Published online Jun 9, 2024. doi: 10.5409/wjcp.v13.i2.93138
Revised: April 9, 2024
Accepted: April 24, 2024
Published online: June 9, 2024
Processing time: 108 Days and 0.8 Hours
Transcranial direct current stimulation (tDCS) is proven to be safe in treating various neurological conditions in children and adolescents. It is also an effective method in the treatment of OCD in adults.
To assess the safety and efficacy of tDCS as an add-on therapy in drug-naive adolescents with OCD.
We studied drug-naïve adolescents with OCD, using a Children’s Yale-Brown obsessive-compulsive scale (CY-BOCS) scale to assess their condition. Both active and sham groups were given fluoxetine, and we applied cathode and anode over the supplementary motor area and deltoid for 20 min in 10 sessions. Reassessment occurred at 2, 6, and 12 wk using CY-BOCS.
Eighteen adolescents completed the study (10-active, 8-sham group). CY-BOCS scores from baseline to 12 wk reduced significantly in both groups but change at baseline to 2 wk was significant in the active group only. The mean change at 2 wk was more in the active group (11.8 ± 7.77 vs 5.25 ± 2.22, P = 0.056). Adverse effects between the groups were comparable.
tDCS is safe and well tolerated for the treatment of OCD in adolescents. However, there is a need for further studies with a larger sample population to confirm the effectiveness of tDCS as early augmentation in OCD in this population.
Core Tip: Transcranial direct current stimulation (tDCS) is a safe treatment modality in the management of obsessive compulsive disorder (OCD) in adolescents. Cathodal stimulation over the supplementary motor area produces insignificant improvement in severity of OCD than sham controls. The improvement following 10 sessions of tDCS is mostly short-lasting and usually goes off by 6 wk.
- Citation: Agrawal A, Agarwal V, Kar SK, Arya A. Transcranial direct current stimulation as early augmentation in adolescent obsessive compulsive disorder: A pilot proof-of-concept randomized control trial. World J Clin Pediatr 2024; 13(2): 93138
- URL: https://www.wjgnet.com/2219-2808/full/v13/i2/93138.htm
- DOI: https://dx.doi.org/10.5409/wjcp.v13.i2.93138
Obsessive compulsive disorder (OCD) is among the most common psychiatric disorders, with a mean age of onset of 19 years and almost a quarter of patients developing the disorder by the age of 10 years[1]. Transcranial direct current stimulation (tDCS) stimulates the brain with electrodes on the scalp. It's safe, affordable, and can activate or suppress cortical activity. Research on its effectiveness in treating OCD is limited to a few studies on adults who are treatment-resistant[2,3]. Even in such patients, it has shown promising results. There is significant heterogeneity between the studies. The electrode placement sites include the supplementary motor area (SMA), orbitofrontal cortex (OFC), and right and left dorsolateral prefrontal cortex (DLPFC)[2,4]. A systemic review of three meta-analyses of tDCS in OCD suggested cathode placement at pre-SMA and anode placement at the extra-cephalic sites as the site with better results. Pre-SMA has been shown to be an effective site for neuromodulation in OCD in repetitive transcranial magnetic stimulation (rTMS) studies as well[4]. By keeping the anode at extracephalic sites the chance of short-circuiting the current flow is reduced. Extra-cephalic sites that have previously been studied include the occipital region, neck, and deltoid. Theoretically, when using deltoid as the extracephalic site, the current will traverse through a pathway involving the striatum, which is a major brain area involved in OCD. The choice of right deltoid was made to save the cardiac electrical conduction from the ill effects of tDCS current. This electrode placement results in modulation of the majority of areas (cortical and sub-cortical) implicated in OCD[5].
tDCS use as early augmentation can lead to an earlier response in OCD with functional recovery. The aim of this randomized controlled trial (RCT) was to assess the efficacy of tDCS as an add-on therapy in drug-naive adolescents with OCD. We hypothesized that utilizing tDCS as an additional therapy with fluoxetine would result in an earlier reduction in obsessive-compulsive symptoms than using fluoxetine with sham tDCS.
This was a randomized, single-blind, sham-controlled trial conducted at a tertiary care centre in India. Adolescents between 10 and 18 years of age attending Outpatient services at King George’s Medical University, Lucknow and diagnosed with moderate OCD based on DSM-5 criteria who were drug naïve (for at least 1 month) were included[6,7]. The diagnosis of OCD and comorbidities was made using the Kiddie Schedule for Affective Disorders and Schizophrenia-Present and Lifetime Version. The Children’s Yale-Brown obsessive-compulsive scale (CY-BOCS) was used to assess the severity of OCD, and cases with a score ≥ 13 were included. Individuals with medical comorbidities requiring priority management, any other psychiatric conditions except major depressive disorder (MDD), intellectual disability, or any contraindication to tDCS were excluded. Revised Child Anxiety and Depression Scale-Hindi adaptation (RCADS-H) was applied to assess anxiety and depressive symptoms. Ethical approval was obtained from the Institutional Ethics Committee (107th ECM II B-Thesis/P16). Written informed consent from the parent/guardian and assent of the adolescent was obtained. The study was registered prospectively with the Clinical Trials Registry of India (CTRI/2021/12/039002). After which recruitment was done between January 2022 and January 2023 with follow up completed by April 2023.
The sample size was calculated through Priori analysis using “G Power: Statistical Power analyses 3.1.9.7” application[8]. Considering an effect size of 0.25, power of 80%, and drop out rate of 20%, the sample size calculated was 30. Participants attending child and adolescent psychiatry outpatient department on specified days of the week were enrolled in the study by investigators (Agarwal V, Kar SK, Arya A). Adolescents who met the inclusion criteria were enrolled in the study by the principal investigator (Agrawal A). Randomization was done using a computer-generated random number table which was generated by the co-investigator (Agarwal V) method in a 1:1 manner in true and sham groups. Blinding of allocation was ensured by giving a sham stimulation as described below. The investigator was aware of the allocation.
Stimulation was provided with tDCS device-Neurostim by Neurosoft, Russia. Two electrodes (size: 5 cm × 5 cm) were placed in the sponge (7 cm × 5 cm) to deliver current after being secured with rubber bands. The cathode was placed on the left SMA, corresponding to FC1 as per the international 10-20 EEG system[9]. During the tDCS session, a current of 2 mA was applied for 20 min with an anode on the right deltoid. The procedure was performed once daily until 10 sessions were completed, with no more than two consecutive days skipped. Adolescents were started on 20 mg of fluoxetine daily, with the dose increased to 40 mg/d after 1 wk. The treating psychiatrist adjusted the dose of fluoxetine during the follow-up period. Adolescents in the sham group were also provided with a brief stimulation after applying electrodes at the same site. Sham stimulation consisted of ramp-up and ramp-down periods of 20 s each during the start and end of the session. During the intervening 20 min, no current was passed. Such stimulation provides somatosensory effects and is recognized as the ‘gold standard’ sham tDCS method[10,11].
We gathered information from adolescents on their demographics and illness. We measured clinical symptoms, CY-BOCS, and RCADS-H scores at different intervals. CY-BOCS is an adapted version of the Yale-Brown Obsessive compulsive scale (Y-BOCS) for use in children and adolescents. Y-BOCS is the gold standard assessment scale for obsessive compulsive symptoms and is widely used in research as well as clinical practice. It has high reliability (α = 0.87) and good validity (r = 0.62)[12]. RCADS-H is the Hindi adaptation of RCADS done by Mishra et al[13]. The original scale has high reliability and moderate validity scores in all subscales except for OCD subscale[14]. To check for side effects, we used a checklist developed from a study done by Eryilmaz et al[15]. Due to coronavirus disease 2019 (COVID-19), assessments were in-person or over the phone. Any minor adverse effects were treated conservatively. We took all COVID-19 precautions.
We used IBM SPSS version 25.0 to analyze data. Descriptive statistics were performed on demographic and clinical variables. We checked for normalcy with the Shapiro-Wilk test. We compared baseline characteristics using the Fisher's exact test for categorical data and the Mann-Whitney U test for numeric variables. Repeated measures ANOVA assessed outcome measure changes. We used Hedges' g for effect size and considered P-values < 0.05 statistically significant.
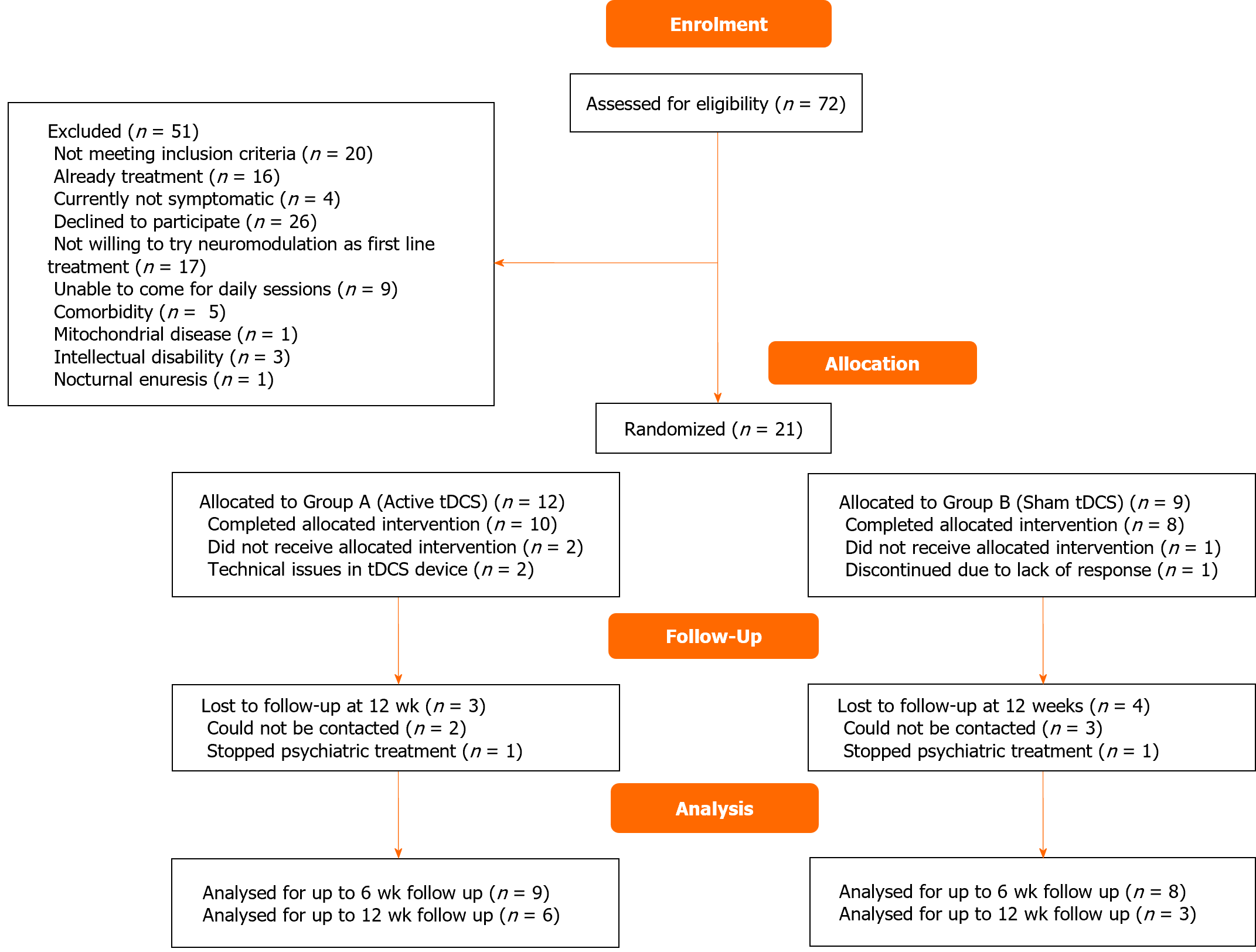
The trial was conducted in 2022 after the clinical trial registry was completed. A total of 72 adolescents with OCD were screened, out of which 51 were excluded. Finally, 21 adolescents were enrolled and randomized; 18 completed the intervention, and 9 followed up till 12 wk (Figure 1). Baseline clinical and demographic characteristics of the groups were comparable (Table 1). Among the active group, the majority had contamination-related obsessions (n = 7, 70%), washing/cleaning compulsions (n = 6, 60%). In the sham group, the majority had aggression-related obsessions (n = 6, 75%) and washing/cleaning compulsions (n = 5, 62.5%).
| Active tDCS (n = 10) | Sham tDCS (n = 8) | P value | |
| Age, yr | 14.4 ± 2.4 | 14.3 ± 2.5 | 0.96 |
| Age at onset, yr | 12.7 ± 3.6 | 12.0 ± 1.9 | 0.69 |
| Duration of symptoms, months | 19.8 ± 19.5 | 28.3 ± 29.2 | 0.68 |
| Gender, male | 5 (50) | 6 (75) | 0.28 |
| Religion, Hindu | 8 (80) | 7 (87.5) | 0.67 |
| Education | |||
| Up to high school | 8 (80) | 6 (75) | 0.8 |
| Up to intermediate | 2 (20) | 2 (25) | |
| Domicile, urban | 6 (60) | 4 (50) | 0.67 |
| Type of family, nuclear | 5 (50) | 5 (62.5) | 0.59 |
| Number of family members | |||
| ≤ 5 | 4 (40) | 5 (62.5) | 0.34 |
| > 5 | 6 (60) | 3 (37.5) | |
| Family income in Rs/month | |||
| ≤ 20000 | 4 (40) | 3 (37.5) | 0.91 |
| > 20000 | 6 (60) | 5 (62.5) | |
| Developmental delay | 2 (20) | - | |
| Past history | |||
| Hypothyroidism | 1 (10) | - | |
| OCD | 2 (20) | - | |
| Depressive episode | - | 1 (12.5) | |
| Family history of psychiatric illness, present | 2 (20) | - | |
| Co-morbidities | |||
| Excoriation | 1 (10) | - | |
| Subnormal intelligence | 1 (10) | - | |
| Major depressive disorder | - | 1 (12.5) | |
| Hospitalization | 2 (20) | - | |
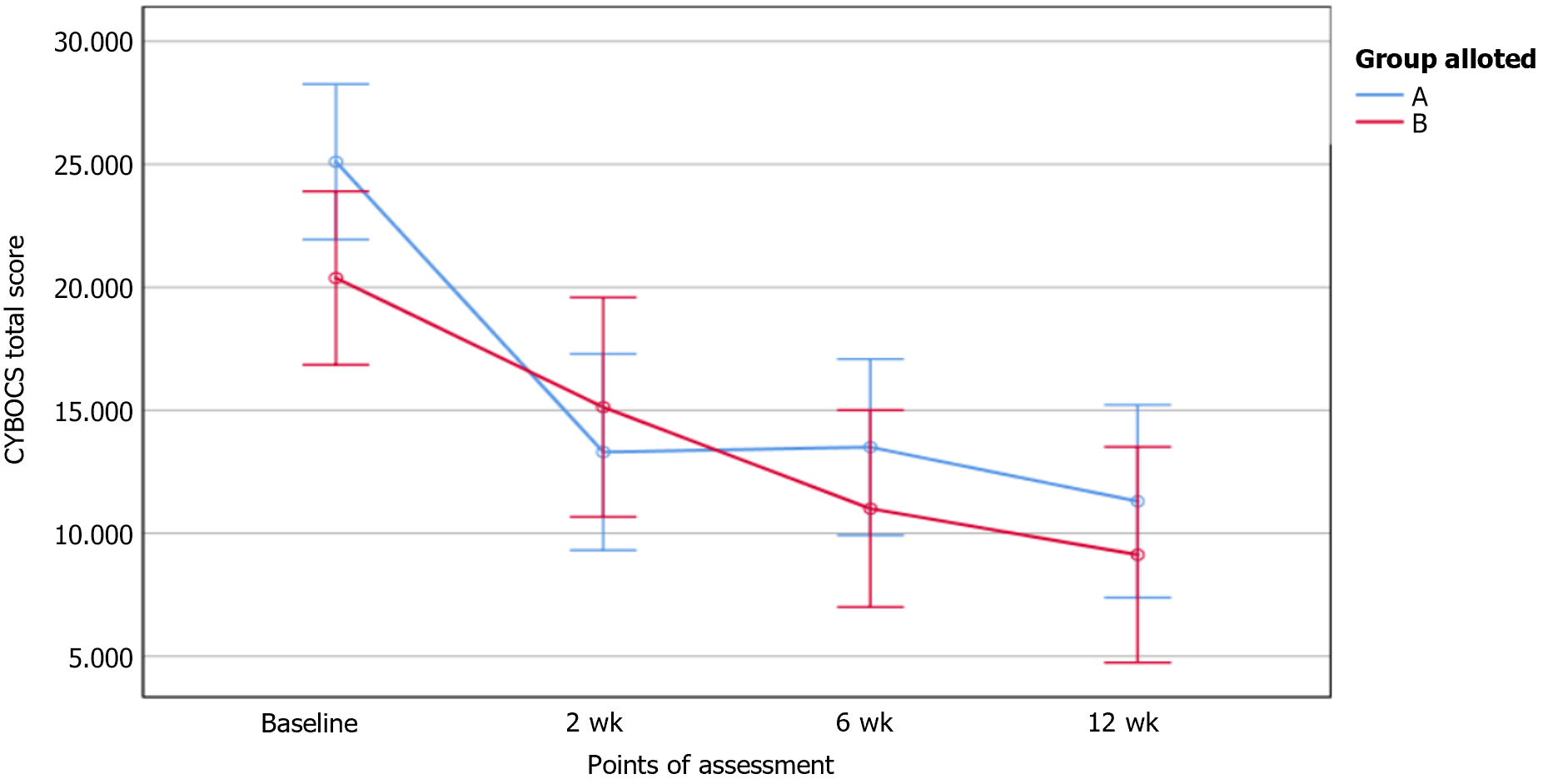
Patients who completed the entire protocol till 12 wk were included in the per protocol (PP) analysis (n = 9, active-6, sham-3). Intention to treat (ITT) analysis was done, with missing data handled using the Last Observation Carried Forward method (n = 18, active-10, sham-8). Total CY-BOCS score in ITT analyses is shown in Figure 2. The total CY-BOCS score was higher in active tDCS (M = 26.83, SD = 6.01) as compared to sham tDCS (M = 22.67, SD = 4.51) but the difference was not statistically significant. (U = 18, P = 0.056). Intergroup differences were not observed in CY-BOCS or RCADS-H scores at any point of assessment. The repeated-measures ANOVA for the CY-BOCS obsession subscale scores and total scores revealed a significant reduction with time in both groups in ITT but only in the active group in PP analysis (Table 2). Significant reduction in CY-BOCS compulsions subscale over time was present in both groups (in ITT and PP analyses).
| Clinical variables | Active tDCS group | Sham tDCS group | Time group interaction (F; P value) |
| CY-BOCS obsession score (0-20) | |||
| Baseline | |||
| ITT | 13.60 (3.06) | 11.38 (3.20) | |
| PP | 15.16 (2.92) | 11.67 (3.51) | |
| 2 wk | |||
| ITT | 7.40 (3.20) | 9.0 (3.21) | |
| PP | 6.17 (3.54) | 9.00 (3.60) | |
| 6 wk | |||
| ITT | 7.60 (2.84) | 6.50 (2.39) | |
| PP | 6.33 (2.73) | 7.67 (2.08) | |
| 12 wk | |||
| ITT | 6.20 (3.29) | 5.75 (2.19) | |
| PP | 4.00 (1.67) | 5.67 (2.08) | |
| Within the group (F; P value) | |||
| ITT | 13.42; < 0.0011 | 39.34; < 0.001 | 2.20; 0.1291 |
| PP | 29.52; 0.0011 | 9.12; 0.091 | 3.77; 0.0511 |
| CY-BOCS compulsion score (0-20) | |||
| Baseline | |||
| ITT | 11.50 (2.99) | 9.0 (3.82) | |
| PP | 11.67 (3.93) | 11.0 (1.00) | |
| 2 wk | |||
| ITT | 5.90 (4.09) | 6.12 (3.00) | |
| PP | 4.0 (4.19) | 7.0 (2.64) | |
| 6 wk | |||
| ITT | 5.90 (3.63) | 4.50 (2.92) | |
| PP | 3.83 (2.71) | 6.67 (2.51) | |
| 12 wk | |||
| ITT | 5.10 (4.07) | 3.38 (2.06) | |
| PP | 2.50 (2.42) | 3.67 (1.52) | |
| Within the group (F; P value) | |||
| ITT | 17.10; < 0.0011 | 17.83; < 0.001 | 1.44; 0.2471 |
| PP | 34.58; < 0.001 | 16.05; 0.0151 | 2.22; 0.115 |
| CY-BOCS total score (0-40) | |||
| Baseline | |||
| ITT | 25.10 (5.06) | 20.38 (4.21) | |
| PP | 26.83 (6.01) | 22.67 (4.51) | |
| 2 wk | |||
| ITT | 13.30 (6.92) | 15.12 (4.39) | |
| PP | 10.17 (7.19) | 16.00 (6.00) | |
| 6 wk | |||
| ITT | 13.50 (5.87) | 11.0 (4.57) | |
| PP | 10.17 (4.26) | 14.33 (4.16) | |
| 12 wk | |||
| ITT | 11.30 (7.18) | 9.12 (3.44) | |
| PP | 6.50 (3.62) | 9.33 (3.51) | |
| Within the group (F; P value) | |||
| ITT | 16.55; < 0.0011 | 40.91; < 0.001 | 2.13; 0.1371 |
| PP | 43.76; < 0.001 | 14.82; 0.0511 | 3.91; 0.061 |
RCADS-H subscale and total scores showed group time interaction to be significant in the MDD subscale [F (3) = 3.55, P = 0.50]. The mean reduction in CY-BOCS subscales and total scores was compared between both groups during the initial 2 wk (Table 3). Response rates at 2 wk were not significantly different [8 out of 10 in the active vs 3 out of 8 in the sham group, Fischer's exact test P = 0.145, number needed to treat = 2.35, 95%CI: (1.2, 127.2)].
| Change in C-YBOCS scores | Active tDCS (n = 10) | Sham tDCS (n = 8) | U value | P value |
| Baseline vs 2 wk | ||||
| Obsessions | 6.2 ± 4.26 | 2.37 ± 0.99 | 18 | 0.056 |
| Compulsions | 5.6 ± 3.75 | 2.87 ± 1.76 | 22 | 0.121 |
| Total | 11.8 ± 7.77 | 5.25 ± 2.22 | 18 | 0.056 |
| Baseline vs 6 wk | ||||
| Obsessions | 7.0 ± 3.90 | 4.87 ± 1.53 | 22.5 | 0.131 |
| Compulsions | 6.5 ± 3.75 | 4.5 ± 2.64 | 25 | 0.312 |
| Total | 11.6 ± 6.93 | 9.37 ± 2.87 | 28 | 0.307 |
| Baseline vs 12 wk | ||||
| Obsessions | 11.2 ± 1.66 | 9.0 ± 4.0 | 27.5 | 0.285 |
| Compulsions | 10.0 ± 2.0 | 7.5 ± 3.2 | 19 | 0.069 |
| Total | 13.8 ± 8.68 | 11.25 ± 3.34 | 29.5 | 0.373 |
No major adverse effects were recorded, and tDCS stimulation was well tolerated. 8 of 10 adolescents in active and 7 of 9 in sham group reported at least one side effect (relative risk = 1.03, number needed to harm = 45.0). For an individual session, 53.3% of active stimulations and 51.4% of sham stimulations resulted in a side effect. The maximum relative risk was for headache (2.14 for per session incidence and 1.56 for per individual incidence). Other side effects commonly reported were numbness, itching, pain at the stimulation site and sedation.
The average dose of fluoxetine and clonazepam was similar in both groups across all assessments. Adjunct medications were Risperidone 0.5 mg/d (2 in the active group, started after completion of tDCS sessions), Melatonin 3 mg (1 in the active group), and Propranolol 20 mg/d (1 in the sham group).
To the best of our knowledge, this is the first study worldwide that: (1) Has utilized tDCS as an early intervention strategy and (2) has studied tDCS in adolescents with OCD[16]. There was a significant reduction in CY-BOCS scores in both groups across the time frame assessed. Despite having a small sample size and single blinding, we did not find any significant intergroup differences in various statistical methods. A number of reasons could explain these findings, including early onset is a known risk factor for poor prognosis, biological differences in early onset OCD could be one of the key reasons. Stimulation parameters that showed positive results had tried twice-daily sessions and longer sessions of up to 30 min. Whether such changes in stimulation parameters could affect response in OCD is yet to be studied. An RCT targeting left OFC with cathodal stimulation also found non-significant changes between active and sham groups, which were controlled for single drug[17]. Another RCT with left DLPFC anodal and right OFC cathodal stimulation along with fluoxetine found non-significant differences with active and sham tDCS[18].
There were no comparator studies available for the adolescent age group. Among studies in adults with OCD, a trial by Silva et al[19] using cathodal SMA stimulation and anodal stimulation over the right supraorbital area showed a significant difference on repeated measures-ANOVA, group time interaction. However, the change in YBOCS scores was noticed in the period between 6 and 12 wk. The authors suggested a delayed effect of tDCS through neuroplasticity. Since we lost a number of patients during this period, we could have missed the delayed effects of tDCS. Multiple studies, including 3 RCTs, however, support the contrary hypothesis of acute effects of tDCS[3,17,20].
Stimulation sites on SMA have shown positive results in a number of studies other than one by Silva et al[19]. An open-label trial using cathodal tDCS over SMA and anodal stimulation over the right supraorbital area in treatment-resistant OCD reported a 26% decrease in Y-BOCS scores[20]. Even anodal stimulation at SMA has reported comparable findings in 25 adults with treatment-resistant OCD[3]. These differences arise from the use of medications, the level of resistance in patients included in the study, and other methodological issues.
A significant difference between groups was observed in the change in depressive symptoms. Comorbid depressive symptoms are common in OCD, and one RCT has reported such a change in depressive symptoms, while another one reported a non-significant reduction in depressive and anxiety symptoms[19,20].
Response in OCD is defined as ≥ 35% reduction in CY-BOCS scores and remission as CY-BOCS ≤ 12 score[21]. Our study found a high response rate of 80% at 2 wk, although the difference with the sham group was not significant. Larger trials have found lower response rates ranging between 15% and 19%[19,20]. The high response rate can be attributed to the selection of drug naïve adolescents and the exclusion of treatment-resistant cases and those with comorbidities. A trial by Yoosefee et al[18], which excluded treatment-resistant cases and used fluoxetine, reported similar response rates in active and sham groups. But they used a lower frequency of sessions (3/wk) and did not target SMA[5]. 5 of 10 adolescents in the active group attained remission, which was sustained till 12 wk. No other studies reported remission in study participants.
One of the reasons for finding no significant difference between the groups could be due to the placebo effect, which has been observed highly in studies of rTMS and tDCS[22]. Participants receive considerable information during the recruitment phase and speculate about its effects[23]. The placebo effect through caregiving, change of environment, and its neurobiological component have been seen in OCD patients as well[24]. The placebo effect in tDCS has been less in treatment-resistant cases[25]. Since the majority of the previous studies have been in such patient populations, the difference in response rates and comparable effects in sham and true groups can be explained by placebo response. Placebo and expectation effects can also change with the wording used to explain participants[26].
Another aspect is that tDCS affects long-term neuroplasticity, which takes several weeks to show its effects. This is particularly important in OCD, as a measurable response can take 8 to 12 wk[27,28]. Meanwhile, the long-term effects of tDCS, particularly on developing brains have not been studied thoroughly. Data from tDCS use in children with neurodevelopmental and motor disorders does not show any serious long-term adverse effects[29]. But long-term follow-up studies in mood and anxiety disorders in children are lacking.
Our study found that tDCS is a safe modality of treatment in adolescents with OCD. tDCS was not found to be effective in this study. Limitations of our study include a small sample size, single blinding, and not conducting twice daily or extended protocols. This highlights the need for further research with robust methodology, controlled drug use, and a larger sample size. The study was also limited by a short follow-up period; therefore, we may have missed out on the long-term effects of tDCS on adolescents, particularly in terms of development. This is another area of research in future studies.
tDCS as a modality for the treatment of OCD in the adolescent age group is safe and well tolerated. However, early intervention with tDCS, may not be effective in adolescents with OCD.
| 1. | Ruscio AM, Stein DJ, Chiu WT, Kessler RC. The epidemiology of obsessive-compulsive disorder in the National Comorbidity Survey Replication. Mol Psychiatry. 2010;15:53-63. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2013] [Cited by in RCA: 1626] [Article Influence: 108.4] [Reference Citation Analysis (1)] |
| 2. | Lefaucheur JP, Antal A, Ayache SS, Benninger DH, Brunelin J, Cogiamanian F, Cotelli M, De Ridder D, Ferrucci R, Langguth B, Marangolo P, Mylius V, Nitsche MA, Padberg F, Palm U, Poulet E, Priori A, Rossi S, Schecklmann M, Vanneste S, Ziemann U, Garcia-Larrea L, Paulus W. Evidence-based guidelines on the therapeutic use of transcranial direct current stimulation (tDCS). Clin Neurophysiol. 2017;128:56-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 829] [Cited by in RCA: 1197] [Article Influence: 133.0] [Reference Citation Analysis (0)] |
| 3. | Gowda SM, Narayanaswamy JC, Hazari N, Bose A, Chhabra H, Balachander S, Bhaskarapillai B, Shivakumar V, Venkatasubramanian G, Reddy YCJ. Efficacy of pre-supplementary motor area transcranial direct current stimulation for treatment resistant obsessive compulsive disorder: A randomized, double blinded, sham controlled trial. Brain Stimul. 2019;12:922-929. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 56] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 4. | Lefaucheur JP, Aleman A, Baeken C, Benninger DH, Brunelin J, Di Lazzaro V, Filipović SR, Grefkes C, Hasan A, Hummel FC, Jääskeläinen SK, Langguth B, Leocani L, Londero A, Nardone R, Nguyen JP, Nyffeler T, Oliveira-Maia AJ, Oliviero A, Padberg F, Palm U, Paulus W, Poulet E, Quartarone A, Rachid F, Rektorová I, Rossi S, Sahlsten H, Schecklmann M, Szekely D, Ziemann U. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS): An update (2014-2018). Clin Neurophysiol. 2020;131:474-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 501] [Cited by in RCA: 1245] [Article Influence: 249.0] [Reference Citation Analysis (0)] |
| 5. | Senço NM, Huang Y, D'Urso G, Parra LC, Bikson M, Mantovani A, Shavitt RG, Hoexter MQ, Miguel EC, Brunoni AR. Transcranial direct current stimulation in obsessive-compulsive disorder: emerging clinical evidence and considerations for optimal montage of electrodes. Expert Rev Med Devices. 2015;12:381-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 50] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 6. | American Psychiatric Association (ed). Obsessive compulsive and related disorder. In: Diagnostic and statistical manual of mental disorders: DSM-5. 5th ed. 2013; 235-264 Available from: https://www.psychiatry.org/File%20Library/Psychiatrists/Practice/DSM/APA_DSM-5-Obsessive-Compulsive-Disorder.pdf. |
| 7. | Lewin AB, Piacentini J, De Nadai AS, Jones AM, Peris TS, Geffken GR, Geller DA, Nadeau JM, Murphy TK, Storch EA. Defining clinical severity in pediatric obsessive-compulsive disorder. Psychol Assess. 2014;26:679-684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 52] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 8. | Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39:175-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26400] [Cited by in RCA: 34901] [Article Influence: 1938.9] [Reference Citation Analysis (0)] |
| 9. | Homan RW, Herman J, Purdy P. Cerebral location of international 10-20 system electrode placement. Electroencephalogr Clin Neurophysiol. 1987;66:376-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 850] [Cited by in RCA: 763] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 10. | Gandiga PC, Hummel FC, Cohen LG. Transcranial DC stimulation (tDCS): a tool for double-blind sham-controlled clinical studies in brain stimulation. Clin Neurophysiol. 2006;117:845-850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1127] [Cited by in RCA: 1271] [Article Influence: 66.9] [Reference Citation Analysis (0)] |
| 11. | Brunoni AR, Schestatsky P, Lotufo PA, Benseñor IM, Fregni F. Comparison of blinding effectiveness between sham tDCS and placebo sertraline in a 6-week major depression randomized clinical trial. Clin Neurophysiol. 2014;125:298-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 81] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 12. | Scahill L, Riddle MA, McSwiggin-Hardin M, Ort SI, King RA, Goodman WK, Cicchetti D, Leckman JF. Children's Yale-Brown Obsessive Compulsive Scale: reliability and validity. J Am Acad Child Adolesc Psychiatry. 1997;36:844-852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1416] [Cited by in RCA: 1307] [Article Influence: 46.7] [Reference Citation Analysis (0)] |
| 13. | Mishra P, Udas A, Pahwa S, Sen S, Haldar K. Adapting a Revised Child Anxiety and Depression Scale for rural India: a pilot, amenable to scale up. 2016;. [DOI] [Full Text] |
| 14. | Chorpita BF, Yim L, Moffitt C, Umemoto LA, Francis SE. Assessment of symptoms of DSM-IV anxiety and depression in children: a revised child anxiety and depression scale. Behav Res Ther. 2000;38:835-855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1074] [Cited by in RCA: 1209] [Article Influence: 48.4] [Reference Citation Analysis (0)] |
| 15. | Eryılmaz G, Sayar GH, Ünsalver BÖ, Gül IG, Özten E, Sağlam E. Adverse Effects of Transcranial Direct Current Stimulation (TDCS) in a Group of Psychiatric Patients. Sch J App Med Sci. 2014; 2: 294-297. Available from: https://www.saspublishers.com/media/articles/SJAMS_21C294-297.pdf. |
| 16. | Lee JC, Kenney-Jung DL, Blacker CJ, Doruk Camsari D, Lewis CP. Transcranial Direct Current Stimulation in Child and Adolescent Psychiatric Disorders. Child Adolesc Psychiatr Clin N Am. 2019;28:61-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 17. | Bation R, Mondino M, Le Camus F, Saoud M, Brunelin J. Transcranial direct current stimulation in patients with obsessive compulsive disorder: A randomized controlled trial. Eur Psychiatry. 2019;62:38-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 18. | Yoosefee S, Amanat M, Salehi M, Mousavi SV, Behzadmanesh J, Safary V, Yoonesi A, Salehi B. The safety and efficacy of transcranial direct current stimulation as add-on therapy to fluoxetine in obsessive-compulsive disorder: a randomized, double-blind, sham-controlled, clinical trial. BMC Psychiatry. 2020;20:570. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 19. | Silva RMFD, Brunoni AR, Goerigk S, Batistuzzo MC, Costa DLDC, Diniz JB, Padberg F, D'Urso G, Miguel EC, Shavitt RG. Efficacy and safety of transcranial direct current stimulation as an add-on treatment for obsessive-compulsive disorder: a randomized, sham-controlled trial. Neuropsychopharmacology. 2021;46:1028-1034. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 33] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 20. | Harika-Germaneau G, Heit D, Chatard A, Thirioux B, Langbour N, Jaafari N. Treating refractory obsessive-compulsive disorder with transcranial direct current stimulation: An open label study. Brain Behav. 2020;10:e01648. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 21. | Mataix-Cols D, Fernández de la Cruz L, Nordsletten AE, Lenhard F, Isomura K, Simpson HB. Towards an international expert consensus for defining treatment response, remission, recovery and relapse in obsessive-compulsive disorder. World Psychiatry. 2016;15:80-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 231] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 22. | Duecker F, Sack AT. Rethinking the role of sham TMS. Front Psychol. 2015;6:210. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 119] [Cited by in RCA: 173] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 23. | Mansur CG, Myczkowki ML, de Barros Cabral S, Sartorelli Mdo C, Bellini BB, Dias AM, Bernik MA, Marcolin MA. Placebo effect after prefrontal magnetic stimulation in the treatment of resistant obsessive-compulsive disorder: a randomized controlled trial. Int J Neuropsychopharmacol. 2011;14:1389-1397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 67] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 24. | Martinotti G, Pettorruso M, Montemitro C, Ekhtiari H, Hanlon CA, Spagnolo PA, Stein E, Di Giannantonio M. Repetitive Transcranial Magnetic Stimulation in Addiction. Dell’Osso B, Di Lorenzo G, editors. Non Invasive Brain Stimulation in Psychiatry and Clinical Neurosciences. Cham: Springer International Publishing 2020; 135-160. [DOI] [Full Text] |
| 25. | Bation R, Poulet E, Haesebaert F, Saoud M, Brunelin J. Transcranial direct current stimulation in treatment-resistant obsessive-compulsive disorder: An open-label pilot study. Prog Neuropsychopharmacol Biol Psychiatry. 2016;65:153-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 65] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 26. | Ray MK, Sylvester MD, Helton A, Pittman BR, Wagstaff LE, McRae TR 3rd, Turan B, Fontaine KR, Amthor FR, Boggiano MM. The effect of expectation on transcranial direct current stimulation (tDCS) to suppress food craving and eating in individuals with overweight and obesity. Appetite. 2019;136:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 27. | Stein DJ, Costa DLC, Lochner C, Miguel EC, Reddy YCJ, Shavitt RG, van den Heuvel OA, Simpson HB. Obsessive-compulsive disorder. Nat Rev Dis Primers. 2019;5:52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 401] [Cited by in RCA: 410] [Article Influence: 68.3] [Reference Citation Analysis (0)] |
| 28. | Knotkova H, Nitsche MA, Polania R. Transcranial Direct Current Stimulation Modulation of Neurophysiological Functional Outcomes: Neurophysiological Principles and Rationale. Knotkova H, Nitsche MA, Bikson M, Woods AJ, editors. Practical Guide to Transcranial Direct Current Stimulation. Cham: Springer International Publishing, 2019; 133-165. [DOI] [Full Text] |
| 29. | Gallop L, Westwood SJ, Lewis Y, Campbell IC, Schmidt U. Effects of transcranial direct current stimulation in children and young people with psychiatric disorders: a systematic review. Eur Child Adolesc Psychiatry. 2023;. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 6.0] [Reference Citation Analysis (0)] |