estimate the frequency of soya cross-allergy in infants diagnosed with CMA and to investigate the spectrum of presentation and outcome of these infants at 2 years of age.
A thorough analysis was undertaken at two tertiary pediatric centers, encompassing clinical records of children below 2 years diagnosed with cow's milk allergy (CMA); spanning from August 2015 to July 2023. The data was segmented into two spans of four years each: One from August 2015 to July 2019 and the 2nd was from August 2019 to July 2023. CMA and Soya allergies were diagnosed by elimination and rechallenge except in infants with food protein-induced enterocolitis syndrome (FPIES) or Anaphylaxis. CMA was not differentiated into IgE-mediated, non-IgE-mediated, or mixed as neither IgE analysis against milk protein /soy protein nor was a skin prick test undertaken at the initial diagnosis. Symptoms and signs at presentation and follow-up records were noted for all the children. Follow-up data was noted for all children up to 2 years of age since diagnosis. An ethical clearance waiver was obtained from a case record analysis perspective.
The study analyzed the case records of 432 infants diagnosed with CMA. In the first four-year period from August 2015 to July 2019, 142 infants were identified, with 27 (19%) showing an allergy to soya protein. In the second half of the same four-year period, a total of 290 infants were studied, and soya allergy was found in 136 babies (47%). This indicates a significant increase (P < 0.0001) in cases of soya protein cross-allergy among infants with CMA (Figure 1). The most common clinical presentations were gastroesophageal reflux disorder (39%), followed by failure to thrive, bloody diarrhea, watery diarrhea, and constipation (Table 1). At 2 years, these infants showed noticeable growth failure compared to infants suffering from CMA alone. Three hundred and ninety-three children (91%) of children in both segments outgrown the CMA and soya cross allergy when the Indian Milk Ladder[3] was introduced after 2 years.
| Clinical features | 1st 4 years (n = 27) | 2nd 4 years (n = 136) | ||
| GERD | 10 | 37 | 48 | 35 |
| Failure to thrive | 7 | 26 | 35 | 26 |
| Bloody diarrhoea | 4 | 15 | 18 | 13 |
| Watery diarrhoea | 4 | 15 | 19 | 14 |
| Constipation | 2 | 7 | 16 | 12 |
Food allergy has been referred to as the second wave of the allergy epidemic, with asthma being the first[4]. The incidence of food protein allergies, especially cow’s milk protein allergies, is on the rise in India. This study found a rise in soya cross-allergy over time, especially post-coronavirus disease 2019 (COVID-19) pandemic. So far, no studies have described this kind of comparative data between two designated periods. Soya allergy among infants with CMA was 19% before August 2019 which increased to 47% after that.
Adverse reactions to soy have been reported in 10%-35% of infants with CMA, regardless of whether or not they were positive or negative for specific IgE antibodies against cow's milk[3]. In particular, infants with multiple food allergies and eosinophilic enterocolitis syndrome react to formulas that include soy protein[5]. Nowak-Wegrzyn et al[6] reported a higher percentage ( 30%-50%) of soya cross allergy in infants with FPIES compared to the non-United States studies[6,7]. The ESPGHAN Committee on Nutrition[8] and the AAP released a commentary and clinical report, respectively, based on studies by Klemola et al[5] and Zeiger et al[9]. They advised against the use of soy infant formula, especially for infants under 6 months old, due to the risk of co-allergy. This recommendation was based on data from a small subgroup of 20 infants[3]. Ahn et al[10], in their study from Korea, depicted a soya protein cross-sensitization in 18.3% of children with IgE-mediated CMA. However, they found that the. prevalence of non-IgE-associated soy protein allergy in CMA children was 42.9%.
As individuals grew older, the prevalence of sensitization to soy decreased. Specifically, it was recorded at 36.8% in the first year of life, 16.4% in the second year, and 13.7% in the third year[11]. In a previous study by this group, a soya cross-allergy was depicted at 16.6% among 60 children below 2 years[12]. In contrast, this present finding of 47% cross-allergy is alarming for the developing world because soya formulas are inexpensive compared to extensively hydrolysed formulas for babies without adequate breast milk to maintain proper nutrition. It is difficult to pinpoint the reason behind this escalation. However, the reduction of the proportion of babies with exclusive breastfeeding, as well as delayed maturation of the immune system after COVID-19-induced hygiene maintenance, can be cited as a few causes. The recent position paper by ESPGHAN recommends the use of soya formulas in such situations especially in cases of IgE-mediated CMA. However, if soya cross allergy is showing an increasing trend; using soya formula in breast milk-deficient babies will invite more nutrition and growth impairment, which was one of the additional presenting features of the babies in the present study (Table 1). This calls for a larger multicenter study involving a substantial number of infants.
The limitations of this study were its retrospective nature and the nondifferentiation of IgE and non-IgE-mediated CMA. So, it could not point out the fractionization of soya cross allergy among the IgE-mediated and non-IgE-mediated CMA population like Ahn et al[10].
In conclusion, this study emphasises the importance of raising awareness and carefully administering soy protein to infants with CMPA in cost-sensitive regions. A multi-center study should be initiated to reappraise this observation and plan better management of CMA in India.
| 1. | Vandenplas Y, Koletzko S, Isolauri E, Hill D, Oranje AP, Brueton M, Staiano A, Dupont C. Guidelines for the diagnosis and management of cow's milk protein allergy in infants. Arch Dis Child. 2007;92:902-908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 239] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 2. | ESPGHAN Committee on Nutrition; Agostoni C, Axelsson I, Goulet O, Koletzko B, Michaelsen KF, Puntis J, Rieu D, Rigo J, Shamir R, Szajewska H, Turck D. Soy protein infant formulae and follow-on formulae: a commentary by the ESPGHAN Committee on Nutrition. J Pediatr Gastroenterol Nutr. 2006;42:352-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 138] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 3. | Hosaagrahara Ramakrishna S, Shah N, Acharyya BC, Durairaj E, Verma L, Sankaranarayanan S, Wadhwa N, Venter C. The Need for Culturally Appropriate Food Allergy Management Strategies: The Indian Milk Ladder. Nutrients. 2023;15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 4. | Prescott S, Allen KJ. Food allergy: riding the second wave of the allergy epidemic. Pediatr Allergy Immunol. 2011;22:155-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 335] [Cited by in RCA: 326] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 5. | Klemola T, Vanto T, Juntunen-Backman K, Kalimo K, Korpela R, Varjonen E. Allergy to soy formula and to extensively hydrolyzed whey formula in infants with cow's milk allergy: a prospective, randomized study with a follow-up to the age of 2 years. J Pediatr. 2002;140:219-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 122] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 6. | Nowak-Wegrzyn A, Berin MC, Mehr S. Food Protein-Induced Enterocolitis Syndrome. J Allergy Clin Immunol Pract. 2020;8:24-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 82] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 7. | Vandenplas Y, Broekaert I, Domellöf M, Indrio F, Lapillonne A, Pienar C, Ribes-Koninckx C, Shamir R, Szajewska H, Thapar N, Thomassen RA, Verduci E, West C. An ESPGHAN Position Paper on the Diagnosis, Management, and Prevention of Cow's Milk Allergy. J Pediatr Gastroenterol Nutr. 2024;78:386-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 63] [Article Influence: 63.0] [Reference Citation Analysis (0)] |
| 8. | Nowak-Węgrzyn A, Katz Y, Mehr SS, Koletzko S. Non-IgE-mediated gastrointestinal food allergy. J Allergy Clin Immunol. 2015;135:1114-1124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 211] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 9. | Zeiger RS, Sampson HA, Bock SA, Burks AW Jr, Harden K, Noone S, Martin D, Leung S, Wilson G. Soy allergy in infants and children with IgE-associated cow's milk allergy. J Pediatr. 1999;134:614-622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 153] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 10. | Ahn KM, Han YS, Nam SY, Park HY, Shin MY, Lee SI. Prevalence of soy protein hypersensitivity in cow's milk protein-sensitive children in Korea. J Korean Med Sci. 2003;18:473-477. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Bocquet A, Dupont C, Chouraqui JP, Darmaun D, Feillet F, Frelut ML, Girardet JP, Hankard R, Lapillonne A, Rozé JC, Simeoni U, Turck D, Briend A; Committee on Nutrition of the French Society of Pediatrics (CNSFP). Efficacy and safety of hydrolyzed rice-protein formulas for the treatment of cow's milk protein allergy. Arch Pediatr. 2019;26:238-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 44] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 12. | Acharyya BC, Bhattacharyya C, Acharyya S. The Profile of Food Protein Allergy in Children below 2 Years in Eastern India–An Observational Study. Adv Res Gastroenterol Hepatol. 2017;4. [DOI] [Full Text] |