Published online Jun 9, 2024. doi: 10.5409/wjcp.v13.i2.92392
Revised: February 11, 2024
Accepted: April 12, 2024
Published online: June 9, 2024
Processing time: 135 Days and 4.1 Hours
Neonatal sepsis is defined as an infection-related condition characterized by signs and symptoms of bacteremia within the first month of life. It is the leading cause of mortality and morbidity among newborns. While several studies have been conducted in other parts of world to assess the usefulness of complete blood count parameters and hemogram-derived markers as early screening tools for neonatal sepsis, the associations between sepsis and its complications with these blood parameters are still being investigated in our setting and are not yet part of routine practice.
To evaluate the diagnostic significance of complete blood cell count hemogram-derived novel markers for neonatal sepsis among neonates attending public hospitals in the southwest region of Oromia, Ethiopia, through a case control study.
A case control study was conducted from October 2021 to October 2023 Socio
In this study, significant increases were observed in the following values in the case group compared to the control group: In white blood cell (WBC) count, neutrophils, monocyte, mean platelet volume (MPV), neutrophils to lymphocyte ratio, monocyte to lymphocyte ratio (MLR), red blood cell width to platelet count ratio (RPR), red blood width coefficient variation, MPV to RPR, and platelet to lymphocyte ratio. Regarding MLR, a cut-off value of ≥ 0.26 was found, with a sensitivity of 68%, a specificity of 95%, a positive predictive value (PPV) of 93.2%, and a negative predictive value (NPV) of 74.8%. The area under the curve (AUC) was 0.828 (P < 0.001). For WBC, a cut-off value of ≥ 11.42 was identified, with a sensitivity of 55%, a specificity of 89%, a PPV of 83.3%, and a NPV of 66.4%. The AUC was 0.81 (P < 0.001). Neutrophils had a sensitivity of 67%, a specificity of 81%, a PPV of 77.9%, and a NPV of 71.1%. The AUC was 0.801, with a cut-off value of ≥ 6.76 (P = 0.001). These results indicate that they were excellent predictors of neonatal sepsis diagnosis.
The findings of our study suggest that certain hematological parameters and hemogram-derived markers may have a potential role in the diagnosis of neonatal sepsis.
Core Tip: It is try to show the importance of complete blood cell count and hemogram-derived markers for the neonatal sepsis, which are simple and accessible relative culture especially in developing countries like Ethiopia.
- Citation: Regassa DA, Nagaash RS, Habtu BF, Haile WB. Diagnostic significance of complete blood cell count and hemogram-derived markers for neonatal sepsis at Southwest Public Hospitals, Ethiopia. World J Clin Pediatr 2024; 13(2): 92392
- URL: https://www.wjgnet.com/2219-2808/full/v13/i2/92392.htm
- DOI: https://dx.doi.org/10.5409/wjcp.v13.i2.92392
A clinical condition known as neonatal sepsis is defined by signs and symptoms of infection and the presence of bacteremia within the first month of life[1]. Neonatal sepsis is the leading cause of mortality and morbidity in newborns[1,2]. It is characterized by the body's systemic inflammatory response to infection. When this occurs within the first 28 d of life, it is referred to as "neonatal sepsis"[3]. Evaluating a baby for suspected infection in neonatal sepsis nurseries is a challenging clinical task[4]. There are two types of neonatal sepsis. Type 1 is known as early-onset sepsis (EOS), which occurs within 0-7 d. The risk factors for EOS include trans-placental, ascending, or intrapartum transmission during the perinatal period before or during birth, up to postnatal day 3[5,6].
Blood culture is the current gold standard for diagnosing newborn sepsis[7]. Prematurity problems, infection, postnatal fluid changes, late umbilical cord clamping, sampling sites, method of delivery, and sample collection timing are all typical factors that alter the neonate's hematological profile. This profile distortion may also be caused by hypothermia, hypoxia, and other prematurity-related issues[8]. Preeclampsia and intrauterine growth restriction, together with prolonged hypoxia, may increase the formation of reticulocytes while decreasing the number and total mass of megakaryocytes and blunting platelet function[9,10].
According to studies from Asia and Africa, around five million neonates die each year, with 1.6 million (20%) dying as a result of sepsis[11]. In wealthy countries, the prevalence of neonatal sepsis ranged from 1 to 5 per 1000 live births[12]. In developing countries, however, the incidence of newborn sepsis has increased from 1.8 to 18 per 1000 live births, with a death rate of 12-68 per 1000 live births[13]. Sepsis also leads to hospitalization and death. The global hospitalization rate for sepsis is predicted to be 19.4 million people, with 14.1 million only surviving hospitalization[14,15]. According to studies, neonate sepsis is a third-stage illness that causes neonatal mortality with a 1%-20% mortality rate after a premature delivery and neonatal encephalopathy (perinatal asphyxia and trauma)[16].
Current diagnostic approaches for neonatal sepsis are poor, resulting in an inability to lead clinical treatment on time, compromising its therapeutic impact. According to a report, the global newborn sepsis death rate ranged from 1.0%-5.0%[17]. According to a 2021 estimate, there were around 2797879 live births and 29608 sepsis cases in 14 middle-income nations. In the total time frame, the random-effects estimation for newborn sepsis incidence was 2824 cases per 100000 live births, with an estimated 17.6% dying[18]. In Ethiopia, the disease is still a major cause of morbidity and mortality[19,20]. The most prevalent diagnosis in the neonatal intensive care unit (NICU) is sepsis, and antibiotics are the most commonly utilized medications in the NICU. The prospects are bleak if antibiotic treatment is postponed until overt clinical symptoms appear. However, overuse of antibiotics can result in a variety of negative effects, including antibiotic resistance. Resistant infections are now responsible for three out of every ten newborn sepsis deaths[21].
Currently, one of the most difficult difficulties confronting clinicians is the early detection of neonatal sepsis[22-24]. If the diagnosis of neonatal sepsis is delayed and treatment is ineffective, it can lead to systemic problems and a high fatality risk. Although blood culture is considered the gold standard diagnostic technique for newborn sepsis, it has several limitations. These are inaccessible in the majority of impoverished nations, have technological issues, and take more than three days to see at least the first preliminary result, with a positivity yield of 30%-70%[25,26]. As a result, the left percent of neonates with sepsis could not be diagnosed by blood culture; the diagnosis of neonatal sepsis is based on clinical assessment, and management is also based on empirical treatment protocol, which usually results in unnecessary hospitalization, increased irrational use of antibiotics, and additional costs for the family[25,26]. Although platelet, lymphocyte, and neutrophil counts are analyzed in a single mode, they may serve as clinical markers of underlying infections such as sepsis and associated immune dysfunctions[27-29]. In recent years, the platelet to lymphocyte ratio (PLR), neutrophil to lymphocyte ratio (NLR), immature to total neutrophil ratio, and immature to mature neutrophil ratio have been identified as prospective markers of systemic inflammation and infectious illness prognosis[27-30].
Because newborns' defense barrier function and immune system development are still insufficient, and the condition of neonatal sepsis advances rapidly, early identification and accurate treatment are critical to lowering death. The NLR and PLR markers derived from blood analysis are now gaining attention in the study of inflammation-related disorders. Furthermore, some research suggests that the NLR and PLR, can be employed as prognostic indicators for cancer and cardiovascular disease[31,32]. Adult sepsis studies revealed that NLR might be employed as a biomarker for assessing systemic inflammation[33]. According to one study, PLR is a good measure for assessing patients' inflammatory response and disease activity[34].
Researchers and previous understandings show a progressive increase in newborn sepsis mortality, morbidity, and economic burden around the world, specifically in developing nations. Though not a replacement for blood culture, hematological parameters and hemogram-derived markers have been proposed as indicators of neonatal sepsis. Though several studies have been conducted to test the usefulness of hematological parameters and hemogram-derived novel markers in neonatal sepsis diagnosis, there is not yet used very commonly in routine practice and results vary extensively among studies. Even though assessment and use of the hemogram-derived markers have infinite considerations in the forecast of diagnosis of neonatal sepsis patients in a simple, rapid, and inexpensive manner. To the best of our knowledge, no published article is in our perspective. Therefore, the goal of this study was to assess complete blood count (CBC) parameters and hemogram-derived novel markers convenience for neonatal sepsis diagnosis among neonates admitted at the NICU of Southwest Shoa Public Hospitals, Ethiopia from October 2021 to October 2023.
A retrospective case control study was conducted at Southwest Shoa Public from October 2021 to October 2023. Tulu Bolo General Hospital and Waliso General Hospitals are the hospitals found in Oromia Regional State and are found in Southwest Shoa Zone central Ethiopia, 47 km apart, southwest of Addis Ababa.
From both hospitals, Waliso referral hospital is located in Woliso town which is the capital city of Southwest Shoa zone at 114 km in the direction of the Southwest from Addis Ababa. It has a latitude and longitude of 8 32′N 37 58′E with an elevation of 2063 m above sea level. Currently, this hospital serves around 1.1 million people by having many departments such as a clinic for patients with tuberculosis, an rapid antiretroviral therapy (ART) clinic, an adult outpatients department, NICU, under-five outpatient departments, antenatal care (ANC), postnatal care, etc.
Tulu Bolo General Hospital is located in Southwest Shoa 90 km from Addis Ababa. Tulu Bolo has a latitude and longitude of 8 40′N 38 13′E with an elevation of 2193 m or 7195 feet above sea level. Also offers chronic care, NICU, emergency services, ART services, surgical, dental, medical services, ophthalmology, pediatrics, gynecology and obstetrics, radiology, physiotherapy, pathology services, pharmacy and laboratory services, and others. Both hospitals are currently giving followup services for neonates that include ICU services, physical examinations, and laboratory services such as determination of CBC parameters, serological, and chemistry. This study obtained needed information in those hospitals' central laboratories providing hematology and immunohematology, parasitology, microbiology, clinical chemistry, and serology services.
The study population of this study consisted of neonates aged 0-28 d. The cases group who showed clinical signs and symptoms of sepsis upon admission to NICU, or who developed sepsis during their hospitalization within the same age interval. The control group consisted of neonates within the same age range who did not show any sign of sepsis. Both groups met the inclusion criteria during the study period. The case group was comprised of neonates aged 0-28 d who were diagnosed with sepsis based on the systematic inflammatory response syndrome (SIRS) criteria. These criteria included the occurrence of at least two of the following: A fever greater than 38 °C or less than 36 °C, a heart rate greater than 90 beats per minute, a respiratory rate greater than 20 breaths per minute, a partial pressure of CO2 less than 32 mmHg, a white blood cell (WBC) count greater than 12000 or less than 40000 per liter. Additionally, these neonates had stayed in the hospital’s ICU for more than 24 h.
The control group consisted of neonates aged 0-28 d who were seen in the out-patient and in-patient departments and did not meet the SIRS criteria in their medical records. These neonates had normal total leukocyte counts, and had not been diagnosed with any infectious disease.
Both the cases and control groups had CBCs taken up to 24 h before admission to the ICU, and this data was archived in the hospital’s database system. All sociodemographic and clinical data were recorded in the computerized system of the hospitals during the study period. To minimize the influence of confounding factors, neonates in both groups with a history of hematological disease, those receiving chemotherapy, glucocorticoids, or antibiotics were excluded.
Additionally, patients with inaccessible or incomplete file information, patients with genetic disease, metabolic disease, congenital heart disease, perinatal asphyxia, and neonates with congenital and chromosomal anomalies were also excluded.
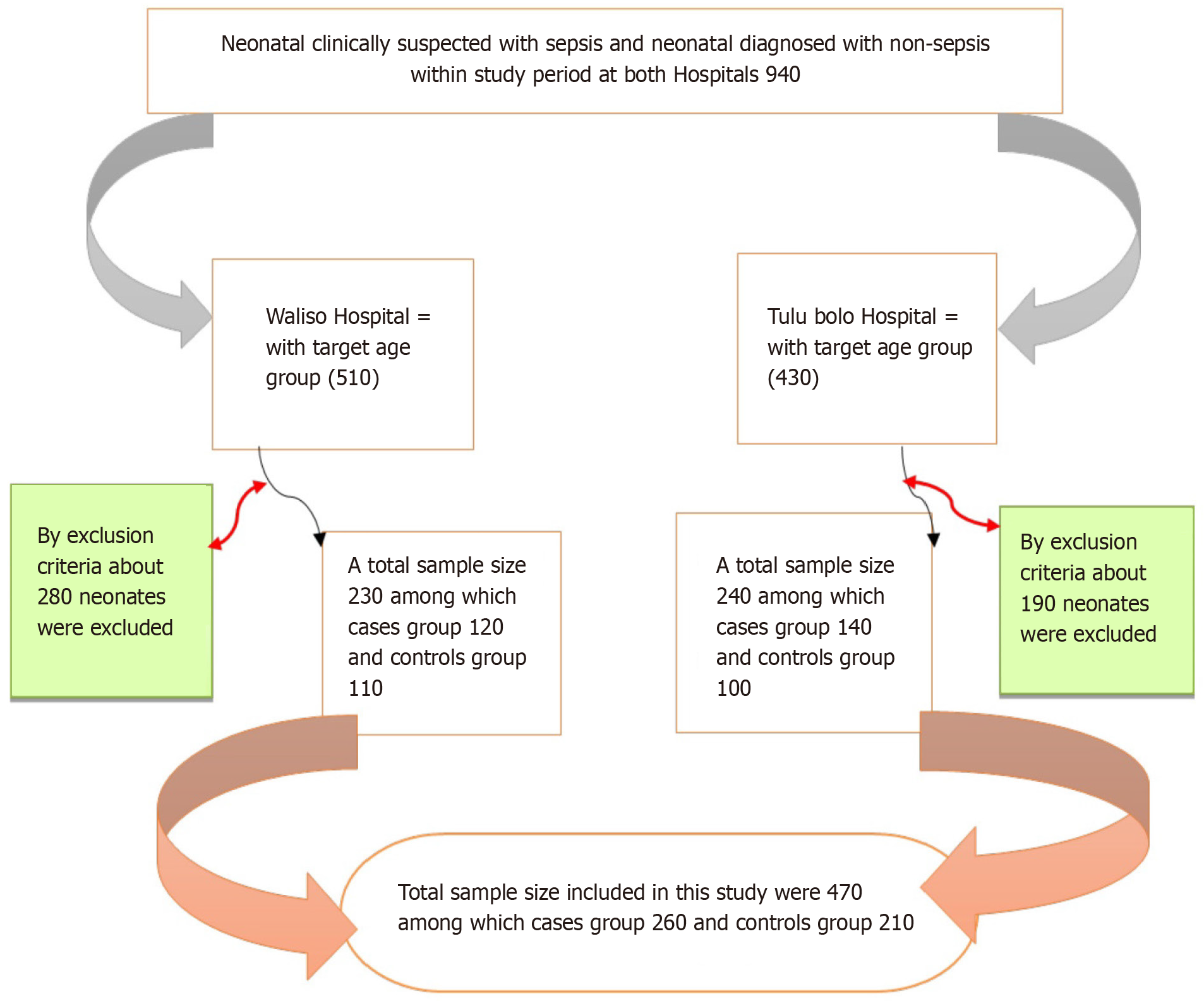
The figure shows the study participant sampling procedure and processes (Figure 1).
Patient information, including age, gender, clinical history, and vital signs (such as body temperature, heart rate, respiratory rate, and systolic and diastolic arterial pressure) was obtained from the medical record department of Southwest Public Hospitals. The cases consisted of patients who were diagnosed with sepsis based on clinical features and laboratory examination findings by a specialist doctor. The control group consisted of healthy neonates who underwent a general check-up, and had no diagnosis of infectious diseases, with normal leukocyte counts on laboratory examinations.
All hematological parameter values were obtained from archived data on a hematological analyzer. The values for NLR, monocyte to lymphocyte ratio (MLR), red blood cell (RBC) width to platelet count ratio (RPR), mean platelet volume (MPV) to platelet count ratio (MPVPCR), and PLR were calculated by the lymphocyte count, dividing the red blood distribution width by the platelet count, calculating the lymphocyte to monocyte count ratio (LMR), dividing the platelet count by the lymphocyte count, calculating the MPV to platelet count, and calculating the MLR count.
To ensure data quality, questionnaires in English were translated into the local language and then retranslated back into English to ensure accuracy and consistency. Two data collectors (two clinical nurses) received a half-day training session on study objectives, data collection procedures, and the importance of maintaining confidentiality to reduce technical and observation bias.
To ensure the quality of the socio-demographic and clinical data, daily checks were conducted by on-site supervisors to ensure completeness and consistency. Codes were used to protect the confidentiality of participants' test results, and all records were stored in a secure, and inaccessible location. Feedback and corrections were provided as necessary throughout the data collection process.
Collected data was checked for completeness and consistency, then entered into Epi-Data version 3.1 (Epi-Data Association, Denmark), and analyzed using Statistical Package for Social Sciences (SPSS) software version 25 (inclusion body myositis SPSS Statistics, United States). Histograms, Kolmogorov-Smirnov, and Shapiro tests were used to check the normality of the data distribution. Results for categorical variables were presented as frequency and percentage. Statistical differences for these variables were determined using the chi-square test.
Since continuous parameters followed a normal distribution in the goodness-of-fit model test, the independent sample t-test was used. Data was reported as mean ± standard deviation. Receiver operating characteristic (ROC) curves were constructed to determine the sensitivity, specificity, cut-off value, area under the curve (AUC), positive predictive value (PPV), and negative predictive value (NPV) of hematogram-derived markers in distinguishing neonates with sepsis from neonates without sepsis. A P-value < 0.05 was considered statistically significant.
Sociodemographic and clinical characteristics data showed that there were no significant differences in age, sex, birth weight, ANC visit, history of diarrhea, respiratory distress, reddish orogastric tube, cardiovascular disturbances, hypoglycemia, abdominal distention, and place of delivery between neonates diagnosed with sepsis and those without sepsis (P > 0.05). However, there were significant differences in the two groups in terms of the residence of mothers, blood pressure, body temperature, gestational age, mode of delivery, history of jaundice, seizure, chorioamnionitis, neonatal reflex, premature membrane rupture, and prolonged premature rupture of membrane (P < 0.05) (Table 1).
| Variables | Categories | Suspected neonatal sepsis | Control group | P value |
| Age in days (mean ± SD) | 11.48 + 8.15 | 11.36 + 8.58 | 0.834 | |
| Sex | Male | 206 (79.2) | 162 (77.1) | 0.585 |
| Female | 54 (20.8) | 48 (22.9) | ||
| Residence of mothers | Urban | 143 (55) | 95 (45.2) | 0.035 |
| Rural | 117 (45) | 115 (54.8) | ||
| Birth weight in grams | < 1500 grams | 27 (10.4) | 21 (10) | 0.979 |
| 1500-2500 grams | 109 (41.9) | 87 (41.4) | ||
| > 2500 grams | 124 (47.7) | 102 (48.6) | ||
| Blood pressure in mmHg | Systolic | 137.57 + 10.86 | 148.87 + 11.077 | < 0.001 |
| Diastolic | 96.79 + 9.29 | 105.94 + 7.672 | ||
| Body temperature in 0C | Hypothermia | 29 (11.2) | 17 (8.1) | < 0.001 |
| Fever | 210 (80.8) | 112 (53.3) | ||
| Normal | 21 (8.1) | 80 (38.1) | ||
| ANC visit | < 4 visit | 108 (41.5) | 82 (39) | 0.584 |
| > 4 visit | 152 (58.5) | 128 (61) | ||
| Gestational age | 28-32 wk | 108 (41.5) | 0 (0) | < 0.001 |
| 33-36 wk | 124 (47.7) | 61 (29) | ||
| 37-41 wk | 28 (10.8) | 149 (71) | ||
| Delivery place | Home | 38 (14.6) | 26 (12.4) | 0.483 |
| Hospital/health facilities | 222 (85.4) | 184 (87.6) | ||
| Mode of delivery | Spontaneous vaginal delivery | 8 (3.1) | 162 (77.1) | < 0.001 |
| Cesarean section | 146 (56.2) | 17 (8.1) | ||
| Forceps extraction | 106 (40.8) | 31 (14.8) | ||
| Respiratory distress | No | 104 (40) | 74 (35.2) | 0.29 |
| Yes | 156 (60) | 136 (64.8) | ||
| Reddish orogastric tube | No | 106 (40.8) | 74 (35.2) | 0.22 |
| Yes | 154 (59.2) | 136 (64.8) | ||
| Jaundice | No | 99 (38.1) | 100 (47.6) | 0.037 |
| Yes | 161 (61.9) | 110 (52.4) | ||
| Seizure | No | 107 (41.2) | 109 (51.9) | 0.02 |
| Yes | 153 (58.8) | 101 (48.1) | ||
| Diarrhea | No | 101 (38.8) | 82 (39) | 0.96 |
| Yes | 159 (61.2) | 128 (61) | ||
| Cardiovascular disturbances | No | 100 (38.5) | 74 (35.2) | 0.472 |
| Yes | 160 (61.5) | 136 (64.8) | ||
| Hypoglycemia Abdominal distention | No | 103 (39.6) | 74 (35.2) | 0.33 |
| Yes | 157 (60.4) | 136 (64.8) | ||
| Chorioamnotic | Negative | 107 (41.2) | 176 (83.8) | < 0.001 |
| Positive | 153 (58.8) | 34 (16.2) | ||
| Premature rupture membrane | Negative | 218 (83.8) | 192 (91.4) | 0.014 |
| Positive | 42 (16.2) | 18 (8.6) | ||
| Duration of premature rupture membrane | < 18h | 11 (26.2) | 13 (72.2) | 0.001 |
| > 18h | 31 (73.8) | 5 (27.8) | ||
| Neonatal reflex | Intact | 88 (33.8) | 148 (70.5) | < 0.001 |
| Depressed | 172 (66.2) | 62 (29.5) | ||
| Age at onset of sepsis | Early onset sepsis | 134 (51.5) | - | |
| Late-onset sepsis | 126 (48.5) | - | ||
| Pulse rate | 100-145 b/m | 67 (25.8) | 101 (48.1) | < 0.001 |
| 146-180 b/m | 193 (74.2) | 109 (51.9) |
High blood pressure, high body temperature, a history of jaundice, seizures, chorioamnionitis, abnormal neonatal reflex, premature membrane rupture, and prolonged premature rupture of the membrane are clinical factors that may be induced in neonates due to sepsis. Some of these factors are directly linked to the alteration of CBC values and hemogram-derived markers, while others are indirectly linked to the alteration of CBC values and hemogram-derived markers.
Regarding the hematological parameters and novel markers derived from the hemogram, there is a significant increase in the values of RBC distribution width coefficient variation (RDWCV), total white cell count, neutrophils, MPV, NLR, MLR, RPR, MPVPCR, and PLR, in the case group compared to the control group (P < 0.05). Conversely, the values of RBC count, absolute lymphocyte count, and platelet count were significantly decreased in the case group compared to the control group (P < 0.05). However, the values of absolute eosinophil count and LMR did not show significant differences between the two groups (P > 0.05) (Table 2).
| Hematological parameters and hemogram-derived markers | Suspected neonatal sepsis | Control group | P value |
| RBC | 3.58 ± 0.8 | 4.46 ± 0.61 | < 0.001 |
| RDWCV | 14.9 ± 2.6 | 13.27 ± 1.67 | < 0.001 |
| WBC | 12.58 ± 3.75 | 8.35 ± 2.77 | < 0.001 |
| NEUTR | 8.66 ± 3.05 | 5.2 ± 2.37 | < 0.001 |
| LYMPH | 1.097 ± 0.64 | 2.23 ± 1.11 | < 0.001 |
| MONO | 2.08 ± 1.59 | 0.29 ± 0.69 | < 0.001 |
| EOS | 0.67 ± 0.38 | 0.6 ± 0.35 | 0.44 |
| PLT | 189.78 ± 56.56 | 244.7 ± 95.3 | < 0.001 |
| MPV | 13.43 ± 5.3 | 10.35 ± 1.06 | < 0.001 |
| NLR | 8.29 ± 18.02 | 3.96 ± 5.59 | < 0.001 |
| PLR | 449.11 ± 996.92 | 161.2 ± 165.4 | < 0.001 |
| MLR | 6.14 ± 16.88 | 0.17 ± 0.57 | < 0.001 |
| RPR | 0.085 ± 0.03 | 0.06 ± 0.02 | < 0.001 |
| LMR | 10.59 ± 20.84 | 53.9 ± 91.36 | 0.061 |
| MPVPCR | 0.081 ± 0.05 | 0.05 ± 0.02 | < 0.001 |
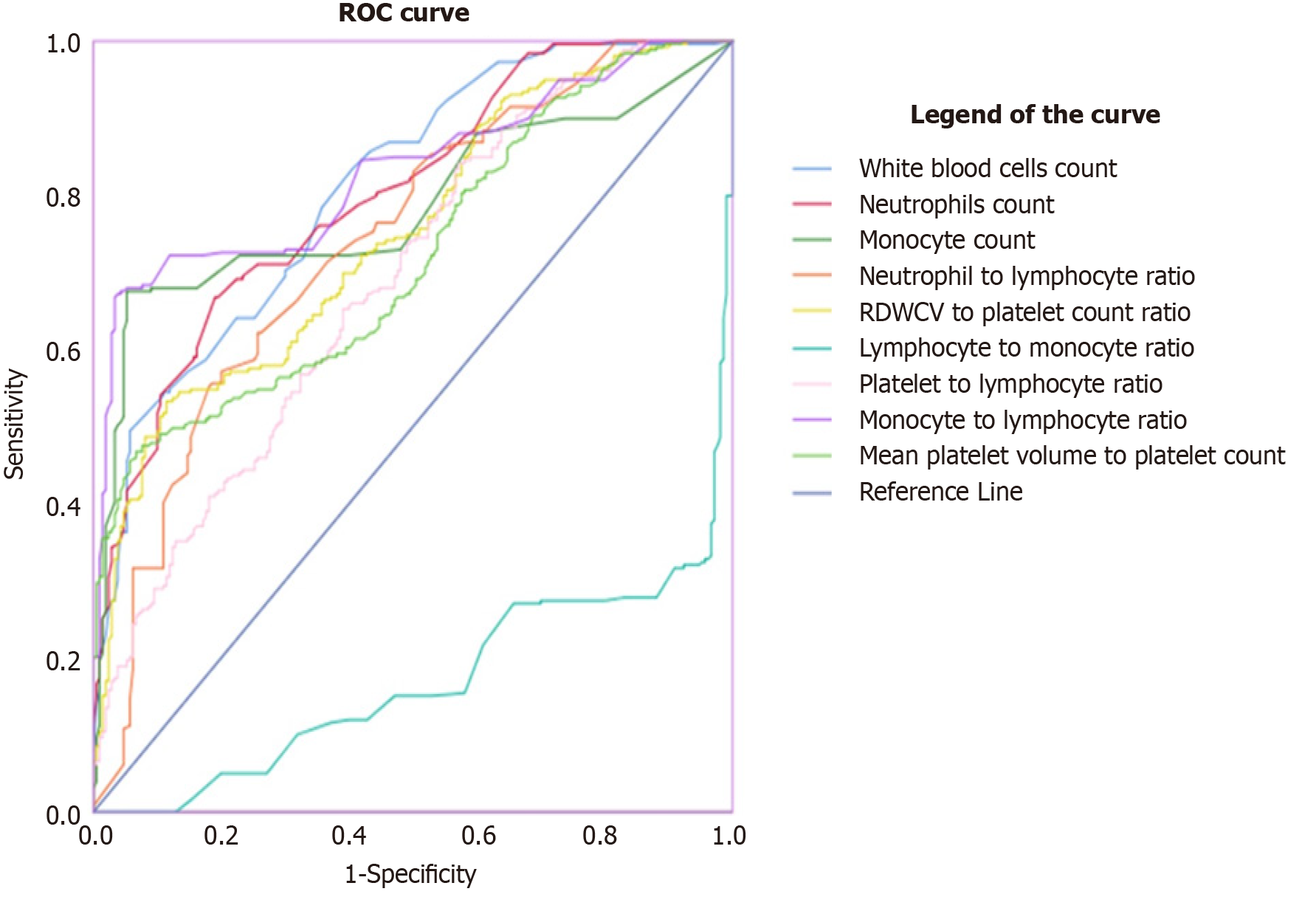
Determination of predictive values of hemogram-derived novel markers as indicators for neonate sepsis among neonates admitted at Southwest Shoa Public Hospitals, Oromia, Ethiopia, by ROC analysis.
An analysis was conducted to determine the efficacy of novel markers derived from the hemogram in predicting neonatal sepsis among neonates admitted to public hospitals in Southwest Shoa, Oromia Ethiopia. The results showed that a WBC cut-off value ≥ 11.42 had a sensitivity of 55%, specificity of 89%, PPV of 83.3%, NPV of 66.4%, and an AUC of 0.81 (P < 0.001). This indicates that WBC can differentiate between neonates suspected of having sepsis and control neonates.
Similarly, neutrophils can distinguish between neonates diagnosed with sepsis and neonates without sepsis with a sensitivity of 67%, specificity of 81%, PPV of 77.9%, NPV of 71.1%, and AUC of 0.801. The cutoff value for neutrophils is ≥ 6.76 (P = 0.001).
The MLR can also differentiate between neonates diagnosed with sepsis and neonates without sepsis. The cutoff value is ≥ 0.26 with a sensitivity of 68%, specificity of 95%, a PPV of 93.2%, NPV of 74.8%, and an AUC of 0.828 (P < 0.001).
Furthermore, the analysis revealed that the NLR can distinguish between neonates diagnosed with sepsis and those without sepsis. The cutoff value is ≥ 4.29, with a sensitivity of 57%, specificity of 80%, PPV of 74%, NPV of 65%, and AUC of 0.74 (P < 0.001). The PLR can differentiate between neonatal sepsis and non- neonatal sepsis at a cutoff value of ≥ 903.7, with a sensitivity of 90%, specificity of 100%, PPV of 100%, NPV of 90%, and an AUC of 0.69 (P < 0.001).
The RPR can distinguish between neonates diagnosed with sepsis and those without sepsis at a cut-off value of ≥ 0.051% with a sensitivity of 82%, specificity of 44%, PPV of 59.4%, NPV of 71%, and AUC of 0.586 at (P < 0.001). However, LMR was unable to differentiate between neonates diagnosed with sepsis and those without sepsis in our data from the ROC analysis (Table 3 and Figure 2).
| Hemogram derived markers | Sensitivity | Specificity | PPV | NPV | Cut-off value | AUC | 95%CI | P value |
| WBC | 55 | 89 | 83.3 | 66.4 | > 11.42 | 0.81 | (0.722-0.848) | < 0.001 |
| NEUTR | 67 | 81 | 77.9 | 71.1 | > 6.76 | 0.801 | (0.763-0.84) | < 0.001 |
| MONO | 67 | 95 | 93.1 | 74.2 | > 0.86 | 0.782 | (0.74-0.825) | < 0.001 |
| NLR | 57 | 80 | 74 | 65 | > 4.29 | 0.740 | (0.695-0.785) | < 0.001 |
| PLR | 90 | 100 | 100 | 90 | > 903.7 | 0.69 | (0.642-0.737) | < 0.001 |
| MPVPCR | 47 | 92 | 85.5 | 63.4 | > 0.187 | 0.718 | (0.673-0.764) | 0.394 |
| MLR | 68 | 95 | 93.2 | 74.8 | > 0.26 | 0.828 | (0.791-0.865) | < 0.001 |
| RPR | 82 | 44 | 59.4 | 71.0 | > 0.051 | 0.586 | (0.534-0.638) | 0.001 |
| LMR | 84 | 53 | 64.1 | 76.8 | < 0.4 | 0.169 | (0.132-0.206) | < 0.001 |
Early diagnosis and therapy are crucial in preventing morbidity and mortality caused by neonatal sepsis. However, there is no excellent biomarker available for accurately evaluated the sensitivity and specificity of diagnostic markers for neonatal sepsis including hematological parameters and hemogram-derived markers.
However, the results of these studies vary widely, the widespread attention given to the use of hemogram derived markers for predicting the diagnosis of neonatal sepsis in a simple, rapid, and inexpensive manner; to the best of our knowledge, no published article is in our perspective.
Current studies have shown that MPV is a marker of activated platelets and is associated with various inflammatory conditions such as diabetes mellitus, cardiovascular disease, peripheral artery disease, and cerebrovascular disease. Increased MPV levels are associated with a low degree of inflammatory status. The MPVPCR has been reported to be prognostic indicator of long-term mortality in several diseases, including ischemic cardiovascular diseases, sepsis, and nonalcoholic fatty liver disease. The RPR has been found to project the severity of liver fibrosis in nonalcoholic fatty liver disease[35]. According to this study, the values of RDWCV, WBC, absolute neutrophil count, and absolute monocyte count, MPV, NLR, MLR, RPR, PLR, and MPVPCR were significantly increased in the case group compared to the control group. Similar results were found in studies conducted in Turkey[28,36,37], China[34,38], Egypt[39-41], and Indonesia[33,42].
On the other hand, studies conducted in Indonesia[33], Turkey[28], Egypt[41], and China[38] found dissimilarities to our findings. This divergence may be attributed to various reasons, such as the treatment received before the CBC was conducted, the manner in which blood pressure was controlled, and the duration of acquired nephrotic syndrome in both current and previous study participants. Failure to control these risk factors may serve as sources for the rise of WBC, monocyte, neutrophil and their derivatives in nephrotic syndrome patients.
A low RBC count is a sign of sepsis, which can be caused by several mechanisms. These mechanisms include functional iron depletion, diminished erythropoietin production, infection, inflammation, RBC loss during sepsis due to pre-existing clinical conditions (such as cancer, liver disease, or renal impairment, as well as new-onset multi-organ dysfunction, especially of the liver and kidney). Disseminated intravascular coagulation (DIC), pathogen-associated hemolysis, hypo-adrenalism, and dietary insufficiency[43].
Similarly, several pathological mechanisms are thought to contribute to low platelet count such as DIC, which leads to the consumption of both platelets and coagulation factors. This paradoxically results in increased bleeding and clotting[44].
The body controls immune-mediated tissue damage by lymphocytes through apoptosis. As a result lymphocyte migration to the site of infection, lymphocytopenia is also observed in sepsis[28]. In line with this concept, our study found that, the values of RBC count, absolute lymphocyte count, and platelet count were significantly decreased in the case group compared to the control group. Consistent findings with our results have also been reported in studies conducted in China[34], Egypt[40,41], and Turkey[37,28].
On the other hand, studies conducted in Turkey[36], Indonesia[42], and Egypt[39] found an increased platelet count. This discrepancy may be due to the fact that in the previous studies the CBC conducted too late. Platelets are recognized as first-line indicators for detecting and responding pathogens, as well as for responding to injury signals in blood vessels and in the extracellular space. Information has revealed that during the initial stage of bacterial infection, there is a significant increase in the number of platelets in the bloodstream, which then deceased disproportionately[45].
Eosinopenia occurs during infection due to increased peripheral eosinophil sequestration, decreased eosinophil production, and eosinophil destruction. The increased release of corticotrophin-releasing hormone in response to inflammatory mediators such as interleukin 1 and 6, and tumor necrosis factor-alpha stimulates the pituitary gland to release adrenocorticotropic hormone, which in turn stimulates the synthesis and release of glucocorticoids that prevent the release of eosinophils from the bone marrow[46,47]. The current study states that the values of absolute eosinophil count; and LMR were not significantly different among the groups. Our study is consistent with the findings of studies conducted in Indonesia[42], Egypt[41], and Turkey[37].
Meanwhile, studies conducted in Turkey[36], and Egypt[39,40] have found that the LMR value is increased in the case group compared to the control group. This inconsistency may be due to the presence of confounding factors and differences in the lifestyle of the caregivers of the participants in the previous and current studies. Lifestyle dynamics could be an unmeasured confounding factor in this context, which could have various effects on multiple organs with cardiovascular and immune implication.
In the current study the ROC analysis showed that WBC, with a cut-off value ≥ 11.42 can differentiate neonates suspected with neonatal septicemia from the control neonates. The sensitivity was 55%, specificity was 89%, PPV was 83.3%, NPV was 66.4%, and the AUC was 0.81 (P < 0.001). Similar results have been reported in studies conducted in Egypt[42], China[48], United States[49,50], and Ethiopia[51].
In cases of severe neonatal infections, there is often an increase in the total leukocyte count. This increase may be due to the secretion of growth factors and cytokines that stimulate bone marrow production. However, virus-infected neonates typically have a normal or slightly reduced WBC count[27].
Also, absolute neutrophils were identified as predictors of neonatal sepsis, with a sensitivity of 67%, a specificity of 81%, a PPV of 77.9%, and a NPV of 71.1% with an AUC of 0.801. The cut-off value was determined to be ≥ 6.76 at significance level of P = 0.001. Similar findings were reported studies conducted in the Detroit, United States[49], Indonesia[52], California, United States[50], and Ethiopia[51].
Dynamic changes in neutrophils and lymphocytes occur during neonatal sepsis. These cells are mobilized from the bone marrow to the site of infection. The apoptosis process these cells is also affected. During sepsis, the lifespan of neutrophils increases due to decreased apoptosis, which is mediated by decreased levels of caspase3 level and activation of NF-kB. Neutrophils play a crucial role as the first-line of defense against invading microbes. They function through phagocytosis and are regulated by various factors at different stages[53].
Monocytes and lymphocytes are types of leukocyte cells that contribute to the immune system and are typically examined in a CBC. Lymphocytes play a role in the adaptive immune system. In the past, decreased lymphocyte counts, or lymphocytopenia, were used as indicators of bacteremia. In cases of sepsis and septic shock, lymphocytopenia can occur due to the migration and redistribution of lymphocyte into the lymphatic system, as well as increased lymphocyte apoptosis[54]. Monocytes, on the other hand, function as antigen-presenting cells and produce cytokines in response to infection. Infection stimulate the immune system, leading to an increase in monocytes and a decrease in lymphocytes[54,55].
As a result of this condition, the MLR is amplified. Therefore, at a cut-off value of ≥ 0.26 as identified as a potential indicators of neonatal septicemia, with a sensitivity of 68%, specificity of 95%, PPV of 93.1%, NPV of 74.8%, and an AUC of 0.828 (P < 0.001). Similar results were found in studies conducted in Indonesia[56] and United States[50].
Additionally, the ROC analysis demonstrated that the NLR can differentiate between neonates diagnosed with sepsis and those without sepsis. The cut-off value for NLR was found to be ≥ 4.29, with a sensitivity of 57%, a specificity of 80%, a PPV of 74%, and a NPV of 65%. The AUC was calculated to be 0.74, with a significance level of P < 0.001. Similar findings were reported in studies conducted in China[38,57], Egypt[39], Turkey[37,28], and California, United States[50].
Sepsis is a systemic inflammatory response disease triggered by infection, and inflammation plays a crucial role in its imitation and development. WBCs and their subpopulations are essential components of the immune system and provide defense against pathogen infections. Numerous clinical studies have shown that neutrophil counts, lymphocyte counts, and the NLR are predictive factors for sepsis. The NLR, in particular, is considered to be more reliable than absolute neutrophil or lymphocyte counts as it takes both into account. The NLR has gained significant attention due to its potential as a new risk factor for sepsis[58].
Studies have suggested that the renowned RPR is effective in human treatment for evaluating and predicting the degree of fibrosis and inflammation in several conditions, including hepatic cirrhosis, acute pancreatitis, severe burns, and acute kidney injury. It has been proposed that high RPR levels are associated with sepsis diagnosis and prognosis in adult and neonatal human patients. In all studies, non-survivors had higher RPR values than survivors. A recent study evaluated RPR as a tool to assist in diagnosing perinatal disease in neonatal thoroughbred foals. The study concluded that foals at risk of developing systemic disease had elevated RPR values[50]. The study identified a cut-off value of ≥ 0.051% with a sensitivity of 82%, specificity of 44%, PPV of 59.4%, NPV of 71% ,and an AUC of 0.586 (P < 0.001) as potential predictors of neonatal sepsis. Similar findings were reported in studies conducted in California, United States[50], and China[57].
The PLR was identified as a predictor of neonatal sepsis diagnosis with a cutoff value of 903.7, sensitivity of 90%, specificity of 100%, PPV of 100%, NPV of 90%, AUC of 0.69, and P < 0.001. Similar reports have been found in studies conducted in China[34,57], Egypt[39,40], Turkey[28,37], and Palembang[59]. Current research also indicates that platelets and lymphocytes play a critical role in the inflammatory progression. The PLR is serve as an indicators of the balance between inflammation and thrombosis. As a result, increased inflammation. Additionally, elevated platelet counts and decreased lymphocyte counts have been shown to be associated with both aggregation and inflammation, thus indicating a higher risk[60].
Our study findings suggest that certain hematological parameters and hemogram-derived marker values were significantly increased in the cases group compared to the control group. On the other hand, some hematological parameters were decreased in the cases group compared to the control group, while others showed no significant difference between the groups. Additionally, certain hematological parameters and hemogram derived markers were identified as potential indicators of neonatal sepsis.
We would like to express our gratitude to the staff members of Waliso General Hospital and Tulu Bolo General Hospital, as well as all the data collectors, study participants, and questionnaire interpreters for their invaluable support in conducting this study.
| 1. | Kliegman RM, Jenson HB, Behrman RE, Stanton BF. Infections of the neonatal. Nelson textbook of pediatrics 18th ed. United States: Philadelphia Saunders, 2007. |
| 2. | Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, Hotchkiss RS, Levy MM, Marshall JC, Martin GS, Opal SM, Rubenfeld GD, van der Poll T, Vincent JL, Angus DC. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315:801-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15803] [Cited by in RCA: 17171] [Article Influence: 1907.9] [Reference Citation Analysis (2)] |
| 3. | Ira Adams-Chapman MD, Barbara J, Stoll MD. Systemic inflammatory response syndrome. Pediatr Infect Dis. 2001;12:5-16. [RCA] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 4. | Movahedian AH, Moniri R, Mosayebi Z. Bacterial culture of neonatal sepsis. Iran J Publ Heal. 2006; 35: 84-89. Available from: https://ijph.tums.ac.ir/index.php/ijph/article/view/2155. |
| 5. | Gonzalez BE, Mercado CK, Johnson L, Brodsky NL, Bhandari V. Early markers of late-onset sepsis in premature neonates: clinical, hematological and cytokine profile. J Perinat Med. 2003;31:60-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 59] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 6. | Cohen-Wolkowiez M, Moran C, Benjamin DK, Cotten CM, Clark RH, Benjamin DK Jr, Smith PB. Early and late onset sepsis in late preterm infants. Pediatr Infect Dis J. 2009;28:1052-1056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 196] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 7. | West BA, Tabansi PN, Ugwu RO, Eneh AU. The predictive value of micro-erythrocyte sedimentation rate in neonatal sepsis in a low-resource country. Pediatr Therapeut. 2013;1-4. [DOI] [Full Text] |
| 8. | Martin RJ, Fanaroff AA, Michele C. Diseases of the fetus and infant. Walsh, Fanaroff and Martin's neonatal-perinatal medicine 10th edition. United States: Philadelphia Elsevier/Saunders, 2015: 11-43. |
| 9. | Bonstein L, Haddad N. Taking a wider view on fetal/neonatal alloimmune thrombocytopenia. Thromb Res. 2017;151 Suppl 1:S100-S102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 10. | Tyagi T, Ahmad S, Gupta N, Sahu A, Ahmad Y, Nair V, Chatterjee T, Bajaj N, Sengupta S, Ganju L, Singh SB, Ashraf MZ. Altered expression of platelet proteins and calpain activity mediate hypoxia-induced prothrombotic phenotype. Blood. 2014;123:1250-1260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 103] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 11. | Aurangzeb B, Hameed A. Neonatal sepsis in hospital-born babies: bacterial isolates and antibiotic susceptibility patterns. J Coll Physicians Surg Pak. 2003;13:629-632. [PubMed] |
| 12. | Ahmed Z, Ghafoor T, Waqar T, Ali S, Aziz S, Mahmud S. Diagnostic value of C- reactive protein and haematological parameters in neonatal sepsis. J Coll Physicians Surg Pak. 2005;15:152-156. [PubMed] |
| 13. | Watson RS, Carcillo JA, Linde-Zwirble WT, Clermont G, Lidicker J, Angus DC. The epidemiology of severe sepsis in children in the United States. Am J Respir Crit Care Med. 2003;167:695-701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 569] [Cited by in RCA: 554] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 14. | Prescott HC, Calfee CS, Thompson BT, Angus DC, Liu VX. Toward Smarter Lumping and Smarter Splitting: Rethinking Strategies for Sepsis and Acute Respiratory Distress Syndrome Clinical Trial Design. Am J Respir Crit Care Med. 2016;194:147-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 247] [Article Influence: 30.9] [Reference Citation Analysis (0)] |
| 15. | Kaukonen KM, Bailey M, Pilcher D, Cooper DJ, Bellomo R. Systemic inflammatory response syndrome criteria in defining severe sepsis. N Engl J Med. 2015;372:1629-1638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 672] [Cited by in RCA: 763] [Article Influence: 76.3] [Reference Citation Analysis (0)] |
| 16. | GBD 2015 Mortality and Causes of Death Collaborators. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388:1459-1544. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4683] [Cited by in RCA: 4356] [Article Influence: 484.0] [Reference Citation Analysis (1)] |
| 17. | Oza S, Lawn JE, Hogan DR, Mathers C, Cousens SN. Neonatal cause-of-death estimates for the early and late neonatal periods for 194 countries: 2000-2013. Bull World Health Organ. 2015;93:19-28. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 200] [Cited by in RCA: 260] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 18. | Fleischmann C, Reichert F, Cassini A, Horner R, Harder T, Markwart R, Tröndle M, Savova Y, Kissoon N, Schlattmann P, Reinhart K, Allegranzi B, Eckmanns T. Global incidence and mortality of neonatal sepsis: a systematic review and meta-analysis. Arch Dis Child. 2021;106:745-752. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 190] [Cited by in RCA: 214] [Article Influence: 53.5] [Reference Citation Analysis (0)] |
| 19. | Shitaye D, Asrat D, Woldeamanuel Y, Worku B. Risk factors and etiology of neonatal sepsis in Tikur Anbessa University Hospital, Ethiopia. Ethiop Med J. 2010;48:11-21. [PubMed] |
| 20. | Ghiorghis B. Neonatal sepsis in Addis Ababa, Ethiopia: a review of 151 bacteremic neonates. Ethiop Med J. 1997;35:169-176. [PubMed] |
| 21. | Laxminarayan R, Matsoso P, Pant S, Brower C, Røttingen JA, Klugman K, Davies S. Access to effective antimicrobials: a worldwide challenge. Lancet. 2016;387:168-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 692] [Cited by in RCA: 809] [Article Influence: 89.9] [Reference Citation Analysis (0)] |
| 22. | Shehab El-Din EM, El-Sokkary MM, Bassiouny MR, Hassan R. Epidemiology of Neonatal Sepsis and Implicated Pathogens: A Study from Egypt. Biomed Res Int. 2015;2015:509484. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 102] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 23. | Chauhan SB, Vaghasia V, Chauhan BB. C-reactive protein (crp) in early diagnosis of neonatal septicemia. NJMR. 2012; 2: 276-278. Available from: https://njmr.in/index.php/file/article/view/784. |
| 24. | Caldas JP, Marba ST, Blotta MH, Calil R, Morais SS, Oliveira RT. Accuracy of white blood cell count, C-reactive protein, interleukin-6 and tumor necrosis factor alpha for diagnosing late neonatal sepsis. J Pediatr (Rio J). 2008;84:536-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 25. | Wienberg GA, D’Angio C. Chapter 36-Laboratory Aids for Diagnosis of Neonatal Sepsis. Infectious Diseases of the Fetus and Newborn Infant. 6th ed. United States: Philadelphia Saunders, 2006: 1207-1222. |
| 26. | Sucilathangam G, Amuthavalli K, Velvizhi G, Ashihabegum MA, Jeyamurugan T, Palaniappan N. Early diagnostic markers for neonatal sepsis: Comparing procalcitonin (PCT) and C-reactive protein (CRP). JCDR. 2012;6:627-631. [DOI] [Full Text] |
| 27. | Ognean ML, Boicean A, Sular F CM. Complete blood count and differential diagnosis of early-onset neonatal sepsis. Rev Rom Med Lab. 2017;25:1-9. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 28. | Can E, Hamilcikan Ş, Can C. The Value of Neutrophil to Lymphocyte Ratio and Platelet to Lymphocyte Ratio for Detecting Early-onset Neonatal Sepsis. J Pediatr Hematol Oncol. 2018;40:e229-e232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 60] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 29. | Russell CD, Parajuli A, Gale HJ, Bulteel NS, Schuetz P, de Jager CPC, Loonen AJM, Merekoulias GI, Baillie JK. The utility of peripheral blood leucocyte ratios as biomarkers in infectious diseases: A systematic review and meta-analysis. J Infect. 2019;78:339-348. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 139] [Cited by in RCA: 167] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 30. | Spoto S, Lupoi DM, Valeriani E, Fogolari M, Locorriere L, Beretta Anguissola G, Battifoglia G, Caputo D, Coppola A, Costantino S, Ciccozzi M, Angeletti S. Diagnostic Accuracy and Prognostic Value of Neutrophil-to-Lymphocyte and Platelet-to-Lymphocyte Ratios in Septic Patients outside the Intensive Care Unit. Medicina (Kaunas). 2021;57. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 31. | Stojkovic Lalosevic M, Pavlovic Markovic A, Stankovic S, Stojkovic M, Dimitrijevic I, Radoman Vujacic I, Lalic D, Milovanovic T, Dumic I, Krivokapic Z. Combined Diagnostic Efficacy of Neutrophil-to-Lymphocyte Ratio (NLR), Platelet-to-Lymphocyte Ratio (PLR), and Mean Platelet Volume (MPV) as Biomarkers of Systemic Inflammation in the Diagnosis of Colorectal Cancer. Dis Markers. 2019;2019:6036979. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 112] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 32. | Qin B, Ma N, Tang Q, Wei T, Yang M, Fu H, Hu Z, Liang Y, Yang Z, Zhong R. Neutrophil to lymphocyte ratio (NLR) and platelet to lymphocyte ratio (PLR) were useful markers in assessment of inflammatory response and disease activity in SLE patients. Mod Rheumatol. 2016;26:372-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 274] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 33. | Djordjevic D, Rondovic G, Surbatovic M, Stanojevic I, Udovicic I, Andjelic T, Zeba S, Milosavljevic S, Stankovic N, Abazovic D, Jevdjic J, Vojvodic D. Neutrophil-to-Lymphocyte Ratio, Monocyte-to-Lymphocyte Ratio, Platelet-to-Lymphocyte Ratio, and Mean Platelet Volume-to-Platelet Count Ratio as Biomarkers in Critically Ill and Injured Patients: Which Ratio to Choose to Predict Outcome and Nature of Bacteremia? Mediators Inflamm. 2018;2018:3758068. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 176] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 34. | Zhang S, Luan X, Zhang W, Jin Z. Platelet-to-Lymphocyte and Neutrophil-to-Lymphocyte Ratio as Predictive Biomarkers for Early-onset Neonatal Sepsis. J Coll Physicians Surg Pak. 2021;31:821-824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 35. | Tekin YK, Tekin G. Mean Platelet Volume-to-Platelet Count Ratio, Mean Platelet Volume-to-Lymphocyte Ratio, and Red Blood Cell Distribution Width-Platelet Count Ratio as Markers of Inflammation in Patients with Ascending Thoracic Aortic Aneurysm. Braz J Cardiovasc Surg. 2020;35:175-180. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 36. | Kurt A, Tosun MS, Altuntaş N. Diagnostic accuracy of complete blood cell count and neutrophil-to-lymphocyte, lymphocyte-to-monocyte, and platelet-to-lymphocyte ratios for neonatal infection. Asian Biomed (Res Rev News). 2022;16:43-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 37. | Arcagok BC, Karabulut B. Platelet to Lymphocyte Ratio in Neonates: A Predictor of Early onset Neonatal Sepsis. Mediterr J Hematol Infect Dis. 2019;11:e2019055. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 38. | Li T, Dong G, Zhang M, Xu Z, Hu Y, Xie B, Wang Y, Xu B. Association of Neutrophil-Lymphocyte Ratio and the Presence of Neonatal Sepsis. J Immunol Res. 2020;2020:7650713. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 39. | Soliman EAG, Hassan MA, Kassem EA, Bashir MA. Diagnostic Values of Platelet to Lymphocyte Ratio and Neutrophil to Lymphocyte Ratio in the Early Diagnosis of Early-Onset Neonatal Sepsis in Full-term Newborns. IJMA. 2022;4:2412-2419. [DOI] [Full Text] |
| 40. | Nasser MM, Afia AA, EL-Khatib GZ, Ibrahim MI. The value of neutrophil to lymphocyte ratio platelet to lymphocyte ratio and procalcitonin level for detecting early-onset neonatal sepsis. Al-Azhar J Ped.. 2020;2:852-874. [DOI] [Full Text] |
| 41. | Ashoura R, Waaela Z, Rashwan NI, Fayed HM. The pattern of systemic inflammatory markers response in neonatal sepsis. SVU-IJMS. 2022;5:252-260. [DOI] [Full Text] |
| 42. | Sekarhandini P, Hidayah D, Moelyo AG. Diagnostic Value of Rodwell Hematological Scoring System Compared to Neutrophil Lymphocyte Count Ratio (NLCR) in Diagnosing Early Onset Neonatal Sepsis. Asia Pac J Paediatr Child Heal. 2020; 3: 119–125. Available from: https://pediatricfkuns.ac.id/publikasi/diagnostic-value-rodwell-hematological-scoring-system-compared-neutrophil-lymphocyte-count. |
| 43. | Vincent JL, Baron JF, Reinhart K, Gattinoni L, Thijs L, Webb A, Meier-Hellmann A, Nollet G, Peres-Bota D; ABC (Anemia and Blood Transfusion in Critical Care) Investigators. Anemia and blood transfusion in critically ill patients. JAMA. 2002;288:1499-1507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1203] [Cited by in RCA: 1164] [Article Influence: 50.6] [Reference Citation Analysis (0)] |
| 44. | Levi M, Toh CH, Thachil J, Watson HG. Guidelines for the diagnosis and management of disseminated intravascular coagulation. British Committee for Standards in Haematology. Br J Haematol. 2009;145:24-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 712] [Cited by in RCA: 690] [Article Influence: 43.1] [Reference Citation Analysis (0)] |
| 45. | Mathews S, Rajan A, Soans ST. Prognostic value of rise in neutrophil to lymphocyte ratio (NLR) and platelet to lymphocyte ratio (PLR) in predicting the mortality in pediatric intensive care. Int J Contemp Pediatr. 2019;6:1052-1058. [DOI] [Full Text] |
| 46. | Bagus E, Kahar H, Wardhani P. Diagnostic values of immature granulocytes, eosinopenia, and I/T ratio in the detection of early-onset neonatal sepsis in neonates with bacterial infection risk. Folia Med. 2014; 50: 43–47. Available from: http://repository.unair.ac.id/id/eprint/127478. |
| 47. | Wilar R. Diagnostic value of eosinopenia and neutrophil to lymphocyte ratio on early onset neonatal sepsis. Korean J Pediatr. 2019;62:217-223. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 48. | Hao YX, Yu JL. Cut-off value of white blood cell count in the diagnosis of early-onset sepsis in neonates. Zhongguo Dang Dai Er Ke Za Zhi. 2020;22:1159-1163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 49. | Romero R, Savasan ZA, Chaiworapongsa T, Berry SM, Kusanovic JP, Hassan SS, Yoon BH, Edwin S, Mazor M. Hematologic profile of the fetus with systemic inflammatory response syndrome. J Perinat Med. 2011;40:19-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 50. | Scalco R, de Oliveira GN, da Rosa Curcio B, Wooten M, Magdesian KG, Hidai ST, Pandit P, Aleman M. Red blood cell distribution width to platelet ratio in neonatal foals with sepsis. J Vet Intern Med. 2023;37:1552-1560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 51. | Sorsa A. Diagnostic Significance of White Blood Cell Count and C-Reactive Protein in Neonatal Sepsis; Asella Referral Hospital, South East Ethiopia. Open Microbiol J. 2018;12:209-217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 52. | Harmansyah H, Alasiry E, Daud D. Absolute Neutrophil Count as Predictor of Early Onset Sepsis. Clin Med Res. 2015;4:87-91. [DOI] [Full Text] |
| 53. | Hotchkiss RS, Monneret G, Payen D. Sepsis-induced immunosuppression: from cellular dysfunctions to immunotherapy. Nat Rev Immunol. 2013;13:862-874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1711] [Cited by in RCA: 1818] [Article Influence: 151.5] [Reference Citation Analysis (0)] |
| 54. | de Jager CP, van Wijk PT, Mathoera RB, de Jongh-Leuvenink J, van der Poll T, Wever PC. Lymphocytopenia and neutrophil-lymphocyte count ratio predict bacteremia better than conventional infection markers in an emergency care unit. Crit Care. 2010;14:R192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 312] [Cited by in RCA: 425] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 55. | Siahaan AE, Silaen JC SLG. Gambaran Profil Hematologi Dalam 24 Jam Pertama Pada Pasien Sepsis Di Unit Neonatus RSUD Dr. Pirngadi Medan Tahun 2017-2018. Nommensen J Med. 2021;6:44-48. [DOI] [Full Text] |
| 56. | Adoe DN, Kardana IM. Validity of eosinophil count and monocyte-lymphocyte ratio for early detection of neonatal sepsis. GSCARR. 2021;8:30-37. [DOI] [Full Text] |
| 57. | Wu J, Huang L, He H, Zhao Y, Niu D, Lyu J. Red Cell Distribution Width to Platelet Ratio Is Associated with Increasing In-Hospital Mortality in Critically Ill Patients with Acute Kidney Injury. Dis Markers. 2022;2022:4802702. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 58. | Sumitro KR, Utomo MT, Widodo ADW. Neutrophil-to-Lymphocyte Ratio as an Alternative Marker of Neonatal Sepsis in Developing Countries. Oman Med J. 2021;36:e214. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 59. | Rizal TS, Irwanto FH, Zainal R, Saleh MI. Correlation of Platelet-Lymphocyte Ratio (PLR) as 28-Day Sepsis Mortality Predictor in Intensive Care Unit of RSMH Palembang. J Anesth And Clin Res 2020. 1:43-62. [DOI] [Full Text] |
| 60. | Wang Z, Peng S, Wang A, Xie H, Guo L, Jiang N, Niu Y. Platelet-lymphocyte ratio acts as an independent predictor of prognosis in patients with renal cell carcinoma. Clin Chim Acta. 2018;480:166-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |