Published online Jun 9, 2025. doi: 10.5409/wjcp.v14.i2.100330
Revised: November 19, 2024
Accepted: January 7, 2025
Published online: June 9, 2025
Processing time: 216 Days and 10.3 Hours
Silver-Russell syndrome (SRS) is a clinically heterogeneous entity characterized by intrauterine and postnatal growth restriction, relative macrocephaly at birth, distinct facial features, and body asymmetry combined with other malformations.
Herein, we describe four individuals with SRS, focusing on their prenatal phenotype, postnatal presentation, diagnosis, and management. All cases had a typical phenotype, including postnatal growth failure, short stature (chronic malnutrition), and protruding forehead. Individually, they presented with feeding difficulties, leg length discrepancy, triangular face, or relative macrocephaly at birth, and each one exhibited distinct SRS features, including motor and/or speech delay, experiencing frequent hypoglycemic episodes. The fact that each patient exhibited a different combination of clinical findings underlines the heterogeneity of the syndrome.
SRS is diagnosed clinically. However, only 60% of cases are genetically confirmed, while most are sporadic. Although SRS is a well-described syndrome, a delayed diagnosis can have grave consequences on a child’s growth. Recombinant human growth hormone treatment is often initiated shortly after the diagnosis. The follow-up requires a multidisciplinary approach.
Core Tip: The present case series questions whether premature delivery should be induced in fetal growth restriction fetuses with the Silver-Russell syndrome (SRS) genotype as it further aggravates growth. The manifestation of SRS symptoms varies greatly in each patient. When premature delivery is induced due to fetal growth restriction, these newborns carry the double burden of SRS and prematurity, making it more difficult for them to catch up with growth and develop properly.
- Citation: Toulia I, Savvidou P, Ververi A, Grammatikopoulou MG, Kosta K, Tziaferi V, Antachopoulos C, Goulis DG, Sotiriadis A, Tsiroukidou K. Clinical and genetic diagnosis and management of Silver-Russell syndrome: Report of four cases. World J Clin Pediatr 2025; 14(2): 100330
- URL: https://www.wjgnet.com/2219-2808/full/v14/i2/100330.htm
- DOI: https://dx.doi.org/10.5409/wjcp.v14.i2.100330
Silver-Russell syndrome (SRS) (Online Mendelian Inheritance in Man #180860) is a rare, clinically heterogeneous syndrome characterized by intrauterine and postnatal growth restriction, distinct facial features, relative macrocephaly at birth, body asymmetry, and other malformations[1,2]. Feeding difficulties, hypoglycemia, and speech delay may appear later in life[2]. The syndrome was first described as an entity by Russell[3] and Silver and Gruskay[4]. According to the Netchine-Harbison clinical scoring system (NH-CSS)[5], its diagnosis is based on clinical features. A patient is suspected to have SRS when four of the six NH-CSS criteria are met[2,5]. Due to genetic heterogeneity, only 60% of cases are genetically confirmed[6], while most are sporadic[1,7-10]. The most common epigenetic and genetic alterations related to SRS are located in the chromosomal region 11p15[7,9,10], with up to 50% of cases presenting hypomethylation at imprinting control region 1 (ICR1)[6].
Fetal growth restriction (FGR) is a significant feature of SRS prenatally and the most prominent ultrasound sign. Other prenatal signs include abnormal placental morphology, oligoamnion, relative macrocephaly, hypospadias, and cleft palate[7]. The management of SRS should be multidisciplinary, involving pediatric endocrinologists, gastroenterologists, dietitians, orthopedic surgeons, a craniofacial team, pediatric neurologists, speech and language therapists, and psychologists[2]. A clinical geneticist’s participation is crucial for diagnosing and counseling the family[2]. In this case series, we present four pediatric patients with SRS according to the case report guidelines[8], focusing on their distinct prenatal phenotype, initial postnatal presentation, diagnosis, and management to underline the heterogeneity in SRS cases.
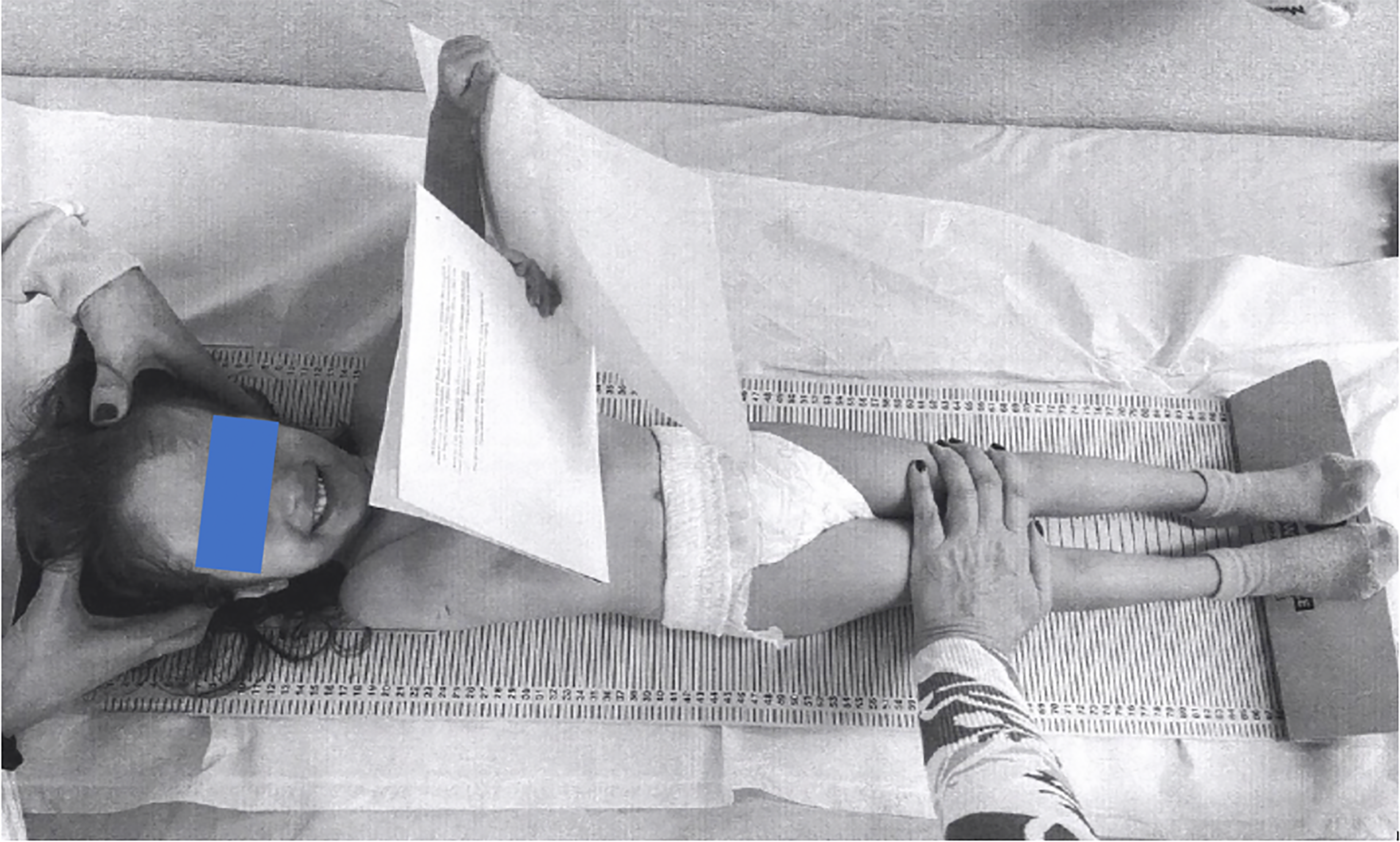
Case 1: A 3-year-old girl (Figure 1) was admitted to the Department of Pediatric Endocrinology, Hippokration Hospital in Thessaloniki, Greece due to feeding difficulties and growth restriction.
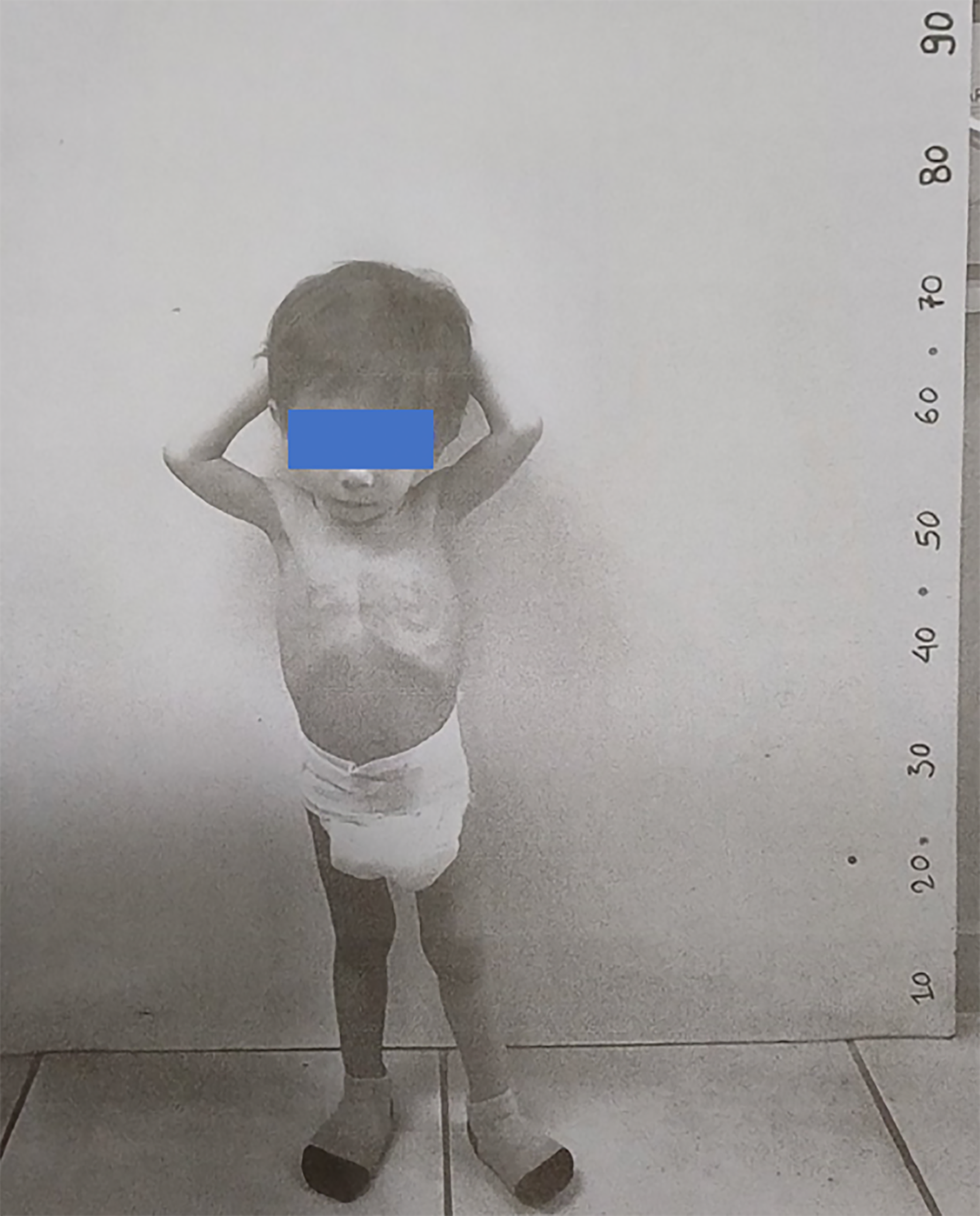
Case 2: A 22-month-old boy (Figure 2) was presented to the Department of Pediatric Endocrinology, Hippokration Hospital in Thessaloniki, Greece with extreme growth failure.
Case 3: A 3-year-old boy presented with short stature and skeletal abnormalities.
Case 4: A 14-month-old boy presented with growth failure, along with a prominent forehead, triangular-shaped head, and mild leg length discrepancy (< 1 cm).
Case 1: During a gastrointestinal infection, the girl presented prolonged hypoglycemia, requiring hospitalization.
Cases 2, 3, and 4: No special histories of present illness were reported.
Case 1: The girl was born at an appropriate-for-gestational age via natural delivery at 37 + 3 gestational weeks.
Case 2: The boy was born small-for-gestational age (SGA), with a birth weight of 1980 g [weight-for-age z-score (WAz): -2.28] and birth length of 43 cm (length-for-age z-score: -2.19), being diagnosed with FGR at 37 gestational weeks (twin pregnancy).
Case 3: The boy was born prematurely and SGA at 33 + 5 gestational weeks, with a birth weight of 1300 g (WAz: -2.14), birth length of 38 cm [height-for-age z-score (HAz): -2.52], and head circumference of 29 cm. He was born via cesarean section due to FGR diagnosed before 37 gestational weeks. Prenatal array comparative genomic hybridization was negative for chromosomal abnormalities.
Case 4: The boy was born prematurely at 30 + 5/7 gestational weeks due to FGR via cesarean section and was admitted to the neonatal intensive care unit due to prematurity and neonatal respiratory distress syndrome.
Case 1: SRS was suspected due to the patient’s facial features (triangular face and prominent frontal bones).
Case 3: The boy’s parents’ methylation-specific multiplex ligation-dependent probe amplification (MS-MLPA) results were normal.
Cases 2 and 4: No personal and family history was reported.
Case 1: The basic condition of the patient included: (1) Body weight (BW): 8000 g; (2) Stature: 80.5 cm; and (3) Body mass index (BMI): 12.3 kg/m2. Z-scores were calculated based on the World Health Organization growth charts[9] using the World Health Organization Anthro software[10], including WAz, which was -2.34, HAz calculated at -2.83, and BMI z-score (BMIz) being -0.62.
Case 2: The basic condition of the patient included: (1) BW: 6300 g; (2) WAz: -5.05; (3) Stature: 69 cm; (4) HAz: -5.53; and (5) BMIz: -2.74. The boy exhibited distinct facial features, including a triangular face, frontal bossing, and hypotelorism. Growth failure and facial characteristics were suggestive of SRS.
Case 3: No special result of physical examination was reported.
Case 4: The basic condition of the patient included: (1) BW: 6880 g; (2) WAz: -3.57; (3) Length: 68.5 cm; (4) Length-for-age z-score: -3.88; and (5) BMIz: -1.97.
Case 1: Targeted genetic testing with MS-MLPA identified maternal disomy of chromosome 7 [upd(7)mat], as seen in other cases[11]. The girl did not exhibit any musculoskeletal disorders. The cardiological examination revealed an aberrant right subclavian artery.
Case 2: Targeted genetic testing with MS-MLPA identified upd(7)mat. The boy did not have any abnormal findings from other systems. Regarding the biochemical profile, reduced glucose and insulin-like growth factor-1 (IGF-1) concentrations (41.3 ng/mL) were detected.
Case 3: Targeted testing for SRS with MS-MLPA revealed hypomethylation at the 11p1.5 ICR1 H19/IGF2 region.
Case 4: During his hospitalization, high concentrations of total cholesterol (198-271 mg/dL) and triglycerides (213-451 mg/dL) were noted, likely attributed to the total parenteral nutrition scheme provided. Targeted genetic testing with MS-MLPA identified hypomethylation at the 11p1.5 ICR1 H19/IGF2 region.
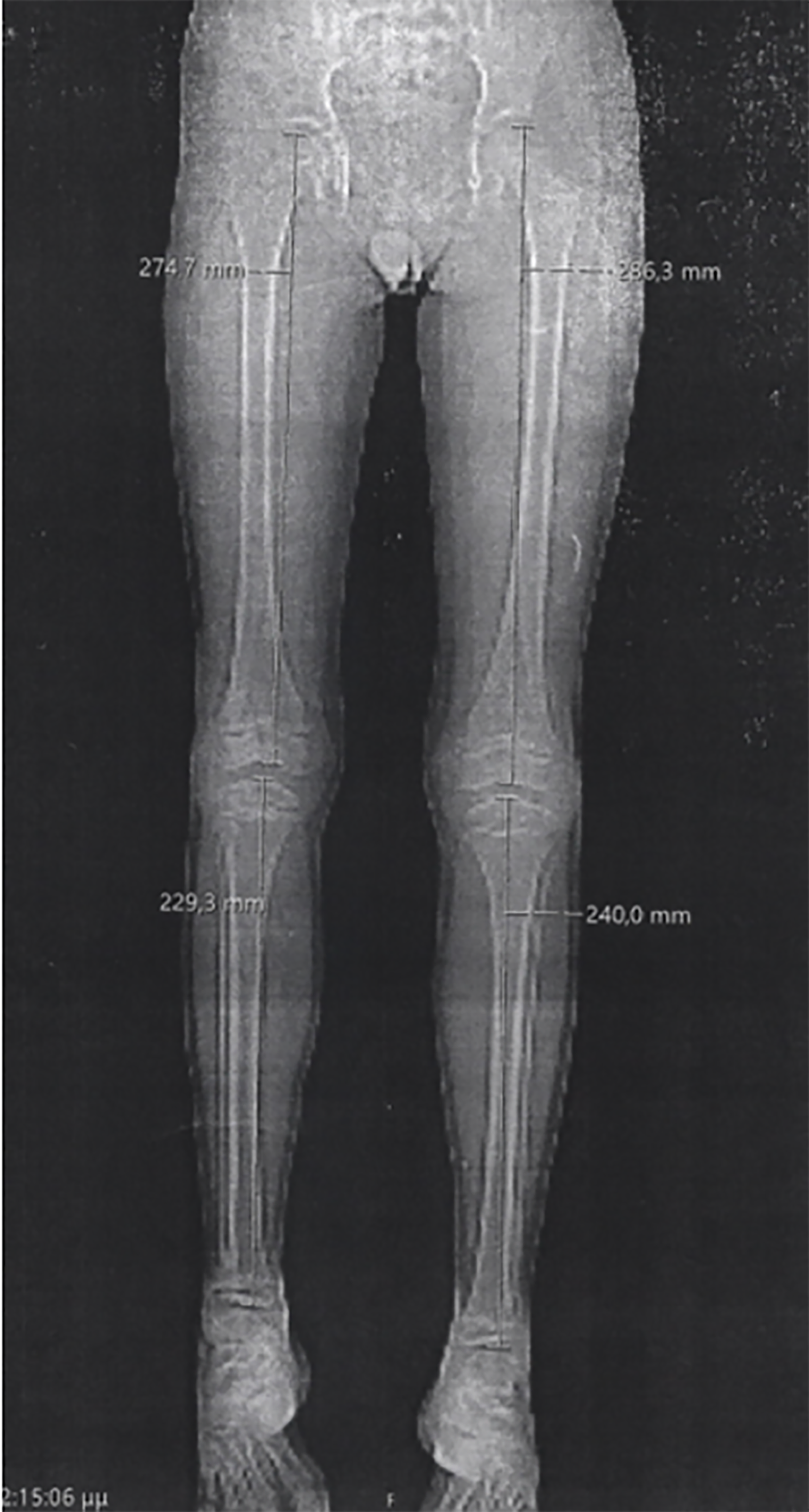
Case 3: The patient presented with short stature and skeletal abnormalities, including a relatively large head in comparison to the rest of the body, a prominent forehead, and anisomelia (> 1 cm leg length discrepancy), as indicated by the lower extremities X-ray (Figure 3).
Cases 1, 2, and 4: Imaging examinations were not performed.
Multidisciplinary management is imperative in SRS. All our patients were followed by pediatric endocrinologists and dieticians to ensure appropriate growth and adherence to ecombinant human growth hormone (rhGH) therapy. A pediatric orthopedic surgeon is involved with our third patient. A clinical geneticist was involved in the diagnosis and family genetic counseling in all cases. Parents and carers were informed about the risk of hypoglycemia, as well as its treatment and prevention.
All four cases were diagnosed as SRS.
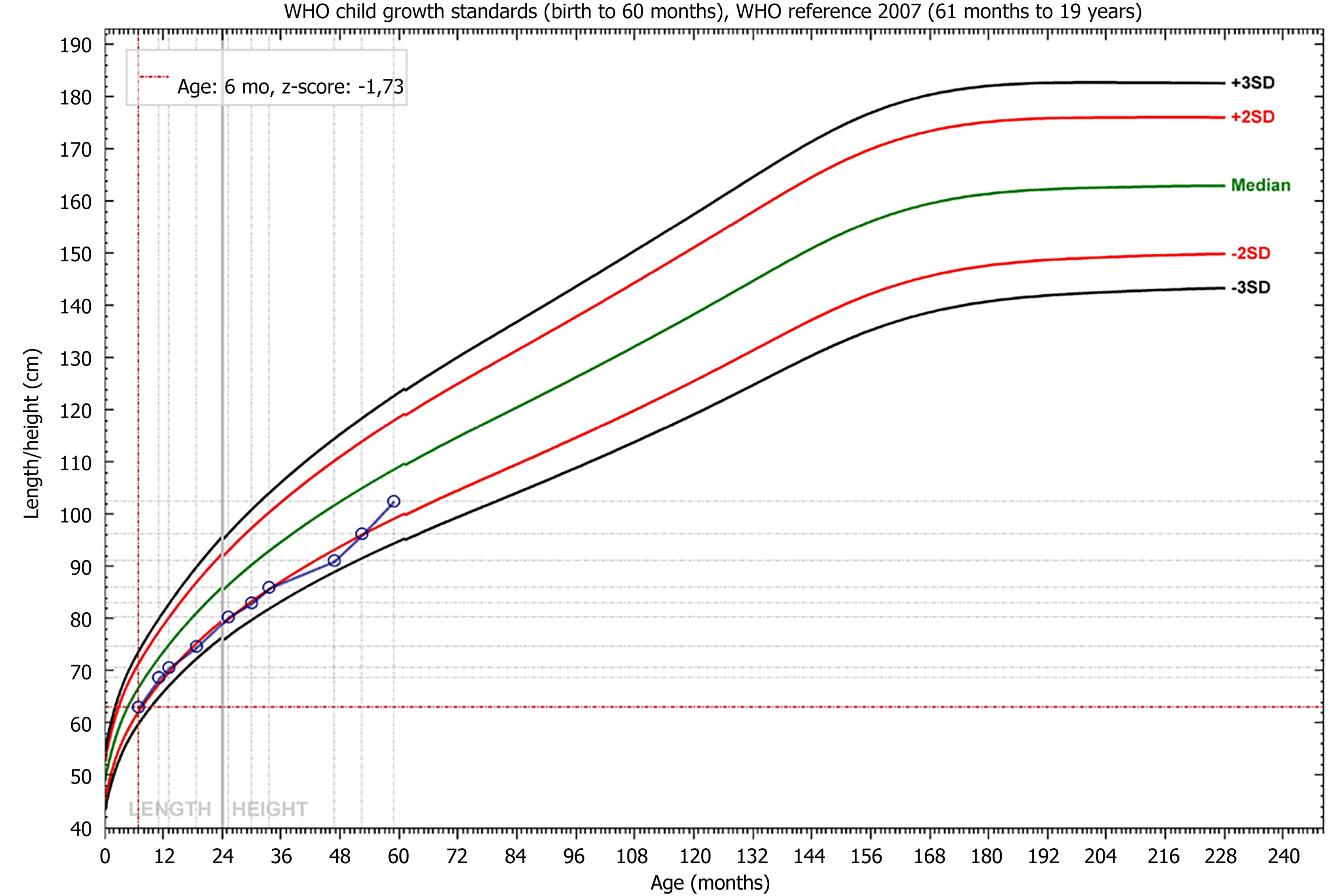
The girl was initiated on rhGH treatment one year after the diagnosis of SRS, with a good response (Figure 4).
Approval for rhGH substitution therapy was asked.
The patient started rhGH treatment at the age of 4 years, showing growth improvement.
According to the guidelines, a recommendation for assessment and management by a pediatric dietitian was given to optimize WHz and correct the caloric deficit-related chronic malnutrition before applying for rhGH treatment approval at two years of age[2].
At the moment, the girl undergoes regular speech therapy sessions due to speech delay, a common neurodevelopmental characteristic of SRS[12].
Currently, the boy is under neurological follow-up due to mild psychomotor delay.
Currently, the boy is on thyroxine treatment due to hypothyroidism and under annual orthopedic follow-up due to anisomelia.
The boy is on a special weight-gain diet to correct for malnutrition and is waiting for the rhGH treatment approval.
Herein, we present four cases of SRS with a typical phenotype, including postnatal growth failure, short stature (chronic malnutrition), and protruding forehead. Two patients (cases 1 and 2) had long-term follow-ups by pediatricians and pediatric gastroenterologists. A dietician provided individualized nutritional instructions. The examination of the food records revealed that feeding difficulties were the main cause of poor caloric intake. Nonetheless, feeding tubes were not prescribed as this is not the standard practice in Greece. For case 1, the improvement in nutritional intake and weight gain after administering rhGH was apparent. The latter improved the child’s eating ability, making her consume more food in less time. Feeding difficulties were also diminished, improving the child’s quality of life. rhGH therapy, as a long-term treatment, improves height and eating ability[13]. In the present cases, the optimal duration of rhGH therapy was determined by combining clinical assessment and IGF-1 concentrations.
In addition, individually, the children presented with leg length discrepancy, triangular face, and relative macrocephaly at birth, as described by the NH-CSS (Table 1). Moreover, each child exhibited distinct features, often described at SRS[1], including motor and/or speech delay and frequent hypoglycemic episodes. The fact that each patient had a different combination of clinical findings underlines the heterogeneity of the syndrome. While most patients with SRS are born SGA[2], case 1 presented herein was born appropriate-for-gestational age. The remaining three individuals presented idiopathic intrauterine growth restriction, but no additional findings were observed on the ultrasound scans. In one of the cases, prenatal screening by array comparative genomic hybridization was normal. According to Eggerman et al[7] and Lee et al[14], who studied 160 fetuses, FGR rarely indicates SRS prenatal molecular testing, probably because it is considered a relatively common, non-specific sign, usually becoming apparent in the third trimester of pregnancy. Additionally, the most common mechanism of SRS, namely, loss of paternal methylation at the 11p1.5 ICR1 H19/IGF2 region, can be mosaic, posing challenges regarding its prompt identification in prenatal testing[7]. Moreover, the region 11p1.5 ICR1 H19/IGF2 is hypomethylated and unstable after chorionic villus sampling (CVS) culturing and may not be reliably identified/excluded by MS-MLPA in CVS[7]. Outside FGR, indications for prenatal SRS testing include trisomy 7 identified in CVS and familial translocations involving the SRS critical regions[7].
| Clinical criteria and individual characteristics | Case 1 | Case 2 | Case 3 | Case 4 |
| SGA (birth weight and/or birth length) | None | Yes | Yes | -1.22 (BW) |
| Postnatal growth failure | Yes | Yes | Yes | Yes |
| Relative macrocephaly at birth | None | NA | Yes | Yes |
| Protruding forehead | Yes | Yes | Yes | Yes |
| Body asymmetry | None | None | Yes | Yes |
| Feeding difficulties and/or low BMI | Yes | Yes | Yes | Yes |
| Age at diagnosis | 3 years | 22 months | 3 years | 15 months |
| Biological sex | Female | Male | Male | Male |
| Body weight, kg | 10.5 | 6.3 | 9.3 | 6.9 |
| WAz1 | -2.34 | -5.05 | -3.90 | -3.57 |
| Stature, cm | 84.8 | 69.0 | 93.0 | 68.5 |
| HAz1 | -2.83 | -5.53 | -1.56 | -3.88 |
| BMI, kg/m2 | 14.6 | 13.0 | 10.8 | 14.4 |
| BMIz1 | -0.62 | -2.74 | -4.55 | -1.97 |
| Pregnancy | Singleton | Twin | Singleton | Singleton |
| FGR | None | Yes | Yes | Yes (early) |
| Delivery | Natural | C-section | C-section | C-section |
| Gestational age at birth, weeks | 37 + 3/7 | 37 | 33 + 5/7 | 30 + 5/7 |
| Birth weight, g | 2440 | 1980 | 1300 | 1110 |
| Birth length, cm | 48 | 43 | 38 | 36 |
| Head circumference at birth, cm | 33 | NA | 30 | 29 |
| Speech delay | Yes | None | None | None |
| Psychomotor delay | None | Yes | None | None |
To our knowledge, there is no association between SRS and spontaneous prematurity. However, two of the four individuals herein underwent premature delivery via cesarean section due to FGR. While FGR can be an indication of premature delivery, the optimal timing for delivery is defined by the computerized cardiotocogram or, if not available or not used, by a combination of Doppler velocimetry indices (mainly ductus venosus before 30 weeks) and conventional cardiotocogram, or biophysical profile[15]. FGR fetuses have a higher rate of conditions associated with prematurity[16] and experience worse neurodevelopmental outcomes. Moreover, neonates with SRS have a priori risk of hypoglycemia, feeding difficulties, and gastrointestinal problems[2], which may be further exacerbated by prematurity. Overall, it is important to thoroughly examine the fetal profile before early induction, given that FGR, due to SRS, does not indicate premature delivery.
When considering these facts, an important question arises regarding the appropriateness of premature delivery of fetuses with FGR due to SRS. When premature delivery is induced, these newborns carry the double burden of SRS and prematurity, making it more difficult to catch up with growth and develop properly. This dilemma was brought to light recently[17,18], highlighting the need for developing evidence-based recommendations on addressing this issue best while aiming for the improved development of the newborns involved and accounting for all maternal-fetal clinical considerations. Despite the presence or absence of features at birth, all patients were diagnosed between the ages of 2 and 3 years, as specific items of the NH-CSS are not assessable until 2 years[19]. Regarding the molecular diagnosis, two of our patients had hypomethylation at the 11p1.5 ICR1 H19/IGF2 region, the most common SRS genetic alteration[6]. The other two had upd(7)mat, described in approximately 10% of SRS cases[6]. Also, this genotype is usually related to a milder SRS phenotype, which might explain why our two patients have not shown any orthopedic disorders.
The four cases presented indicate the great heterogeneity in SRS characteristics and symptoms. In parallel, an important question arises from the current data regarding the appropriateness of premature delivery of fetuses with FGR due to SRS. When premature delivery is induced, these newborns carry the double burden of SRS and prematurity, making it more difficult to catch up with growth and develop properly.
| 1. | Carlson S. Russell-Silver Syndrome. In: Sarwark JF, Carl RL. Orthopaedics for the Newborn and Young Child. Switzerland: Springer Cham, 2023. [DOI] [Full Text] |
| 2. | Wakeling EL, Brioude F, Lokulo-Sodipe O, O'Connell SM, Salem J, Bliek J, Canton AP, Chrzanowska KH, Davies JH, Dias RP, Dubern B, Elbracht M, Giabicani E, Grimberg A, Grønskov K, Hokken-Koelega AC, Jorge AA, Kagami M, Linglart A, Maghnie M, Mohnike K, Monk D, Moore GE, Murray PG, Ogata T, Petit IO, Russo S, Said E, Toumba M, Tümer Z, Binder G, Eggermann T, Harbison MD, Temple IK, Mackay DJ, Netchine I. Diagnosis and management of Silver-Russell syndrome: first international consensus statement. Nat Rev Endocrinol. 2017;13:105-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 268] [Cited by in RCA: 333] [Article Influence: 41.6] [Reference Citation Analysis (0)] |
| 3. | Russell A. A syndrome of intra-uterine dwarfism recognizable at birth with cranio-facial dysostosis, disproportionately short arms, and other anomalies (5 examples). Proc R Soc Med. 1954;47:1040-1044. [PubMed] |
| 4. | Silver HK, Gruskay FL. Syndrome of congenital hemihypertrophy and elevated urinary gonadotropins; occurrence in a seven-year-old boy. AMA J Dis Child. 1957;93:559-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 5. | Azzi S, Salem J, Thibaud N, Chantot-Bastaraud S, Lieber E, Netchine I, Harbison MD. A prospective study validating a clinical scoring system and demonstrating phenotypical-genotypical correlations in Silver-Russell syndrome. J Med Genet. 2015;52:446-453. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 118] [Cited by in RCA: 151] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 6. | Ishida M. New developments in Silver-Russell syndrome and implications for clinical practice. Epigenomics. 2016;8:563-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 7. | Eggermann T, Brioude F, Russo S, Lombardi MP, Bliek J, Maher ER, Larizza L, Prawitt D, Netchine I, Gonzales M, Grønskov K, Tümer Z, Monk D, Mannens M, Chrzanowska K, Walasek MK, Begemann M, Soellner L, Eggermann K, Tenorio J, Nevado J, Moore GE, Mackay DJ, Temple K, Gillessen-Kaesbach G, Ogata T, Weksberg R, Algar E, Lapunzina P. Prenatal molecular testing for Beckwith-Wiedemann and Silver-Russell syndromes: a challenge for molecular analysis and genetic counseling. Eur J Hum Genet. 2016;24:784-793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 8. | Gagnier JJ, Kienle G, Altman DG, Moher D, Sox H, Riley D; CARE Group*. The CARE Guidelines: Consensus-based Clinical Case Reporting Guideline Development. Glob Adv Health Med. 2013;2:38-43. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 314] [Cited by in RCA: 652] [Article Influence: 59.3] [Reference Citation Analysis (0)] |
| 9. | de Onis M, Garza C, Onyango AW, Rolland-Cachera MF; le Comité de nutrition de la Société française de pédiatrie. [WHO growth standards for infants and young children]. Arch Pediatr. 2009;16:47-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 208] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 10. | World Health Organization. WHO Anthro for Personal Computers, version 3.2.2, 2011: Software for Assessing Growth and Development of the World’s Children. [cited 15 December 2024]. Available from: http://www.who.int/childgrowth/software/en/. |
| 11. | Behnecke A, Hinderhofer K, Jauch A, Janssen JW, Moog U. Silver-Russell syndrome due to maternal uniparental disomy 7 and a familial reciprocal translocation t(7;13). Clin Genet. 2012;82:494-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | Ribeiro EHP, Haduo MDH, Ribeiro CDC, Lamônica DAC. Silver-Russell syndrome: clinical, neurodevelopmental and communication characteristics: clinical case studies. Codas. 2021;34:e20200273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | Blissett J, Harris G, Kirk J. Effect of growth hormone therapy on feeding problems and food intake in children with growth disorders. Acta Paediatr. 2000;89:644-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 14. | Eggermann T, Spengler S, Gogiel M, Begemann M, Elbracht M. Epigenetic and genetic diagnosis of Silver-Russell syndrome. Expert Rev Mol Diagn. 2012;12:459-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 15. | Lees CC, Stampalija T, Baschat A, da Silva Costa F, Ferrazzi E, Figueras F, Hecher K, Kingdom J, Poon LC, Salomon LJ, Unterscheider J. ISUOG Practice Guidelines: diagnosis and management of small-for-gestational-age fetus and fetal growth restriction. Ultrasound Obstet Gynecol. 2020;56:298-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 255] [Cited by in RCA: 453] [Article Influence: 90.6] [Reference Citation Analysis (0)] |
| 16. | Francis JH, Permezel M, Davey MA. Perinatal mortality by birthweight centile. Aust N Z J Obstet Gynaecol. 2014;54:354-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 61] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 17. | Bujorescu DL, Ratiu A, Citu C, Gorun F, Gorun OM, Crisan DC, Cozlac AR, Chiorean-Cojocaru I, Tunescu M, Popa ZL, Folescu R, Motoc A. Appropriate Delivery Timing in Fetuses with Fetal Growth Restriction to Reduce Neonatal Complications: A Case-Control Study in Romania. J Pers Med. 2023;13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 18. | Lausman A, Kingdom J. How and when to recommend delivery of a growth-restricted fetus: A review. Best Pract Res Clin Obstet Gynaecol. 2021;77:119-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 19. | Darneau D, Giabicani E, Netchine I, Pham A. Perinatal features of children with Silver-Russell syndrome due to 11p15 loss of methylation. Front Pediatr. 2024;12:1367433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |