Published online Dec 9, 2024. doi: 10.5409/wjcp.v13.i4.91638
Revised: August 30, 2024
Accepted: September 25, 2024
Published online: December 9, 2024
Processing time: 303 Days and 4.3 Hours
Globally, obesity and diabetes mellitus (DM) are emergent public health concerns in the adolescent population. India, home to the largest adolescent population and the second largest diabetes cohort is experiencing rapid but unplanned urba
To determine prevalence and determinants of obesity and hyperglycaemia and their association among community-dwelling older adolescents (15-19 years) in India.
This cross-sectional analysis from the national family health survey-5 included data of 258028 adolescents aged 15-19 across India (2019-2021). The survey employed stratified two-stage sampling, with systematic random sampling in rural and urban areas. Statistical analysis included descriptive statistics, bivariate, and multivariable logistic regression, employing generalized linear models.
The weighted prevalence of DM was 1.09% including 0.77% [95% confidence interval (CI): 0.72-0.83] previously diagnosed and 0.32% (95%CI: 0.29-0.35) newly diagnosed cases detected on survey screening. On adjusted analysis, increasing age, higher education levels, higher wealth index, and overweight/obesity were the factors significantly associated with presence of DM. Only 61% of the adolescents with previously diagnosed DM were on anti-diabetes treatment. The weighted prevalence of overweight/obesity among older adolescents was 6.9% with significantly higher odds in the male sex, having higher educational levels, urban residence, and those with a higher wealth index.
Nearly one in hundred older adolescents in India have diabetes, with one in three undiagnosed. Strengthening DM screening and treatment access among adolescents through public health programs is urgently warranted.
Core Tip: In resource-limited settings, 3 in 10 older adolescents with diabetes (DM) are undiagnosed due to lack of screening while only 6 in 10 older adolescents previously diagnosed with DM utilize anti-diabetes medication. Primary care physicians including paediatricians in outpatient settings should necessarily screen older adolescents with family history or those who are overweight or obese for DM. Furthermore, they should advise older adolescents to engage in regular physical activity and exercise to maintain normal body weight even in the absence of hyperglycaemia. Finally, medication adherence in older adolescents with DM should be assessed during each appointment accompanied with regular counselling.
- Citation: Maheshwari V, Basu S. Prevalence of obesity, determinants, and its association with hyperglycaemia among community dwelling older adolescents in India. World J Clin Pediatr 2024; 13(4): 91638
- URL: https://www.wjgnet.com/2219-2808/full/v13/i4/91638.htm
- DOI: https://dx.doi.org/10.5409/wjcp.v13.i4.91638
Obesity and hyperglycaemia are rapidly emerging as critical public health concerns globally among adolescents. High body mass index (BMI) is positively correlated with Type 2 diabetes mellitus (T2DM) in adolescents, with estimates indicating a 13-fold higher risk of T2DM in obese adolescents compared to those with normal BMI[1-4]. Obesity in adolescence is also a strong predictor of obesity in adulthood[5].
India has the largest population of adolescents worldwide[6] and also the second largest diabetes mellitus (DM) cohort globally[7]. Increasing urbanization, consumption of unhealthy diets and decreased physical activity patterns[8,9] since childhood renders adolescents particularly susceptible to both increased BMI and lifestyle disorders especially T2DM. Genetic predisposition also enhances the risk, especially in the Indian population, which is more prone to insulin resistance and thereby T2DM even at lower levels of obesity compared to Caucasian populations[10].
Previous research has indicated the need for region-specific data in understanding the burden and determinants of diabetes in the adolescent population[11]. The prevalence of childhood obesity and overweight in India as per pooled evidence is 8.4%, and 12.4% respectively[12], although most of the existing data is derived from studies with small sample sizes and single-centre studies that lack representativeness while lacking generalizability. Furthermore, the linkage of adolescent BMI with hyperglycaemia and their sociodemographic and behavioural determinants have not been adequately explored in Indian health settings. We therefore conducted this study with the objectives of determining the prevalence and determinants of obesity and hyperglycaemia and their association among community-dwelling older adolescents (15-19 years) in India using data from a nationally representative dataset.
This study utilized data from the national family health survey-5 (NFHS-5), a nationally representative large-scale, multistage survey conducted in a representative sample of households across India between 2019 and 2021. NFHS-5 survey collected comprehensive information on India’s population and health from 707 districts, 28 states, and eight union territories. The survey employed a stratified, two-stage sampling design to ensure the representativeness of the sample. In rural regions, primary sampling units (PSUs) were villages selected using probability proportional to size (PPS). Conversely, in urban areas, census enumeration blocks were chosen as PSUs through PPS systematic sampling. During the second stage, households were randomly chosen using systematic random sampling after a comprehensive mapping and household listing of the selected PSUs. Detailed information pertaining to the sampling, survey tools and data collection is available elsewhere[13].
The present study included adolescents aged 15-19 years as the target population to estimate the prevalence of DM in this age-group. The study sample was derived from the NFHS-5 household dataset, which included a representative sample of households from across the country.
In NFHS-5, all individuals aged 15 years and older were invited to participate in a finger-stick blood glucose assessment[13]. Trained health investigators conducted random or fasting blood glucose testing using the Accu-Chek Performa glucometer along with glucose test strips. Threshold values were employed to identify DM using a random glucose test result of ≥ 200 mg/dL for individuals who were not in a fasting state and ≥ 126 mg/dL for individuals who reported fasting for ≥ 8 hours prior to the test.
An individual was categorized as having a previous DM diagnosis if they had answered affirmatively to the question, “Told high blood glucose on two or more occasions by doctor or health professionals?”. Similarly, an individual was classified as using DM medication if they had indicated “yes” in response to the question, “Currently taking any prescribed medicine to lower blood glucose?”. In our analysis, an individual was considered to have DM if they either exhibited elevated blood glucose levels, were previously diagnosed with DM, or were using medication to reduce their blood glucose levels. Further, we assessed treatment-seeking behaviour among adolescents with previously diagnosed DM, based on whether they reported taking medication to lower blood glucose.
The major socio-demographic variables considered, and their categories included age, sex (male or female), education levels (no education, primary, secondary or higher education), wealth index (poorest to richest), urban or rural residence, and household religion (Hindu, Muslim or others). Lifestyle factors included tobacco consumption (self-reported as yes or no) and alcohol use (self-reported as yes or no) among the adolescents. The type of healthcare facility accessed by the participant in the last 3 months was queried and categorized into four groups: None, public, private, and others (nongovernmental organization along with other facilities). In NFHS-5, the Seca 213 stadiometer was used to measure height and the Seca 874 digital scale was used to measure the weight of the participants. The World Health Organization growth reference standard was applied to ascertain the BMI z-scores in the adolescents[14]. For the current analysis, individuals were grouped into three categories: Overweight/obese (z-score > + 1 SD), normal (between + 1 and - 2 SD), and thin (> - 2 SD).
Descriptive statistics were employed to summarize the characteristics of the study participants. The mean and SD were reported for continuous variables, while frequency and percentages were reported for categorical variables. Bivariate analysis was conducted to examine the associations between the independent variables and the presence of DM among the adolescents.
We utilized the modified Poisson regression approach[15] to identify the determinants of DM among the adolescents. The model was fit using a generalized linear model with the Poisson family. Both unadjusted and adjusted rate ratios (RR) were reported along with their 95% confidence intervals (CI). Similar analysis was conducted to identify the predictors of overweight/obesity among adolescents. For treatment-seeking behaviour, multiple logistic regression was used to estimate odds ratios (ORs) and 95%CI for each predictor variable while adjusting for potential confounders. Adjusted models included variables that showed a significant association (P < 0.05) in the bivariate analysis, and model fit was assessed using appropriate tests. Pre-specified sampling weights were applied throughout the analysis to account for the survey design using the “svy” suffix. P value < 0.05 was considered statistically significant. Data analysis was conducted using Stata version 15.1 (StataCorp, College Station, TX, United States).
NFHS-5 was conducted in compliance with ethical guidelines and received approval from the ethics review board of the international institute of population sciences, Mumbai, India. Informed consent was obtained from all study participants, and data confidentiality was maintained throughout the analysis. We obtained the datasets through written permission from the Department of Homeland Security (DHS) which also approved the study proposal for this secondary data analysis. The NFHS-5 dataset is an anonymous publicly accessible dataset devoid of any personally identifiable information regarding the participants.
The NFHS-5 dataset included a total of 258028 adolescents aged 15-19 years. Table 1 presents the demographic characteristics of the study participants. The mean (± SD) age of the adolescents was 16.99 (1.40) years, with nearly half (50.14%) being females. More than two-thirds of the adolescents resided in rural areas (70.4%), were Hindus by religion (80%) and had secondary education (83.93%). Most of the adolescents had a normal BMI (82.74%) and did not consume tobacco (96.19%) and alcohol (98.89%).
| Variables | Males, n = 1291271 | Females, n = 1289011 | Total, n = 2580282 |
| Age in years, mean (SD) | 16.97 (1.39) | 17.00 (1.40) | 16.99 (1.40) |
| Respondents’ education, n = 257984 | |||
| No education | 4185 (43.39) | 5533 (56.61) | 9718 (3.86) |
| Primary education | 7442 (51.08) | 7315 (48.92) | 14757 (5.77) |
| Secondary education | 110891 (50.33) | 108314 (49.67) | 219205 (83.93) |
| Higher education | 6589 (46.51) | 7715 (53.49) | 14304 (6.44) |
| Religion, n = 139062 | |||
| Hindu | 12631 (12.08) | 91715 (87.92) | 104346 (79.95) |
| Muslim | 2116 (11.03) | 17549 (88.97) | 19665 (15.69) |
| Others | 1903 (12.86) | 13148 (87.14) | 15051 (4.36) |
| Residence | |||
| Urban | 31058 (52.74) | 28303 (47.26) | 59361 (29.64) |
| Rural | 98069 (48.65) | 100598 (51.35) | 198667 (70.36) |
| Wealth index | |||
| Poorest | 29734 (47.99) | 31166 (52.01) | 60900 (21.87) |
| Poorer | 30774 (48.73) | 31748 (51.27) | 62522 (22.37) |
| Middle | 26967 (49.64) | 26970 (50.36) | 53937 (20.69) |
| Richer | 22600 (50.53) | 22173 (49.47) | 44773 (18.8) |
| Richest | 19052 (53.45) | 16844 (46.55) | 35896 (16.26) |
| Tobacco consumption, n = 257580 | |||
| No | 118792 (48.36) | 126756 (51.64) | 245548 (96.19) |
| Yes | 9920 (86.28) | 2112 (13.72) | 12032 (3.81) |
| Alcohol usage, n = 257610 | |||
| No | 125356 (49.39) | 128255 (50.61) | 253611 (98.89) |
| Yes | 3380 (87.2) | 619 (12.8) | 3999 (1.11) |
| BMI, n = 134346 | |||
| Normal | 12333 (10.78) | 100459 (89.22) | 112792 (82.74) |
| Thin | 2333 (18.39) | 10600 (81.61) | 12933 (10.37) |
| Overweight/obese | 1211 (13.47) | 7410 (86.53) | 8621 (6.88) |
| Health seeking behaviour in past 3 months, n = 139058 | |||
| None | 13733 (13.01) | 91180 (86.99) | 104913 (74.07) |
| Public facility | 1629 (8.06) | 19130 (91.94) | 20759 (14.13) |
| Private facility | 1268 (10.04) | 11740 (89.96) | 13008 (11.48) |
| Other | 20 (4.44) | 358 (95.56) | 378 (0.32) |
The weighted prevalence of DM among the adolescents aged 15-19 years was estimated as per the operational definition with previously diagnosed DM observed among 0.77% (95%CI: 0.72-0.83) participants, while 0.32% (95%CI: 0.29-0.35) were newly diagnosed with DM during survey screening. Overall, 1.09% (95%CI: 1.02-1.15) of adolescents had evidence of DM, as determined by either elevated blood glucose levels, self-reported status of the disease or anti-diabetes medication usage.
A binary logistic regression analysis was performed to identify the predictors of DM among the adolescents (Table 2). Upon unadjusted analysis, increasing age, higher education levels, higher wealth index and overweight/obesity were the factors associated with significantly higher odds of having DM among the adolescents. In this study, adjusted RR (aRR) for age was 1.09 (95%CI: 1.02-1.15) indicating that for every 1-year increase in age among the adolescents, the rate of DM increases by 8%. Similarly, a higher rate of having DM was found among those having overweight/obesity (aRR = 1.85; 95%CI: 1.46-2.34).
| Variables | DM absent, n = 221556 | DM present, n = 2359 | Unadjusted RR (95%CI) | Adjusted RR (95%CI) |
| Age in years, mean (SD) | 16.98 (1.40) | 17.11 (1.40) | 1.07 (1.02-1.11)a | 1.09 (1.02-1.15)a |
| Sex | ||||
| Male | 105161 (98.87) | 1168 (1.13) | Reference | - |
| Female | 116395 (98.95) | 1191 (1.05) | 0.93 (0.83-1.04) | |
| Respondents’ education | ||||
| No education | 7785 (99.24) | 73 (0.76) | Reference | Reference |
| Primary education | 12239 (98.95) | 142 (1.05) | 1.38 (0.97-1.96) | 1.14 (0.74-1.75) |
| Secondary education | 189093 (98.91) | 1987 (1.09) | 1.43 (1.07-1.91)a | 1.31 (0.91-1.88) |
| Higher education | 12414 (98.83) | 156 (1.17) | 1.53 (1.09-2.15)a | 1.13 (0.73-1.76) |
| Religion | ||||
| Hindu | 98145 (98.95) | 1006 (1.05) | Reference | - |
| Muslim | 17820 (98.78) | 210 (1.22) | 1.17 (0.95-1.44) | |
| Others | 14248 (98.85) | 151 (1.15) | 1.10 (0.81-1.49) | |
| Residence | ||||
| Urban | 49616 (98.88) | 562 (1.12) | Reference | - |
| Rural | 171940 (98.93) | 1797 (1.07) | 0.96 (0.84-1.11) | |
| Wealth index | ||||
| Poorest | 52225 (99.05) | 486 (0.95) | Reference | Reference |
| Poorer | 54247 (98.86) | 612 (1.14) | 1.19 (1.01-1.41)a | 1.22 (0.97-1.53) |
| Middle | 47012 (98.86) | 523 (1.14) | 1.20 (1.01-1.42)a | 1.21 (0.97-1.52) |
| Richer | 38521 (98.89) | 428 (1.11) | 1.16 (0.98-1.39) | 1.11 (0.88-1.41) |
| Richest | 29551 (98.93) | 310 (1.07) | 1.12 (0.93-1.36) | 1.19 (0.92-1.54) |
| Tobacco consumption | ||||
| No | 211095 (98.92) | 2242 (1.08) | Reference | - |
| Yes | 10087 (98.67) | 115 (1.33) | 1.23 (0.96-1.57) | |
| Alcohol usage | ||||
| No | 217809 (98.91) | 2332 (1.09) | Reference | - |
| Yes | 3402 (99.12) | 25 (0.88) | 0.81 (0.47-1.38) | |
| BMI | ||||
| Normal | 110800 (99.01) | 1088 (0.99) | Reference | Reference |
| Thin | 12659 (98.78) | 153 (1.22) | 1.24 (0.99-1.56) | 1.24 (0.99-1.56) |
| Overweight/obese | 8377 (98.13) | 141 (1.87) | 1.90 (1.50-2.40)b | 1.85 (1.46-2.34)b |
| Health seeking behaviour in past 3 months | ||||
| None | 97890 (98.92) | 993 (1.08) | Reference | - |
| Public facility | 19697 (98.78) | 242 (1.22) | 1.14 (0.93-1.39) | |
| Private facility | 12261 (99.1) | 128 (0.9) | 0.84 (0.66-1.06) | |
| Other | 361 (99.4) | 4 (0.6) | 0.56 (0.18-1.73) |
Among adolescents previously diagnosed with DM (n = 1744), 60.84% (95%CI: 57.38-64.20) reported taking anti-diabetes medications to lower their blood glucose levels (Table 3). Upon unadjusted logistic regression, adolescents of higher age, of highest educational status, of middle and richer wealth indices and those utilizing a health facility in the past 3 months had significantly higher odds of not taking anti-diabetes treatment despite being previously diagnosed with the condition. Upon adjusted analysis, middle wealth index (aOR = 2.13; 95%CI: 1.20-3.78), utilization of public facility (aOR = 1.76; 95%CI: 1.14-2.71) and private facility (aOR = 1.99; 95%CI: 1.14-3.47) showed significantly higher odds of not taking anti-diabetes treatment as compared to their counterparts.
| Variables | On treatment, n = 1088 | Not on treatment, n = 656 | Unadjusted OR (95%CI) | Adjusted OR (95%CI) |
| Age in years, mean (SD) | 17.00 (1.39) | 17.24 (1.42) | 1.13 (1.03-1.24)a | 1.08 (0.95-1.24) |
| Sex | ||||
| Male | 553 (63.81) | 308 (36.19) | Reference | - |
| Female | 535 (57.93) | 348 (42.07) | 1.28 (0.98-1.67) | |
| Respondents’ education | ||||
| No education | 38 (70.58) | 16 (29.42) | Reference | Reference |
| Primary education | 66 (57.18) | 44 (42.82) | 1.80 (0.75-4.29) | 1.61 (0.53-4.88) |
| Secondary education | 922 (61.92) | 547 (38.08) | 1.48 (0.70-3.09) | 1.10 (0.44-2.78) |
| Higher education | 62 (48.58) | 48 (51.42) | 2.54 (1.07-6.04)a | 1.77 (0.59-5.32) |
| Religion | ||||
| Hindu | 445 (59.12) | 289 (40.88) | Reference | - |
| Muslim | 99 (63.13) | 54 (36.87) | 0.84 (0.48-1.47) | |
| Others | 83 (53.26) | 43 (46.74) | 1.27 (0.63-2.55) | |
| Residence | ||||
| Urban | 238 (60.22) | 172 (39.78) | Reference | - |
| Rural | 850 (61.09) | 484 (38.91) | 0.96 (0.68-1.37) | |
| Wealth index | ||||
| Poorest | 262 (69.36) | 110 (30.64) | Reference | Reference |
| Poorer | 271 (60.54) | 177 (39.46) | 1.48 (0.97-2.24) | 1.12 (0.64-1.97) |
| Middle | 225 (55.31) | 158 (44.69) | 1.83 (1.21-2.77)a | 2.13 (1.20-3.78)a |
| Richer | 183 (54.72) | 130 (45.28) | 1.87 (1.22-2.87)a | 1.45 (0.80-2.62) |
| Richest | 147 (65.45) | 81 (34.55) | 1.19 (0.75-1.90) | 1.17 (0.62-2.19) |
| Tobacco consumption | ||||
| No | 1029 (60.67) | 627 (39.33) | Reference | - |
| Yes | 57 (63.97) | 29 (36.03) | 0.87 (0.48-1.57) | |
| Alcohol usage | ||||
| No | 1075 (60.87) | 649 (39.13) | Reference | - |
| Yes | 11 (45.38) | 7 (54.62) | 1.87 (0.46-7.56) | |
| BMI | ||||
| Normal | 506 (59.10) | 311 (40.90) | Reference | - |
| Thin | 71 (60.64) | 46 (39.36) | 0.94 (0.55-1.59) | |
| Overweight/obese | 56 (59.81) | 33 (40.19) | 0.97 (0.54-1.74) | |
| Health seeking behaviour in past 3 months | ||||
| None | 484 (63.34) | 255 (36.66) | Reference | Reference |
| Public facility | 97 (48.34) | 87 (51.66) | 1.85 (1.20-2.83)a | 1.76 (1.14-2.71)a |
| Private facility | 44 (47.45) | 44 (52.55) | 1.91 (1.11-3.30)a | 1.99 (1.14-3.47)a |
| Other | 2 (100) | 0 (0) | - | - |
The weighted prevalence of obesity among the older adolescents in this study was 1.70% (95%CI: 1.58-1.82), while for overweight was 5.20% (95%CI: 5.01-5.39). A binary logistic regression analysis was performed to identify the predictors of overweight and/or obesity among the adolescents (Table 4). Upon unadjusted analysis, all the variables included in the regression model were found to be significantly associated with overweight/obesity. Upon adjusted analysis, male sex (aRR = 1.10; 95%CI: 1.01-1.20), secondary education (aRR = 1.28; 95%CI: 1.06-1.54), higher education (aRR = 1.26; 95%CI: 1.02-1.56), Muslim religious household (aRR = 1.21; 95%CI: 1.10-1.34), other (non-Hindu) religions (aRR = 1.18; 95%CI: 1.04-1.33), urban residence (aRR = 1.28; 95%CI: 1.18-1.38), increasing wealth index (aRR for richest = 3.24; 95%CI: 2.86-3.67) and undiagnosed DM (aRR = 2.43; 95%CI: 1.82-3.24) were the significant predictors of overweight/obesity among the older adolescents.
| Variables | Normal/thin, n = 125725 | Overweight/obesity, n = 8621 | Unadjusted RR (95%CI) | Adjusted RR (95%CI) |
| Age in years, mean (SD) | 16.99 (1.40) | 17.03 (1.42) | 1.02 (1.001-1.04)a | 1.01 (0.99-1.03) |
| Sex | ||||
| Male | 14666 (92.11) | 1211 (7.89) | 1.17 (1.06-1.29)a | 1.10 (1.01-1.20)a |
| Female | 111059 (93.25) | 7410 (6.75) | Reference | Reference |
| Respondents’ education | ||||
| No education | 5068 (96.24) | 213 (3.76) | Reference | Reference |
| Primary education | 7092 (95.48) | 343 (4.52) | 1.20 (0.97-1.50) | 1.08 (0.86-1.35) |
| Secondary education | 106285 (92.96) | 7397 (7.04) | 1.87 (1.57-2.24)b | 1.28 (1.06-1.54)a |
| Higher education | 7266 (91.25) | 667 (8.75) | 2.33 (1.91-2.85)b | 1.26 (1.02-1.56)a |
| Religion | ||||
| Hindu | 93663 (93.5) | 6088 (6.5) | Reference | Reference |
| Muslim | 16969 (91.78) | 1353 (8.22) | 1.27 (1.15-1.40)b | 1.21 (1.10-1.34)b |
| Others | 13385 (90.81) | 1086 (9.19) | 1.41 (1.25-1.60)b | 1.18 (1.04-1.33)a |
| Residence | ||||
| Urban | 26192 (89.28) | 3028 (10.72) | 1.97 (1.84-2.12)b | 1.28 (1.18-1.38)b |
| Rural | 99533 (94.57) | 5593 (5.43) | Reference | Reference |
| Wealth index | ||||
| Poorest | 31167 (96.82) | 1013 (3.18) | Reference | Reference |
| Poorer | 31730 (95.26) | 1615 (4.74) | 1.49 (1.33-1.67)b | 1.45 (1.29-1.63)b |
| Middle | 26511 (93.11) | 1911 (6.89) | 2.17 (1.93-2.43)b | 2.00 (1.77-2.25)b |
| Richer | 21175 (90.65) | 2001 (9.35) | 2.94 (2.63-3.29)b | 2.49 (2.22-2.80)b |
| Richest | 15142 (87.17) | 2081 (12.83) | 4.04 (3.61-4.51)b | 3.24 (2.86-3.67)b |
| Tobacco consumption | ||||
| No | 122540 (93.08) | 8446 (6.92) | Reference | Reference |
| Yes | 3108 (95.33) | 168 (4.67) | 0.67 (0.53-0.86)a | 0.96 (0.74-1.25) |
| Alcohol usage | ||||
| No | 124667 (93.1) | 8563 (6.9) | Reference | Reference |
| Yes | 992 (95.62) | 52 (4.38) | 0.64 (0.42-0.96)a | 0.78 (0.50-1.21) |
| Incident DM | ||||
| No | 124060 (93.21) | 8430 (6.79) | Reference | Reference |
| Yes | 331 (81.82) | 57 (18.18) | 2.68 (1.97-3.62)b | 2.43 (1.82-3.24)b |
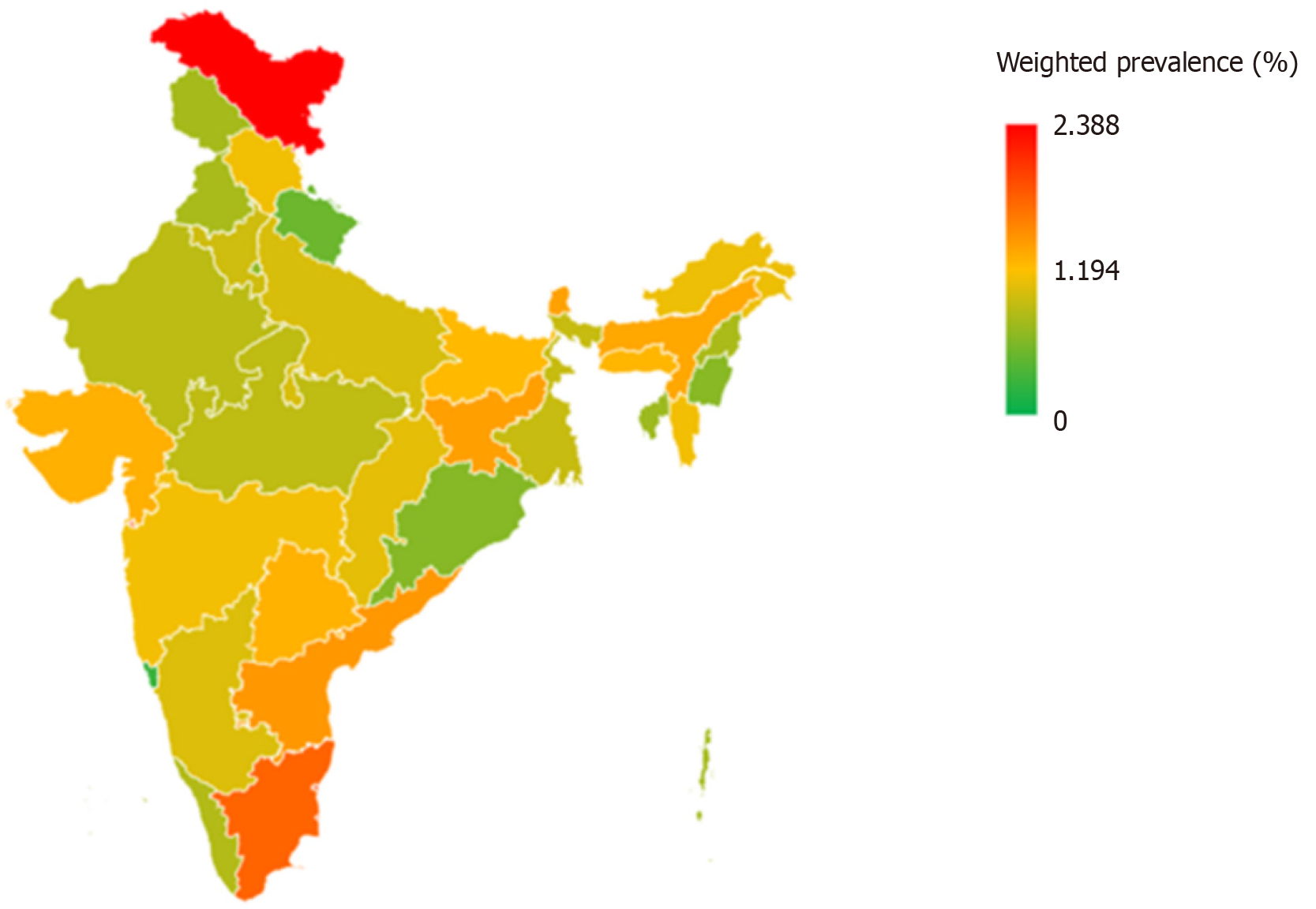
Figure 1 depicts the prevalence of DM among adolescents across various states and UTs of India. The highest prevalence was found in the UT of Ladakh (2.39%), followed by Dadra and Nagar Haveli (1.96%), Puducherry (1.94%) and the state of Tamil Nadu (1.76%). Lowest prevalence was found in Chandigarh (0.0%) and Goa (0.28%).
The present study observed a significant association with obesity/overweight and presence of DM in older adolescents in India corroborating the evidence from previous studies[16-18]. However, the prevalence of DM observed in this study (1.09%), was nearly eight times lower compared to an estimation using the glycated haemoglobin method in another large-scale national survey[16]. Furthermore, one in three adolescents with DM were undiagnosed and detected only on screening further indicative of the high burden of unrecognized DM in this vulnerable population which frequently remains asymptomatic. The study findings signify the need for screening older adolescents in India using the glycated haemoglobin method to obtain higher yields and achieve early detection to ensure timely initiation of effective anti-diabetes treatment to prevent or delay the onset of DM related microvascular and macrovascular complications[19,20].
In this study, each year increase from age of 15 onwards was associated with an 8% higher rate of DM indicating that as older adolescents transition into young adulthood, there is increased risk of developing DM particularly in the presence of overweight and obesity. Although increasing age is a well-established risk factor for DM worldwide[21], the increased risk of DM in late adolescence signifies a public health hazard that requires focused interventions for awareness generation and public health screening, strategies which are currently restricted to the > 30 age-group in the existing national program for non-communicable diseases in India[22]. Furthermore, in this study, the risk of DM was higher in the affluent adolescent subgroup possessing higher educational levels and having a higher wealth index which also correlated with their higher risk of obesity. Previously, epidemiological surveys have shown that affluent populations in long-term collaborations in low- and middle-income countries are likely to exhibit sedentary lifestyle that contribute to an increased risk of lifestyle disorders including DM[23]. The present study findings suggest a similar pattern of risk for DM may have occurrence even in the adolescent age-groups, although the absence of data on exercise and physical activity precludes estimation of the association of sedentarism on risk of DM.
The present study found that nearly 61% of the adolescents previously diagnosed with DM reported taking their prescribed anti-diabetes medications. Surprisingly, those who accessed either public or private health facilities in the past 3 months had significantly lower odds of utilizing anti-diabetes medication suggestive of missed opportunities and necessitating increased sensitization of healthcare providers for focusing on medication adherence in the adolescent patients with DM. The burden of overweight and obesity observed among adolescents in this study is also comparatively higher than estimates from the comprehensive national nutrition survey (2016-2018)[24]. We also observed male sex to be significantly associated with a higher risk of overweight/obesity compared to females, a finding consistent with previous studies conducted elsewhere[17,25,26]. Adolescents living in urban areas also had significantly higher odds of overweight and obesity suggestive of the effect of the urban environment characterized by increased accessibility to processed and unhealthy food options, coupled with sedentary lifestyles[27,28]. The strong association between DM and overweight/obesity underscores the potential deleterious long-term health consequences associated with adolescent obesity with emphasis on the need for early initiation of public health interventions to mitigate this risk.
Ensuring universal access to healthcare services in low-resource settings regardless of socioeconomic status or geographic location, is crucial in managing and mitigating the burden of DM in younger populations who are conventionally not screened for elevated blood sugar. We observed significant regional variations in the prevalence of DM across Indian states and territories suggestive of differential access and availability of healthcare services, variable efficiency of public health systems, and cultural and lifestyle factors heterogeneity contributing to the burden of obesity and hyperglycaemia in Indian adolescent populations. Consequently, tailored interventions that consider these regional differences will be instrumental in addressing this public health problem.
The strengths of the study include the large sample size and national representativeness with estimation of outcomes using standardized procedures by trained field personnel. However, the study also has certain limitations. Firstly, the cross-sectional nature of the NFHS-5 data restricts our ability to establish causality due to lack of temporal association. Longitudinal studies would enable investigating the causal pathways and confirming the directionality of these associations. Secondly, the reliance on self-reported data for certain variables, such as DM diagnosis and treatment, may introduce recall bias. Third, diagnosis of DM was based mostly on random blood sugar levels instead of glycated haemoglobin and fasting blood glucose levels, thereby reducing the sensitivity resulting in underestimation of the DM case burden. Third, the clinical relevance of the study is diminished by the small effect sizes and the inability to differentiate between type of DM, especially T1DM and T2DM cases which are distinct conditions with divergent risk factor profile and management strategies.
Based on the study’s findings, we recommend primary care physicians including paediatricians in outpatient settings should necessarily screen older adolescents with family history or those who are overweight or obese for DM using either the fasting blood glucose test or the glycated haemoglobin method. Furthermore, they should advise older adolescents to engage in regular physical activity and exercise to maintain normal body weight even in the absence of hyperglycaemia. Finally, medication adherence in older adolescents with DM should be assessed during each appointment with appropriate counselling to achieve optimal adherence.
Nearly one in hundred older adolescents in India have DM, with significantly elevated risk of the disease in overweight and obese individuals. One in three adolescents with DM remain undiagnosed, while four in 10 adolescents with previously diagnosed DM are currently not on anti-diabetes treatment. Individuals from higher wealth quintile and those from urban areas have significantly elevated risk of obesity and DM. Tailored strategies for strengthening screening, confirmation of diagnosis, and adherence to anti-diabetes therapy of adolescents with DM warrant early incorporations in India’s national health programmes.
The authors would like to thank the DHS program for providing the NFHS-5 datasets.
| 1. | WHO. Obesity and overweight. [cited September 23 2024]. Available from: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight. |
| 2. | Gurnani M, Birken C, Hamilton J. Childhood Obesity: Causes, Consequences, and Management. Pediatr Clin North Am. 2015;62:821-840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 192] [Article Influence: 19.2] [Reference Citation Analysis (1)] |
| 3. | Sahoo K, Sahoo B, Choudhury AK, Sofi NY, Kumar R, Bhadoria AS. Childhood obesity: causes and consequences. J Family Med Prim Care. 2015;4:187-192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 724] [Cited by in RCA: 977] [Article Influence: 97.7] [Reference Citation Analysis (0)] |
| 4. | He QX, Zhao L, Tong JS, Liang XY, Li RN, Zhang P, Liang XH. The impact of obesity epidemic on type 2 diabetes in children and adolescents: A systematic review and meta-analysis. Prim Care Diabetes. 2022;16:736-744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 5. | Simmonds M, Llewellyn A, Owen CG, Woolacott N. Predicting adult obesity from childhood obesity: a systematic review and meta-analysis. Obes Rev. 2016;17:95-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1440] [Cited by in RCA: 1244] [Article Influence: 138.2] [Reference Citation Analysis (0)] |
| 6. | UNICEF. Adolescent development and participation. [cited September 23 2024]. Available from: https://www.unicef.org/india/what-we-do/adolescent-development-participation. |
| 7. | Pradeepa R, Mohan V. Epidemiology of type 2 diabetes in India. Indian J Ophthalmol. 2021;69:2932-2938. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 186] [Article Influence: 46.5] [Reference Citation Analysis (0)] |
| 8. | Hu FB. Globalization of diabetes: the role of diet, lifestyle, and genes. Diabetes Care. 2011;34:1249-1257. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1153] [Cited by in RCA: 1255] [Article Influence: 89.6] [Reference Citation Analysis (0)] |
| 9. | Weisman A, Fazli GS, Johns A, Booth GL. Evolving Trends in the Epidemiology, Risk Factors, and Prevention of Type 2 Diabetes: A Review. Can J Cardiol. 2018;34:552-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 107] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 10. | Ali O. Genetics of type 2 diabetes. World J Diabetes. 2013;4:114-123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 188] [Cited by in RCA: 206] [Article Influence: 17.2] [Reference Citation Analysis (23)] |
| 11. | Atre S, Deshmukh S, Kulkarni M. Prevalence of type 2 diabetes mellitus (T2DM) in India: A systematic review (1994-2018). Diabetes Metab Syndr. 2020;14:897-906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 12. | Ranjani H, Mehreen TS, Pradeepa R, Anjana RM, Garg R, Anand K, Mohan V. Epidemiology of childhood overweight & obesity in India: A systematic review. Indian J Med Res. 2016;143:160-174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 196] [Cited by in RCA: 136] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 13. | International Institute for Population Sciences. National Family Health Survey (NFHS-5) INDIA Report. [cited September 23 2024]. Available from: https://iipsindia.ac.in/content/national-family-health-survey-nfhs-5-india-report. |
| 14. | de Onis M, Onyango AW, Borghi E, Siyam A, Nishida C, Siekmann J. Development of a WHO growth reference for school-aged children and adolescents. Bull World Health Organ. 2007;85:660-667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4196] [Cited by in RCA: 5406] [Article Influence: 318.0] [Reference Citation Analysis (0)] |
| 15. | Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159:702-706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5384] [Cited by in RCA: 7220] [Article Influence: 343.8] [Reference Citation Analysis (0)] |
| 16. | Kumar P, Srivastava S, Mishra PS, Mooss ETK. Prevalence of pre-diabetes/type 2 diabetes among adolescents (10-19 years) and its association with different measures of overweight/obesity in India: a gendered perspective. BMC Endocr Disord. 2021;21:146. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 17. | Seema S, Rohilla KK, Kalyani VC, Babbar P. Prevalence and contributing factors for adolescent obesity in present era: Cross-sectional Study. J Family Med Prim Care. 2021;10:1890-1894. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 18. | Praveen PA, Tandon N. Childhood obesity and type 2 diabetes in India. WHO South East Asia J Public Health. 2016;5:17-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 19. | Cade WT. Diabetes-related microvascular and macrovascular diseases in the physical therapy setting. Phys Ther. 2008;88:1322-1335. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 733] [Cited by in RCA: 640] [Article Influence: 37.6] [Reference Citation Analysis (0)] |
| 20. | Chawla A, Chawla R, Jaggi S. Microvasular and macrovascular complications in diabetes mellitus: Distinct or continuum? Indian J Endocrinol Metab. 2016;20:546-551. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 539] [Cited by in RCA: 606] [Article Influence: 67.3] [Reference Citation Analysis (0)] |
| 21. | GBD 2021 Diabetes Collaborators. Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: a systematic analysis for the Global Burden of Disease Study 2021. Lancet. 2023;402:203-234. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1683] [Cited by in RCA: 1763] [Article Influence: 881.5] [Reference Citation Analysis (18)] |
| 22. | Government of India Press Information Bureau. Update on treatment of Diabetes. [cited September 23 2024]. Available from: https://pib.gov.in/pib.gov.in/Pressreleaseshare.aspx?PRID=1944600. |
| 23. | Owen N, Sparling PB, Healy GN, Dunstan DW, Matthews CE. Sedentary behavior: emerging evidence for a new health risk. Mayo Clin Proc. 2010;85:1138-1141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 513] [Cited by in RCA: 579] [Article Influence: 38.6] [Reference Citation Analysis (0)] |
| 24. | Pandurangi R, Mummadi MK, Challa S, Reddy NS, Kaliaperumal V, Khadar Babu C, Telikicherla UR, Pullakandham R, Geddam JJB, Hemalatha R. Burden and Predictors of Malnutrition Among Indian Adolescents (10-19 Years): Insights From Comprehensive National Nutrition Survey Data. Front Public Health. 2022;10:877073. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 25. | Shah B, Tombeau Cost K, Fuller A, Birken CS, Anderson LN. Sex and gender differences in childhood obesity: contributing to the research agenda. BMJ Nutr Prev Health. 2020;3:387-390. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 125] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 26. | Al-Haifi AR, Al-Awadhi BA, Al-Dashti YA, Aljazzaf BH, Allafi AR, Al-Mannai MA, Al-Hazzaa HM. Prevalence of overweight and obesity among Kuwaiti adolescents and the perception of body weight by parents or friends. PLoS One. 2022;17:e0262101. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 27. | Bren d’Amour C, Pandey B, Reba M, Ahmad S, Creutzig F, Seto K. Urbanization, processed foods, and eating out in India. Global Food Security. 2020;25:100361. [RCA] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 28. | Squillacioti G, De Petris S, Bellisario V, Borgogno Mondino EC, Bono R. Urban environment and green spaces as factors influencing sedentary behaviour in school-aged children. Urban For Urban Green. 2023;88:128081. [DOI] [Full Text] |