Published online Jun 9, 2025. doi: 10.5409/wjcp.v14.i2.100975
Revised: January 8, 2025
Accepted: January 14, 2025
Published online: June 9, 2025
Processing time: 193 Days and 22.8 Hours
Recently, the gut microbiota has been identified as a significant risk factor associated with metabolic disorders related to obesity. Advances in high-throu
Core Tip: Obesity is a global pandemic with severe implications for children's health, and is associated with metabolic syndrome. This letter evaluates the importance of understanding changes in the gut microbiota of obese children and the factors influencing research outcomes. We discuss Pan et al's perspective on Li et al's research, highlighting relevant factors that need further exploration and suggesting future research directions to clarify the role of the gut microbiota in childhood obesity.
- Citation: Nguyen Tran T, Luong TV, Nguyen NVD, Dang HNN. Complex relationship between childhood obesity and the gut microbiota. World J Clin Pediatr 2025; 14(2): 100975
- URL: https://www.wjgnet.com/2219-2808/full/v14/i2/100975.htm
- DOI: https://dx.doi.org/10.5409/wjcp.v14.i2.100975
Obesity, characterized by an abnormal or excessive accumulation of adipose tissue, has become a global epidemic with rapidly increasing prevalence rates. In children and adolescents, obesity not only affects physical health and development but also increases the risk of severe metabolic disorders such as type 2 diabetes, hypertension, dyslipidemia, nonalcoholic fatty liver disease, and psychological issues[1]. Obesity in children often arises from a complex interplay of factors, including genetics, the environment, and behavior. Among environmental factors, the gut microbiota has emerged as a significant element in the development of obesity.
The gut microbiota, which comprises approximately 1–2 kg of body weight and is regarded as a "special organ", consists of trillions of microorganisms, including bacteria, fungi, archaea, and viruses. In terms of cell numbers, the gut microbiota outnumbers the body's cells by a factor of 10[2]. Genetically, it harbors nearly 100 times more genetic material than the human genome does[3]. The gut microbiota, often referred to as the "second human genome", plays a crucial role in the body’s physiological, metabolic, and immune processes. Over the past decade, the link between the gut microbiota and the development of obesity has gradually become clearer[4]. Research indicates that the gut microbiota can influence obesity through mechanisms such as energy absorption regulation, appetite modulation, fat storage, chronic inflammation, and circadian rhythms[5].
The advancement of PCR and next-generation sequencing techniques has enabled researchers to use 16S rRNA molecular markers to classify and identify bacterial species within the gut microbiota[6]. A recent study by Li et al[7] highlighted changes in the composition and structure of the gut microbiota in obese children and evaluated the strengths and limitations of this research.
This letter discusses the current research findings, provides an objective assessment, and proposes a more comprehensive approach to studying the relationship between gut microbiota and obesity in children.
Previous studies have indicated that the gut microbiota functions as a crucial endocrine organ in maintaining the body's energy balance and immune function. Although there is a significant amount of inconsistent data, it is clear that obesity is characterized by alterations in the gut microbiota composition between obese and nonobese individuals[1,2,4,5,8].
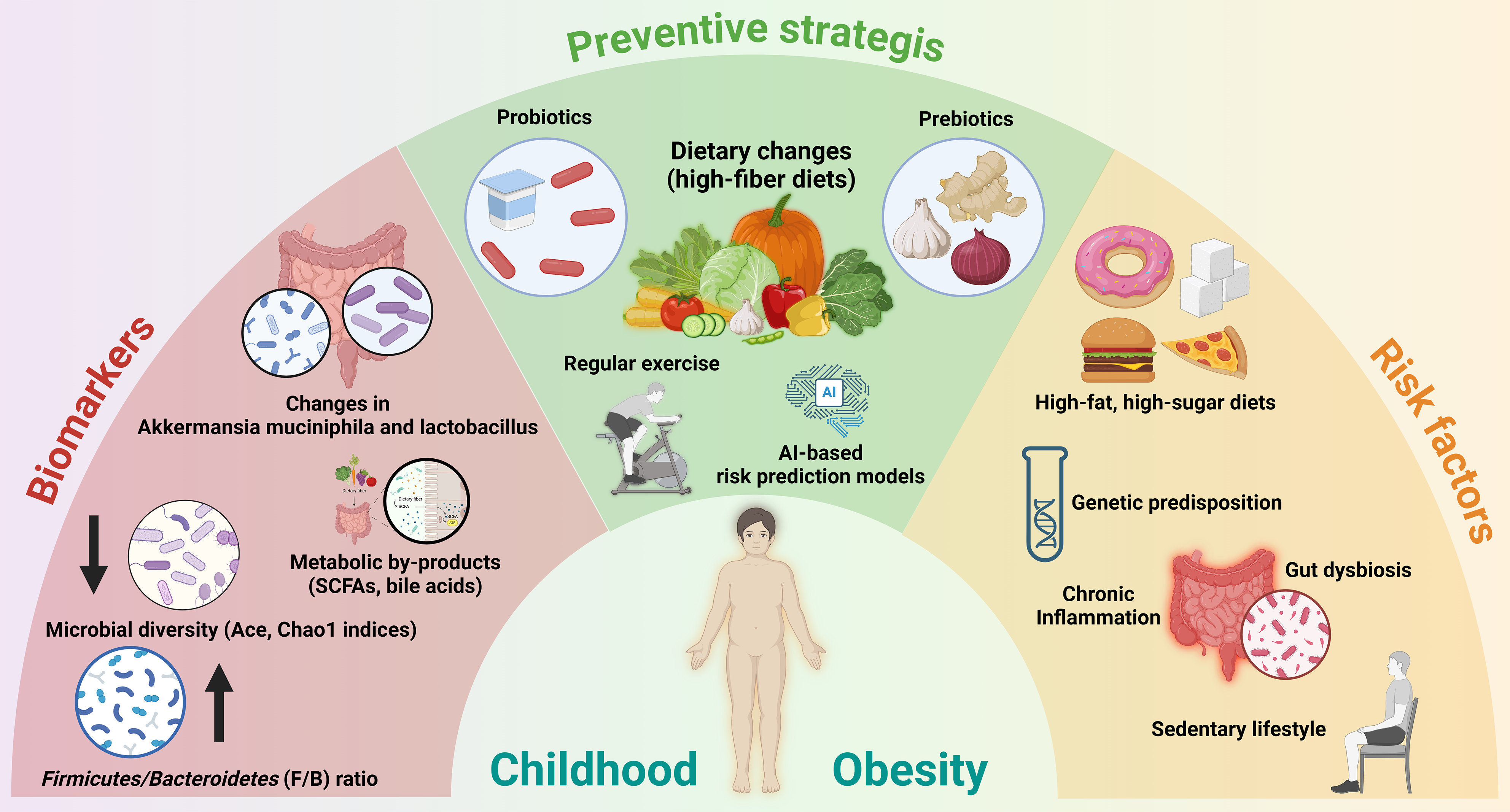
The human gut microbiota is primarily composed of five main phyla: (1) Bacteroidetes; (2) Firmicutes; (3) Actinobacteria; (4) Proteobacteria; and (5) Verrucomicrobia, with Bacteroidetes and Firmicutes being the predominant ones[2,8]. An overgrowth of Firmicutes and a reduction in Bacteroidetes have traditionally been recognized as key biomarkers of obesity[9]. These biomarkers, along with associated risk factors and prevention strategies, are visually summarized in Figure 1, highlighting their interconnected roles in childhood obesity.
The relationship between the gut microbiota and obesity in adults has been established through numerous studies. However, this relationship in children remains less explored, particularly with respect to early-life factors that contribute to obesity. The study by Li et al[7] offers key insights into the differences in the gut microbiota between obese and normal-weight children.
A study by Li et al[7], which was conducted on a group of children aged 8-12 years, divided them into two groups, obese and normal-weight, based on body mass index while ensuring age and sex matching. This study employed 16S rRNA gene sequencing technology in the hypervariable V3 and V4 regions to analyze the diversity and composition of the gut microbiota. The results revealed that the abundance-based coverage estimator (Ace) and Chao1 indices were lower in the obese group than in the control group, whereas the differences in the Shannon and Simpson indices between the two groups were not statistically significant[7].
Analyses of differences in the gut microbiota composition revealed that in the obese group, Prevotella and Firmicutes were predominant, whereas Bacteroides and Sanguibacteroides were predominant in the normal-weight group. The study by Li et al[7] also mentioned that the reduction of Bacteroidetes resulted in a higher Firmicutes/Bacteroidetes ratio in obese children, a point we address in Figure 1.
A review by Pan et al[10] discussed how Li et al[7] interpreted their research results and assessed the support for the conclusions based on the data. Pan et al[10] also evaluated key results and common issues when interpreting new study findings, such as their alignment with previous research and the novel discoveries made.
A higher Firmicutes/Bacteroidetes ratio has been observed in obese children in several studies. However, some studies have not reported significant changes or have even reported a decrease in this ratio[11]. These discrepancies may arise from different research methodologies, including sample handling, DNA extraction, selection of amplification regions on the 16S rRNA gene, and bioinformatics analysis methods. Studies often use different sample sizes, leading to variability in results. Further research with larger sample sizes and standardized analytical methods is needed to clarify this relationship and its clinical applications.
In addition to the Firmicutes/Bacteroidetes ratio, the abundance of Firmicutes is related to energy absorption and storage in obese children[12]. However, the inconsistent findings regarding the diversity of Firmicutes across studies need to be addressed. While Li et al[7] reported increased Firmicutes abundance in obese children, recent studies in South Korea and China reported that the difference in Firmicutes diversity between obese and normal-weight children was not statistically significant[13,14].
A study by Li et al[7] focused on influencing factors and used 16S rRNA gene sequencing. However, the study had limitations, including sample size, study duration, and a lack of analysis of lifestyle and environmental factors. Future studies should increase the sample size and utilize advanced microbiological techniques to enhance our understanding of how gut microbiota influences childhood obesity.
Recent studies highlight the significant role of microbial imbalance in the gut microbiota in relation to obesity in children. While the Firmicutes/Bacteroidetes ratio remains a key factor, recent research also points to changes in other microbial species, such as Akkermansia muciniphila and Lactobacillus, which may impact fat accumulation and appetite[1,15].
Genetic factors have been found to interact with the gut microbiota, significantly influencing obesity risk. For instance, studies indicate that genetic variants associated with metabolism can modify the distribution and function of gut microbes. These changes are particularly notable in their impact on obesity risk in children, suggesting a complex interplay between genetic predispositions and microbial environments[16-19].
Recent studies have emphasized the impact of diet on gut microbiota development and obesity. Diets high in fiber and probiotics can positively influence the microbiota, whereas high-sugar and high-fat diets may disrupt microbial balance and contribute to obesity[4,20].
Emerging studies highlight the role of the gut microbiota in obesity development through its impact on the immune system. An imbalance in gut microbes can trigger low-grade chronic inflammation, which is a known risk factor for obesity. By modifying the microbiota, it may be possible to enhance immune responses and thereby reduce the risk of obesity[3,5,8,21].
The effectiveness of probiotics and prebiotics in modifying the gut microbiota to prevent obesity has been demonstrated in recent findings. Specific strains of probiotics have shown potential in regulating body weight and improving health metrics related to obesity in children[22,23].
Environmental and lifestyle factors, including maternal diet during pregnancy, exercise habits, and environmental exposures, play crucial roles in shaping the gut microbiota and influencing obesity risk in children[24-26]. Recognizing these impacts highlights the importance of integrating dietary and lifestyle modifications into comprehensive obesity management strategies. This approach not only addresses immediate health concerns but also aligns with the growing understanding of the intricate relationship between gut microbiota and childhood obesity, emphasizing the need for informed prevention and treatment plans.
When investigating the relationship between obesity and the gut microbiota, innovative approaches can shed light on unexplored aspects and open new opportunities for research and intervention. Some creative methods and strategies are as follows.
The application of omics technologies enhances our understanding of the complex interactions within the gut microbiota and its impact on obesity. Starting with metagenomics, this approach analyzes the entire genome of the gut microbiota, offering a detailed view of microbial diversity and functionality, far surpassing the insights provided by traditional 16S rRNA-based analysis. Building on this foundation, metabolomics further explores the metabolic products from both the gut microbiota and the host body, identifying specific metabolites associated with obesity and illustrating how microbes influence metabolic pathways. Finally, proteomics complements these findings by analyzing proteins in the gut and serum, shedding light on the interactions between microbes and the human body and pinpointing protein factors that may play roles in obesity. Together, these omics technologies provide a comprehensive picture of how the gut microbiota contributes to obesity, suggesting potential pathways for therapeutic intervention.
Animal models, including mice and pigs, are utilized to explore the effects of gut microbiota on obesity, alongside potential interventions like dietary modifications or probiotics. Complementing these studies, artificial human gut microbiota models are created in vitro to test various factors that influence obesity development within a controlled environment. This dual approach allows for a comprehensive analysis of obesity-related microbial dynamics.
The exploration of new strains of probiotics and types of prebiotics is pivotal in modulating the gut microbiota beneficially for obesity management. The 2023 clinical practice guidelines issued by the American Academy of Pediatrics emphasize the critical role of probiotics and prebiotics in modulating gut microbiota to enhance obesity management in children[27]. Research should prioritize the identification of specific species and nutrients most effective in optimizing gut health. Following this, there should be an emphasis on testing refined diets, such as plant-based or low-carbohydrate options, that are designed to enhance the balance of the gut microbiota.
Investigating how human genetic variants influence the gut microbiota and obesity development offers significant insights into the personalized connection between genes and microbiota. This research approach aims to uncover the unique interactions that may inform more tailored and effective strategies for preventing and managing obesity based on individual genetic profiles.
Studying the multidimensional interactions between the gut microbiota, the immune system, and other physiological factors enriches our understanding of obesity's pathophysiology. Additionally, investigating the effects of environmental, social, and psychological factors further reveals how these external elements influence the gut microbiota and obesity development, highlighting a complex network of biological and environmental interactions.
Big data analysis, utilizing advanced tools such as machine learning and artificial intelligence, allows for a more comprehensive analysis of microbiota and biological data. These technologies are capable of detecting patterns and relationships that traditional methods may overlook. For example, recent advancements in machine learning, such as DeepHealthNet, have introduced predictive frameworks specifically tailored for adolescent obesity, providing personalized feedback that aids in more effective health management[28].
Building on this technological foundation, the development of predictive models using microbiota data and related factors is a natural progression. These models are designed to assess obesity risk and gauge the effectiveness of various interventions, enhancing our ability to predict and manage obesity with greater precision and personalization. Together, these approaches represent a significant leap forward in understanding and addressing the complex dynamics of obesity.
Exploring the gut-brain axis reveals how the gut microbiota might influence eating behaviors and body weight regulation. By examining the intricate communication between the gut and the brain, researchers are gaining deeper insights into the mechanisms through which the microbiota affects obesity. This knowledge is critical for developing more targeted and effective treatment and prevention strategies. Utilizing innovative research methods to study these interactions, we can better understand the complex interplay at work and potentially unlock new approaches to managing obesity.
Recent studies have clarified the relationship between childhood obesity and the gut microbiota, with a key indicator being the increased Firmicutes/Bacteroidetes ratio in obese children. However, the current data remain inconsistent, and further research with larger sample sizes and standardized methods is needed to confirm these findings. The complex relationship between obesity and the gut microbiota highlights the need for a multidimensional approach to address this issue. These insights provide opportunities for dietary and lifestyle interventions, as well as new treatment strategies aimed at modulating the gut microbiota and managing obesity more effectively. To develop comprehensive and personalized solutions for childhood obesity, future studies should integrate genetic, environmental, and advanced technological factors and prioritize longitudinal study designs and comprehensive profiling of microbiota and metabolism. By bridging these research gaps, we can develop more effective prevention and treatment strategies, ultimately improving metabolic health and well-being for children worldwide.
| 1. | Cho KY. Association of gut microbiota with obesity in children and adolescents. Clin Exp Pediatr. 2023;66:148-154. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 2. | Liu BN, Liu XT, Liang ZH, Wang JH. Gut microbiota in obesity. World J Gastroenterol. 2021;27:3837-3850. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 98] [Cited by in RCA: 286] [Article Influence: 71.5] [Reference Citation Analysis (22)] |
| 3. | Islam MM, Islam MM, Rahman MA, Ripon MAR, Hossain MS. Gut microbiota in obesity and related complications: Unveiling the complex interplay. Life Sci. 2023;334:122211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 4. | Geng J, Ni Q, Sun W, Li L, Feng X. The links between gut microbiota and obesity and obesity related diseases. Biomed Pharmacother. 2022;147:112678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 168] [Article Influence: 56.0] [Reference Citation Analysis (0)] |
| 5. | Zhuang Z, Zhou P, Wang J, Lu X, Chen Y. The Characteristics, Mechanisms and Therapeutics: Exploring the Role of Gut Microbiota in Obesity. Diabetes Metab Syndr Obes. 2023;16:3691-3705. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 6. | García-Gamboa R, Díaz-Torres O, Senés-Guerrero C, Gradilla-Hernández MS, Moya A, Pérez-Brocal V, Garcia-Gonzalez A, González-Avila M. Associations between bacterial and fungal communities in the human gut microbiota and their implications for nutritional status and body weight. Sci Rep. 2024;14:5703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 7. | Li XM, Lv Q, Chen YJ, Yan LB, Xiong X. Association between childhood obesity and gut microbiota: 16S rRNA gene sequencing-based cohort study. World J Gastroenterol. 2024;30:2249-2257. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (10)] |
| 8. | Gomes AC, Hoffmann C, Mota JF. The human gut microbiota: Metabolism and perspective in obesity. Gut Microbes. 2018;9:308-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 303] [Article Influence: 43.3] [Reference Citation Analysis (2)] |
| 9. | Indiani CMDSP, Rizzardi KF, Castelo PM, Ferraz LFC, Darrieux M, Parisotto TM. Childhood Obesity and Firmicutes/Bacteroidetes Ratio in the Gut Microbiota: A Systematic Review. Child Obes. 2018;14:501-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 266] [Article Influence: 38.0] [Reference Citation Analysis (1)] |
| 10. | Pan Y, Jiao FY. Link between childhood obesity and gut microbiota. World J Gastroenterol. 2024;30:3560-3563. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1] [Cited by in RCA: 1] [Article Influence: 1.0] [Reference Citation Analysis (1)] |
| 11. | Magne F, Gotteland M, Gauthier L, Zazueta A, Pesoa S, Navarrete P, Balamurugan R. The Firmicutes/Bacteroidetes Ratio: A Relevant Marker of Gut Dysbiosis in Obese Patients? Nutrients. 2020;12:1474. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 418] [Cited by in RCA: 1294] [Article Influence: 258.8] [Reference Citation Analysis (0)] |
| 12. | Porras D, Nistal E, Martínez-Flórez S, Pisonero-Vaquero S, Olcoz JL, Jover R, González-Gallego J, García-Mediavilla MV, Sánchez-Campos S. Protective effect of quercetin on high-fat diet-induced non-alcoholic fatty liver disease in mice is mediated by modulating intestinal microbiota imbalance and related gut-liver axis activation. Free Radic Biol Med. 2017;102:188-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 287] [Cited by in RCA: 373] [Article Influence: 46.6] [Reference Citation Analysis (0)] |
| 13. | Chen X, Sun H, Jiang F, Shen Y, Li X, Hu X, Shen X, Wei P. Alteration of the gut microbiota associated with childhood obesity by 16S rRNA gene sequencing. PeerJ. 2020;8:e8317. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 82] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 14. | Shin S, Cho KY. Altered Gut Microbiota and Shift in Bacteroidetes between Young Obese and Normal-Weight Korean Children: A Cross-Sectional Observational Study. Biomed Res Int. 2020;2020:6587136. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 15. | Zhou Q, Zhang Y, Wang X, Yang R, Zhu X, Zhang Y, Chen C, Yuan H, Yang Z, Sun L. Gut bacteria Akkermansia is associated with reduced risk of obesity: evidence from the American Gut Project. Nutr Metab (Lond). 2020;17:90. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 99] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 16. | Concepción-Zavaleta MJ, Quiroz-Aldave JE, Durand-Vásquez MDC, Gamarra-Osorio ER, Valencia de la Cruz JDC, Barrueto-Callirgos CM, Puelles-León SL, Alvarado-León EJ, Leiva-Cabrera F, Zavaleta-Gutiérrez FE, Concepción-Urteaga LA, Paz-Ibarra J. A comprehensive review of genetic causes of obesity. World J Pediatr. 2024;20:26-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Reference Citation Analysis (0)] |
| 17. | Novelli G, Cassadonte C, Sbraccia P, Biancolella M. Genetics: A Starting Point for the Prevention and the Treatment of Obesity. Nutrients. 2023;15:2782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 21] [Reference Citation Analysis (0)] |
| 18. | Al-Humadi AW, Alabduljabbar K, Alsaqaaby MS, Talaee H, le Roux CW. Obesity Characteristics Are Poor Predictors of Genetic Mutations Associated with Obesity. J Clin Med. 2023;12:6396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 19. | Fitch AK, Malhotra S, Conroy R. Differentiating monogenic and syndromic obesities from polygenic obesity: Assessment, diagnosis, and management. Obes Pillars. 2024;11:100110. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 20. | Livantsova EN, Leonov GE, Starodubova AV, Varaeva YR, Vatlin AA, Koshechkin SI, Korotkova TN, Nikityuk DB. Diet and the Gut Microbiome as Determinants Modulating Metabolic Outcomes in Young Obese Adults. Biomedicines. 2024;12:1601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 21. | Colangeli L, Escobar Marcillo DI, Simonelli V, Iorio E, Rinaldi T, Sbraccia P, Fortini P, Guglielmi V. The Crosstalk between Gut Microbiota and White Adipose Tissue Mitochondria in Obesity. Nutrients. 2023;15:1723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 22. | Joseph N, Clayton JB, Hoops SL, Linhardt CA, Mohd Hashim A, Mohd Yusof BN, Kumar S, Amin Nordin S. Alteration of the Gut Microbiome in Normal and Overweight School Children from Selangor with Lactobacillus Fermented Milk Administration. Evol Bioinform Online. 2020;16:1176934320965943. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 23. | Borka Balas R, Meliț LE, Lupu A, Lupu VV, Mărginean CO. Prebiotics, Probiotics, and Synbiotics-A Research Hotspot for Pediatric Obesity. Microorganisms. 2023;11:2651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 24. | Laforest-Lapointe I, Becker AB, Mandhane PJ, Turvey SE, Moraes TJ, Sears MR, Subbarao P, Sycuro LK, Azad MB, Arrieta MC. Maternal consumption of artificially sweetened beverages during pregnancy is associated with infant gut microbiota and metabolic modifications and increased infant body mass index. Gut Microbes. 2021;13:1-15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 44] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 25. | Kern T, Blond MB, Hansen TH, Rosenkilde M, Quist JS, Gram AS, Ekstrøm CT, Hansen T, Stallknecht B. Structured exercise alters the gut microbiota in humans with overweight and obesity-A randomized controlled trial. Int J Obes (Lond). 2020;44:125-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 79] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 26. | Aragón-Vela J, Solis-Urra P, Ruiz-Ojeda FJ, Álvarez-Mercado AI, Olivares-Arancibia J, Plaza-Diaz J. Impact of Exercise on Gut Microbiota in Obesity. Nutrients. 2021;13:3999. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 45] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 27. | Hu P, Samuels S, Sharifi M. Overview of the Treatment of Pediatric Obesity and the 2023 Clinical Practice Guidelines. Pediatr Clin North Am. 2024;71:919-926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 28. | Jeong JH, Lee IG, Kim SK, Kam TE, Lee SW, Lee E. DeepHealthNet: Adolescent Obesity Prediction System Based on a Deep Learning Framework. IEEE J Biomed Health Inform. 2024;PP. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |