Published online Dec 9, 2024. doi: 10.5409/wjcp.v13.i4.91971
Revised: August 4, 2024
Accepted: October 8, 2024
Published online: December 9, 2024
Processing time: 293 Days and 5.4 Hours
Childhood malnutrition contributes over half of the childhood mortality around the world, predominantly in South-Asian and sub-Saharan countries.
To summarize the childhood malnutrition epidemiology along with the comorbid factors associated with it and its management within the community.
The data collection process involved conducting a comprehensive search using specific keywords such as child nutrition disorders and India with Boolean operators. The search was conducted in the Scopus and PubMed electronic databases.
Inadequate energy consumption initiates pathological alterations in the form of growth retardation, fat, visceral, and muscle loss, a reduction in basal metabolic rate, and a significant reduction in total energy expenditure. It has become evident that malnutrition shows an increased prevalence and incidence rate, despite available guidelines for the management of malnutrition.
Malnutrition can be a major player in the establishment of severe infections that result in significant post discharge mortalities in children. Future trials are required to fill the prime gaps in knowledge regarding the identification of other contributory factors in the pathogenesis of malnutrition and post-discharge infection. New biomarkers for early detection of malnutrition should be the priority of the scientific community for the early management of malnutrition.
Core Tip: Childhood malnutrition, a leading cause of global childhood mortality, especially in South-Asian and sub-Saharan countries, demands urgent attention. This review consolidates malnutrition epidemiology, its associated factors, and community-based management. Despite available guidelines, inadequate energy intake leads to severe complications. Addressing knowledge gaps and identifying new biomarkers are pivotal for effective early detection and management of malnutrition-induced severe infections in children.
- Citation: Mishra M, Rao YK, Shrivastav D, Tripathi P, Singh DD. Indian perspective on childhood malnutrition: Prevalence, pathophysiology, risk factors, and prevention. World J Clin Pediatr 2024; 13(4): 91971
- URL: https://www.wjgnet.com/2219-2808/full/v13/i4/91971.htm
- DOI: https://dx.doi.org/10.5409/wjcp.v13.i4.91971
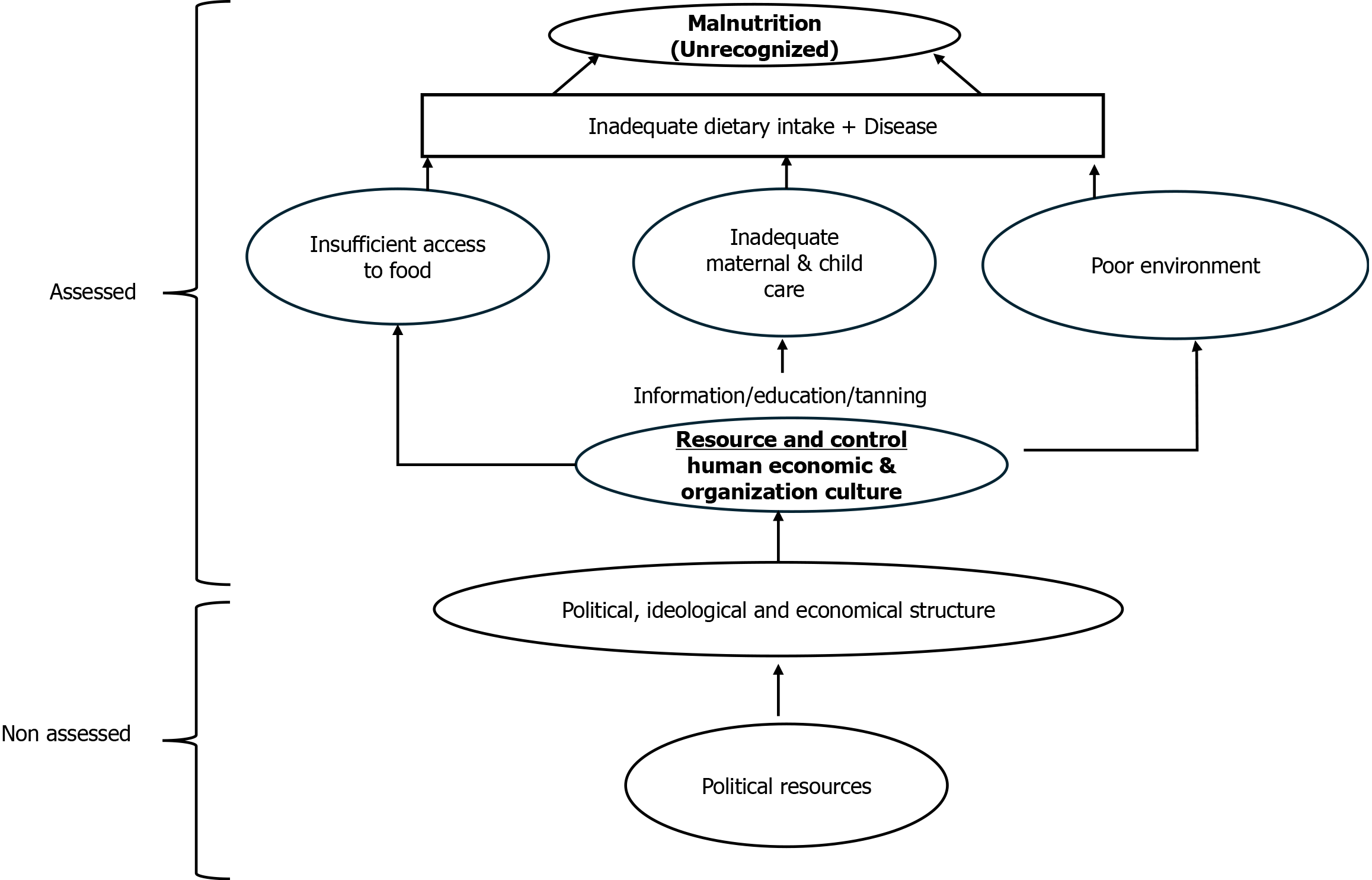
Acute malnutrition is prevalent among children aged below 5 years and affects 47 million children, along with 1 million deaths annually worldwide. According to the United Nations Children's Fund (UNICEF) 2024 report, in East Asia and the Pacific, 59 million (45%) young children are not getting the nutrition and variety they need to grow and develop to their full potential because of a lack of a balanced diet[1,2]. Nutrition has been a global priority for children for many years. For decades, affected children with acute malnutrition were managed by supplementation of fortified milk products. The breakthrough introduction of ready-to-use therapeutic foods (RUTF) and ready-to-use supplementary foods (RUSF) changed the scenario of acute malnutrition management completely after endorsement from the World Health Organization (WHO) and the United Nations through the Community Management of Acute Malnutrition (CMAM) Programme[3]. In many countries, only a few nutrition indicators are regularly tracked, and even fewer pay attention to the diversity of symptoms of malnutrition. Over the past ten years, India has seen considerable improvements in several health metrics. Inequality and uneven development have increased during the past ten years, especially in the fields of child nutrition, education, and health (Figure 1).
In low- and middle-income countries, malnutrition is a significant public health issue. Around 165 million under-five children are stunted, 52 million are wasted, and 17 million are severely wasted worldwide[4]. Asia is home to more than two-thirds of the wasted children and more than half of the stunted children.
A wide range of clinical disorders, including wasting or stunting, marasmus, kwashiorkor, and micronutrient deficiencies, fall under the umbrella term of malnutrition. A weight-for-height Z-score (WHZ) that is more than three SDs below the mean, a mid-upper arm circumference (MUAC) that is less than 115 mm, or the development of nutritional oedema are all considered signs of severe acute malnutrition (SAM). Marasmus is the medical term for moderate acute malnutrition (MAM) or SAM without bilateral pitting oedema. The term "kwashiorkor" is used when there is bilateral pitting oedema. Despite enormous efforts and progress in maternal and child malnutrition, a significant burden of SAM persists. In a recent study, it was reported that a slight decrease in wasting is seen in countries with low income (from 15.9% to 14.2%) while a slight increase in wasting was found in countries with middle income (from 3.3% to 4.7%). In total, 50 million children aged under 5 years remain wasted worldwide[5,6]. In a recent report, it was seen that the disease burden of SAM is likely to aggravate during the pandemic of coronavirus disease 2019, showing 6.7 million additional children at risk of wasting in the year 2020[7]. The present review assessed the prevalence and pathophysiology of malnutrition and focused on current management approaches for such affected children.
A comprehensive search was conducted using PubMed and Scopus, applying keywords such as "child nutrition disorders," "India," "malnutrition," and "risk factors," with Boolean operators to refine results. The inclusion criteria focused on peer-reviewed articles, clinical studies, and reports published between relevant years, addressing malnutrition’s prevalence, risk factors, pathophysiology, and management. Data were synthesized under key themes, including prevalence, management strategies, and developmental consequences.
For several previous years, the malnutrition prevalence has decreased, however, it is around 155 million stunted children and 52 million wasted children worldwide[8].
Currently, India is on the way to reducing to target for impaired growth and development of children although approximate 34.7% of < 5 years of children are still under the phase of stunning which is much greater than the Asia region (21.8%) In the case of wasting, nothing has achieved by India, In India 17.3% of children under 5 years of age affected, which is higher than the average for the Asia region (8.9%)[9]. According to the fifth in the series of surveys, the National Family Health Survey (NFHS) 2019-2021, In India neither the population’s neither health nor nutritional status have appreciably improved. The most recent statistics show that 19.3 percent of children are wasted, 7.7 percent are highly wasted, and 35.5 percent are stunted. In addition, 3.4% of children are overweight, which is higher than the NFHS-4 average of 2.1%[10]. According to NFHS-4, the prevalence of anemia in children under the age of five has dramatically increased, going from 58.6 percent to 67.1 percent. In India, it has been seen that 57% of women are anemic at childbearing age[11]. The double burden of malnutrition has been studied in numerous ways, including underweight and obesity in mothers, obesity and thinness in children[12]. SAM is a major death factor and increases the case fatality rate in children who already have complications of common illnesses like diarrhea and pneumonia[13]. For the management of SAM, WHO and UNICEF recommend two major approaches: (1) Hospital-based approach - for clinical management as per WHO criteria; and (2) Home-based approach - the use of RUTF or medical nutrition therapy as part of integrated public health response to acute malnutrition without medical complications[14].
Criteria for the assessment or diagnosis of malnutrition include specific measures such as the WHZ, MUAC, and the presence of bilateral pitting edema. Children with a WHZ below -2 or -3 standard deviations from the WHO child growth standards are considered wasted or severely wasted, respectively. Additionally, a MUAC of less than 115 mm in children aged 6–59 months is a key indicator of SAM.
General physiological changes: SAM induces a series of adaptive responses in the body. Growth restriction occurs as the body prioritizes essential functions over growth. There's a significant loss of fat, muscle, and visceral mass as the body catabolizes these tissues for energy. The basal metabolic rate decreases as a survival mechanism to conserve energy, leading to an overall reduction in energy expenditure[15,16].
Hormonal and metabolic alterations: Malnutrition causes substantial alterations in the endocrine system. It leads to reduced production of several crucial hormones, including insulin, triiodothyronine, and insulin-like growth factor-1. These hormonal changes subsequently impact the body's ability to regulate blood sugar and ultimately affect cell growth. Conversely, growth hormone and cortisol levels rise, promoting the breakdown of tissues for energy and maintaining blood glucose levels. These hormonal shifts contribute to the catabolic state characteristic of malnutrition[17,18].
Electrolyte imbalances: Malnutrition disrupts the body's electrolyte balance. Sodium retention occurs, while intracellular potassium is depleted. In kwashiorkor, a form of severe malnutrition, cell membrane permeability increases, exacerbating these imbalances. The activity of the glycoside-sensitive, energy-dependent sodium pump is reduced, further compromising cellular homeostasis[19].
Immune system effects: The immune system is severely impacted by malnutrition. Atrophy of the thymus, lymph nodes, and tonsils impairs cellular immunity. There's a reduction in CD4 clusters of differentiation, although CD8-T cells remain relatively normal. The loss of delayed hypersensitivity, poor phagocytic activity, and lower levels of secretory IgA collectively weaken the body's defense against pathogens, increasing susceptibility to infections[20].
Neurological effects: Malnutrition has profound effects on the developing brain. It leads to a decrease in the number of neurons, synapses, dendritic arborization, and myelination, resulting in an overall reduction in brain size and thinning of the cerebral cortex. These structural changes translate into functional deficits, including delays in cognitive and motor development. Critically, if malnutrition occurs after 3-4 years of age, some of these neurological impacts may become permanent, underscoring the importance of early intervention[21-23].
Facility based management of children with SAM: Currently, SAM-affected children in India are cared for by Nutrition Rehabilitation Centers (NRCs); however, due to the dearth of NRCs, most SAM children never receive any treatment[23]. Most SAM children admitted to NRCs do not have any medical conditions. There is already a way to help and enhance more SAM kids in India[24]. To ensure that children with SAM receive quick and high quality care, efforts are being made at the national level to identify the procedures that need to be followed. The facility-based care is implemented through a network of 262 NRCs[25]. Admission requires either edema, MUAC 115 mm, or a W/H score of less than -3Z. The appetite test is administered to all children, for 14-21 days, the children are housed in the facility, and they are given locally produced F-75 and F100[26]. The youngsters are freed after recovering a normal appetite and weight. A child is registered under the ICDS program after being released, and home visits are utilized to monitor the kid's well-being[27].
Community based management of children with SAM: Except for those experiencing complications, no SAM child needs to be admitted to the hospital. It has been discovered that home based management with RUTF is linked to better outcomes than traditional hospital therapy[28]. There is sufficient evidence that such SAM children can be successfully managed at the home level, and it has been noted that between 60% and 90% of SAM cases, identified by active case finding in the community, are without medical issues[29]. Since children experience fewer hospital-acquired infections and obtain continuity of care after discharge, home-based management of SAM children using medical nutrition therapy provides many more benefits[30]. It also benefits mother by giving them more time to spend with their families and lowering the possibility of siblings being neglected. Additionally, mothers can receive guidance on better feeding and care techniques in their local environments while also managing other family obligations[31].
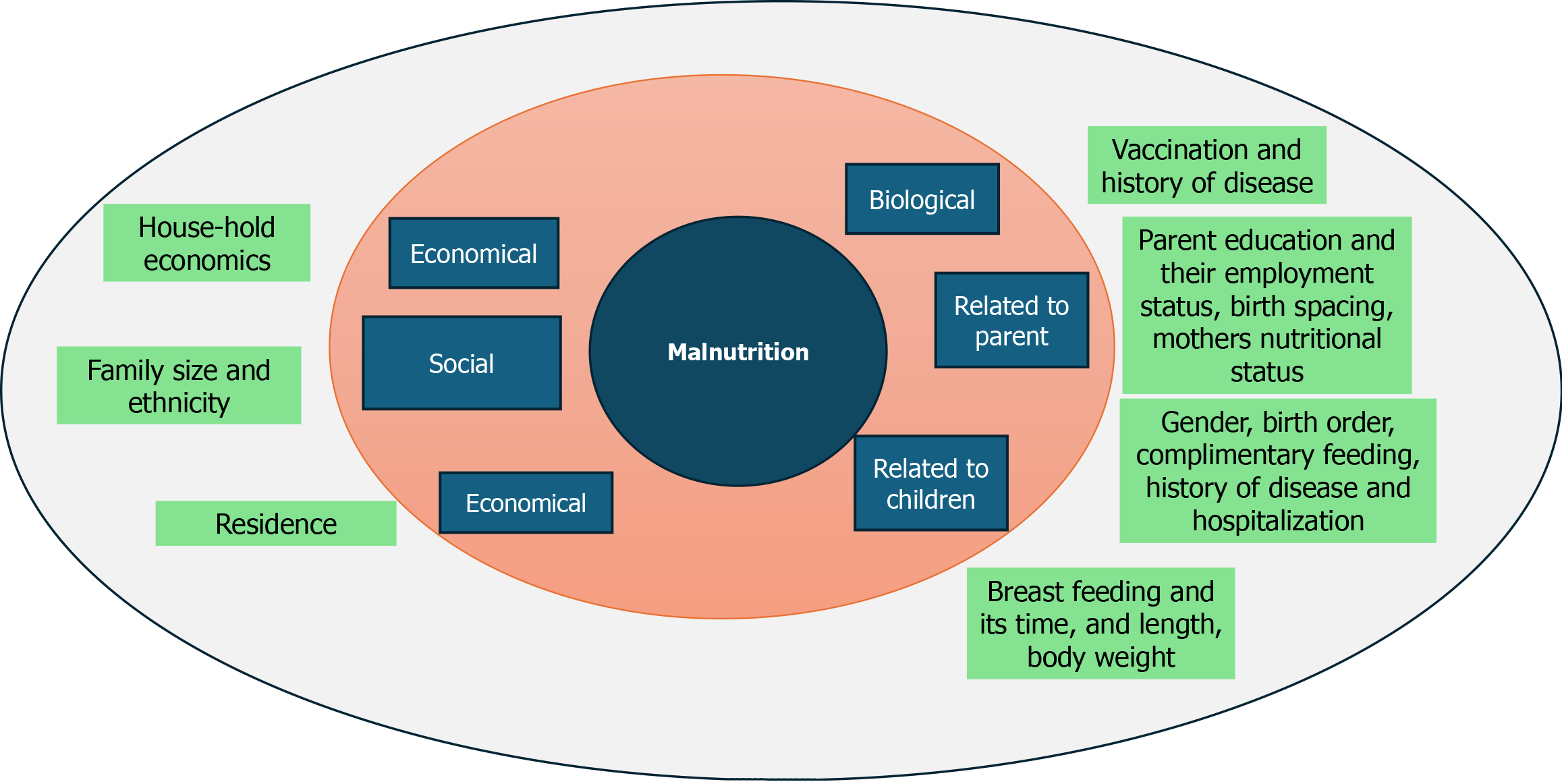
Possible risk factors for SAM: Factors like low birth weight, sociodemographic traits, inadequate nutrition, improper feeding techniques, incomplete immunization, political and environmental instability, emergency situations, and a high prevalence of infectious diseases are considered risk factors for SAM (Figure 2)[32,33].
Research indicates that children suffering from SAM face a higher risk of developmental issues compared to those who receive adequate breast milk and additional nutritional supplements[34]. The risk factors such as feeding practices, water availability, sanitation, and hygiene conditions plays a critical roles in occurrence of SAM in infant and young child[35]. Islam et al[36] have established that when caregiver’s education level is low, child stands a higher risk of becoming wasted[36,37]. Children of illiterate parents have a higher risk of SAM especially when it has to do with the caregiver[38].
Beginning at conception, disease and insufficient food intake are major contributors to early childhood malnutrition, with the first 24 months of life having the highest risk of a fall in length-for-weight[39]. More than 200 million children under the age of five are thought to be living below their full developmental potential on a global scale[40]. A child who is malnourished starts a "vicious cycle" in which she/he becomes more prone to illness and infection, which can subsequently cause malnutrition to increase[41]. Illness can temporarily reduce infants' appetite and nutrient absorption, while increasing catabolism, which diverts nutrients from growth to the immune response, potentially hindering psychomotor and cognitive development[42].
Giving adequate nutrition and disease prevention: Due to its importance to the early childhood developmental phase, exclusive breastfeeding should not be compromised in the fight against SAM[32]. However, preventative intervention is propelled by nutrition education. Encouragement of supplementing programs and treatment of micronutrient deficiencies by dietary approaches such as dietary diversity through home gardens or other techniques like micronutrient fortification[43].
Therapeutic foods for SAM prevention: F75 and F100 are exclusive dairy products that are typically utilized in inpatient settings to treat SAM. Inpatient treatment facilities administer the F75 to children who need to be stabilized. Children receiving inpatient care typically get 80 to 100 kcal/kg/day across eight to twelve meals per day for three to seven days[44]. The F100, which is administered during the recovery stage of inpatient therapy for SAM, provides children with around 100-200 kcal/kg/day for three-four weeks[45]. F75s and F100s are not kept at a temperature of 25 °C for very long since they need some preparation, as well as because of their high moisture content[44].
In addition, these foods are not distributed to caretakers for home preparation (UNICEF catalog). Community-based treatment methods have benefited enormously from the creation of ready-to-use foods (RUFs)[46]. Unlike F75s and F100s, RUTFs are ready to eat without preparation and are more nutrient-dense than typical household foods, with a very low moisture content that makes them resistant to microbial growth[47].
To treat MAM and SAM, treatment centers provide a variety of items, including formulated biscuits, bars, and pastes like RUTFs, RUSFs, LNS (Lipid-based nutritional supplements and specialized products like Plumpy Sup, Plumpy'Nut, and Plumpy'Doz. Specifically, Plumpy'Doz and Plumpy Sup aimed at treating MAM, and Plumpy'Nut intended for treating both MAM and SAM in infants. Children receive one to two 92 g sachets daily for MAM and two to three sachets per day for SAM treatment. Plumpy'Nut is formulated to meet all a child's daily nutritional needs for SAM treatment (Nutriset), while families may also receive Super Cereal Plus (formerly Corn Soy Blend Plus Plus or CSB++) as part of their food rations to help prevent MAM (Table 1)[48].
| List of items |
| RUTFs like formulated bars, pastes, or biscuits |
| RUSFs |
| LNS |
| Plumpy’Doz, Plumpy Sup, and Plumpy’Nut, which are common RUFs used to prevent or treat MAM and SAM, are given to supplement children’s diets: |
| Plumpy’Doz, designed to prevent or treat MAM in infants |
| Plumpy Sup, designed to treat MAM in infants |
| Plumpy’Nut, designed to treat MAM or SAM in infants |
Moderate malnutrition is treated with RUTF: Due to strong evidence supporting their effectiveness in treating SAM, RUFs are now being considered for use in supplemental feeding programs aimed at managing moderate malnutrition[48]. Given its success in expanding coverage through decentralized community-based care, the CMAM model may find wider application in supplemental feeding programs. RUTF was initially created as a therapeutic meal for severe malnutrition, but it has also been used to treat moderate and mild malnutrition. More recently, it has been extensively disseminated to at-risk groups in order to prevent malnutrition[46]. The nutritional content and micronutrient profile of RUTF and RUSF are quite similar. For treating SAM, RUTF must meet the full daily nutritional requirements of the child, with the dosage adjusted based on weight. In cases of moderate malnutrition, RUTF is administered as a standard 500 kcal/day ration, regardless of the child’s weight, to supplement their daily diet[46].
SAM is a life-threatening condition requiring immediate attention, affecting millions of children worldwide. Understanding its impact is crucial for early action and prevention. Factors such as infant feeding practices, hygiene, and caregiver education significantly influence the risk of SAM. Childhood malnutrition starts a detrimental cycle leading to heightened vulnerability to illnesses, impairing physical and cognitive development. It's evident that over 200 million children globally aren't reaching their developmental potential, with malnutrition significantly hindering learning capabilities[49].
Preventative strategies against SAM encompass a multi-pronged approach involving adequate nutrition, disease prevention, and educational interventions. Exclusive breastfeeding, coupled with nutritional education, plays a pivotal role. Therapeutic foods like F75 and F100, administered in inpatient settings, aid stabilization and recovery, respectively, but face challenges in distribution and preparation. RUFs, such as RUTFs, exhibit promise in community-based therapy due to their ease of use and high nutrient density. These RUFs, including Plumpy'Nut and others, are proving effective in preventing and treating various degrees of malnutrition, contributing to children's nutritional needs[50]. Furthermore, RUTFs have shown potential in treating moderate malnutrition, expanding their role beyond severe cases. The success of the CMAM approach indicates the possibility of broader applications in supplemental feeding programs. The nutritional equivalence of RUTFs and RUSFs is highlighted, offering insight into dosage and application, thereby broadening their usage in addressing varying degrees of malnutrition[51].
SAM remains a critical issue in low- and middle-income countries, despite ongoing efforts. Innovative management strategies and reliable biomarkers for early detection and treatment are urgently needed. Addressing the dual burden of malnutrition-undernutrition and overnutrition-among vulnerable populations, especially women and children, is crucial. The review emphasizes the importance of enhanced public health policies, targeted nutrition programs, and community-based interventions to mitigate malnutrition's impact and improve health outcomes. A comprehensive approach is essential to reduce malnutrition's prevalence and its long-term effects on global health.
The authors would like to thank Amity Institute of Biotechnology, Amity University Rajasthan Jaipur, and India. GSVM Medical College, Kanpur, India, and, MLB Medical College, Jhansi, India. The authors also acknowledge the DST-FIST-AIMT project at Amity Institute Microbial Technology and the DST-PURSE project at Amity University Rajasthan for providing necessary facilities.
| 1. | Govender I, Rangiah S, Kaswa R, Nzaumvila D. Malnutrition in children under the age of 5 years in a primary health care setting. S Afr Fam Pract (2004). 2021;63:e1-e6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 2. | Stevens GA, Beal T, Mbuya MNN, Luo H, Neufeld LM; Global Micronutrient Deficiencies Research Group. Micronutrient deficiencies among preschool-aged children and women of reproductive age worldwide: a pooled analysis of individual-level data from population-representative surveys. Lancet Glob Health. 2022;10:e1590-e1599. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 224] [Cited by in RCA: 228] [Article Influence: 76.0] [Reference Citation Analysis (0)] |
| 3. | Duke T. Levels and trends in child mortality estimation. Arch Dis Child. 2024;109:620-621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 4. | Sigh S, Roos N, Chhoun C, Laillou A, Wieringa FT. Ready-to-Use Therapeutic Foods Fail to Improve Vitamin A and Iron Status Meaningfully during Treatment for Severe Acute Malnutrition in 6-59-Month-old Cambodian Children. Nutrients. 2023;15:905. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 5. | Bassaganya-Riera J, Berry EM, Blaak EE, Burlingame B, le Coutre J, van Eden W, El-Sohemy A, German JB, Knorr D, Lacroix C, Muscaritoli M, Nieman DC, Rychlik M, Scholey A, Serafini M. Goals in Nutrition Science 2020-2025. Front Nutr. 2020;7:606378. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 6. | Hitchings MDT, Berthé F, Aruna P, Shehu I, Hamza MA, Nanama S, Steve-Edemba C, Grais RF, Isanaka S. Effectiveness of a monthly schedule of follow-up for the treatment of uncomplicated severe acute malnutrition in Sokoto, Nigeria: A cluster randomized crossover trial. PLoS Med. 2022;19:e1003923. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 7. | Victora CG, Christian P, Vidaletti LP, Gatica-Domínguez G, Menon P, Black RE. Revisiting maternal and child undernutrition in low-income and middle-income countries: variable progress towards an unfinished agenda. Lancet. 2021;397:1388-1399. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 343] [Cited by in RCA: 380] [Article Influence: 95.0] [Reference Citation Analysis (0)] |
| 8. | Headey D, Heidkamp R, Osendarp S, Ruel M, Scott N, Black R, Shekar M, Bouis H, Flory A, Haddad L, Walker N; Standing Together for Nutrition consortium. Impacts of COVID-19 on childhood malnutrition and nutrition-related mortality. Lancet. 2020;396:519-521. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 218] [Cited by in RCA: 224] [Article Influence: 44.8] [Reference Citation Analysis (0)] |
| 9. | India State-Level Disease Burden Initiative Malnutrition Collaborators. The burden of child and maternal malnutrition and trends in its indicators in the states of India: the Global Burden of Disease Study 1990-2017. Lancet Child Adolesc Health. 2019;3:855-870. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 194] [Cited by in RCA: 182] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 10. | Sahu SK, Kumar SG, Bhat BV, Premarajan KC, Sarkar S, Roy G, Joseph N. Malnutrition among under-five children in India and strategies for control. J Nat Sci Biol Med. 2015;6:18-23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 11. | Jha A, Chandrakar A. A Comparative Study of National Family Health Survey-4 and National Family Health Survey-5 of Nutritional Indicators in Chhattisgarh. Cureus. 2024;16:e55524. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 12. | Kinyoki D, Osgood-Zimmerman AE, Bhattacharjee NV; Local Burden of Disease Anaemia Collaborators, Kassebaum NJ, Hay SI. Anemia prevalence in women of reproductive age in low- and middle-income countries between 2000 and 2018. Nat Med. 2021;27:1761-1782. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 118] [Cited by in RCA: 92] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 13. | Popkin BM, Corvalan C, Grummer-Strawn LM. Dynamics of the double burden of malnutrition and the changing nutrition reality. Lancet. 2020;395:65-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 954] [Cited by in RCA: 760] [Article Influence: 152.0] [Reference Citation Analysis (0)] |
| 14. | Rodríguez L, Cervantes E, Ortiz R. Malnutrition and gastrointestinal and respiratory infections in children: a public health problem. Int J Environ Res Public Health. 2011;8:1174-1205. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 230] [Cited by in RCA: 202] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 15. | Fischer M, JeVenn A, Hipskind P. Evaluation of muscle and fat loss as diagnostic criteria for malnutrition. Nutr Clin Pract. 2015;30:239-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 16. | Keller U. Nutritional Laboratory Markers in Malnutrition. J Clin Med. 2019;8:775. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 199] [Cited by in RCA: 405] [Article Influence: 67.5] [Reference Citation Analysis (0)] |
| 17. | Kasprzak A. Insulin-Like Growth Factor 1 (IGF-1) Signaling in Glucose Metabolism in Colorectal Cancer. Int J Mol Sci. 2021;22:6434. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 107] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 18. | Martins VJB, de Albuquerque MP, Sawaya AL. Endocrine Changes in Undernutrition, Metabolic Programming, and Nutritional Recovery. In: Preedy V, Patel V, editors. Handbook of Famine, Starvation, and Nutrient Deprivation. Cham: Springer, 2017. [DOI] [Full Text] |
| 19. | Raza M, Kumar S, Ejaz M, Azim D, Azizullah S, Hussain A. Electrolyte Imbalance in Children With Severe Acute Malnutrition at a Tertiary Care Hospital in Pakistan: A Cross-Sectional Study. Cureus. 2020;12:e10541. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 20. | Morales F, Montserrat-de la Paz S, Leon MJ, Rivero-Pino F. Effects of Malnutrition on the Immune System and Infection and the Role of Nutritional Strategies Regarding Improvements in Children's Health Status: A Literature Review. Nutrients. 2023;16:1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 63] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 21. | Cakir M, Senyuva S, Kul S, Sag E, Cansu A, Yucesan FB, Yaman SO, Orem A. Neurocognitive Functions in Infants with Malnutrition; Relation with Long-chain Polyunsaturated Fatty Acids, Micronutrients Levels and Magnetic Resonance Spectroscopy. Pediatr Gastroenterol Hepatol Nutr. 2019;22:171-180. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 22. | Cusick SE, Georgieff MK. The Role of Nutrition in Brain Development: The Golden Opportunity of the "First 1000 Days". J Pediatr. 2016;175:16-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 345] [Cited by in RCA: 356] [Article Influence: 39.6] [Reference Citation Analysis (0)] |
| 23. | Tandon M, Quereishi J, Prasanna R, Tamboli AF, Panda B. Performance of Nutrition Rehabilitation Centers: A Case Study from Chhattisgarh, India. Int J Prev Med. 2019;10:66. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 24. | Kumar B, Shrivastava J, Satyanarayana S, Reid AJ, Ali E, Zodpey S, Agnani M. How effective is the integration of facility and community-based management of severe acute malnutrition in India? Public Health Action. 2013;3:265-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 25. | Kumar P, Sinha RK, Daniel A, Shah H, Sriswan R, Kokane A, Mohapatra A, Kashyap V, Goel AK, Kumar V, Kiran A, Arlappa N, Joshi A, Nayak RR, Singh M, Salasibew M, Ghosh S, Pawar SM, Mishra P, Tiwari K, Bhattacharjee S, Saiyed F, Patel TS, Nayak PK, Sahoo SK, Prajapati M, Sinha S, de Wagt A. Effectiveness of community-based treatment programs for treatment of uncomplicated severe acute malnourished children aged 6-59 months using locally produced nutrient dense foods: protocol for a multicentric longitudinal quasi-experimental study. BMC Nutr. 2021;7:85. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 26. | Grellety E, Golden MH. Severely malnourished children with a low weight-for-height have a higher mortality than those with a low mid-upper-arm-circumference: I. Empirical data demonstrates Simpson's paradox. Nutr J. 2018;17:79. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 27. | Chakraborty R, Joe W, ShankarMishra U, Rajpal S. Integrated child development service (ICDS) coverage among severe acute malnourished (SAM) children in India: A multilevel analysis based on national family health survey-5. PLoS One. 2024;19:e0294706. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 28. | Schoonees A, Lombard MJ, Musekiwa A, Nel E, Volmink J. Ready-to-use therapeutic food (RUTF) for home-based nutritional rehabilitation of severe acute malnutrition in children from six months to five years of age. Cochrane Database Syst Rev. 2019;5:CD009000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 29. | Jones KD, Berkley JA. Severe acute malnutrition and infection. Paediatr Int Child Health. 2014;34 Suppl 1:S1-S29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 99] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 30. | Williams PCM, Berkley JA. Guidelines for the treatment of severe acute malnutrition: a systematic review of the evidence for antimicrobial therapy. Paediatr Int Child Health. 2018;38:S32-S49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 31. | Poduval J, Poduval M. Working mothers: how much working, how much mothers, and where is the womanhood? Mens Sana Monogr. 2009;7:63-79. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 43] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 32. | Mukuku O, Mutombo AM, Kamona LK, Lubala TK, Mawaw PM, Aloni MN, Wembonyama SO, Luboya ON. Predictive Model for the Risk of Severe Acute Malnutrition in Children. J Nutr Metab. 2019;2019:4740825. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 33. | David SM, Pricilla RA, Paul SS, George K, Bose A, Prasad JH. Risk factors for severe acute malnutrition among children aged 6-59 months: A community-based case-control study from Vellore, Southern India. J Family Med Prim Care. 2020;9:2237-2243. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 34. | Abeshu MA, Lelisa A, Geleta B. Complementary Feeding: Review of Recommendations, Feeding Practices, and Adequacy of Homemade Complementary Food Preparations in Developing Countries - Lessons from Ethiopia. Front Nutr. 2016;3:41. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 97] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 35. | Sachdeva S, Vijayaran M. Nutritional rehabilitation using energy dense local food as ready to use therapeutic food in hospitalized malnourished children: Case for primary prevention at grass root levels. J Med Trop. 2014;16:22. [DOI] [Full Text] |
| 36. | Islam MM, Alam M, Tariquzaman M, Kabir MA, Pervin R, Begum M, Khan MM. Predictors of the number of under-five malnourished children in Bangladesh: application of the generalized poisson regression model. BMC Public Health. 2013;13:11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 53] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 37. | Patel MP, Sandige HL, Ndekha MJ, Briend A, Ashorn P, Manary MJ. Supplemental feeding with ready-to-use therapeutic food in Malawian children at risk of malnutrition. J Health Popul Nutr. 2005;23:351-357. [PubMed] |
| 38. | Sanghvi J, Mehta S, Kumar R. Predicators for weight gain in children treated for severe acute malnutrition: a prospective study at nutritional rehabilitation center. ISRN Pediatr. 2014;2014:808756. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 39. | Reinhardt K, Fanzo J. Addressing Chronic Malnutrition through Multi-Sectoral, Sustainable Approaches: A Review of the Causes and Consequences. Front Nutr. 2014;1:13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 100] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 40. | Grantham-McGregor S, Cheung YB, Cueto S, Glewwe P, Richter L, Strupp B; International Child Development Steering Group. Developmental potential in the first 5 years for children in developing countries. Lancet. 2007;369:60-70. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2295] [Cited by in RCA: 1717] [Article Influence: 95.4] [Reference Citation Analysis (0)] |
| 41. | Schaible UE, Kaufmann SH. Malnutrition and infection: complex mechanisms and global impacts. PLoS Med. 2007;4:e115. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 527] [Cited by in RCA: 562] [Article Influence: 31.2] [Reference Citation Analysis (0)] |
| 42. | Dewey KG, Mayers DR. Early child growth: how do nutrition and infection interact? Matern Child Nutr. 2011;7 Suppl 3:129-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 157] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 43. | Nair MK, Augustine LF, Konapur A. Food-Based Interventions to Modify Diet Quality and Diversity to Address Multiple Micronutrient Deficiency. Front Public Health. 2015;3:277. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 76] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 44. | Lanyero B, Namusoke H, Nabukeera-Barungi N, Grenov B, Mupere E, Michaelsen KF, Mølgaard C, Christensen VB, Friis H, Briend A. Transition from F-75 to ready-to-use therapeutic food in children with severe acute malnutrition, an observational study in Uganda. Nutr J. 2017;16:52. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 45. | Das JK, Salam RA, Saeed M, Kazmi FA, Bhutta ZA. Effectiveness of Interventions for Managing Acute Malnutrition in Children under Five Years of Age in Low-Income and Middle-Income Countries: A Systematic Review and Meta-Analysis. Nutrients. 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 57] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 46. | Brixi G. Innovative optimization of ready to use food for treatment of acute malnutrition. Matern Child Nutr. 2018;14:e12599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 47. | Bitew ZW, Ayele EG, Worku T, Alebel A, Alemu A, Worku F, Yesuf A. Determinants of mortality among under-five children admitted with severe acute malnutrition in Addis Ababa, Ethiopia. Nutr J. 2021;20:94. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 48. | Medoua GN, Ntsama PM, Ndzana AC, Essa'a VJ, Tsafack JJ, Dimodi HT. Recovery rate of children with moderate acute malnutrition treated with ready-to-use supplementary food (RUSF) or improved corn-soya blend (CSB+): a randomized controlled trial. Public Health Nutr. 2016;19:363-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 49. | Sokhela H, Govender L, Siwela M. Complementary Feeding Practices and Childhood Malnutrition in South Africa: The Potential of Moringa Oleifera Leaf Powder as a Fortificant: A Narrative Review. Nutrients. 2023;15:2011. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 50. | Akinmoladun OF, Bamidele OP, Jideani VA, Nesamvuni CN. Severe Acute Malnutrition: The Potential of Non-Peanut, Non-Milk Ready-to-Use Therapeutic Foods. Curr Nutr Rep. 2023;12:603-616. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 51. | Fetriyuna F, Purwestri RC, Jati IRAP, Setiawan B, Huda S, Wirawan NN, Andoyo R. Ready-to-use therapeutic/supplementary foods from local food resources: Technology accessibility, program effectiveness, and sustainability, a review. Heliyon. 2023;9:e22478. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |