Published online Dec 9, 2024. doi: 10.5409/wjcp.v13.i4.94156
Revised: June 6, 2024
Accepted: July 2, 2024
Published online: December 9, 2024
Processing time: 231 Days and 21.2 Hours
Diazoxide is the sole approved drug for congenital hyperinsulinism; however, diuretic administration and vigilant monitoring are crucial to prevent and pro
The manuscript describes a patient diagnosed with congenital hyperinsulinism (CHI) treated with diazoxide not overlapping with diuretic. He resulted in su
Diuretic administration and vigilant monitoring of diazoxide therapy are crucial to prevent and promptly identify potentially life-threatening adverse effects.
Core Tip: Diazoxide can be effective in treating congenital hyperinsulinism, but its use requires diuretic administration and careful surveillance to prevent and promptly diagnose potential life-threatening adverse effects. Although diazoxide cardiopulmonary side effects were previously described in sporadic case reports, the United States Food and Drug Administration (FDA) has clearly listed them as severe adverse events since 2015. Despite this, numerous reports were published after the FDA statement, describing the increasing rate of these diazoxide side effects. Further studies are needed to identify the underlying mechanism and precise predisposing factors.
- Citation: Pajno R, Visconti C, Bucolo C, Guarneri MP, Del Barba P, Silvani P, Gregnanin M, Barera G. Diazoxide toxicity in congenital hyperinsulinism: A case report. World J Clin Pediatr 2024; 13(4): 94156
- URL: https://www.wjgnet.com/2219-2808/full/v13/i4/94156.htm
- DOI: https://dx.doi.org/10.5409/wjcp.v13.i4.94156
Congenital hyperinsulinism (CHI) comprises a rare group of hypoketotic hypoglycemia disorders, characterized by abnormal insulin secretion leading to transient or persistent hypoglycemia[1]. While transient forms are linked to perinatal factors (i.e. Infant of diabetic mother, intrauterine growth retardation, maternal toxemia, birth asphyxia and perinatal stress) and can resolve rapidly (lasting less than 6 months), persisting forms of CHI typically persist beyond 6 months of age and are often associated with mutations in genes involved in regulating pancreatic β-cell function (e.g. ABCC8, KCNJ11, GLUD1, GCK, HADH, SLC16A1, HNF4A, HNF1A, HK1, PGM1, PMM2) or genetic syndromes (e.g. Beckwith Wiedemann); however in some of these forms the exact genetic cause remains unknown[2]. If misdiagnosed, this condition can lead to irreversible brain damage[3]. The treatment of CHI depends on the underlying condition and can be medical, surgical or both. Currently, the only approved treatment for CHI is diazoxide, though some patients may be resistant or intolerant, necessitating alternative options[4,5]. Surgical intervention may be curative in focal forms of CHI[6], while others can benefit from administration of continuous enteral glucose or drugs such as octreotide or its long-acting analogs[7]. This report aims to describe a rare side effect of diazoxide that, though documented in literature, lacks defined incidence, pathogenesis, and timing of appearance.
The manuscript describes a patient diagnosed with CHI treated with diazoxide not overlapping with diuretic. He resulted in sudden respiratory distress and therefore was transferred to the Neonatal Intensive Care Unit (NICU). The cardiological evaluation showed pericardial effusion and left ventricular myocardial hypertrophy, absent before. In suspicion of an iatrogenic effect of diazoxide it was progressively reduced until stop while introducing diuretic treatment, with resolution of symptoms
The patient was referred to our center at the age of 45 days of life (see History of past illness) for the suspicion of diazoxide-resistant CHI.
Once admitted, we conducted glucose monitoring without altering the ongoing treatment (diazoxide 15mg/kg/day). We opted not to reintroduce thiazide, considering the baby’s age and the normal findings of the recent cardiac ultrasound scan (US). The baby showed poor glycemic control (average glycemia 40-60 mg/dL), so a second-line therapy with subcutaneous octreotide was started. Octreotide was administered at an initial dosage of 5 mcg/kg/day in three daily administrations. After four days, the patient developed tachypnea [respiratory rate (RR) = 70 breaths/min], intercostal, subcostal and jugular retractions, tachycardia (180-190 bpm), diuresis contraction (0.6-0.7 mL/kg/h) and mild increase in body weight, with adequate oxygen saturation in room air. Due to respiratory distress, high-flow oxygen therapy (maximum flow 10 L/min, maximum FiO2 0.25%) was initiated. Considering the acute presentation and the uncertain etiology of the symptoms, the infant was transferred to the NICU for intensive observation.
The patient was born at 39 gestational weeks through eutocic delivery; he weighed 3300 g (41st weight percentile, -0.24 standard deviation score) and measured 51 cm in length (66th length percentile, 0.4 standard deviation score). The preg
At two hours of life, the infant exhibited hyporeactivity and difficulty in sucking. A blood gas analysis revealed a blood glucose level of 11 mg/dL. Consequently, early enteral feeding with both breast and formula milk was initiated. Despite these measures, persistent hypoglycemia necessitated his transfer to the NICU. Intravenous (IV) infusion therapy with a 10% glucose solution was initiated, but hypoglycemia persisted. Subsequently, IV infusion with glucose 33% was administered via an umbilical venous catheter, gradually increased to 50%, achieving an IV glucose infusion rate (GIR) of 10. 4 mg/kg/min. Simultaneously, enteral feeding was supplemented first with fortified breast milk, then with formula milk containing a high-calorie glucolipid preparation, and finally with maltodextrins, resulting in an increased GIR of 15.3 mg/kg/min. On the 4th day of life, blood tests and a glucagon test during hypoglycemia strongly suggested CHI (Table 1).
| Glycemia (mg/dL) | Insulin (mcU/mL) | C-peptide (ng/mL) | ACTH (pg/mL) | Cortisol (mcg/dL) | GH (ng/mL) | Glucagon test (mg/dL) |
| 46 (nv > 70) | 14.4 (nv < 1.25) | 2 (nv < 0.5) | 17.1 (nv 4.7-48.8) | 17 (nv > 18) | 7.76 (nv > 8) | Glycemia 36-85 |
Due to the high suspicion of CHI, subsequent genetic analysis was carried out and identified a heterozygous variant, c.4477C>Tp. (Arg1493Trp), in the ABCC8 gene, inherited from the father and classified as a pathogenetic variant. On 22nd day of life, diazoxide and thiazide treatment was initiated, with diazoxide gradually increased to 15 mg/kg/day (administered in three daily doses), resulting in a partial improvement in the glycemic profile. IV glucose infusion was no longer required, but the patient still experienced blood sugar levels < 70 mg/dL 2-3 times/day, leading to suspicion of a resistant-diazoxide form. For this reason the patient was transferred to our center at the age of 45 days of life (see history of present illness).
The patient is the only child of non-consanguineous parents from India. No family history of endocrinopathies described nor pancreatic diseases.
After four days of second-line therapy with subcutaneous octreotide the patient developed tachypnea (RR = 70 breaths/min), intercostal, subcostal and jugular retractions, tachycardia (180-190 bpm), diuresis contraction (0.6-0.7 mL/kg/h) and mild increase in body weight, with adequate oxygen saturation in room air. Due to respiratory distress, high-flow oxygen therapy (maximum flow 10 L/min, maximum FiO2 0.25%) was initiated.
Urgent laboratory-instrumental tests were performed due to the clinical conditions: Cell blood count, electrolytes, renal-liver function and inflammatory indexes, blood culture resulted negative, blood gas analysis showed respiratory alkalosis, leading us to exclude sepsis and infectious etiologies.
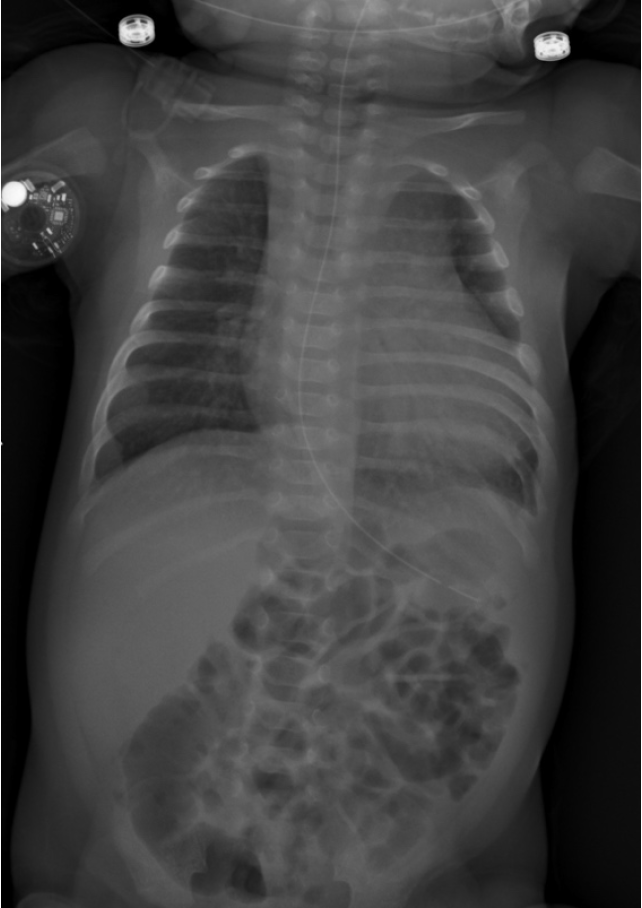
The chest X-ray revealed an enlargement of the cardiac image beyond normal limits with clear lungs and pleural spaces (Figure 1).
Subsequent cardiological evaluation with US showed pericardial effusion in the anterolateral site (0.5-0.7 cm), with no pulmonary hypertension signs, and left ventricular myocardial hypertrophy.
After consultation with our pediatric anesthesiologist and cardiologist, we suspected cardiovascular iatrogenic side-effect of diazoxide.
We diagnosed a diazoxide’s side effect; in particular we diagnosed a cardiovascular side effect considering the patient’s history and clinical manifestations and the cardiological evaluation.
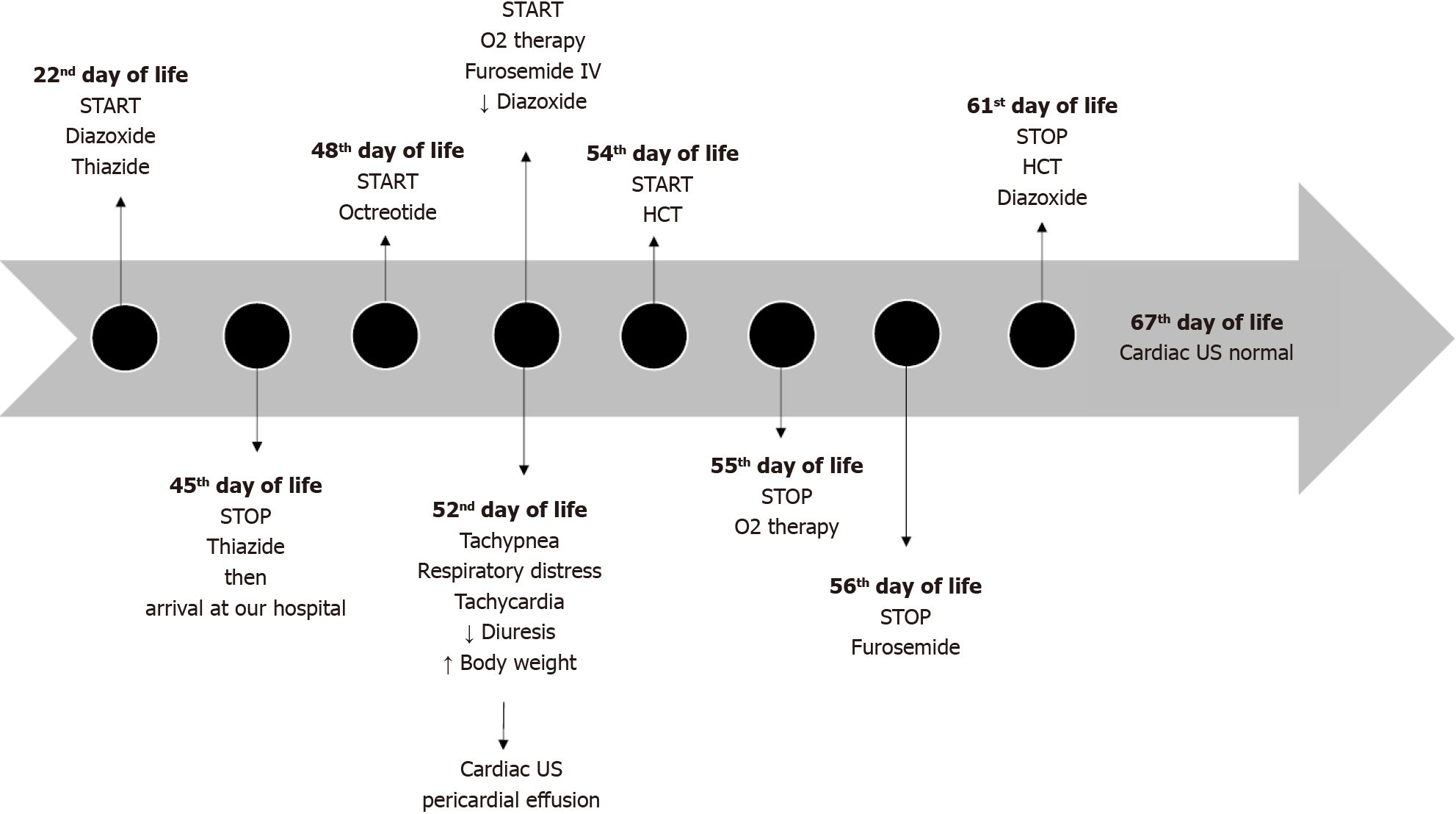
On 52nd day of life therapy with IV furosemide was initiated and the diazoxide dosage was progressively reduced until stopped. Octreotide therapy was gradually increased. Furosemide was continued for five days, and hydrochlorothiazide was initiated on the third day, continuing for a total of eight days (Figure 2).
During the permanence in NICU, the infant continued meals with formula integrated with maltodextrins via bottle and nasogastric tube. After three days form therapies editing, the patient’s conditions improved, prompting the suspension of oxygen support. Daily monitoring by echocardiography revealed a progressive reduction of the pericardial effusion, culminating in complete resolution after 15 days from the onset of symptoms and four days after the complete suspension of diazoxide therapy.
Once clinically stabilized, an 18 fluoro-diydroxy-phenylalanine positron emission tomography/computed tomography (PET/CT) was performed to differentiate between a focal or diffuse form of CHI. The PET/CT highlighted the presence of a single focal accumulation of the tracer located in the pancreatic tail, consistent with a focal form of hyperinsulinism. At the age of four months, the patient underwent a distal pancreatectomy with histological confirmation of a focal form of nesidioblastosis, resulting in a curative operation. The patient’s glycemic profile, after surgery, became normal and he did not require anymore administration of glucose, nor other drugs. Nowadays, he’s an infant with adequate neurological development. Moreover he presented a complete regression of the cardiological manifestations.
Diazoxide currently remains the only drug approved by the United States Food and Drug Administration (FDA) for treatment of CHI[8]. However, over the last 50 years, severe adverse events (SAEs) related to diazoxide have been reported, including necrotizing enterocolitis[9], pericardial effusion[10-13], pulmonary hypertension, respiratory de
The physiological effect of diazoxide is to bind to the SUR1 subunit of the KATP channels keeping them open and thereby inhibiting insulin secretion[18]. In addition, it also binds to the SUR2 subunit expressed in the cardiac muscle, smooth muscles, skeletal muscles and the brain. Due to its action on renal arterioles and renal tubular system, it also has antidiuretic and antihypertensive effects[19,20]. Thus, the off-target effects of diazoxide through its binding to the SUR2 subunit of the KATP channel may explain the most serious side effects (pulmonary hypertension, pericardial effusion and congestive heart failure) even though their physiopathology is not well defined: It remains unclear whether they are caused by primary damage to the cardiovascular system or by a secondary effect due to fluid retention. To reduce the risk of SAEs, the European Society for Pediatric Endocrinology suggests performing a cardiopulmonary assessment (cardiac examination and US) in all infants before starting diazoxide and one week after the beginning of the treatment, even if in the absence of signs of fluid overload[8,21]. They also strongly recommend starting thiazide diuretics at the same time of diazoxide due to risk of fluid retention and pulmonary hypertension[22]. Furthermore, fluid restriction before diazoxide treatment and routine surveillance during the treatment course are advised. These clinical practice guidelines for dosing and monitoring adverse events in infants treated with diazoxide can be highly useful for clinicians treating CHI patients. In line with these recommendations, it is crucial to discontinue the treatment promptly if a diazoxide-related side effect is suspected. Other previous publications describe different diazoxide collateral effects[22] such as hypertrichosis, bone marrow suppression (neutropenia and thrombocytopenia), hyperuricemia and gastrointestinal symptoms (poor appetite and vomiting). While these collateral effects are more frequent and well-known, they are generally considered minor and rarely require discontinuation of the drug.
When the patient was admitted to our center, he was only taking diazoxide. At the age of 45 days, thiazide treatment was discontinued while diazoxide was continued. Before suspending thiazide, cardiac US was normal. We chose to continue diazoxide based on the baby’s age and the normal cardiac ultrasound. After three days in our center, we observed that the patient was diazoxide resistant and therefore octreotide was initiated (48 days of life). When the patient became symptomatic (at 52 days of life), he was transferred to NICU due to his serious clinical conditions. The patient was stabilized with High Flow Nasal Cannula and diuretic treatment. We conducted a comprehensive evaluation (blood tests and instrumental work-up) to understand the etiology of the acute symptoms. We considered the possibility of drug side effects and two factors made octreotide side effects more probable. Firstly, the timing of symptom onset: The patient had been taking diazoxide for over a month, while octreotide had only been initiated for 4 days. Additionally, the patient did not have any risk factors for diazoxide side effects, such as IV fluid administration, transient hyperinsulinism, abnormal cardiac ultrasound findings, or prematurity. The most serious octreotide side effect is necrotizing entero colitis, which we ruled out through abdominal X-rays and observation over the following days. However, guidelines re
Diazoxide can be effective in treating CHI, but its use requires diuretic administration and careful surveillance to prevent and promptly diagnose potential life-threatening adverse effects. Although diazoxide cardiopulmonary side effects were previously described in sporadic case reports, the FDA has clearly listed them as SAEs since 2015. Despite this, numerous reports were published after the FDA statement, describing the increasing rate of these diazoxide side effects. Further studies are needed to identify the underlying mechanism and precise predisposing factors of diazoxide cardiopulmonary side effects. In current clinical practice, it is crucial to adhere to guidelines when initiating diazoxide, and if there is suspicion of cardiopulmonary side effects, the treatment should be promptly interrupted. Additionally, for patients with baseline comorbidities such as congenital heart disease or intolerance to diazoxide, alternative therapies for hyper
| 1. | Demirbilek H, Hussain K. Congenital Hyperinsulinism: Diagnosis and Treatment Update. J Clin Res Pediatr Endocrinol. 2017;9:69-87. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 74] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 2. | Galcheva S, Demirbilek H, Al-Khawaga S, Hussain K. The Genetic and Molecular Mechanisms of Congenital Hyperinsulinism. Front Endocrinol (Lausanne). 2019;10:111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 70] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 3. | Banerjee I, Raskin J, Arnoux JB, De Leon DD, Weinzimer SA, Hammer M, Kendall DM, Thornton PS. Congenital hyperinsulinism in infancy and childhood: challenges, unmet needs and the perspective of patients and families. Orphanet J Rare Dis. 2022;17:61. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 4. | Banerjee I, Salomon-Estebanez M, Shah P, Nicholson J, Cosgrove KE, Dunne MJ. Therapies and outcomes of congenital hyperinsulinism-induced hypoglycaemia. Diabet Med. 2019;36:9-21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 92] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 5. | Sikimic J, Hoffmeister T, Gresch A, Kaiser J, Barthlen W, Wolke C, Wieland I, Lendeckel U, Krippeit-Drews P, Düfer M, Drews G. Possible New Strategies for the Treatment of Congenital Hyperinsulinism. Front Endocrinol (Lausanne). 2020;11:545638. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 6. | Barthlen W, Varol E, Empting S, Wieland I, Zenker M, Mohnike W, Vogelgesang S, Mohnike K. Surgery in Focal Congenital Hyperinsulinism (CHI) - The "Hyperinsulinism Germany International" Experience in 30 Children. Pediatr Endocrinol Rev. 2016;14:129-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 7. | McMahon AW, Wharton GT, Thornton P, De Leon DD. Octreotide use and safety in infants with hyperinsulinism. Pharmacoepidemiol Drug Saf. 2017;26:26-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 8. | De Leon DD, Arnoux JB, Banerjee I, Bergada I, Bhatti T, Conwell LS, Fu J, Flanagan SE, Gillis D, Meissner T, Mohnike K, Pasquini TLS, Shah P, Stanley CA, Vella A, Yorifuji T, Thornton PS. International Guidelines for the Diagnosis and Management of Hyperinsulinism. Horm Res Paediatr. 2024;97:279-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 4] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 9. | Theodorou CM, Hirose S. Necrotizing enterocolitis following diazoxide therapy for persistent neonatal hypoglycemia. J Pediatr Surg Case Rep. 2020;52. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 10. | Silvani P, Camporesi A, Mandelli A, Wolfler A, Salvo I. A case of severe diazoxide toxicity. Paediatr Anaesth. 2004;14:607-609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 11. | Maffre I, Vincenti M, Dalla Vale F, Amouroux C, Werner O, Meilhac A, de Barry G, Amedro P. Diazoxide Causality Assessment of a Pericardial Effusion in a Child with Kabuki Syndrome. J Clin Res Pediatr Endocrinol. 2019;11:218-219. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | Avatapalle B, Banerjee I, Malaiya N, Padidela R. Echocardiography monitoring for diazoxide induced pericardial effusion. BMJ Case Rep. 2012;2012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | Hastings LA, Preddy J, McCready M, Neville K, Verge CF. Pericardial Effusion Associated with Diazoxide Treatment for Congenital Hyperinsulinism. Horm Res Paediatr. 2020;93:206-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 14. | Timlin MR, Black AB, Delaney HM, Matos RI, Percival CS. Development of Pulmonary Hypertension During Treatment with Diazoxide: A Case Series and Literature Review. Pediatr Cardiol. 2017;38:1247-1250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 15. | Kylat RI. Pulmonary hypertension occurring with diazoxide use in a preterm infant with hypoglycemia. Drug Healthc Patient Saf. 2019;11:7-10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 16. | US Food and Drug Administration. FDA drug safety communication: FDA warns about a serious lung condition in infants and newborns treated with Proglycem (diazoxide). 2015. Available from: https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-warns-about-serious-lung-condition-infants-and-newborns-treated#:~:text=The%20U.S.%20Food%20and%20Drug%20Administration%20%28FDA%29%20is,treated%20with%20Proglycem%20%28diazoxide%29%20for%20low%20blood%20sugar. |
| 17. | Thornton P, Truong L, Reynolds C, Hamby T, Nedrelow J. Rate of Serious Adverse Events Associated with Diazoxide Treatment of Patients with Hyperinsulinism. Horm Res Paediatr. 2019;91:25-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 18. | Shyng S, Ferrigni T, Nichols CG. Regulation of KATP channel activity by diazoxide and MgADP. Distinct functions of the two nucleotide binding folds of the sulfonylurea receptor. J Gen Physiol. 1997;110:643-654. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 216] [Cited by in RCA: 218] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 19. | Taylor RM, Rubin AA. Studies on the renal pharmacology of diazoxide (an antidiuretic benzothiadiazine). J Pharmacol Exp Ther. 1964;144:284-292. [PubMed] |
| 20. | Fine LG, Weber H. Effect of diazoxide on renal handling of sodium in the rat. Clin Sci Mol Med. 1975;49:277-282. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 21. | Brar PC, Heksch R, Cossen K, De Leon DD, Kamboj MK, Marks SD, Marshall BA, Miller R, Page L, Stanley T, Mitchell D, Thornton P. Management and Appropriate Use of Diazoxide in Infants and Children with Hyperinsulinism. J Clin Endocrinol Metab. 2020;105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 22. | Herrera A, Vajravelu ME, Givler S, Mitteer L, Avitabile CM, Lord K, De León DD. Prevalence of Adverse Events in Children With Congenital Hyperinsulinism Treated With Diazoxide. J Clin Endocrinol Metab. 2018;103:4365-4372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 62] [Article Influence: 8.9] [Reference Citation Analysis (0)] |